Abstract
Background:
The aim of this study was to investigate the effect of Hemiscorpius lepturus venom on leukocytes and the leukocyte subgroups in peripheral blood of rat.
Methods:
In this experimental study, sixty N-Mari rats were divided into three groups of 20 rats. Then the rats in each group were divided into four subgroups based on the blood sampling time that was 2, 6, 24 and 48 hours after the venom injection, respectively. The control group did not receive anything, however, the first and the second experimental groups received 0.1 and 0.01mg/kg of venom, subcutaneously. In accordance with a designated four sampling times, the blood sampling was carried out in three groups. After RBC lysis, the leukocytes and leukocyte sub-populations were determined and counted using appropriate hematological standard methods.
Results:
The leukocyte and the neutrophil count at two (P<0.05), six (P<0.01) and 24 (P<0.05) hours after the venom injection showed a significant decline compared with the control group, this decrease was significant at the dose of 0.1 mg/kg until 48 hours after the venom injection (P<0.05). The lymphocyte count showed a significant decline throughout the all hours of the experiment, compared with the control group (P<0.05).
Conclusion:
Leukocytes are probably affected by the cytotoxicity effect of the H. lepturus venom in a dose-dependent manner. This could be a wakeup call for the medical staff to perform quick and accurate treatment in the least time possible.
Keywords: Hemiscorpius lepturus, Venom, Leucocyte, Rat
Introduction
Scorpion envenomation is one of the main problems in the public health system in many countries in the world. This involves 2.3 billion inhabitants in the areas with the scorpion sting threat (Chippaux et al. 2008). In 2008, the annual incidence of scorpion stings was 1.200.000 leading to 3250 deaths (Chip-paux et al. 2008, Khoobdel et al. 2013). This number demonstrated a relative growth in a report released in 2012 and exceeded than annually 1,500,000 scorpion stings, however, the mortality rate due to the scorpion stings showed a significant decrease and fell into 2600 deaths per year (Jean-Philippe 2012).
The highest number of scorpion sting in the world has been allocated to Iran after Mexico (Osnaya-Romero et al. 2001, Dehghani and Fathi 2012). The annually 42,500 scorpion stings and 20 following deaths have been reported from 2001 to 2009, in Iran (Celis et al. 2007). In the Middle East, among the 52 known species of scorpions, the most dangerous scorpions are reported in Iran (Celis et al. 2007). The scorpion sting has been reported from all of the provinces of Iran, however, the most common incidence rates have been detected in Khuzestan, with incidences of 541 per 100000 individuals (Dehghani et al. 2009, Rafizadeh et al. 2014).
Hemiscorpius lepturus belongs to the Hemiscorpiidae family, and is the most medically important and a dangerous scorpion in Khuzestan, Iran and the world (Shahbazzadeh et al. 2007). Hemiscorpius lepturus has been responsible for 15 % of the scorpion sting bite cases, however, it is the leading cause of 89 % of deaths followed by the scorpion sting (Pipelzadeh et al. 2007). The lethality arising from this scorpion is approximately 60 times higher than the average for the remaining venomous scorpion stings in the region (Pipelzadeh et al. 2007).
The venom of H. lepturus leads to acute renal failure, thrombocytopenia and microangiopathic hemolytic anemia, known as the nephrotoxic, hepatotoxic and hemolytic complications of the scorpion venom (Valavi et al. 2008). Envenomation by H. lepturus is characterized by various local and systemic signs. The local signs vary from erythema to necrosis, while the patient feels no pain. On the other hand, the nephrotoxicity is the most important systemic complication that if left untreated could result in severe renal, cardiac and pulmonary failure (Pipelzadeh et al. 2007). Consequently, it may damage the intestinal lamina propria (Mojgan Heidarpour et al. 2011). The venom may induce severe pathological damages in target organs such as skin, blood cells, central nervous system (CNS), and cardiovascular system (Seyedian et al. 2010). It leads to an increase in liver enzymes Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT) and Alkaline Phosphatase (ALP), indicating the severe hepatic damage (Pipelzadeh et al. 2006). Khodadadi et al. showed that the H. lepturus causes more RBC lysis and LDH increase, comparing with the complications of envenomation resulting from the stings of two other species of scorpions, Mesobuthus eupeus and Androctonus crassicauda (Khodadadi et al. 2012).
