Abstract
CecropinXJ is a cationic antimicrobial peptide originally isolated from the larvae of Bombyx mori. The anticancer effect of cecropinXJ has been reported in various tumor cells, including leukemia, gastric and esophageal cancer cells. However, the activity of cecropinXJ on hepatocellular carcinoma (HCC) and its underlying mechanism have not been investigated to date. Therefore, the present study investigated the efficacy and associated mechanism of cecropinXJ in Huh-7 cells. Flow cytometric analysis was performed to determine the presence of cell cycle arrested and apoptotic cells. CecropinXJ significantly inhibited the growth of Huh-7 cells in a dose- and time-dependent manner. CecropinXJ treatment for 24 h induced S cell cycle arrest and apoptosis, in addition to loss of the mitochondrial membrane potential, in hepatoma cells. CecropinXJ induced HCC cell apoptosis by activating caspase-3 and poly(ADP-ribose) polymerase. Furthermore, cecropinXJ downregulated the expression of B-cell lymphoma 2 (Bcl-2), while upregulated the expression of Bcl-2-associated death promoter and Bcl-2-associated X protein. In conclusion, the results of the present study suggest that cecropinXJ may be an active anti-HCC agent and provide novel insights into the mechanism of cecropinXJ.
Keywords: antimicrobial peptide, hepatocellular carcinoma, apoptosis, cell cycle
Introduction
Hepatocellular carcinoma (HCC) is a prevalent type of cancer worldwide, with >600,000 individuals succumbing to the disease each year (1). In China, HCC exhibits an incidence of 30.3 cases per 100,000 individuals (2). Similar to other solid tumors, the main curative therapy for HCC is surgery, which generally is only successful if the cancer is diagnosed at an early stage (3). The conventional chemotherapies and radiotherapies used to treat advanced or late-stage HCC tumors, despite being reasonably effective, have also demonstrated various side effects, including hepatotoxicity (4) and hematotoxicity (5), and only a small percentage of patients may have the chance to undergo surgery for radical therapy, which complicates the safe administration of systemic therapy.
Numerous types of cancer cells and microorganisms have relatively more anionic phospholipids in the outer layer of their external membrane compared with normal eukaryotic cells. Several studies have demonstrated that certain antimicrobial peptides (AMPs) are more cytotoxic against transformed cells than against non-transformed cells (6,7). In addition, certain AMPs, when administered locally to solid tumors, exhibit anticancer activity (8). The cecropins, which were first isolated by Boman et al (9) from Hyalophora cecropia pupae (9,10), are a family of AMPs. To date, >20 types of cecropins have been identified, some of which have been reported to possess antitumor activities against various cancer cells, including bladder cancer (11), HCC (12), gastric carcinoma (13), fibrosarcoma (14) and leukemia cells (15). The mechanisms of action of cecropins against prokaryotic cells have been widely investigated (16). Cecropins exert their cytolytic activity by folding into an amphipathic helix, following selective binding and insertion into the target membrane, leading to the breakdown of the membrane structure, thus causing leakage of the cell contents and resulting in cell death (17). In eukaryotic cells, cecropins target non-polar lipid cell membranes, whereby they form transmembrane channels, which leads to irreversible cytolysis and eventually cell death (18). In addition, certain cecropins translocate spontaneously across eukaryotic membranes into the cytoplasm, whereby they depolarize inner mitochondrial membranes, thus causing disruption of the mitochondrial membrane potential, release of mitochondrial cytochrome c (cyt c) and induction of apoptosis (19).
CecropinXJ, a polypeptide composed of 33 amino acid residues, is a cationic AMP isolated from the larvae of Bombyx mori (20). CecropinXJ exhibits 98% homology with cecropin B (20). In the present study, the cecropinXJ gene was cloned into the pYES2/CT/α-Factor expression vector, and expressed in Saccharomyces cerevisiae (21).
CecropinXJ was previously demonstrated to exhibit various antibacterial activities (22). In addition, previous studies have reported that cecropinXJ is able to inhibit the proliferation and induce the apoptosis of tumor cells (23), although its antitumor mechanism remains unclear. In the present study, the cytotoxicity and mechanism of cecropinXJ against the human HCC cell line Huh-7 was investigated. The results revealed that cecropinXJ suppressed the proliferation and induced the apoptosis of Huh-7 cells in vitro through mitochondrial apoptosis pathways, suggesting that cecropinXJ is a potential anticancer drug.
