Abstract
Objective
The aim of this study was to characterize the relationship between serum low-density lipoprotein cholesterol (LDL-c) and subsequent depressive symptoms onset in postmenopausal women. We secondarily assessed serum high-density lipoprotein (HDL-c), total cholesterol, and triglycerides.
Methods
This population-based prospective cohort study utilizes data from 24,216 50-79 year old participants of the Women’s Health Initiative, which originally ran from 1993-2005 and has since incorporated two extension studies, with the most recent culminating in 2015. Fasting lipids were measured for all participants at baseline, and for a subset through six years of follow-up. Depressive symptoms were characterized using the Burnam eight-item scale for depressive disorders (Center for Epidemiologic Studies-Depression/Diagnostic Interview Schedule short form) at baseline and over follow-up, using a cutpoint of 0.06 to indicate presence of depressive symptoms.
Results
The lowest quintile of LDL-c was associated with an increased risk of subsequent depressive symptoms (HR=1.25, CI 1.05-1.49, p=0.01) and follow-up analyses demonstrated that the elevated risk appeared to be confined to the lowest decile (LDL-c <100 mg/dL). Further, this elevated risk was moderated by lipid-lowering drug treatment. Elevated risk was demonstrated among those who reported no lipid-lowering medication use (HR=1.23, CI 1.03-1.47, p=0.02), but not among those reporting use (HR=0.65, CI 0.18-2.29, p=0.50).
Conclusion
Among postmenopausal women, untreated serum LDL-c below 100 mg/dL was associated with an increased risk of developing depressive symptoms. No excess risk was observed in those attaining LDL-c <100 mg/dl with lipid-lowering therapy. These findings have important implications for risk assessment, treatment considerations, and mechanistic insight.
Keywords: WHI, menopause, depression, mood, cholesterol, LDL
Introduction
Observation of a relationship between low cholesterol and suicide has led to the exploration of low serum cholesterol as a clinically-relevant biomarker for suicide risk,1 which is relevant to the assessment of depression risk among older individuals due to the close link between suicide and depression among this population subset.2 Cholesterol is thought to play a role in depression pathophysiology via serotonergic function: serotonin transporter activity is modulated by cell membrane cholesterol, depletion of which results in diminished serotonin transporter function.3 Although studies have largely demonstrated an inverse association between serum total cholesterol and depression,4 these findings have not been consistently replicated.5, 6
Inconsistencies in the association between depression and cholesterol have raised interest in the roles of individual lipid fractions, particularly serum LDL-c. LDL-c is the main component of the total cholesterol measurement, demonstrating a strong correlation with total cholesterol (r=0.91).7 In this sense, total cholesterol serves as an imperfect surrogate, and as such would be expected to demonstrate greater inconsistency than would occur when using LDL-c itself.Several studies demonstrate a cross-sectional relationship between low LDL-c and depressive symptoms;8, 9 although these findings suggest utility of serum LDL-c as a clinical marker, the temporal relationship between serum LDL-c and depressive symptoms is not well-understood, leaving it challenging to determine whether low serum LDL-c precedes the development of depression or if depression leads to decreases in cholesterol via nutritional or metabolic changes.10
As serum cholesterol levels vary with age and gender, it is important to study the relationship between cholesterol and depression in various subgroups. Although several studies have been conducted among all-female cohorts,11-13 to date this association has not been adequately assessed among women who have completed the menopausal transition, such as the members of the Women’s Health Initiative (WHI) cohort. The Women’s Health Initiative provides an opportunity to evaluate the temporal relationship between serum lipid fractions and depressive symptoms among a large cohort of postmenopausal women and provide evidence towards the distinction between cholesterol as a predictor of depression rather than a consequence or epiphenomenon.10 In our primary analysis, we will assess the associations between low density lipoprotein cholesterol (LDL-c) and subsequent depressive symptoms, and secondarily assess other lipid measures (total cholesterol, high density lipoprotein cholesterol (HDL-c), and triglycerides). We will also evaluate the potential for effect modification by lipid-lowering medication use in the relationship between LDL-c and subsequent development of depressive symptoms.
Methods
Study Sample
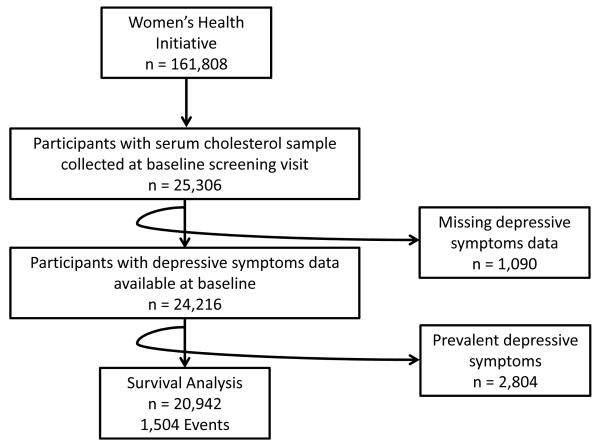
The WHI consisted of three concurrent clinical trials and an observational study, including a total of 161,808 postmenopausal women between the ages of 50-79 at baseline. Participants were enrolled into the WHI between 1993 and 1998 over the course of three screening visits.14 A number of Women’s Health Initiative ancillary studies included biomarkers; of these, serum lipid data was collected as a component of both the SNP Health Association Resource (SHARe) CVD Biomarkers study and the European American Hormone Trial (EA HT) Biomarkers study. The SHARe study included 12,007 African American and Latina members of the WHI study cohort.15, 16 The EA HT Biomarkers study consisted of 5,060 participants from the Genome-wide Association Studies of Treatment in Randomized Clinical Trials (GARNET) study and 7,479 members of the Women’s Health Initiative Memory Study (WHIMS). In addition, serum lipids were collected for a randomly-selected subset of 8.6% of hormone trial participants and 4.3% of dietary modification participants, oversampled for racial/ethnic minorities.17 Our study was conducted using a 24,216-participant subset of the WHI for whom both depressive symptom and serum lipid data was available– participant selection is described in greater detail in Figure 1. To account for sampling differences between studies, all multivariable analyses were adjusted for race/ethnicity, age, region, and WHI treatment assignment.
Figure 1.
Cohort Selection. Our sample of 24,216 participants was selected from the broader Women’s Health Initiative cohort with serum cholesterol and depressive symptoms assessed at baseline The 20 942 not depressed at baseline were utilized for survival analysis.
Cholesterol Fractions
Fasting blood samples were originally collected as a part of WHI screening and enrollment, centrifuged, and serum and plasma were frozen at −70°C; serum total cholesterol, HDL-c, and triglyceride values were subsequently obtained via direct assay and LDL-c values were derived using the Friedewald formula (total cholesterol-HDL cholesterol-triglyceride/5).18, 19 LDL-c values could not be calculated for 470 participants (1.9%) and were omitted from analysis. Cholesterol fractions were measured for all included participants at baseline, and among a 10-15% subsample at years 1, 3, and 6.
