Abstract
Background
β-Glucanase is one of the most extensively used biocatalysts in biofuel, food and animal feed industries. However, the poor thermostability and low catalytic efficiency of most reported β-glucanases limit their applications. Currently, two strategies are used to overcome these bottlenecks, i.e., mining for novel enzymes from extremophiles and engineering existing enzymes.
Results
A novel endo-β-1,3-1,4-glucanase of GH16 (Tlglu16A) from the thermophilic fungus Talaromyces leycettanus JCM12802 was produced in Pichia pastoris and characterized. For potential industrial applications, recombinant TlGlu16A exhibits favorable enzymatic properties over most reported glucanases, i.e., remarkable stability over a wide pH range from 1.0 to 10.0 and superior activity on glucan substrates (up to 15,197 U/mg). The only weakness of TlGlu16A is the thermolability at 65 °C and higher. To improve the thermostability, the enzyme thermal stability system was then used to engineer TlGlu16A through optimization of residual charge–charge interactions. Eleven mutants were constructed and compared to the wild-type TlGlu16A. Four mutants, H58D, E134R, D235G and D296K, showed longer half-life time at 80 °C (31, 7, 25, 22 vs. 0.5 min), and two mutants, D235G and D296K, had greater specific activities (158.2 and 122.2 %, respectively) and catalytic efficiencies (kcat/Km, 170 and 114 %, respectively).
Conclusions
The engineered TlGlu16A has great application potentials from the perspectives of enzyme yield and properties. Its thermostability and activity were apparently improved in the engineered enzymes through charge optimization. This study spans the genetic, functional and structural fields, and provides a combination of gene mining and protein engineering approaches for the systematic improvement of enzyme performance.
Electronic supplementary material
The online version of this article (doi:10.1186/s13068-016-0544-8) contains supplementary material, which is available to authorized users.
Keywords: Talaromyces leycettanus JCM12802; Endo-β-1,3-1,4-glucanase; Thermostability improvement; High specific activity; Charge–charge interaction
Background
β-Glucans are linear polymers of β-D-glycosyl residues and represent the most important constituent of endosperm cell walls in cereals (barley, wheat and rye) [1, 2]. Endoglucanases are the enzymes that specifically cleave the mixed (1 → 3) or (1 → 4) linkages of β-D-glucan [3] and are classified into endo-1,3-1,4-β-glucanase (lichenase; EC 3.2.1.73), endo-1,3-β-glucanase (laminarinase; EC 3.2.1.39) and endo-1,3(4)-β-glucanase (EC 3.2.1.6) based on their modes of action [4, 5]. β-Glucanases are important commercial biocatalysts in various industries. For example, the accretion of exogenous β-glucanases can reduce the undesirable effects of barley β-glucan in the process of mashing in the brewing industry and can enhance the β-glucan digestibility in poultry feedstuffs [6–8]; endoglucanase is also used in biomass conversion to bioethanol in combination with xylanase and has application potential in bioenergy production [9].
A large proportion of β-glucanases are unstable during the high-temperature processes. To improve the thermostability of glucanases, either mining new genetic resources of thermophiles, engineering the protein, or optimizing application procedures is the most common practice [10, 11]. Thermophilic bacteria like Fibrobacter [12], Streptomyces [13], Bacillus [14, 15] and Alicyclobacillus [16] have been reported to produce high-temperature active glucanases, and thermophilic fungi are another source of glucanase genes, such as Paecilomyces spp. [17], Thermoascus aurantiacus CBMAI-756 [18] and Talaromyces leycettanus [19]. The thermophilic Talaromyces spp. are known to be potential industrial enzyme producers, as they have been reported to secrete various kinds of hydrolytic enzymes such as mannanase [19] and α-galactosidase [20]. However, no β-glucanase of GH16 has been reported from this genus yet.
Protein engineering is an important tactic to acquire thermostable enzymes, including but not limited to augmenting the number of disulfide bridges, hydrogen bonds, or salt bridges, introducing ionic bonds [21] or cation–π interactions [22] and replacing the N terminus [23]. For example, Wang et al. [24] employed directed evolution to construct a hyperthermostable xylanase mutant with an increased half-life of >9 times (about 228 min); Wintrode et al. [25] utilized DNA shuffling to create a protease mutant with increased melting temperature (Tm) of 25 °C and half-life improvement of 1200-fold at 60 °C. However, along with the improvement of enzyme thermostability, enzyme activity often decreases at various degrees. How to improve the enzyme properties without activity loss is always the biggest challenge.