Materials and methods
Preparation of the AMP cecropinXJ and reagents
CecropinXJ of B. mori was prepared using the S. cerevisiae eukaryotic pYES2/CT/α-Factor expression system (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and purified with Ni-nitrilotriacetic acid agarose, as previously reported (21). The concentration of purified recombinant cecropinXJ protein was determined with a Bradford protein assay kit (BioTek China, Beijing, China). Prior to use, the peptide was dissolved in Dulbecco's modified Eagle's medium (DMEM) (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at a concentration of 50 mmol/l, and sterilized by filtration through a 0.22-µm filter.
Fetal bovine serum (FBS) was obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA), while dimethyl sulfoxide (DMSO) was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was acquired from, 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] was obtained from Sigma-Aldrich (St. Louis, MO, USA), cell cycle staining solution was purchased from Multi Sciences (Lianke) Biotech Co., Ltd. (Hangzhou, China) and Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining apoptosis detection kit was acquired from BestBio (Shanghai, China). Monoclonal rabbit anti-caspase 3 (#9664), monoclonal rabbit anti-poly(ADP-ribose) polymerase (PARP; #9532), monoclonal rabbit anti-cytochrome c (#11940), monoclonal mouse anti-B-cell lymphoma 2 (Bcl-2; #15071), monoclonal mouse anti-Bcl-2-associated death promoter (Bad; #9296), monoclonal rabbit anti-Bcl-2-associated X protein (Bax; #14796), monoclonal rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; #2118) (dilution, 1:1,000) were all obtained from Cell Signaling Technology, Inc., Danvers, MA, USA. CytoBuster™ protein extraction reagent was purchased from Novagen (Merck Millipore, Darmstadt, Germany), bicinchoninic acid (BCA) assay kit was acquired from Beyotime Institute of Biotechnology (Haimen, China) and enhanced chemiluminescence detection kit was obtained from CWbio. Co. Ltd. (Beijing, China).
Cell culture
The human HCC cell line Huh-7 was purchased from the Chinese Academy of Medical Sciences (Beijing, China). Cells were cultured in DMEM supplemented with 10% FBS, 100 µg/ml streptomycin and 100 U/ml penicillin in a humidified atmosphere of 5% CO2 in air at 37°C.
Cell viability assay
To evaluate the effects of cecropinXJ on the proliferation of Huh-7 cells, cell viability was measured by MTT assay. Huh-7 cells in the logarithmic phase of growth were collected, seeded in 96-well plates at a density of 2×103 cells/well and cultured overnight. Following 24 h, Huh-7 cells were treated with or without cecropinXJ at various concentrations (1, 5, 10 and 50 µmol/l) for 0, 24, 48, 72, 96 and 120 h. Bovine serum albumin (10 µmol/l; Beijing Solarbio Science & Technology Co., Ltd.) served as a negative control, while 10 µmol/l cisplatin (Beijing Solarbio Science & Technology Co., Ltd.) served as a positive control. Upon incubation, the culture medium was removed, and 100 µl MTT solution (5 mg/ml) was added to each well, followed by incubation at 37°C for 4 h. Then, 150 µl DMSO was added to each well, and the plates were incubated at 37°C for additional 10 min. Absorbance was measured at 540 and 655 nm using a 96-well microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the ratio of optical density (OD)540/655 was determined. Cell viability (%) was calculated as (ODtreatment-ODblank)/(ODcontrol-ODblank) × 100.
Cell cycle analysis of cecropinXJ effects on Huh-7 cells by flow cytometry
Huh-7 cells at 5×105 cells/ml were inoculated into 100-mm dishes and incubated at 37°C for 24 h. Once the medium had been removed, cells were treated for 24 h with cecropinXJ at a final concentration of 5, 10 and 50 µmol/l. Untreated cells served as the control. Upon culture, both floating and adherent cells were collected, washed with cold phosphate-buffered saline (PBS; Beijing Solarbio Science & Technology Co., Ltd.) (pH 7.4) and fixed with 75% ethanol overnight at −20°C. Cells were then treated with DNA staining solution at 37°C in the darkness for 30 min. Samples were analyzed by fluorescence-activated cell sorting (FACS) with a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Cell cycle analysis was performed using the ModFit LT™ 3.0 DNA analysis software (Verity Software House, Inc., Topsham, ME, USA).