Depressive Symptoms
All participants included in this study had baseline depressive symptom data available. One-year follow-up data was collected for 16,636 participants, and for some follow-up extended up to the 19th study year. Baseline depressive symptoms were assessed at the second screening visit, a mean of 21 days following baseline lipid assessment. Depressive symptoms were assessed via the 8-item shortened combined Center for Epidemiologic Studies-Depression (CES D)/Diagnostic Interview Schedule (DIS), developed by Burnam et al. in 1988 using participants from the Los Angeles Epidemiologic Catchment Area Study and the Psychiatric Screening Questionnaires for Primary Care Patients study for use in the National Study of Medical Care Outcomes.20 The algorithm used by Burnam et al. multiplies each of the 8 items by a coefficient based on predictive ability and then rescales the point scores to a score between 0 and 1, with higher scores representing greater symptom severity. This study uses a score of 0.06 as the cut-point for presence of depressive symptoms, as established by Burnam et al. and used in previous WHI analyses.21-24 This scale has been used in a variety of studies, sometimes under other names, including the Brief Screening Instrument, the Medical Outcomes Study Depression Screen, the 8-item Rand Screening Instrument, and the Rand Short Depression Screener.25-33 In published studies of the WHI cohort, it has been referenced as the Burnam 8-item scale for depressive disorders,21, 22, 24 or more simply, the Burnam score.
As a sensitivity analysis, we subsequently expanded our event definition to include either antidepressant use (defined as use of tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, or other antidepressants), suicide, or Burnam score greater than 0.06 as an event. Antidepressant use was not considered in our primary definition due to the potential for antidepressant medications to be prescribed off-label for the treatment of vasomotor symptoms following menopause34-36 and because antidepressant use may modulate lipid fractions.37, 38
Selected Covariates
To account for the effects of confounding, the covariates selected for inclusion are those that have been previously determined or are otherwise likely to be associated with depressive symptomatology or serum cholesterol levels: WHI treatment assignment, age, education, race/ethnicity, marital status, family income, health insurance, BMI, blood pressure, heart disease, stroke, peripheral artery disease, smoking status, alcohol intake, leisure-time physical activity, diabetes, cancer, colitis, thyroid disease, and cholesterol-lowering medications. Covariates were assessed via questionnaire response or clinical measurement.14 Diabetes was identified via self-report of diabetes diagnosis or treatment.39 Coronary heart disease includes the adjudicated report of myocardial infarction, coronary artery disease, bypass or angioplasty, or angina. BMI was categorized based on the World Health Organization (WHO) clinical cutpoints of underweight (BMI <18.5), normal (BMI 18.5-24.9), overweight (BMI 25.0-29.9), obesity I (BMI 30.0-34.9), obesity II (BMI 35.0-39.9), and obesity III (BMI ≥40.0),40 and systolic and diastolic blood pressure were categorized based on clinical hypertension guidelines.41 Leisure-time physical activity was derived via questionnaire response and converted into MET-hr/wk based on established criteria.42 Alcohol consumption was categorized based on national guidelines for women (none: 0 servings per week, moderate: 1-7 servings per week, heavy: >7 servings per week).43 Lipid-lowering medication use was defined as reported use of at least one of the following medications during the study period: antihyperlipidemics, HMG CoA reductase inhibitors (statins), nicotinic acid derivatives, intestinal cholesterol absorption inhibitors, fibric acid derivatives, or bile sequestrants.
Statistical Analysis
All analyses were conducted using SAS 9.3. Scatterplots and regression diagnostics were used to assess distribution of the data. Study participants were contrasted across selected covariates by presence/absence of depressive symptoms, using chi-square analysis (Table 1). To most accurately characterize the nature of the relationship between serum cholesterol and depressive symptoms, several models were examined. In primary analysis, each cholesterol measure was evaluated using cholesterol quintiles derived based on study data, with the highest quintile designated as the reference category. We primarily assessed the associations between depressive symptoms and LDL-c, and secondarily assessed HDL-c, total cholesterol, and triglycerides.
Table 1.
Baseline Participant Characteristics by Depressive Symptoms
| Depressive Symptoms | ||||
|---|---|---|---|---|
| Yes n = 2804 |
No n = 20942 |
|||
| Variable | missing | % | % | p-value |
| age | 0 | <0.001 | ||
| <50-59 | 42.4 | 29.8 | ||
| 60-69 | 40.6 | 45.2 | ||
| 70-79+ | 17.0 | 24.9 | ||
| region | 0 | <0.001 | ||
| Northeast | 21.3 | 20.7 | ||
| South | 36.7 | 32.3 | ||
| Midwest | 18.1 | 22.4 | ||
| West | 23.9 | 24.6 | ||
| ethnicity | 0 | <0.001 | ||
| Am. Indian | 0.7 | 0.4 | ||
| Asian | 1.0 | 1.7 | ||
| Black | 38.8 | 34.3 | ||
| Hispanic | 22.5 | 13.4 | ||
| White | 36.6 | 49.7 | ||
| Other | 0.4 | 0.4 | ||
| HRT participant | 0 | <0.001 | ||
| Yes | 49.2 | 55.7 | ||
| No | 50.8 | 44.3 | ||
| Dietary Modification participant | 0 | 0.33 | ||
| Yes | 34.6 | 35.5 | ||
| No | 65.4 | 64.5 | ||
| Ca/D participant | 0 | <0.001 | ||
| Yes | 36.9 | 42.2 | ||
| No | 63.1 | 57.8 | ||
| Observational study participant | 0 | <0.001 | ||
| Yes | 31.1 | 24.6 | ||
| No | 68.9 | 75.4 | ||
| Diastolic BP | 6 | 0.31 | ||
| <90 mmHg | 90.8 | 91.3 | ||
| >=90 mmHg | 9.2 | 8.7 | ||
| Systolic BP | 0 | 0.0006 | ||
| <=120 mmHg | 35.6 | 32.2 | ||
| 120-140 mmHg | 41.8 | 42.8 | ||
| >140 mmHg | 22.6 | 25.0 | ||
| Lipid-lowering medication | 94 | 0.027 | ||
| Yes | 2.5 | 3.3 | ||
| No | 97.5 | 96.7 | ||
| Antidepressant medication | 94 | <0.001 | ||
| Yes | 4.9 | 1.4 | ||
| No | 95.1 | 98.6 | ||
| CHD | 0 | 0.60 | ||
| Yes | 7.3 | 7.0 | ||
| No | 92.7 | 93.0 | ||
| Stroke | 0 | 0.61 | ||
| Yes | 5.3 | 5.5 | ||
| No | 94.7 | 94.5 | ||
| Peripheral artery disease | 0 | 0.0055 | ||
| Yes | 1.8 | 1.2 | ||