During a five-year study performed in Ahvaz, it was described that 90 % of patients whom were admitted in hospitals due to the general condition worsening, were stung by H. lepturus (Mir Dehghan et al. 2001).
The immune system cells are distributed throughout the body, from the outer most points to the deepest organs and tissues, such as blood, bone marrow, thymus and spleen. Despite this diversity, the major group of immune cells is the peripheral blood leukocytes. These cells apply a comprehen-sive supervision on different organs and tissues through the regular circulation from blood to lymph, from inside of the vessels to the outside and from the interior of the organs to the blood, resulting in protection of the body against the pathological factors (Vodjgani 2012). The results of an experimental study aiming the investigation of the effect of envenomation by H. lepturus on the hematological indices three days after injection of the venom suggested that the leukocyte number has been increased over the normal range, however, there was not any significant difference with the control group (Dehghani et al. 2012). An increase in the peripheral leukocyte count had been demonstrated during the investigation of the blood among H. lepturus scorpion sting victims (Chitnis et al. 1993). While comparative studies to investigate the effects of H. lepturus venom on hematologic parameters and vital organs of the body have been done, but so far, any experimental research on the effect of the scorpion venom on the immune system cells and its various sub-groups in the early hours after the sting, and further-more determination and investigation of the period and the intensity of the envenomation have not carried out yet.
Therefore, the aim of the present study was to investigate the effect of H. lepturus envenomation on blood leukocytes and their subgroups in the early hours after the sting. The results of this study can help physicians, health officials and the medical staff to fast and accurate treatment of the victims of H. lepturuss sting and prevention of complications of the scorpion venom on important factors of the immune system.
Materials and Methods
Animals
Sixty male rats from N-Mari species (weight range of 300–350 grams) purchased from Pasteur Institute of Iran (Tehran), were used during the study. The animals were kept in standard cages in animal house at the School of Pharmacy of Ahvaz Jundishapur University of Medical Sciences. Rats were housed in temperature-controlled rooms (22–25 °C) with constant relative humidity (40–70%) and 12h/12h light/dark cycle before doing experimental protocols.
The study was performed in accordance with the principles of laboratory care established by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Venom Preparation
The scorpion electroshocked venom was provided as a lyophylized powder from Pasteur Institute of Iran, (Venom and Therapeutic Biomolecule Lab, Biotechnology Res. Center, Tehran, Iran). The concentration of crude venom protein was determined by using BradFord method (Bradford, 1976). The 0.1 and 0.01 concentrations of the venom prepared in distilled water. The injection volume was 0.1 ml.
Grouping of Animals and Sampling
The animals were randomly divided into three groups of 20 rats in each. The control group did not receive any thing; however, the first and the second groups received H. lepturus venom at the concentrations of 0.1 and 0.01 mg/kg/BW subcutaneously. Thereafter, the animals of each group (N= 20) were divided into four subgroups (N= 5), with respect of the four blood sampling time, that were two, six, 24 and 48 hours after the venom injection, the animals were then kept in separate cages.
Experimental Studies
The animals of each subgroup were anesthetized with ketamine and xylazine (Alfasan, Holland). The blood samples were obtained from the animal’s heart amounted to 0.5–2 ml by syringe. Soon after the sampling, the blood was maintained in glasses containing the anticoagulant, EDTA (Ethylene-Diamine-Tetra-Acetic acid), and the leukocytes were counted using the diluent solution, Marcanu (RBC lysis buffer) and the Neobar slide (hemocytometer) by using the light microscope (Olympus, 3H-Z-Japan), In order to count and determine the leukocyte subgroups (including neutrophils, lymphocytes, monocytes and eosinophils) the appropriate peripheral blood smears were prepared on the microscope slides and then fixed by means of the water-free methanol. Then Giemsa staining (Merck-Germany) was carried out by the diluted stain with the rate of 1/10. And finally, the differential counting was performed using a 100x lens microscope (Mahbod 2008, Mansouri et al. 2015).