Apoptosis rate determined by flow cytometry
Huh-7 cells at 5×105 cells/ml were inoculated into 60-mm dishes and incubated at 37°C for 24 h. Upon removal of the medium, 2 ml complete DMEM with cecropinXJ at a final concentration of 5, 10 and 50 µmol/l was added to each dish, and cells were incubated for 12, 24 and 48 h. Untreated cells served as the control. Subsequently, cells were collected following digestion with 0.25% trypsin, washed with PBS (two times) and suspended in 400 µl binding buffer. Cell suspensions were stained with 5 µl Annexin V-FITC and 10 µl PI, according to the manufacturer's protocol, prior to be subjected to flow cytometry analysis in a (BD Biosciences). The results are presented as the percentage of Annexin V+ cells (mean ± standard error).
Measurement of the mitochondrial membrane potential (Δψm)
Changes in the Δψm during apoptosis were measured using DiOC6(3), which is a lipophilic cationic dye. Huh-7 cells (5×105 cells/ml) were cultured in 100-mm dishes in the absence or presence of cecropinXJ for 24 h at a concentration of 0, 5, 10 and 50 µmol/l. Subsequently, cells were incubated with 40 nmol/l DiOC6(3) for 15 min at 37°C. Cells were then washed twice in PBS and fluorescence was measured using a fluorescence spectrophotometer with an excitation wavelength of 482 nm and an emission wavelength of 504 nm.
Western blotting
Huh-7 cells were incubated with 0, 1, 5, 10 and 50 µmol/l cecropinXJ for 24 h, followed by two washes with ice-cold PBS. The adherent and floating cells were next harvested and lysed in 100 µl CytoBuster™ protein extraction reagent on ice for 15 min. Following centrifugation at 12,000 rpm 4°C for 10 min, the protein concentration of the supernatant was determined by BCA assay. The protein lysates (40 µg/lane) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes. Upon being washed with Tris-buffered saline and Tween 20 (TBS-T) buffer (20 mM Tris-HCl, 150 mM NaCl and 0.05% Tween 20), the membranes were blocked with 5% skimmed milk at room temperature for 1 h, and then incubated with the aforementioned primary antibodies (1:2,000) overnight at 4°C. Subsequently, membranes were washed with TBS-T and incubated with the corresponding HRP-conjugated secondary antibodies for 2 h at room temperature. Upon washing with TBS-T, the membranes were exposed using an ECL detection kit (CWbio. Co. Ltd.). Proteins were visualized using the Odyssey® CLx Imaging system (Li-Cor, Lincoln, NE, USA).
Statistical analysis
All results were confirmed in ≥3 independent experiments. Data are expressed as the mean ± standard deviation. Differences between two sample means were assessed by Student's t test. P<0.05 was considered to indicate a statistically significant difference.
Results
CecropinXJ suppressed Huh-7 cell proliferation and decreased cell viability
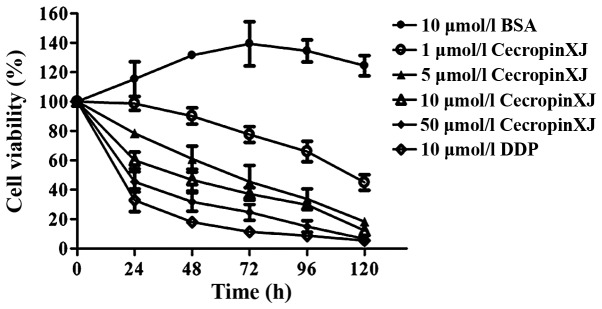
The results of MTT assay revealed that B. mori cecropinXJ inhibited the proliferation of Huh-7 cells in a dose- and time-dependent manner. CecropinXJ treatment for 24 h suppressed the growth of Huh-7 cells, and this inhibitory effect was enhanced by increasing concentrations of cecropinXJ. CecropinXJ at a concentration of 50 µmol/l significantly inhibited the proliferation of Huh-7 cells, with an inhibitory rate of ≤53% (P<0.05). Furthermore, the inhibition effects of 50 µmol/l cecropinXJ on Huh-7 cells was similar to those caused by 10 µmol/l cisplatin (Fig. 1).