Optimization of residual charge–charge interactions is a structure-based rational design approach that has been proven to be an effective method for thermostability improvement [26, 27]. However, its extensive application has been limited by underdeveloped bioinformatic tools. Tanford and Kirkwood [28] initially set up the TK model to calculate the contribution of each single charged residue to overall stability. This model was then improved by introducing solvent accessibility (SA) [29], Gibbs free energy [30] and electrostatic interactions [31]. Using a suite of enzyme redesign algorithms, enzyme thermal stability system (ETSS) [32] was developed to refine the calculation of the TK-SA model and surface charge–charge interaction analysis. By replacing positively charged residues with negatively charged or neutral ones, the Eij value is decreased and the enzyme thermostability would be improved. A previous study has shown that ETSS is efficient in the improvement of thermostability and catalytic efficiency of a GH28 endopolygalacturonase [33]. In this study, we identified a novel endoglucanase of GH16 and employed the ETSS to engineer the protein for better industrial performance.
Results and discussion
Gene cloning and sequence analysis
A gene fragment, 553 bp in length, was amplified from the genomic DNA of T. leycettanus JCM12802 using the degenerate primers [34]. BLASTx analysis indicated that the 553 bp fragment had the uppermost deduced amino acid sequence identity (80 %) to an uncharacterized endo-β-glucanase of GH16 (XM013476233). The DNA and cDNA of the complete gene (Tlglu16A) were obtained by thermal asymmetric interlaced (TAIL)-PCR and RT-PCR, respectively. Sequence analysis manifested that the open reading frame (ORF) of endoglucanase gene (Tlglu16A) consists of 1198 bp that is interrupted by two introns (61 and 69 bp, respectively, in length). The deduced TlGlu16A consists of 355 amino acid residues and shares the maximum identity of 76 % with a putative endo-1,3(4)-β-glucanase from Rasamsonia emersonii CBS 393.64 (KKA25075), 72 % identity with the functionally characterized endo-1,3(4)-β-glucanase from Penicillium sp. C1 (AFC38442) and 64 % identity with the structurally resolved glucanase from Paecilomyces thermophila (3WDT). SignalP analysis indicated that the deduced TlGlu16A had a predicted 20-residue signal peptide. The pI value and the calculated molecular mass of the mature protein were estimated to be 4.69 and 35.5 kDa, respectively. Two predicted N-glycosylation sites, Asn277 and Asn309, were identified. The deduced TlGlu16A harbors a single module, the catalytic domain of GH16. The conserved motif EIDIIE of GH16 fungal glucanases was identified, and Glu112 and Glu117 were the conjectural nucleophile and acid/base catalytic residues, respectively (Additional file 1).
Expression, purification and identification of TlGlu16A
Escherichia coli, Bacillus subtilis, Pichia pastoris, Saccharomyces cerevisiae, Aspergillus oryzae and Trichoderma reesei represent the most widely used heterologous expression systems [35]. Of them, the P. pastoris system is strong as a powerful promoter, in the secretion of eukaryotic proteins, correct folding of heterologous proteins and high-level expression at low cost [36, 37]. Using this system, a large number of glucanases have been successfully expressed in P. pastoris [38]. In this study, we produced recombinant TlGlu16A in P. pastoris GS115 competent cells. After 48 h induction with methanol at 30 °C, a transformant showing the highest β-glucanase activity on barley β-glucan (tested at pH 5.0, 50 °C and 10 min) in 1.5 mL tube culture was selected for high cell density fermentation in a 15-L fermentor. The β-glucanase activity reached 22,450 U/mL after 144 h of methanol induction. The total secreted proteins reached a relatively high titer of 1.64 g/L, 90 %, which was verified to be recombinant TlGlu16A (data not shown). This expression level was greater than that of β-glucanases produced in P. pastoris, including Bgl7A (370 mg/L) from Bispora sp. MEY-1 [39], Bgl (0.8 g/L) from Paenibacillus sp. F-40 [40] and even the codon optimized β-1,3-1,4-glucanase (250 mg/L) from B. licheniformis [15].
Recombinant TlGlu16A in the culture supernatants was purified to electrophoretic homogeneity by anion exchange chromatography. The purified enzyme showed a single band with an apparent molecular mass of 43 kDa on SDS-PAGE (Additional file 2), which is higher than the calculated molecular weight of the protein (35. 5 kDa). Three peptides of the purified recombinant TlGlu16A, DHTNVASGSGR, GSIPADISSGS and VYQDTAEST, were identified by the MALDI-TOF/MS analysis, which completely corresponded to the deduced amino acid sequence of TlGlu16A. The result indicates that the single band on SDS-PAGE is purified recombinant TlGlu16A indeed, and N-glycosylation might occur in TlGlu16A during heterologous expression in P. pastoris. After deglycosylation with Endo H, recombinant TlGlu16A showed a molecular mass of 35 kDa, which is consistent with the predicted size.