| No | 98.2 | 98.8 | ||
| Diabetes ever | 19 | <0.001 | ||
| Yes | 12.4 | 8.9 | ||
| No | 87.6 | 91.1 | ||
| Cancer ever | 203 | <0.001 | ||
| Yes | 8.3 | 5.2 | ||
| No | 91.7 | 94.8 | ||
| Thyroid disease ever | 0 | 0.15 | ||
| Yes | 21.0 | 19.8 | ||
| No | 79.0 | 80.2 | ||
| Colitis ever | 0 | 0.032 | ||
| Yes | 1.4 | 1.0 | ||
| No | 98.6 | 99.0 | ||
| BMI | 178 | <0.001 | ||
| underweight <18.5 | 0.6 | 0.5 | ||
| normal 18.5-24.9 | 19.6 | 24.5 | ||
| overweight 25.0-29.9 | 31.6 | 35.3 | ||
| obesity I 30.0-34.9 | 26.5 | 23.4 | ||
| obesity II 35.0-39.9 | 13.2 | 10.6 | ||
| obesity III 40.0+ | 8.5 | 5.7 | ||
| Alcohol servings per week | 76 | <0.001 | ||
| none | 56.8 | 51.2 | ||
| 7 or fewer | 36.9 | 39.9 | ||
| more than 7 | 6.3 | 8.9 | ||
| Leisure-time physical activity (MET hr/wk) | 1048 | <0.001 | ||
| less than 7.5 | 19.2 | 26.8 | ||
| 7.5 to 15 | 18.7 | 20.7 | ||
| 15 or more | 62.1 | 52.5 | ||
| Smoking | 315 | <0.001 | ||
| never smoked | 45.6 | 52.9 | ||
| past smoker | 38.8 | 38.8 | ||
| current smoker | 15.6 | 8.3 | ||
| Any Health Insurance | 352 | <0.001 | ||
| Yes | 85.0 | 93.8 | ||
| No | 15.0 | 6.2 | ||
| Income | 795 | <0.001 | ||
| <$20,000 | 38.0 | 22.4 | ||
| $20k to 49,999 | 39.0 | 46.2 | ||
| $50k or more | 18.3 | 28.8 | ||
| don’t know | 4.7 | 2.6 | ||
| Education | 184 | <0.001 | ||
| no h.s. diploma | 16.9 | 8.1 | ||
| h.s. diploma or some college | 60.0 | 57.2 | ||
| bachelor’s degree or higher | 23.1 | 34.7 | ||
| Marital Status | 145 | <0.001 | ||
| never married | 4.5 | 4.4 | ||
| divorced | 26.6 | 19.4 | ||
| widowed | 23.4 | 21.1 | ||
| married | 44.0 | 53.6 | ||
| marriage-like | 1.5 | 1.5 | ||
Cross-Sectional Analysis
We used logistic regression to evaluate the cross-sectional relationships between baseline serum cholesterol quintiles and prevalent depressive symptoms, using the highest quintile as the reference value. To explore the potential for dose-response, models were re-evaluated modeling serum lipids as continuous variables. For each serum lipid measure, a preliminary bivariate analysis was conducted, followed by more extensive covariate adjustment to allow for assessment of the impact of additional covariates on the relationship between depressive symptoms and cholesterol. Models were adjusted for age, WHI trial arm, ethnicity, U.S. region, marital status, income, education, insurance coverage, heart disease, stroke, peripheral artery disease, diabetes, cancer, thyroid disease, colitis, cholesterol-lowering medications, systolic blood pressure, diastolic blood pressure, BMI, smoking status, alcohol consumption, and physical activity level.
Survival Analysis
Time-to-onset of depressive symptoms was assessed using semi-parametric survival models. Extended Cox regression analyses were modeled using the SAS PROC PHREG function. These models assumed proportional hazards, which was tested by statistical evaluation of time-dependence and by graphical analysis. Including only participants for whom depressive symptoms were not present at baseline (N=20,942), we evaluated the potential for differences in event timing due to the effects of serum lipid fractions. For the purpose of this analysis, onset of depressive symptoms was considered to be a non-repeatable event, and participants who did not have an event were censored five years after the last lipid assessment, up to year 11 of follow-up.
Serum lipid fractions were modeled as time-varying effects, for which missing values were assumed to be unchanged from the most recent previous measurement. In primary analysis, the final serum lipid measurement was carried forward as a fixed effect for an additional five years, creating a total follow-up time of up to 11 years (mean 4.87±3.07). A sensitivity analysis was next conducted to challenge the assumption that the final serum lipid measurement may be relevant for as long as five years; the exposure window was shortened to only one additional year of follow-up after the final lipid measurement, creating a total follow-up period of up to 7 years (mean 3.89±2.34).
Due to their potential to fluctuate over time, BMI, blood pressure, and cholesterol-lowering medication use were modeled as time-varying effects. Baseline values for the remaining covariates - WHI treatment assignment, age, education, ethnicity, marital status, family income, health insurance, heart disease, stroke, peripheral artery disease, thyroid disease, colitis, smoking status, alcohol intake, physical activity, diabetes, and cancer – were also included in the adjusted model.
Use of a lipid-lowering medication was selected as the sole a priori effect moderator and was assessed through inclusion of an interaction term. Significant findings were pursued with survival analysis stratified by lipid-lowering medication use. To account for potential differences between lipid-lowering medication types and given the current relevance of statins as a first-line treatment,44 a sensitivity analysis was next conducted in which the definition of lipid-lowering medication use was restricted to include statin users only.
Results
Cross-Sectional Analyses
2,804 (11.8%) participants had depressive symptoms at baseline. Participants with depressive symptoms were more likely to be younger than 60, be African-American or Latina, reside in the southern United States, earn less than $20,000 per year, report more leisure-time physical activity, have peripheral artery disease, a history of cancer, BMI greater than 30, a history of colitis, or systolic blood pressure below 120 mmHg. They were also less likely to be married, use lipid-lowering medications, drink alcohol, or have health insurance or a college degree (Table 1). In cross-sectional analysis, there was no association between baseline LDL-c quintiles and depressive symptoms in unadjusted or multivariable-adjusted analyses. Other lipid fractions demonstrated significant differences across quintiles in unadjusted analyses, but this appears to be largely accounted for by confounding, as no such association remained following multivariable adjustment (Table 2). In analysis of serum lipids modeled as continuous variables, neither LDL-c, HDL-c, total cholesterol, or triglycerides demonstrated any linear, quadratic, or cubic relationship to depressive symptoms.
Table 2.