Figure 1.
Effects of different concentrations of cecropinXJ and incubation times on the viability of treated Huh-7 cells. The results are expressed as the mean ± standard deviation of three independent experiments. BSA, bovine serum albumin; DDP, cisplatin.
Detection of tumor cell apoptosis rate by flow cytometry
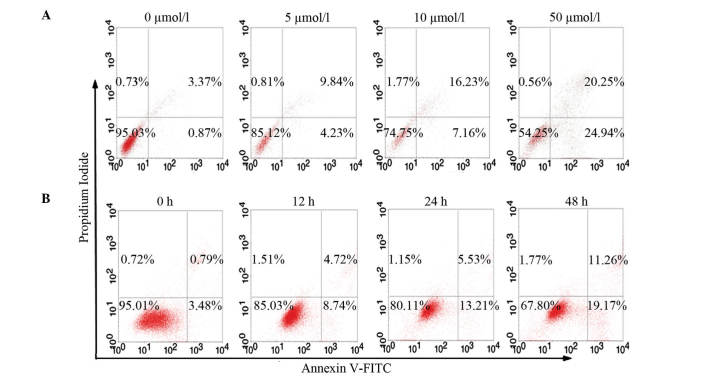
Since cell viability was significantly inhibited by cecropinXJ, it was critical to determine which type of cell death was induced by this AMP in Huh-7 cells (P<0.05). For that purpose, an Annexin V/PI assay. Flow cytometry assay with Annexin V/PI double staining revealed that cecropinXJ induced Huh-7 cell apoptosis. The apoptosis rate increased, whereas the number of necrotic cells did not significantly increase with cecropinXJ concentration or time. CecropinXJ treatment (5–50 µmol/l) for 24 h increased Huh7 cell apoptosis at both early and late stages, in a dose-dependent manner. The percentage of total apoptotic cells significantly increased from 3.76±0.53% (untreated) to 15.24±0.31% at 5 µmol/l cecropinXJ, 23.13±0.26% at 10 µmol/l cecropinXJ and 44.77±0.42% at 50 µmol/l cecropinXJ (Fig. 2). Furthermore, the number of apoptotic cells increased with 10 µmol/l cecropinXJ treatment for 12 h, and was significantly elevated at 24 h (P<0.05) (Fig. 2). This result indicated that cecropinXJ induced apoptotic cell death in Huh-7 cells.
Figure 2.
CecropinXJ induced apoptosis in Huh-7 cells. Huh-7 cells were treated with (A) different concentrations of cecropinXJ (0, 5, 10 and 50 µmol/l) for 24 h, or with (B) 10 µmol/l cecropinXJ for 0, 12, 24 and 48 h. Cells were stained with Annexin V-fluorescein isothiocyanate and propidium iodide, and analyzed by fluorescence-activated cell sorting. The number of apoptotic cells (Annexin V+) was indicated as the percentage of gated cells. (B) The percentage of apoptotic cells was expressed as the mean ± standard deviation of triplicate samples. FITC fluorescein isothiocyanate, PI, propidium iodide.
Cell cycle analysis of cecropinXJ on Huh-7 cells by flow cytometry
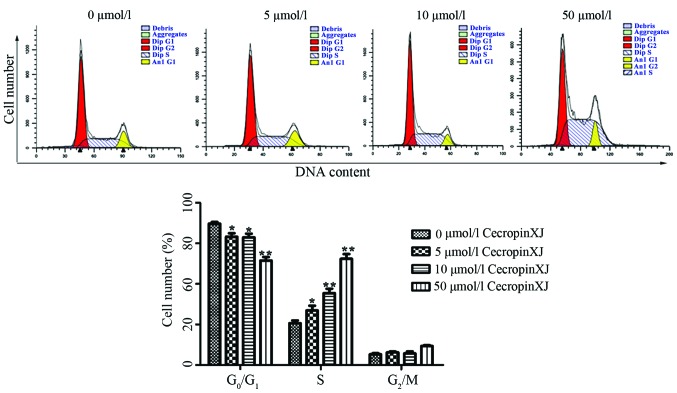
Flow cytometry was used to determine the effects of cecropinXJ on cell cycle distribution (Fig. 3). Upon treatment with cecropinXJ for 24 h, the percentage of S-phase cells was significantly higher in the treated group than in the control group (P<0.05). These results suggested that cecropinXJ arrested the cell cycle at the S phase in vitro.