Statistical Analysis
Data were analyzed using the SPSS ver. 13 (version 13, SPSS Inc, Chicago, IL) and the statistical tests of ANOVA and LSD. Data were considered significant statistically when P< 0.05 as presented in Fig. 1–5.
Fig. 1.

The comparison of the Mean±SD of white blood cells (× 10 per ml3) in peripheral blood of rat between the groups receiving venom with the concentration of 0.01 and 0.1 mg/kg and the control group at different times
*Significant difference (P< 0.05) between the experimental and control groups
** Significant difference (P< 0.01) between the experimental and control groups
Fig. 5.

The comparison of the Mean±SD of eosino-phil (× 10 per ml3) in peripheral blood of rat between the groups receiving venom with the concentration of 0.01 and 0.1 mg/kg and the control group at different times
Fig. 3.
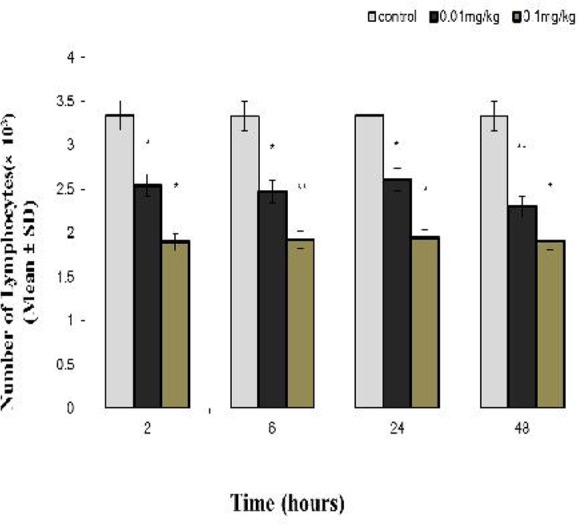
The comparison of the Mean±SD of lymphocytes (× 10 per ml3) in peripheral blood of rat between the groups receiving venom with the concentration of 0.01 and 0.1 mg/kg, and the control group at different times
* Significant difference (P< 0.05) between the experimental and control groups
Significant difference (P< 0.01) between the experimental and control groups
Results
The Effect of H. lepturus Venom on Leukocyte Count
The scorpion venom has led to a significant reduction of leukocytes in the venom-receiving group with the dose of 0.01 mg/kg at two and 24 hours after the injection, compared with the control group (P< 0.05). This decrease has been maximum of six hours after the injection (P< 0.01). After 24 hours from the injection, the effect of the venom has subsided; and after 48 h there was no any significant difference with the control group (P> 0.05). The toxicity effect of the venom is intensified with the increase of the injected venom concentration, so that in spite of a decrease in venom effect in the group receiving 0.01 mg/kg after 48 hours, the decrease in toxicity effect is still significant in the group receiving 0.1 mg/kg compared with the control group (P< 0.01) (Fig. 1).
The Effect of the H. lepturus Venom on the Peripheral Blood Neutrophil Count
The neutrophil mean count showed a significant decline in the venom receiving group with the dose of 0.01 mg/kg at two, 24 and 48 hours (P< 0.05), and six hours after the injection, compared with the control group (Fig. 2).
Fig. 2.

The comparison of the Mean±SD of neutrophils (× 10 per ml3) in peripheral blood of rat between the groups receiving venom with the concentration of 0.01 and 0.1 mg/kg and the control group at different times
* Significant difference (P< 0.05) between the experimental and control groups
** Significant difference (P< 0.01) between the experimental and control groups
The Effect of H. lepturus Venom on the Peripheral Blood lymphocyte Count
The mean of the peripheral blood lymphocyte count in the venom receiving group of 0.01 mg/kg at two, six and 24 hours (P< 0.05), and 48 hours (P< 0.01) after the injection demonstrated a significant decline compared with the control group, respectively.