Cross-Sectional Analysis of Depressive Symptoms by Cholesterol Quintiles
| Unadjusted | Multivariable-adjusted a | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | |
| LDL Cholesterol | 0.23b | 0.39b | ||||
|
| ||||||
| 175+ mg/dL | reference | reference | ||||
| 153 – 174 mg/dL | 0.92 | 0.82-1.05 | 0.21 | 0.97 | 0.85-1.12 | 0.70 |
| 134 - 152 mg/dL | 0.90 | 0.79-1.01 | 0.083 | 0.90 | 0.79-1.04 | 0.14 |
| 114 – 133 mg/dL | 0.89 | 0.79-1.01 | 0.064 | 0.89 | 0.78-1.03 | 0.11 |
| < 114 mg/dL | 0.98 | 0.87-1.11 | 0.78 | 0.97 | 0.85-1.12 | 0.69 |
|
| ||||||
| HDL Cholesterol | <0.0001b | 0.95b | ||||
|
| ||||||
| 66+ mg/dL | reference | reference | ||||
| 56 – 65 mg/dL | 1.13 | 1.00-1.28 | 0.05 | 1.05 | 0.91-1.20 | 0.52 |
| 50 - 55 mg/dL | 1.18 | 1.04-1.35 | 0.012 | 1.01 | 0.88-1.18 | 0.85 |
| 43 – 49 mg/dL | 1.21 | 1.07-1.38 | 0.0026 | 1.01 | 0.87-1.16 | 0.94 |
| < 43 mg/dL | 1.43 | 1.26-1.62 | <0.0001 | 1.04 | 0.90-1.21 | 0.60 |
|
| ||||||
| Total Cholesterol | 0.063b | 0.38b | ||||
|
| ||||||
| 260+ mg/dL | reference | reference | ||||
| 236 – 259 mg/dL | 0.95 | 0.84-1.08 | 0.46 | 0.98 | 0.85-1.12 | 0.73 |
| 216 – 235 mg/dL | 0.88 | 0.77-0.99 | 0.04 | 0.89 | 0.77-1.02 | 0.09 |
| 195 - 215 mg/dL | 0.95 | 0.84-1.08 | 0.42 | 0.92 | 0.80-1.06 | 0.24 |
| < 195 mg/dL | 1.05 | 0.93-1.19 | 0.43 | 0.99 | 0.86-1.13 | 0.83 |
|
| ||||||
| Triglycerides | 0.0022b | 0.08b | ||||
|
| ||||||
| 185+ mg/dL | reference | reference | ||||
| 136 – 184 mg/dL | 1.00 | 0.89-1.13 | 0.96 | 1.07 | 0.93-1.22 | 0.35 |
| 106 – 135 mg/dL | 0.95 | 0.84-1.07 | 0.38 | 1.04 | 0.91-1.20 | 0.56 |
| 80 – 105 mg/dL | 0.81 | 0.72-0.92 | 0.0014 | 0.88 | 0.76-1.02 | 0.09 |
| < 80 mg/dL | 0.86 | 0.76-0.98 | 0.022 | 0.98 | 0.84-1.14 | 0.78 |
adjusted for age, WHI trial arm, ethnicity, U.S. region, marital status, income, education, insurance coverage, heart disease, stroke, peripheral artery disease, diabetes, cancer, thyroid disease, colitis, cholesterol-lowering medications, systolic blood pressure, diastolic blood pressure, BMI, smoking status, alcohol consumption, and leisure-time physical activity
linear trend across quintiles
Survival Analyses
A total of 1,504 depressive events were observed over follow-up in those who were free of depression at baseline. In an unadjusted analysis, participants in the lowest quintile of LDL-c at baseline, corresponding to serum LDL-c levels below 114 mg/dL, had a higher risk of subsequent development of depressive symptoms relative to those in the highest quintile (LDL-c ≥175 mg/dL ) (HR=1.25, CI 1.07-1.47, p=0.006) (Table 3); these findings persisted after covariate adjustment (HR=1.25, CI 1.05-1.49, p=0.01). Similar multivariable findings were observed after expanding the event definition to include onset of at least one of the following: Burnam score greater than 0.06, antidepressant use, or suicide (2,054 events) (HR=1.40, CI 1.21-1.63, p<0.0001), as well as when analyses were conducted using the shortened follow-up period of 7 years (1,463 events) (HR 1.26, 1.05-1.50, p=0.01) (Table 4). To examine for a potential threshold effect, the lowest LDL-c quintile was recategorized into two deciles (<100 mg/dL and 100-114 mg/dL, respectively) and for comparison evaluated using the highest quintile (≥175mg/dL) again as the reference category. In multivariable-adjusted analysis, the effect of LDL-c persisted in the lowest decile (HR=1.33, CI 1.09-1.63, p=0.006), but was not significant in the next lowest decile (HR=1.16, CI 0.94-1.44, p=0.18).
Table 3.
Hazard Analysis for 11-year Follow-up
| Unadjusted | Multivariable-adjusteda | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazards Ratio | 95% CI | p-value | Hazards Ratio | 95% CI | p-value | |
| LDL Cholesterol | ||||||
|
| ||||||
| 175+ mg/dL | reference | reference | ||||
| 153-174 mg/dL | 1.10 | 0.93-1.30 | 0.27 | 1.20 | 1.00-1.43 | 0.055 |
| 134-152 mg/dL | 1.06 | 0.90-1.25 | 0.50 | 1.16 | 0.97-1.39 | 0.11 |
| 114-133 mg/dL | 1.06 | 0.90-1.25 | 0.49 | 1.09 | 0.90-1.30 | 0.40 |
| <114 mg/dL | 1.25 | 1.07-1.47 | 0.0055 | 1.25 | 1.05-1.49 | 0.014 |
|
| ||||||
| HDL Cholesterol | ||||||
|
| ||||||
| 66+ mg/dL | reference | reference | ||||
| 56 – 66 mg/dL | 1.04 | 0.89-1.21 | 0.63 | 1.00 | 0.85-1.18 | 0.99 |
| 50 - 56 mg/dL | 1.04 | 0.89-1.22 | 0.61 | 0.92 | 0.78-1.10 | 0.38 |
| 43 – 50 mg/dL | 1.00 | 0.85-1.16 | 0.95 | 0.82 | 0.69-0.98 | 0.03 |
| < 43 mg/dL | 1.09 | 0.92-1.28 | 0.31 | 0.81 | 0.67-0.98 | 0.029 |
|
| ||||||
| Total Cholesterol | ||||||
|
| ||||||
| 260+ mg/dL | reference | reference | ||||
| 236 – 259 mg/dL | 0.99 | 0.84-1.18 | 0.94 | 1.09 | 0.91-1.31 | 0.33 |
| 216 – 235 mg/dL | 1.10 | 0.94-1.30 | 0.23 | 1.17 | 0.98-1.40 | 0.08 |
| 195 - 215 mg/dL | 1.05 | 0.89-1.24 | 0.58 | 1.07 | 0.89-1.28 | 0.48 |
| < 195 mg/dL | 1.15 | 0.98-1.36 | 0.08 | 1.11 | 0.93-1.33 | 0.23 |
|
| ||||||
| Triglycerides | ||||||
|
| ||||||
| 185+ mg/dL | reference | reference | ||||
| 136 – 184 mg/dL | 0.93 | 0.80-1.09 | 0.39 | 0.957 | 0.81-1.13 | 0.61 |
| 106 – 135 mg/dL | 0.91 | 0.78-1.07 | 0.25 | 1.004 | 0.85-1.19 | 0.97 |
| 80 – 105 mg/dL | 0.92 | 0.78-1.07 | 0.28 | 1.041 | 0.87-1.24 | 0.65 |
| < 80 mg/dL | 0.80 | 0.68-0.95 | 0.0089 | 0.962 | 0.79-1.17 | 0.69 |
Adjusted for WHI trial arm, BMI, lipid-lowering medication, blood pressure, smoking status, coronary heart disease, stroke, peripheral artery disease, age, U.S. region, ethnicity, marital status, health insurance, alcohol consumption, leisure-time physical activity, cancer, income, education, diabetes, thyroid disease, and colitis
Table 4.