Figure 3.
CecropinXJ arrested the cell cycle of Huh-7 cells at the S phase. Huh-7 cells were treated with different concentrations of cecropinXJ (0, 5, 10 and 50 µmol/l) for 24 h. (A) Subsequently, cells were stained with DNA staining solution, and analyzed by fluorescence-activated cell sorting. (B) The percentage of cells in the G0/G1, S and G2/M phases of the cell cycle was expressed as the mean ± standard deviation of triplicate samples. *P<0.05 and **P<0.01. Dip, diploid; Anl, aneupl.
CecropinXJ caused the loss of the Δψm and the release of cyt c in Huh-7 cells
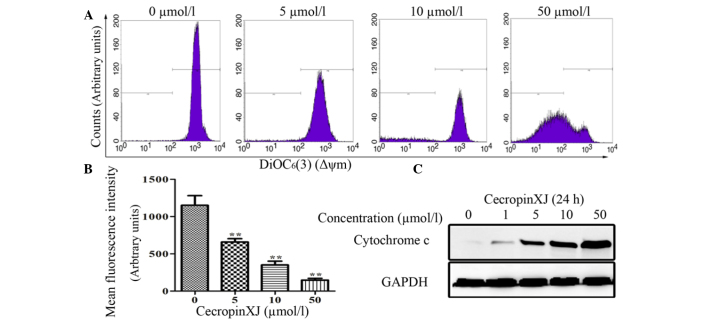
Since the loss of the Δψm acts as a key regulator in the intrinsic apoptosis pathway (24), it was next examined whether the Δψm was affected by cecropinXJ (Fig. 4). The mean fluorescence intensity of cells treated with 5, 10 and 50 µmol/l cecropinXJ for 24 h decreased to 658.17±44.57, 350.07±52.23 and 147.27±23.99, respectively, compared with the untreated control (1,196±76.12) (Fig. 4B). CecropinXJ also induced the release of cyt c into the cytosol (Fig. 4C) and triggered the intrinsic apoptosis pathway. This suggests that cecropinXJ may initiate apoptosis through depolarization of the Δψm.
Figure 4.
CecropinXJ caused the loss of the Δψm and the release of cyt c. Huh-7 cells treated with 5, 10 and 50 µmol/l cecropinXJ for 24 h were trypsinized to evaluate the Δψm by 3,3-dihexyloxacarbocyanine iodide staining. (A) The counts represent the percentages of cells with depolarized mitochondria. The results of a representative experiment are shown. (B) Data are presented as the mean ± standard deviation of the percentage of cells that retained polarized mitochondria from three different experiments. Statistical analyses indicated a significant (**P<0.01 vs. 0 µmol/l cecropinXJ) decrease in the number of cells with depolarized membrane following cecropinXJ treatment (C) Western blot analysis of cyt c release following treatment of cells with 0, 1, 5, 10 and 50 µmol/l cecropinXJ for 24 h. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Δψm, mitochondrial membrane potential; cyt c, cytochrome c.
CecropinXJ induced caspase-dependent apoptosis and regulated the expression of Bcl-2-family proteins in Huh-7 cells
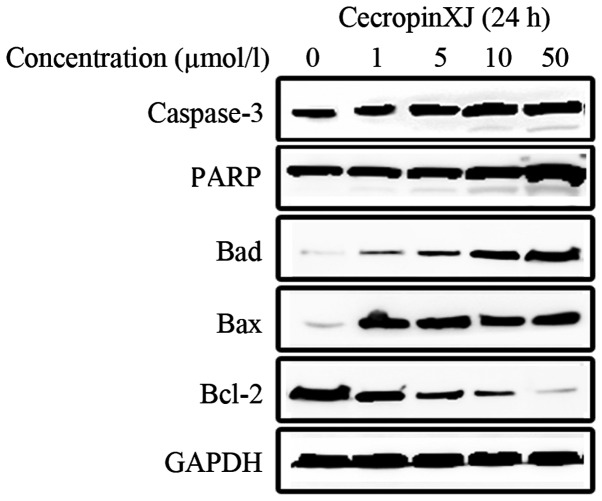
To further investigate whether cecropinXJ induced apoptosis in Huh-7 cells through the caspase-dependent pathway, the present study investigated whether caspase-3 and PARP were cleaved in cecropinXJ-treated cells. According to the results of western blot analysis, the above enzymes were cleaved in a dose-dependent manner (Fig. 5), indicating that cecropinXJ induced caspase-dependent apoptosis in the Huh-7 HCC cell line. Bcl-2-family members are important in the mitochondrial pathway of apoptosis, and are divided into pro- and anti-apoptotic family members, according to whether they promote or inhibit apoptosis (25). As indicated in Fig. 5, the pro-apoptotic proteins Bad and Bax were upregulated, whereas the anti-apoptotic protein Bcl-2 was downregulated, following treatment with cecropinXJ.