The Effect of H. lepturus Venom on the Peripheral Blood Eosinophil and Monocytes Count
The comparison of the mean and standard deviation of the eosinophil and monocytes of the peripheral blood in the case and control groups showed no significant differences (P> 0.05) (Figs. 4, 5).
Fig. 4.

The comparison of the Mean±SD of monocytes (× 10 per ml3) in peripheral blood of rat between the groups receiving venom with the concentration of 0.01 and 0.1 mg/kg and the control group at different times
Discussion
The present study was aimed to investigate the effect of H. lepturus venom on leukocytes and their subgroups in peripheral blood of rats. Leukocytes are considered as the major group of immune cells. These cells apply a comprehensive supervision on different organs and tissues, through the regular circulation of blood to lymph, from inside of the vessels to the outside and from the interior of the tissues to the blood, resulting in a protection of the body against the pathological factors. In general, leukocytes consist of various groups of cells including lymphocytes, monocytes and granulocytes (Vodjgani 2012).
The clinical syndrome induced by H. lepturus sting is different from the stings by the other scorpions existing in Iran and the world, and exhibits more severe manifestations. Lack of local pain or being a mild pain after the sting, cutaneous manifestations such as erythema, swelling and necrosis at the sting site, the red blood cell lysis, and nephrotoxicity, including hemoglobinuria, proteinuria and hematuria are some manifestations among scorpion sting victims (Radmansh 1990, Radmanesh 1998, Pipelzadeh et al. 2006).
The results of the present study demonstrated those leukocytes are affected by the venom after two hours, exhibiting sensitivity, decrease in leukocytes continues for six hours after the venom injection. If leukocytes were evaluated after 12 hours, it was possible to notice a decreasing trend, due to prolongation of the presence of the venom into the body. Probably these variations in leukocytes are the results of cytotoxic effects of H. lepturus venom, which leads to white blood cell lysis and destruction (Shayesteh et al. 2012). There is no any considerable experimental or clinical study in this field, and the decrease in leukocytes in the early hours is one of the new findings of this research. Lack of the related reports might be due to the difference in evaluation time of the hematological indices in various studies (Dehghani et al 2005, Dehghani et al. 2012), it has been the result of the use of the anti-venom in human researches, as well (Chitnis et al. 1993, GhafourianBoroujerdnia and Mohebbi 2008). In the present study, the leukocyte count trend increased after 24 hours of venom injection, and there was not any significant difference in the venom-receiving group of 0.01 mg/kg with the control group.
The bone marrow has been probably capable to reconstruct white blood cells. Various studies have reported leukocytosis as well as the major clinical signs of the envenomation by H. lepturus, after other symptom such as hemoglobinuria, hematuria and proteinuria (Chitnis et al. 1993, Ghafourian Boroujerdnia and Mohebbi 2008). Therefore, the reconstruction of leukocytes observed in this study is in agreement with the previous findings (Dehghani et al. 2005, Dehghani et al. 2012). However, the more venom concentration, the more decline in leukocyte count, the slower WBC reconstruction, so that the leukocyte count has not reached to the normal range after 48 hours of envenomation with 0.1 mg/kg. According to the present study, after two hours, the percent of the blood neutrophils has been changed from the normal range of 61 % to 35.2 %. This reduction has been more severe after six hours and has fallen down to 25.5 %.
Neutrophils make up the most abundant population of white blood cells, and mediate the primary stages of inflammatory response. They are the most effective phagocytes in peripheral blood and have a major role in defense against the extracellular factors (Abbas et al. 2011). These mature cells migrate to the inflammation site just four hours after the antigen entrance, and have the capability to invade the antigen. Phagocytosis of the particles and waste products in neutrophils is associated with a series of biochemical events and morphological changes in the cell (Vodjgani 2012). The majority of the neutrophils at the inflammation site will be wiped out by other cells, such as macrophages, after the phagocytosis of the invader. Therefore, this can explain the rapid decrease in neutrophils.