Hazard Analysis for 7-year Follow-up
| Unadjusted | Multivariable-adjusteda | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazards Ratio | 95% CI | p-value | Hazards Ratio | 95% CI | p-value | |
| LDL Cholesterol | ||||||
|
| ||||||
| 175+ mg/dL | reference | reference | ||||
| 153-174 mg/dL | 1.11 | 0.91-1.31 | 0.25 | 1.19 | 0.99-1.43 | 0.06 |
| 134-152 mg/dL | 1.08 | 0.91-1.27 | 0.43 | 1.17 | 0.97-1.40 | 0.09 |
| 114-133 mg/dL | 1.07 | 0.91-1.27 | 0.42 | 1.10 | 0.91-1.32 | 0.32 |
| <114 mg/dL | 1.27 | 1.08-1.49 | 0.0039 | 1.26 | 1.05-1.50 | 0.012 |
|
| ||||||
| HDL Cholesterol | ||||||
|
| ||||||
| 66+ mg/dL | reference | reference | ||||
| 56 – 66 mg/dL | 1.04 | 0.90-1.21 | 0.60 | 1.02 | 0.86-1.20 | 0.86 |
| 50 - 56 mg/dL | 1.04 | 0.88-1.22 | 0.64 | 0.93 | 0.78-1.11 | 0.44 |
| 43 – 50 mg/dL | 1.00 | 0.85-1.17 | 0.98 | 0.83 | 0.70-1.00 | 0.05 |
| < 43 mg/dL | 1.09 | 0.93-1.29 | 0.30 | 0.81 | 0.67-0.98 | 0.034 |
|
| ||||||
| Total Cholesterol | ||||||
|
| ||||||
| 260+ mg/dL | reference | reference | ||||
| 236 – 259 mg/dL | 1.03 | 0.87-1.22 | 0.74 | 1.13 | 0.94-1.35 | 0.21 |
| 216 – 235 mg/dL | 1.12 | 0.95-1.32 | 0.17 | 1.20 | 1.00-1.44 | 0.05 |
| 195 - 215 mg/dL | 1.06 | 0.89-1.25 | 0.52 | 1.09 | 0.91-1.31 | 0.37 |
| < 195 mg/dL | 1.18 | 1.00-1.39 | 0.05 | 1.14 | 0.95-1.36 | 0.17 |
|
| ||||||
| Triglycerides | ||||||
|
| ||||||
| 185+ mg/dL | reference | reference | ||||
| 136 – 184 mg/dL | 0.94 | 0.80-1.10 | 0.44 | 0.97 | 0.81-1.15 | 0.70 |
| 106 – 135 mg/dL | 0.91 | 0.78-1.07 | 0.27 | 1.01 | 0.85-1.20 | 0.95 |
| 80 – 105 mg/dL | 0.93 | 0.79-1.09 | 0.37 | 1.05 | 0.88-1.26 | 0.56 |
| < 80 mg/dL | 0.81 | 0.69-0.96 | 0.014 | 0.97 | 0.80-1.17 | 0.74 |
Adjusted for WHI trial arm, BMI, lipid-lowering medication, blood pressure, smoking status, coronary heart disease, stroke, peripheral artery disease, age, U.S. region, ethnicity, marital status, health insurance, alcohol consumption, leisure-time physical activity, cancer, income, education, diabetes, thyroid disease, and colitis
There was suggestion of a significant interaction between the lowest quintile of serum LDL-c (< 114 mg/dL) and use of lipid-lowering medication on outcome (χ2=4.32, p=0.04). In subsequent stratified analysis, the association between low LDL-c and development of depressive symptoms in the lowest LDL-c quintile relative to the highest quintile was confined solely to the nonmedicated group (HR=1.23, CI 1.03-1.47, p=0.02), with no such association observed among those who reported lipid-lowering medication use (HR=0.65, CI 0.18-2.29, p=0.50). Further, this association was found to be restricted to the lowest decile of LDL-c (<100 mg/dL) in those reporting no lipid-lowering medication use (HR= 1.32, CI 1.07-1.62, p=0.009), with no association seen among those reporting lipid-lowering medication use (HR=0.28, CI 0.25-3.86, p=0.27). These effects persisted after narrowing the definition of lipid-lowering medication use to include only statin users.
In analysis of HDL-c using 11 years of follow-up data, low HDL-c was associated with a lower risk of developing depressive symptoms over the follow-up period for the two lowest quintiles, relative to the highest quintile (≥66 mg/dL) (HDL-c <43 mg/dL (HR=0.81, CI 0.67-0.98, p=0.03), HDL-c 43-50 mg/dL (HR=0.82, CI 0.69-0.98, p=0.02)) only after multivariate adjustment. Findings remained significant after secondarily expanding the event definition to include depressive symptoms, antidepressant use, or suicide (HDL-c <43 mg/dL (HR=0.73, CI 0.62-0.86, p=0.0002), HDL-c 43-50 mg/dL (HR=0.76, CI 0.65-0.88, p=0.0004). Similar relationships were also observed in the sensitivity analysis (7-year follow-up) with significant findings in the lowest HDL-c quintile (<43 mg/dL) (HR=0.81, CI. 0.67-0.98, p=0.03) and marginally significant findings in the next lowest quintile (HDL-c 43-50 mg/dL), relative to the highest (HR=0.83, CI 0.70-1.00, p=0.05). No significant associations were demonstrated between total cholesterol or triglycerides and the onset of depressive symptoms.
Discussion
We found evidence for an association between low serum LDL-c and subsequent depressive symptoms. Follow-up analyses suggest this increased risk appears to be restricted to individuals with serum LDL-c is below 100 mg/dL. The association between low serum LDL-c and subsequent depressive symptoms appears to be moderated by lipid-lowering treatment, such that risk was not associated with treatment of dyslipidemia. There was some suggestion of a small, however non-significant, protective effect of lipid-lowering medication; while this may be due in part to healthy user bias,45 the magnitude of the difference makes this an unlikely sole explanation for the demonstrated interaction, which is corroborated by several studies failing to show any significant association between medically-lowered serum lipids and depression.46-50
The association between low LDL-c and subsequent depressive symptoms in the absence of lipid-lowering therapy suggests that low LDL-c, while predictive of depression risk, is not necessarily causative, and may be supportive of the presence of an unknown third factor that both causes low serum LDL-c and increases the risk of developing depression. To this end, the role of circadian dysregulation may warrant further investigation. The suprachiasmatic nuclei, located within the hypothalamus and responsible for coordinating the sleep-wake cycle to various physiological processes,51, 52 has been separately linked to both lipid metabolism53, 54 and depression,55-57 however the precise nature of this relationship is unclear. Additionally, animal studies suggest a link between cholesterol depletion and altered serotonin1A receptor function, which underscores the importance of LDL due to its function in delivery of cholesterol to the cell membrane.58, 59
Overall, these findings are consistent with studies across multiple other studies that included men8, 9 and younger women,13 suggesting that the findings from this cohort of post-menopausal women may be largely generalizable. Altogether, individuals who have low levels of LDL-c without the use of lipid-lowering treatments may represent a subgroup at risk for depression. Given the presumed heterogeneity of major depression, this at-risk group may represent a more homogeneous risk, thus potentially proving useful for mechanistic study.