Figure 5.
Western blot analysis of the activation of apoptosis-related enzymes and expression of proteins of the Bcl-2 family. Huh-7 cells were treated with 0, 1, 5, 10 and 50 µmol/l cecropinXJ for 24 h, and their cellular extracts were analyzed by western blotting for detection of the cleaved forms of caspase-3 and poly(ADP-ribose) polymerase, and the expression levels of Bcl-2, Bcl-2-associated death promoter and Bcl-2-associated X protein, in order to evaluate whether cecropinXJ induced cell apoptosis through the caspase-dependent pathway. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PARP, poly(ADP-ribose) polymerase; Bcl-2, B-cell lymphoma 2; Bad, Bcl-2-associated death promoter; Bax, Bcl-2-associated X protein.
Discussion
AMPs are a kind of small peptides that specifically interact with membranes and affect the proliferation and apoptosis of various tumor cells (26,27). AMPs have gained great attention due to their antitumor effects (28). Since Moore et al (29) first discovered the antitumor activity of cecropin B against mammalian cancer cells, other studies have reported the cyototoxicity of cecropin-family members on gastric, bladder, liver and other types of cancer cells, but without causing damage to human normal cells (30). Thus, cecropins may be good candidates for the development of antitumor agents. In recent years, a number of studies have reported that AMPs could inhibit the growth of HCC, including Musca domestica cecropin (12), melittin (31) and PR-39 (32). In the present study, cecropinXJ inhibited the proliferation of Huh-7 cells, and the inhibitory rate of 50 µmol/l cecropin on HCC was 36.6±0.1%, which was higher than that of M. domestica cecropin (12).
Previous studies have demonstrated that antitumor drugs generally inhibit tumor proliferation through the induction of apoptosis in sensitive tumor cells, and their antitumor effects are associated with the drug-induced activation of apoptosis in the tumor cells. Therefore, the induction of apoptosis to treat tumors has become a novel target for the development of antitumor drugs, and constitutes a novel direction in tumor pharmacology research. In the present study, flow cytometry revealed that cecropinXJ induced apoptosis in Huh-7 HCC cells.
The mechanism of cecropins-induced apoptosis in vitro has been previously reported (11). Cecropin A, a 37-residue linear AMP produced by the cecropia moth, is able to induce apoptosis in HL-60 cells through a signaling mechanism that is mediated by the mitochondria but is independent of caspase activation (19). In the present study, the Δψm decreased following cecropinXJ treatment, which also resulted in the release of cyt c into the cytoplasm of Huh-7 cells, suggesting that cecropinXJ-mediated apoptosis may be associated with mitochondrial dysfunction. To further understand the mechanism of cell apoptosis mediated by cecropinXJ, its effects on the expression of caspase-family and Bcl-2-family proteins were examined. The results indicated that caspase-3 and PARP were cleaved, while Bad and Bax were upregulated and Bcl-2 was downregulated in a time-dependent manner, indicating that cecropinXJ induced caspase-dependent apoptosis in the Huh-7 HCC cell line. These findings suggested the existence of a common pathway that involves the activation of the family of proteolytic enzymes known as caspases (33). One of the pathways that lead to caspase activation is triggered by cyt c, following its release from the mitochondria into the cytoplasm, which is called the ‘intrinsic’ pathway of apoptosis (34). Bcl-2 blocks the release of cyt c from the mitochondria (35), suggesting that the mitochondria are the principal sites for apoptotic regulation by the Bcl-2 family.