Enzymatic properties of purified recombinant TlGlu16A
All β-glucanase activities were assayed with barley β-glucan as the substrate. The purified TlGlu16A displayed the optimal activity at pH 4.5 when assayed at 60 °C for 10 min (Fig. 1a), which falls within the pH range of fungal β-glucanases (pH 4.0–7.0) from Cochliobolus carbonum [41], T. emersonii [42], Rhizopus microsporus var. microsporus [6] and P. thermophile [43]. Nevertheless, the pH stability of TlGlu16A is broader than most bacterial β-1,3-1,4-glucanases (pH 6.0–7.5). It retained more than 65 % of its original activity after being incubated in different buffers with a pH range of 1.0–10 (Fig. 1b) at 37 °C for 1 h. Therefore, TlGlu16A would remain highly active and stable in the digestive tract of animals.
Fig. 1.
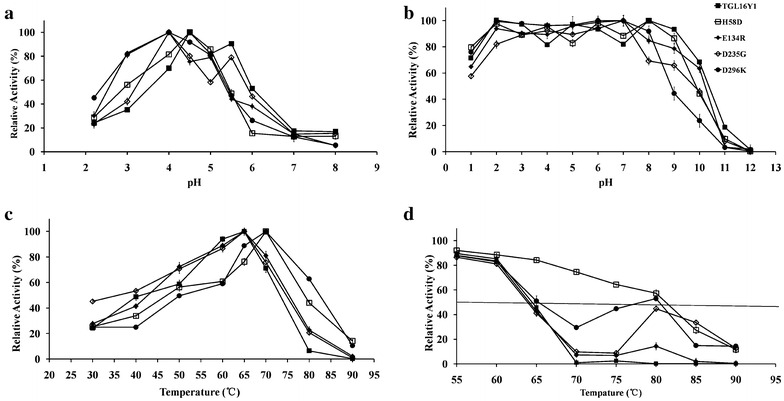
Enzymatic properties of wild-type TlGlu16A and its mutants. a pH-dependent activity profiles. b pH stability. c Temperature-dependent activity profiles. d Enzyme inactivation at different temperatures for 30 min
The optimal temperature of most reported β-1,3-1,4-glucanases ranges from 40 to 65 °C [4, 6, 8]. The optimal temperature of TlGlu16A was determined to be 65 °C on barley β-glucan in 50 mM citrate buffer (pH 4.5) (Fig. 1c), only a little lower than that of β-glucanases from Laetiporus sulphureus var. miniatus [44] and P. thermophile [43]. The enzyme exhibited good thermostability at 60 °C for 1 h (Fig. 2), but lost activity rapidly with the half-lives of 25, 3, 1 and 0.5 min at 65, 70, 75 and 80 °C (Table 1), respectively. Most fungal β-1,3-1,4-glucanases are denatured at 65 °C and higher, such as the enzymes from R. microsporus var. microsporus (<1 min at 80 °C) [6], P. thermophila (13 min at 80 °C) [17] and T. emersonii CBS 814.70 (25 min at 80 °C) [45]. The only known exception is the β-1,3-1,4-glucanase from L. sulphureus var. miniatus that has a half-life of 60 min at 80 °C [7]. Thermostability is an essential parameter of enzymes for utilization in high-temperature industries (50–70 °C for the malting and 65–90 °C for feed pelleting) [7]. From the industrial point of view, the thermostability of TlGlu16A should be improved.
Fig. 2.
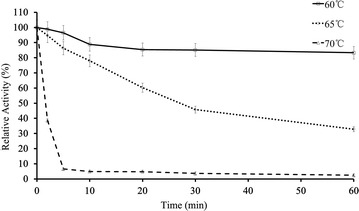
Thermostability of TlGlu16A at 60, 65 and 70 °C
Table 1.