Additionally, lower levels of HDL-c were generally found to be associated with a reduced risk of developing depressive symptoms, relative to higher levels. These findings contradict those of studies demonstrating an association between low HDL-c and depression,6, 60-62 although they are consistent with those of several smaller cross-sectional studies9, 63 and a meta-analysis.4 Because this study’s findings regarding HDL-c run in contrast to the greater body of existing literature and the majority of existing studies on the association between HDL-c and depression are cross-sectional, the findings of this study underscore the need for cholesterol fractions to be addressed separately and for additional research to be conducted to further clarify the relationship between HDL-c and depression before mechanistic insight can be drawn.
Notable strengths of this study include the sample size and duration of follow-up, as well as the utilization of biomarkers to assess cholesterol levels. The study also assesses the new onset of depressive symptoms prospectively, mitigating the potential for reverse-causality – namely, the potential for depression subsequently lowering cholesterol levels. Depressive symptoms were assessed systematically but not at a high intensity over follow-up. Our time-to-event analyses thus can only approximate the onset of depressive symptoms and it is possible that clinically significant depressive symptoms between assessments could have been missed. Presumably any such incomplete ascertainment of exposure would not bias results away from the null hypothesis. Further, data on clinician-diagnosis of Major Depressive Disorder were not available as a component of WHI data collection and the Burnam scale is not sufficient for the diagnosis of Major Depressive Disorder, which is ideally made by a structured clinical interview. For this reason, we have referred to our outcome as depressive symptoms throughout. Baseline prevalence of depressive symptoms in this study sample was 11.8%, which is within the range of that which would be expected based on the general population prevalence of major depression.64
A few findings in cross-sectional analyses differ from what would be anticipated. Subjects with depression at baseline reported greater participation in leisure time physical activity. Self-reported physical activity is commonly over-reported65 and differential over-reporting is possible. Because WHI calculation of MET-hours captures the perceived difficulty of activities, it is possible that those with depressive symptoms, who are more likely to perceive activities as strenuous, were more likely to over-report their activity. Future study with directly measured physical activity would be useful to determine if depressed participants differentially over-report when using self-reported activity measures that focus on difficulty. Participants with depressive symptoms were also less likely to use alcohol. Several recent studies have similarly failed to show a positive association between alcohol use and depression in women.66-68
Depression is independently associated with vascular disease69 and vascular mortality70, 71 and may convey risk of magnitude similar to conventional risk factors.72 Those with depression may thus reflect a neglected at-risk group. Yet, those with psychiatric disorders such as recurrent major depression are under-screened and under-treated for dyslipidemia,73, 74 only further contributing to health disparity. Our findings suggest there is no reason for concern that treatment of lipids may worsen depression is one factor contributing to the under-screening and under-treatment of dyslipidemia in those with depression. Similarly, our data do not support the concern that treatment-induced low LDL-c worsens the risk of depressive symptoms.
Low serum LDL-c (< 100 mg/dL) is associated with the subsequent onset of depressive symptoms in postmenopausal women. There does not appear to be any such association for low LDL-c levels in the setting of lipid-lowering medications; to this end, clinicians should not underutilize lipid-lowering agents in those with or at-risk of depression. The presence of very low LDL-c without treatment may represent a biomarker associated with depression risk and warrants further study.
Clinical Points.
Cross-sectional studies suggest LDL-c may play a role in depression pathophysiology, however the directionality of this relationship is unclear.
LDL-c < 100 mg/dL is associated with incident depressive symptoms in postmenopausal women and may represent a biomarker warranting further investigation.
No association among lipid-lowering medication users suggests clinicians should not underutilize lipid-lowering agents in those with or at-risk of depression.
Acknowledgements
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Dr. Fiedorowicz is supported by the National Institutes of Health (1K23MH083695-01A210, P01HL014388). Dr. Payne is supported by an NIH Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12 grant (#HD043446). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring institutions.
Footnotes
Conflict of Interest
Dr. Robinson has received research grants to institution from Amarin, Amgen, Astra-Zeneca, Daiichi-Sankyo, Genetech/Hoffman La Roche, Glaxo-Smith Kline, Merck, Regeneron/Sanofi, and Zinfandel/Takeda and serves as a consultant for Amgen, Hoffman LaRoche, Merck, Pfizer, and Sanofi. No other authors (JEP, JGF, MEP, WHC) have conflicts to disclose.
WHI Investigators:
The manuscript has been approved by the Women’s Health Initiative (WHI) Publications and Presentations Committee. The following is a short list of WHI investigators:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx
REFERENCES
- 1.Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry research. 2007 Mar 30;150(2):187–191. doi: 10.1016/j.psychres.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conwell Y, Duberstein PR, Caine ED. Risk factors for suicide in later life. Biological psychiatry. 2002 Aug 1;52(3):193–204. doi: 10.1016/s0006-3223(02)01347-1. [DOI] [PubMed] [Google Scholar]
- 3.Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001 Sep 4;40(35):10507–10513. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- 4.Shin JY, Suls J, Martin R. Are cholesterol and depression inversely related? A meta-analysis of the association between two cardiac risk factors. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2008 Aug;36(1):33–43. doi: 10.1007/s12160-008-9045-8. [DOI] [PubMed] [Google Scholar]
- 5.Nakao M, Yano E. Relationship between major depression and high serum cholesterol in Japanese men. The Tohoku journal of experimental medicine. 2004 Dec;204(4):273–287. doi: 10.1620/tjem.204.273. [DOI] [PubMed] [Google Scholar]
- 6.Liang Y, Yan Z, Cai C, Jiang H, Song A, Qiu C. Association Between Lipid Profile and Depressive Symptoms Among Chinese Older People: Mediation by Cardiovascular Diseases? International journal of behavioral medicine. 2013 Oct 18; doi: 10.1007/s12529-013-9358-2. [DOI] [PubMed] [Google Scholar]
- 7.Kardys I, Oei HH, van der Meer IM, Hofman A, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase A2 and measures of extracoronary atherosclerosis: the Rotterdam Study. Arteriosclerosis, thrombosis, and vascular biology. 2006 Mar;26(3):631–636. doi: 10.1161/01.ATV.0000201289.83256.cf. [DOI] [PubMed] [Google Scholar]