Jalali et al. 2011 aiming to investigate the effect of H. lepturus on the serum levels of cytokines IL-1β, IL-6, IL-8 and TNF-α, demonstrated a direct relation between the worsening of the patient’s general condition and the above-mentioned cytokines. IL-8 is a chemotactic protein that is known as NAP-1 or the attractive and activator of neutrophils (Vodjgani 2012). The increase of this cytokine leads to fever and hyperthermia, which is probably due to neutrophil aggregation, pathogen killing and the death of the two kinds of cells (Taraz 2008). TNF-α enhances the production of some particular serum proteins such as amyloid A, through affecting hepatocytes. This cytokine suppresses stem cell division, which may lead to neutropenia (Vodjgani 2012). Therefore, the severe decrease in neutrophils in the group receiving high doses of H. lepturus venom will be reasonable. In the following 24 hours, the neutrophil count especially in the venom-receiving group of 0.01 mg/kg has approached the normal range. Regarding the neutrophilia, the neutrophil compensation could be explained. Through the neutrophilia the conversion of marginal neutrophils into circulating neutrophils could be noticed. These findings are consistent with the results of the research carried out by Ghafourian and Mohebby on patients with scorpion referred to the hospital bite (Ghafourian and Mohebbi 2008).
In the present study, lymphocytes have decreased during the first hours after the venom injection, and this reduction was significant even after 48 hours. One of the fractions extracted from the venom of H. lepturus may reduce the lymphocyte count (Bigdeli et al. 2006). Lymphocytes make up around 20 to 40 % of the leukocytes, and 99 % of the cells in the lymph, respectively. Lymphocytes are considered as the most important specific immune cells. The process of recognition and processing of antigen by lymphocytes as well as the clonal expansion requires the time, and the precise determination of the mechanism of the effect of the venom of H. lepturus on lymphocytes cannot be explained during the first 48 hours, perhaps due to immunological reasons. In the present study, the blood lymphocyte decrease was dose-dependent, which could probably be the result of the direct effect of the venom on the lymphocytes, which leads to disruption and lysis of these cells. In fact, it could be concluded that the venom of H. lepturus has a lymphotoxic effect (Ghafourian and Mohebbi 2008). In the present research, the peripheral blood eosinophil count in the control group showed no significant difference with the experimental groups. Eosino-phil consists of 2 % of leukocytes found in the normal situation in tissues, especially the epithelium of the respiratory tract, gastrointestinal tract and genitourinary tract. These cells have a weak phagocytic ability, and increase mainly in type-1 hypersensitivity responses and parasitic infections, as well. Therefore, the lack of their considerable change could be reasonable.
Conclusion
The venom of the scorpion H. lepturus effect on the leukocytes in the early hours, as well as increasing the concentration of the toxin, its destructive power is increased. In other word, the venom effect is dose dependent. The scorpion venom may induce the release of bradykinin, prostaglandin and corticosteroids. However, the essential role of these factors is to intensify the inflammation, but recently the natural corticosteroid hormones or similar synthetic substances are used to alleviate the inflammatory reactions against the allograft transplantation and immune system suppression.
Therefore, the extraction of the useful fractions of H. lepturus venom for natural induction of corticosteroids and decreasing leukocytes may be useful in the treatment of some types of leukemia and the graft surgeries, as well. It is recommended to investigate this issue in the future studies. However, the severe and dose-dependent reduction of the immune cells in the first hours after the injection of H. lepturus venom could be an alarm for health officials and medical staff to perform quick and accurate treatment in the least possible time, and to prevent of complications of the scorpion venom is the body’s vital organs.
Acknowledgements
The results provided are from MD thesis of Neda Ganjalikhanhakemi approved with number\ 469 and implemented in the Student Research Committee, and funded by the Research Deputy Vice-Chancellor for Research Affairs of AJUMS. The authors appreciate and thank this Deputy Vice-Chancellor for financial support, particularly the Research Consultation Center for technical support and Dr Delavar Shahbazzadeh and Miss Sara Ali-Akbari due to their unsparing cooperation. The authors declare that there are no conflicts of interest.