Half-lives of wild-type TlGlu16A and its mutants for thermal inactivation
| Enzyme | t 1/2 (min) at | |||
|---|---|---|---|---|
| 65 °C | 70 °C | 75 °C | 80 °C | |
| TlGlu16A | 25 | 3 | 1 | 0.5 |
| H58D | 77 | 56 | 37 | 31 |
| E134R | 41 | 3 | 4.5 | 7 |
| D235G | 39 | 4 | 3.5 | 25 |
| D296K | 60 | 15 | 30 | 22 |
The enzyme activity was assayed at each optimal condition for 10 min
The influences of chemical reagents and metal ions on the TlGlu16A activity were tested at concentrations of 5 and 10 mM. Except for Ag+, Mn2+, Cu2+ and SDS that partially inhibited the glucanase activity of TlGlu16A (Table 2), most chemicals had no effect on the TlGlu16A activity. Of them, β-mercaptoethanol enhanced the enzymatic activity up to 1.30- and 1.46-fold at both tested concentrations. Co2+ and EDTA reinforced the β-glucanase activity at the concentration of 10 mM by 1.21- and 1.20-fold, respectively, which was different from most β-glucanases. The great resistance to most metal ions and chemicals widens the potential application spectrum of TlGlu16A in more fields such as detergent and paper industries.
Table 2.
Effects of metal ions and chemical reagents on the TlGlu16A activity
| Chemical | Relative activity (%)a | Chemical | Relative activity (%) | ||
|---|---|---|---|---|---|
| 5 mM | 10 mM | 5 mM | 10 mM | ||
| None | 100.0 ± 0.9 | 100.0 ± 0.8 | Fe3+ | 106.2 ± 2.1 | 99.6 ± 1.9 |
| Na+ | 110.8 ± 1.8 | 120.4 ± 1.5 | Pb2+ | 95.6 ± 2.7 | 91.8 ± 2.8 |
| Co2+ | 116.6 ± 1.8 | 121.2 ± 1.7 | Mn2+ | 87.2 ± 1.2 | 66.1 ± 2.1 |
| K+ | 109.3 ± 2.1 | 119.6 ± 2.7 | Cu2+ | 86.1 ± 2.4 | 48.5 ± 1.8 |
| Ca2+ | 108.8 ± 1.3 | 114.9 ± 1.3 | Ag+ | 78.4 ± 1.9 | 1.2 ± 0.7 |
| Ni2+ | 108.3 ± 1.6 | 117.7 ± 2.1 | β-Mercaptoethanol | 130.8 ± 3.0 | 146.3 ± 2.4 |
| Mg2+ | 106.5 ± 1.7 | 112.0 ± 2.1 | EDTA | 110.3 ± 2.5 | 120.0 ± 2.1 |
| Cr3+ | 103.1 ± 1.6 | 116.0 ± 1.2 | SDS | 64.7 ± 3.1 | 64.6 ± 1.0 |
| Zn2+ | 101.4 ± 2.1 | 111.3 ± 1.8 | |||
aValues represent mean ± SD (n = 3) relative to the untreated control samples
TlGlu16A had high specific activities toward barley β-glucan (15,197 ± 153 U/mg), lichenan (12,770 ± 98 U/mg) and laminarin (763 ± 87 U/mg), higher than the counterparts from P. thermophila (11,938 U/mg) [17], P. pinophilum C1 (12,622 U/mg) [34], Paecilomyces sp. FLH30 (8649 U/mg) [46], F. succinogenes (5180 U/mg) [37], Bispora sp. MEY-1 (4040 U/mg) [39], Paenibacillus sp. F-40 (3076 U/mg) [40], B. subtilis MA139 (728.79 U/mg) [21], and Aspergillus niger (63.83 U/mg) [22] with barley β-glucan as the substrate. Considering its great specific activity and high expression level as well as simple processing procedure, TlGlu16A could be a desirable candidate for use in industrial applications with broad applicability.
Substrate specificity, hydrolysis properties and kinetics of TlGlu16A
The specific activities of the purified recombinant TlGlu16A toward different substrates were determined. Similar to most fungal β-1,3-1,4-glucanases from thermophilic R. miehei [47], A. japonicas [48], Melanocarpus sp. [49] and R. microsporus var. microsporus [6], TlGlu16A exhibited rigid substrate specificity (lichenan, barley β-glucan and laminarin), and had no activity on carboxymethyl cellulose-sodium (CMC-Na), carob bean gum, Avicel, 4-nitrophenyl β-d-cellobioside (pNPC), 4-nitrophenyl-α-d-galactopyranoside (pNPG) and birchwood xylan. Thus, the enzyme was classified as a β-1,3-1,4-glucanase on the strength of its substrate specificity. However, TlGlu16A differs from the β-1,3-1,4-glucanases from Laetiporus sulphureus var. miniatus and R. miehei DSM 1330 that exhibited a higher relative activity toward laminarin (15–33 vs. 5 % against that of barley β-glucan) [50].