- 8.Aijanseppa S, Kivinen P, Helkala EL, Kivela SL, Tuomilehto J, Nissinen A. Serum cholesterol and depressive symptoms in elderly Finnish men. International journal of geriatric psychiatry. 2002 Jul;17(7):629–634. doi: 10.1002/gps.666. [DOI] [PubMed] [Google Scholar]
- 9.Igna CV, Julkunen J, Vanhanen H, Keskivaara P, Verkasalo M. Depressive symptoms and serum lipid fractions in middle-aged men: physiologic and health behavior links. Psychosomatic medicine. 2008 Nov;70(9):960–966. doi: 10.1097/PSY.0b013e318189a942. [DOI] [PubMed] [Google Scholar]
- 10.Fiedorowicz JG, Haynes WG. Cholesterol, Mood, and Vascular Health: Untangling the Relationship: Does Low Cholesterol Predispose to Depression and Suicide, or Vice Versa? Current Psychiatry. 2010;9(7) [PMC free article] [PubMed] [Google Scholar]
- 11.Horsten M, Wamala SP, Vingerhoets A, Orth-Gomer K. Depressive symptoms, social support, and lipid profile in healthy middle-aged women. Psychosomatic medicine. 1997 Sep-Oct;59(5):521–528. doi: 10.1097/00006842-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Henderson VW, Guthrie JR, Dennerstein L. Serum lipids and memory in a population based cohort of middle age women. Journal of neurology, neurosurgery, and psychiatry. 2003 Nov;74(11):1530–1535. doi: 10.1136/jnnp.74.11.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang CY, Egleston BL, Gabriel KP, et al. Depressive symptoms and serum lipid levels in young adult women. Journal of behavioral medicine. 2013 Apr;36(2):143–152. doi: 10.1007/s10865-012-9409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Design of the Women's Health Initiative clinical trial and observational study. 1. Vol. 19. Controlled clinical trials The Women's Health Initiative Study Group; Feb, 1998. pp. 61–109. [DOI] [PubMed] [Google Scholar]
- 15.Carty CL, Johnson NA, Hutter CM, et al. Genome-wide association study of body height in African Americans: the Women's Health Initiative SNP Health Association Resource (SHARe) Human molecular genetics. 2012 Feb 1;21(3):711–720. doi: 10.1093/hmg/ddr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CT, Fernandez-Rhodes L, Brzyski RG, et al. Replication of loci influencing ages at menarche and menopause in Hispanic women: the Women's Health Initiative SHARe Study. Human molecular genetics. 2012 Mar 15;21(6):1419–1432. doi: 10.1093/hmg/ddr570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA : the journal of the American Medical Association. 2004 Jun 23;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 18.Bray PF, Larson JC, Lacroix AZ, et al. Usefulness of baseline lipids and C-reactive protein in women receiving menopausal hormone therapy as predictors of treatment-related coronary events. The American journal of cardiology. 2008 Jun 1;101(11):1599–1605. doi: 10.1016/j.amjcard.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossouw JE, Cushman M, Greenland P, et al. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the women's health initiative trials of hormone therapy. Archives of internal medicine. 2008 Nov 10;168(20):2245–2253. doi: 10.1001/archinte.168.20.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Medical care. 1988 Aug;26(8):775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Goveas JS, Espeland MA, Hogan P, et al. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women's Health Initiative MRI Study. Journal of affective disorders. 2011 Jul;132(1-2):275–284. doi: 10.1016/j.jad.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertone-Johnson ER, Powers SI, Spangler L, et al. Vitamin D intake from foods and supplements and depressive symptoms in a diverse population of older women. The American journal of clinical nutrition. 2011 Oct;94(4):1104–1112. doi: 10.3945/ajcn.111.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persons JE, Robinson JG, Ammann EM, et al. Omega-3 fatty acid biomarkers and subsequent depressive symptoms. International journal of geriatric psychiatry. 2013 Dec 11; doi: 10.1002/gps.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women's Health Initiative Observational Study. Journal of the American Geriatrics Society. 2012 May;60(5):854–861. doi: 10.1111/j.1532-5415.2012.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McQuellon RP, Wells M, Hoffman S, et al. Reducing distress in cancer patients with an orientation program. Psycho-oncology. 1998 May-Jun;7(3):207–217. doi: 10.1002/(SICI)1099-1611(199805/06)7:3<207::AID-PON304>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 26.Samsa GP, Cohen SJ, Goldstein LB, et al. Knowledge of risk among patients at increased risk for stroke. Stroke; a journal of cerebral circulation. 1997 May;28(5):916–921. doi: 10.1161/01.str.28.5.916. [DOI] [PubMed] [Google Scholar]
- 27.Lynch DJ, Tamburrino MB, Nagel R. Telephone counseling for patients with minor depression: preliminary findings in a family practice setting. The Journal of family practice. 1997 Mar;44(3):293–298. [PubMed] [Google Scholar]
- 28.Houghton A, Bowling A, Clarke KD, Hopkins AP, Jones I. Does a dedicated discharge coordinator improve the quality of hospital discharge? Quality in health care : QHC. 1996 Jun;5(2):89–96. doi: 10.1136/qshc.5.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson RE, Hornbrook MC, Nichols GA. Replicating the chronic disease score (CDS) from automated pharmacy data. Journal of clinical epidemiology. 1994 Oct;47(10):1191–1199. doi: 10.1016/0895-4356(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 30.Kemper KJ, Osborn LM, Hansen DF, Pascoe JM. Family psychosocial screening: should we focus on high-risk settings? Journal of developmental and behavioral pediatrics : JDBP. 1994 Oct;15(5):336–341. [PubMed] [Google Scholar]
- 31.Mulrow CD, Williams JW, Jr., Gerety MB, Ramirez G, Montiel OM, Kerber C. Case-finding instruments for depression in primary care settings. Annals of internal medicine. 1995 Jun 15;122(12):913–921. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Watson JM, Kemper KJ. Maternal factors and child's health care use. Soc Sci Med. 1995 Mar;40(5):623–628. doi: 10.1016/0277-9536(94)e0112-6. [DOI] [PubMed] [Google Scholar]
- 33.Duncan PW, Samsa GP, Weinberger M, et al. Health status of individuals with mild stroke. Stroke; a journal of cerebral circulation. 1997 Apr;28(4):740–745. doi: 10.1161/01.str.28.4.740. [DOI] [PubMed] [Google Scholar]
- 34.Archer DF, Seidman L, Constantine GD, Pickar JH, Olivier S. A double-blind, randomly assigned, placebo-controlled study of desvenlafaxine efficacy and safety for the treatment of vasomotor symptoms associated with menopause. American journal of obstetrics and gynecology. 2009 Feb;200(2):172, e171–110. doi: 10.1016/j.ajog.2008.09.877. [DOI] [PubMed] [Google Scholar]
- 35.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2011 Jan 19;305(3):267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013 Jan;20(1):38–46. doi: 10.1097/GME.0b013e318274699f. [DOI] [PubMed] [Google Scholar]
- 37.Hummel J, Westphal S, Weber-Hamann B, et al. Serum lipoproteins improve after successful pharmacologic antidepressant treatment: a randomized open-label prospective trial. The Journal of clinical psychiatry. 2011 Jul;72(7):885–891. doi: 10.4088/JCP.09m05853blu. [DOI] [PubMed] [Google Scholar]
- 38.Raeder MB, Bjelland I, Emil Vollset S, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. The Journal of clinical psychiatry. 2006 Dec;67(12):1974–1982. doi: 10.4088/jcp.v67n1219. [DOI] [PubMed] [Google Scholar]