In conclusion, the present study has demonstrated that cecropinXJ possesses antitumor activity against human HCC Huh-7 cells in vitro. The mechanism of cecropinXJ-induced apoptosis involves the loss of the Δψm, the release of cyt c from the mitochondria and the activation of caspase-3 and PARP, which suggests that cecropinXJ induced cell apoptosis possibly via the mitochondrial pathway. Therefore cecropinXJ may be a potential candidate for the treatment of HCC.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (no. 31500752), the Doctoral Start-up Fund of Xinjiang University (no. BS150241) and the High-Tech Research and Development Program of Xinjiang (no. 201110101).
References
- 1.Somboon K, Siramolpiwat S, Vilaichone RK. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac J Cancer Prev. 2014;15:3567–3570. doi: 10.7314/APJCP.2014.15.8.3567. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. The incidences and mortalities of major cancers in China, 2009. Chin J Cancer. 2013;32:106–112. doi: 10.5732/cjc.013.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Zolfagharzadeh F, Roshan VD. Pretreatment hepatoprotective effect of regular aerobic training against hepatic toxicity induced by doxorubicin in rats. Asian Pac J Cancer Prev. 2013;14:2931–2936. doi: 10.7314/APJCP.2013.14.5.2931. [DOI] [PubMed] [Google Scholar]
- 5.Sostelly A, Henin E, Chauvenet L, Hardy-Bessard AC, Jestin-Le Tallec V, Kirsher S, Leyronnas C, Ligeza-Poisson C, Ramdane S, Salavt J, et al. Can we predict chemo-induced hematotoxicity in elderly patients treated with pegylated liposomal doxorubicin? Results of a population-based model derived from the DOGMES phase II trial of the GINECO. J Geriatr Oncol. 2013;4:48–57. doi: 10.1016/j.jgo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Cruciani RA, Barker JL, Zasloff M, Chen HC, Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci USA. 1991;88:3792–3796. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin XB, Li XB, Zhu JY, Lu XM, Shen J, Chu FJ, Mei HF. The target of Musca domestica cecropin on human hepatocellular carcinoma BEL-7402 cells. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2011;29:271–273. (In Chinese) [PubMed] [Google Scholar]
- 8.Jin XB, Wang YJ, Liang LL, Pu QH, Shen J, Lu XM, Chu FJ, Zhu JY. Cecropin suppresses human hepatocellular carcinoma BEL-7402 cell growth and survival in vivo without side-toxicity. Asian Pac J Cancer Prev. 2014;15:5433–5436. doi: 10.7314/APJCP.2014.15.13.5433. [DOI] [PubMed] [Google Scholar]
- 9.Boman HG, Nilsson-Faye I, Paul K, Rasmuson T. Insect immunity. I. Characteristics of an inducible cell-free antibacterial reaction in hemolymph of Samia cynthia pupae. Infect Immun. 1974;10:136–145. doi: 10.1128/iai.10.1.136-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner H, Hultmark D, Engström A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 11.Suttmann H, Retz M, Paulsen F, Harder J, Zwergel U, Kamradt J, Wullich B, Unteregger G, Stöckle M, Lehmann J. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008;8:5. doi: 10.1186/1471-2490-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin X, Mei H, Li X, Ma Y, Zeng AH, Wang Y, Lu X, Chu F, Wu Q, Zhu J. Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim Biophys Sin (Shanghai) 2010;42:259–265. doi: 10.1093/abbs/gmq021. [DOI] [PubMed] [Google Scholar]
- 13.Pan WR, Chen PW, Chen YL, Hsu HC, Lin CC, Chen WJ. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J Dairy Sci. 2013;96:7511–7520. doi: 10.3168/jds.2013-7285. [DOI] [PubMed] [Google Scholar]
- 14.Lin WJ, Chien YL, Pan CY, Lin TL, Chen JY, Chiu SJ, Hui CF. Epinecidin-1, an antimicrobial peptide from fish (Epinephelus coioides) which has an antitumor effect like lytic peptides in human fibrosarcoma cells. Peptides. 2009;30:283–290. doi: 10.1016/j.peptides.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Hui L, Leung K, Chen HM. The combined effects of antibacterial peptide cecropin A and anti-cancer agents on leukemia cells. Anticancer Res. 2002;22:2811–2816. [PubMed] [Google Scholar]