References
- Abbas AK, Lichtman HH, Pillai S. (2011) Cellular and Molecular Immunology. 7th ed PA: Elsevier Health Sciences; Philadelphia. [Google Scholar]
- Bigdeli MR, Taghavi-Moghadam A. (2006) Extraction of proteinous components from the venom of scorpion Hemiscorpious lepturus and evaluation of some their toxic effects in mice. Jundishapur Sci Med J. 5(2): 538– 542. [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72: 248– 254. [DOI] [PubMed] [Google Scholar]
- Celis A, Gaxiola-Robles R, Sevilla-Godinez E, de Orozco Valerio MJ, Armas J. (2007) Trends in mortality from scorpion stings in Mexico, 1979–2003. Rev Panam Salud Publica. 21( 6): 373– 380. [DOI] [PubMed] [Google Scholar]
- Chippaux JP, Goyffon M. (2008) Epidemiology of scorpionism: a global appraisal. Acta Trop. 107( 2): 71– 79. [DOI] [PubMed] [Google Scholar]
- Chitnis PA, Maraghi Sh, Vazirianzadeh B. (1993) Epidemiological and laboratory study on scorpion stings in Khuzestan Province. J Med Fac Guilan Univ Med Sci. 2: 5– 12. [Google Scholar]
- Dehghani R, Valayi N. (2005) Review on scorpions’ taxonomy and Iranian scor-pions’ key identification. Feiz. 32: 73– 92. [Google Scholar]
- Dehghni R, Dinparast Djadid N, Shahbazzadeh D, Bigdelli S. (2009) Introducing Compsobuthus matthiesseni (Birula, 1905) scorpion as one of the major stinging scorpions in Khuzestan, Iran. Toxicon. 54: 272– 275. [DOI] [PubMed] [Google Scholar]
- Dehghani R, Fathi B. (2012) Scorpion sting in Iran: a review. Toxicon. 60( 5): 919– 933. [DOI] [PubMed] [Google Scholar]
- Dehghani R, Khamehchian T, Vazirianzadeh B, Vatandoost H, Moravvej SA. (2012) Toxic effects of scorpion, Hemiscorpius lepturus (Hemiscorpiidae) venom on mice. J Anim Plant Sci. 22(3): 593– 596. [Google Scholar]
- Dehghani R, Khamehchian T, Tirgari S, Vatandoost H, Rasi Y, Rafeenejad J. (2005) Study of the Effect of Hemiscorpius lepturus Venom on Levels of WBC, RBCc and Hematocrit in Rats. J Shaheed Sadoughi Univ Med Sci. 13( 1): 66– 70. [Google Scholar]
- Jalali A, Pipelzadeh MH, Taraz M, Khodadadi A, Makvandi M, Rowan EG. (2011) Serum TNF-α levels reflect the clinical severity of envenomation following a Hemiscorpius lepturus sting. Eur Cytokine Netw. 22(1): 5– 10. [DOI] [PubMed] [Google Scholar]
- Jean-Philippe Ch. (2012) Emerging options for the management of scorpion stings. Drug Des Devel Ther. 6: 165– 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi A, Pipelzadeh MH, Vazirianzadeh V, Pipelzadeh M, Sharifat M. (2012) An in vitro comparative study upon the toxic properties of the venoms from Hemiscorpius lepturus, Androctonuscrassicauda and Mesobuthuseupeus scorpions. Toxicon. 60( 3): 385– 390. [DOI] [PubMed] [Google Scholar]
- Khoobdel M, Zahraei-Salehi T, Nayeri-Fasaei B, Khosravi M, Omidian Z, Motedayen M, Akbari A. (2013) Purification of the Immunogenic Fractions and Determination of Toxicity in Mesobuthus eupeus (Scorpionida: Buthidae) Venom. J Arthropod-Borne Dis. 7( 2): 139– 146. [PMC free article] [PubMed] [Google Scholar]
- Mahbod A. (2008) Principles, Practical Application and Interpretation of Tests in Hematology. 4th Ed Eshraghiye Pub, Tehran. [Google Scholar]
- Ghafourian Boroujerdnia M, Mohebby Z. (2008) A survey on comparison of Hemiscorpius lepturus and black scorpion effects on peripheral blood sub-populations of patients with scorpion bite under 15 years old referred to Ahwaz Abuzar Hospital. Toxicol Lett. 180: S131. [Google Scholar]