- 39.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5(3):240–247. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Physical status: the use and interpretation of anthropometry. Vol. 854. Report of a WHO Expert Committee; World Health Organization: 1995. pp. 1–452. (World Health Organization technical report series). [PubMed] [Google Scholar]
- 41.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA : the journal of the American Medical Association. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 42.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise. 1993 Jan;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 43.McGuire S, U.S. Department of Agriculture. U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2010. 7th Edition. U.S. Government Printing Office; Washington, DC: Jan, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adv Nutr. 2011 May;2(3):293–294. doi: 10.3945/an.111.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12; [Google Scholar]
- 45.Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009 Apr 21;119(15):2051–2057. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide & life-threatening behavior. 2002 Fall;32(3):333–346. doi: 10.1521/suli.32.3.333.22177. [DOI] [PubMed] [Google Scholar]
- 47.Huffman JC, Stern TA. Neuropsychiatric consequences of cardiovascular medications. Dialogues in clinical neuroscience. 2007;9(1):29–45. doi: 10.31887/DCNS.2007.9.1/jchuffman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994 Feb 5;308(6925):373–379. doi: 10.1136/bmj.308.6925.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahebzamani FM, D'Aoust RF, Friedrich D, Aiyer AN, Reis SE, Kip KE. Relationship among low cholesterol levels, depressive symptoms, aggression, hostility, and cynicism. Journal of clinical lipidology. 2013 May-Jun;7(3):208–216. doi: 10.1016/j.jacl.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 50.O'Neil A, Sanna L, Redlich C, et al. The impact of statins on psychological wellbeing: a systematic review and meta-analysis. BMC medicine. 2012;10:154. doi: 10.1186/1741-7015-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hickie IB, Naismith SL, Robillard R, Scott EM, Hermens DF. Manipulating the sleep-wake cycle and circadian rhythms to improve clinical management of major depression. BMC medicine. 2013;11:79. doi: 10.1186/1741-7015-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep medicine. 2007 Dec;8(Suppl 3):27–33. doi: 10.1016/j.sleep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Wang F, Zhang X, Wang J, et al. LGR4 acts as a link between the peripheral circadian clock and lipid metabolism in liver. Journal of molecular endocrinology. 2013 Dec 18; doi: 10.1530/JME-13-0042. [DOI] [PubMed] [Google Scholar]
- 54.Lee YJ, Han DH, Pak YK, Cho SH. Circadian regulation of low density lipoprotein receptor promoter activity by CLOCK/BMAL1, Hes1 and Hes6. Experimental & molecular medicine. 2012 Nov 30;44(11):642–652. doi: 10.3858/emm.2012.44.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendlewicz J. Disruption of the circadian timing systems: molecular mechanisms in mood disorders. CNS drugs. 2009;23(Suppl 2):15–26. doi: 10.2165/11318630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Gouin JP, Connors J, Kiecolt-Glaser JK, et al. Altered expression of circadian rhythm genes among individuals with a history of depression. Journal of affective disorders. 2010 Oct;126(1-2):161–166. doi: 10.1016/j.jad.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li JZ, Bunney BG, Meng F, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jun 11;110(24):9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pucadyil TJ, Chattopadhyay A. Cholesterol modulates the antagonist-binding function of hippocampal serotonin1A receptors. Biochimica et biophysica acta. 2005 Aug 1;1714(1):35–42. doi: 10.1016/j.bbamem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Pucadyil TJ, Chattopadhyay A. Cholesterol depletion induces dynamic confinement of the G-protein coupled serotonin(1A) receptor in the plasma membrane of living cells. Biochimica et biophysica acta. 2007 Mar;1768(3):655–668. doi: 10.1016/j.bbamem.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Tedders SH, Fokong KD, McKenzie LE, Wesley C, Yu L, Zhang J. Low cholesterol is associated with depression among US household population. Journal of affective disorders. 2011 Dec;135(1-3):115–121. doi: 10.1016/j.jad.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 61.Lehto SM, Niskanen L, Tolmunen T, et al. Low serum HDL-cholesterol levels are associated with long symptom duration in patients with major depressive disorder. Psychiatry and clinical neurosciences. 2010 Jun;64(3):279–283. doi: 10.1111/j.1440-1819.2010.02079.x. [DOI] [PubMed] [Google Scholar]
- 62.van Reedt Dortland AK, Giltay EJ, van Veen T, van Pelt J, Zitman FG, Penninx BW. Associations between serum lipids and major depressive disorder: results from the Netherlands Study of Depression and Anxiety (NESDA) The Journal of clinical psychiatry. 2010 Jun;71(6):729–736. doi: 10.4088/JCP.08m04865blu. [DOI] [PubMed] [Google Scholar]
- 63.Gary TL, Crum RM, Cooper-Patrick L, Ford D, Brancati FL. Depressive symptoms and metabolic control in African-Americans with type 2 diabetes. Diabetes care. 2000 Jan;23(1):23–29. doi: 10.2337/diacare.23.1.23. [DOI] [PubMed] [Google Scholar]
- 64.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA : the journal of the American Medical Association. 2003 Jun 18;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 65.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. The international journal of behavioral nutrition and physical activity. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi NG, Dinitto DM. Heavy/binge drinking and depressive symptoms in older adults: gender differences. International journal of geriatric psychiatry. 2011 Aug;26(8):860–868. doi: 10.1002/gps.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perera B, Torabi M, Kay NS. Alcohol use, related problems and psychological health in college students. International journal of adolescent medicine and health. 2011;23(1):33–37. doi: 10.1515/ijamh.2011.006. [DOI] [PubMed] [Google Scholar]
- 68.Payne ME, Hybels CF, Bales CW, Steffens DC. Vascular nutritional correlates of late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2006 Sep;14(9):787–795. doi: 10.1097/01.JGP.0000203168.28872.21. [DOI] [PubMed] [Google Scholar]
- 69.Fiedorowicz JG, He J, Merikangas KR. The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. Journal of psychosomatic research. 2011 Feb;70(2):145–154. doi: 10.1016/j.jpsychores.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laursen TM, Munk-Olsen T, Nordentoft M, Mortensen PB. Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. The Journal of clinical psychiatry. 2007 Jun;68(6):899–907. doi: 10.4088/jcp.v68n0612. [DOI] [PubMed] [Google Scholar]
- 71.Westman J, Hallgren J, Wahlbeck K, Erlinge D, Alfredsson L, Osby U. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ open. 2013;3(4) doi: 10.1136/bmjopen-2012-002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002 Mar 5;105(9):1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 73.Kreyenbuhl J, Dickerson FB, Medoff DR, et al. Extent and management of cardiovascular risk factors in patients with type 2 diabetes and serious mental illness. The Journal of nervous and mental disease. 2006 Jun;194(6):404–410. doi: 10.1097/01.nmd.0000221177.51089.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. Journal of affective disorders. 2007 Sep;102(1-3):145–151. doi: 10.1016/j.jad.2007.01.006. [DOI] [PubMed] [Google Scholar]