- 16.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 17.Chou HT, Wen HW, Kuo TY, Lin CC, Chen WJ. Interaction of cationic antimicrobial peptides with phospholipid vesicles and their antibacterial activity. Peptides. 2010;31:1811–1820. doi: 10.1016/j.peptides.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Chen HM, Wang W, Smith D, Chan SC. Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B1 and B2, on liposomes, bacteria, and cancer cells. Biochim Biophys Acta. 1997;1336:171–179. doi: 10.1016/S0304-4165(97)00024-X. [DOI] [PubMed] [Google Scholar]
- 19.Cerón JM, Contreras-Moreno J, Puertollano E, de Cienfuegos GÁ, Puertollano MA, de Pablo MA. The antimicrobial peptide cecropin A induces caspase-independent cell death in human promyelocytic leukemia cells. Peptides. 2010;31:1494–1503. doi: 10.1016/j.peptides.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Li JY, Zhang FC, Ma ZH. Prokaryotic expression of cecropin gene isolated from the silkworm Bombyx mori Xinjiang race and antibacterial activity of fusion cecropin. Acta Entomologica Sinica. 2004;47:407–411. (In Chinese) [Google Scholar]
- 21.Xia L, Liu Z, Ma J, Sun S, Yang J, Zhang F. Expression, purification and characterization of cecropin antibacterial peptide from Bombyx mori in Saccharomyces cerevisiae. Protein Expr Purif. 2013;90:47–54. doi: 10.1016/j.pep.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Xia L, Zhang F, Liu Z, Ma J, Yang J. Expression and characterization of cecropinXJ, a bioactive antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in Escherichia coli. Exp Ther Med. 2013;5:1745–1751. doi: 10.3892/etm.2013.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia L, Wu Y, Kang S, Ma J, Yang J, Zhang F. CecropinXJ, a silkworm antimicrobial peptide, induces cytoskeleton disruption in esophageal carcinoma cells. Acta Biochim Biophys Sin (Shanghai) 2014;46:867–876. doi: 10.1093/abbs/gmu070. [DOI] [PubMed] [Google Scholar]
- 24.Cerón JM, Contreras-Moreno J, Puertollano E, de Cienfuegos GÁ, Puertollano MA, de Pablo MA. The antimicrobial peptide cecropin A induces caspase-independent cell death in human promyelocytic leukemia cells. Peptides. 2010;31:1494–1503. doi: 10.1016/j.peptides.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Chen YL, Li JH, Yu CY, Lin CJ, Chiu PH, Chen PW, Lin CC, Chen WJ. Novel cationic antimicrobial peptide GW-H1 induced caspase-dependent apoptosis of hepatocellular carcinoma cell lines. Peptides. 2012;36:257–265. doi: 10.1016/j.peptides.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Huh JE, Kang JW, Nam D, Baek YH, Choi DY, Park DS, Lee JD. Melittin suppresses VEGF-A-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. J Nat Prod. 2012;75:1922–1929. doi: 10.1021/np300446c. [DOI] [PubMed] [Google Scholar]
- 28.Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci. 2005;62:784–790. doi: 10.1007/s00018-005-4560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore AJ, Devine DA, Bibby MC. Preliminary experimental anticancer activity of cecropins. Pept Res. 1994;7:265–269. [PubMed] [Google Scholar]
- 30.Chernysh S, Kim SI, Bekker G, Pleskach VA, Filatova NA, Anikin VB, Platonov VG, Bulet P. Antiviral and antitumor peptides from insects. Proc Natl Acad Sci USA. 2002;99:12628–12632. doi: 10.1073/pnas.192301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Gu W, Zhang C, Huang XQ, Han KQ, Ling CQ. Growth arrest and apoptosis of the human hepatocellular carcinoma cell line BEL-7402 induced by melittin. Onkologie. 2006;29:367–371. doi: 10.1159/000094711. [DOI] [PubMed] [Google Scholar]
- 32.Ohtake T, Fujimoto Y, Ikuta K, Saito H, Ohhira M, Ono M, Kohgo Y. Proline-rich antimicrobial peptide, PR-39 gene transduction altered invasive activity and actin structure in human hepatocellular carcinoma cells. Br J Cancer. 1999;81:393–403. doi: 10.1038/sj.bjc.6690707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]