- Mansouri E, Kooti W, Bazvand M, Ghasemi Boroon M, Amirzargar A, Afrisham R, Afzalzadeh MR, Ashtary-Larky D, Jalali N. (2015) The Effect of Hydro-Alcoholic Extract of Foeniculum vulgare Mill on Leukocytes and Hematological Tests in Male Rats. Jundishapur J Nat Pharm Prod. 10( 1): e18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir Dehghan MM, Motlagh ME, Chomeili B. (2001) A 5-year study of scorpion stings in children referred to Abuzar Pediatric Center in Ahwaz, 1994–1999. Urmia Med J. 12( 2): 138– 174. [Google Scholar]
- Heidarpour M, Ennaifer E, Ahari H, Srairi-Abid N, Borchani L, Khalili Gh. (2011) Histopathological changes induced by Hemiscorpius lepturus scorpion venom in mice. Toxicon. 3( 59): 373– 378. [DOI] [PubMed] [Google Scholar]
- Osnaya-Romero N, de Jesus Medina-Hernández T, Flores-Hernández SS, León-Rojas G. (2001) Clinical symptoms observed in children envenomated by scorpion stings, at the children’s hospital from the State of Morelos, Mexico. Toxicon. 39( 6): 781– 785. [DOI] [PubMed] [Google Scholar]
- Pipelzadeh MH, Jalali A, Taraz M, Pourabbas R, Zaremirakabadi A. (2007) An epidemiological and clinical study on scorpionism by the Iranian scorpion Hemiscorpius lepturus. Toxicon. 50( 7): 984– 992. [DOI] [PubMed] [Google Scholar]
- Pipelzadeh M, Dezfulian A, Jalali M, Mansouri A. (2006) In vitro and in vivo studies on some toxic effects of the venom from Hemiscorpious lepturus scorpion. Toxicon. 48: 93– 103. [DOI] [PubMed] [Google Scholar]
- Radmanesh M. (1998) Cutaneus manifestation of Hemiscorpius lepturus sting: a clinical study. Int J Dermatol. 37: 500– 507. [DOI] [PubMed] [Google Scholar]
- Radmansh M. (1990) Clinical study of Hemiscorpion lepturus in Iran. J Trop Med Hyg. 93: 327– 332. [PubMed] [Google Scholar]
- Rafizadeh S, Rafinejad J, Rassi Y. (2013) Epidemiology of Scorpionism in Iran during 2009. J Arthropod-Borne Dis. 7( 1): 66– 70. [PMC free article] [PubMed] [Google Scholar]
- Seyedian R, Pipelzadeh MH, Jalali A, Kim E, Lee H, Kang Ch. (2010) Enzymatic analysis of Hemiscorpius lepturus scorpion venom using zymography and venom specific antivenin. Toxicon. 56 ( 4): 521– 525. [DOI] [PubMed] [Google Scholar]
- Shahbazzadeh D, Srairi-Abid N, Feng W, Ram N, Borchani L, Ronjat M. (2007) Hemicalcin a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem J. 404: 89– 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayesteh AA, Zamiri N, Peymani P, Zargani FJ, Lankarani KB. (2011) A novel management method for disseminated intravascular coagulation like syndrome after a sting of Hemiscorpius lepturus: a case series. Trop Biomed. 28( 3): 518– 523. [PubMed] [Google Scholar]
- Taraz M. (2008) Study of serum levels of cytokines IL-1β, IL-6, IL-8 and TNF-α in Hemiscorpius lepturus envenomed children referred to Ahwaz City hospitals. [MD Dissertation]. School of Pharmacy, Ahwaz Jondishapur University of Medical Sciences, Iran. [Google Scholar]
- Valavi E, Alemzadeh Ansari MJ. (2008) Hemolytic uremic syndrome following Hemiscorpius lepturus (scorpion) sting. Indian J Nephrol. 18( 4): 166– 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodjgani M. (2012) Immunology. 8th ed Jahad Daneshgahi Publication, Tehran. [Google Scholar]