The kinetic values of TlGlu16A for barley β-glucan and lichenan are shown in Table 3. The substrate affinity of TlGlu16A (5.74 mg/mL) is similar to that of the β-glucanases produced by thermoacidophilic Alicyclobacillus sp. A4 [16] and B. licheniformis UEB CF [50], much lower than that of the β-1,3-1,4-glucanase from R. microsporus var. microsporus (19.8 mg/mL) [6], but higher than those of the β-1,3-1,4-glucanases from thermophilic Malbranchea cinnamomea [43] and R. miehei [42].
Table 3.
Specific activities and kinetics of wild-type TlGlu16A and its mutants with barley β-glucan and lichenan as the substrate
| Enzymes | Barley β-glucan | Lichenan | ||||||
|---|---|---|---|---|---|---|---|---|
| Specific activity (U/mg) | V max (μmol/min·mg) | V max (μmol/min·mg) | k cat/K m (mL/s·mg) | Specific activity (U/mg) | K m (mg/mL) | K m (mg/mL) | K cat/K m (mL/s·mg) | |
| TlGlu16A | 15,197 ± 153 | 5.74 ± 0.63 | 38,314 ± 187 | 3944 ± 156 | 12,770 ± 98 | 3.69 ± 0.54 | 13,447 ± 112 | 3643 ± 115 |
| H58D | 15,066 ± 113 | 2.52 ± 0.75 | 22,936 ± 113 | 5384 ± 127 | 13,408 ± 87 | 3.45 ± 0.46 | 27,100 ± 198 | 4637 ± 99 |
| E134R | 11,996 ± 79 | 5.04 ± 0.76 | 26,810 ± 154 | 3144 ± 143 | 7715 ± 88 | 2.64 ± 0.93 | 13,351 ± 167 | 2988 ± 198 |
| D235G | 24,040 ± 102 | 5.41 ± 1.01 | 61,350 ± 187 | 6701 ± 154 | 13,886 ± 102 | 9.32 ± 0.98 | 50,761 ± 201 | 3221 ± 154 |
| D296K | 18,574 ± 103 | 3.35 ± 0.43 | 25,381 ± 201 | 4479 ± 163 | 14,488 ± 101 | 7.39 ± 1.41 | 22,669 ± 175 | 3067 ± 103 |
Values are means ± standard deviations
The hydrolytic degradation products of barley β-glucan and lichenan by TlGlu16A were exhibited on thin layer chromatography (TLC) (Fig. 3). Tetrasaccharide and trisaccharide were the main products at the initial stage (10 min). Along with the extended reaction time, trisaccharide and tetrasaccharide were further hydrolyzed into monosaccharides and disaccharides. In combination with the hydrolysis products, it suggested that TlGlu16A had an endotype mode of action, and the enzyme is expected to be an endo-β-1,3-1,4-glucanase that specifically cleaves the β-1,4 linkages adjacent to β-1,3 glycosidic bonds and generates primarily tetrasaccharide, trisaccharide and disaccharide [42, 43].
Fig. 3.
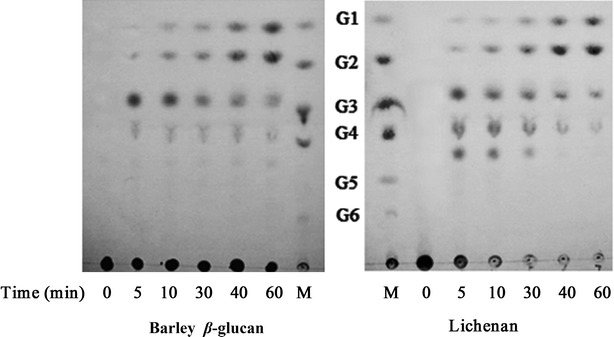
TLC analysis of the hydrolytic products of barley β-glucan and lichenan by purified TlGlu16A. Glucose (G1), cellobiose (G2), cellotriose (G3), cellotetraose (G4), cellopentaose (G5) and cellohexaose (G6) were used as the standards (M)
In view of its optimal activity at acidic and high expression level, mesothermal conditions and high specific activity as well as simple processing method, TlGlu16A would represent an excellent candidate for utilization in the animal feed industry.
Selection of the mutagenesis sites in TlGlu16A
ETSS calculation of total interaction energy (Eij) between charged amino acids of point i and j of wild-type TlGlu16A was conducted (Additional file 3). Mutation sites were selected following the three criteria [27] as shown below: (1) residues with high positive Eij values were taken as priorities; (2) prior residues far from the catalytic center were preferred to retain the activity; and (3) multiple-sequence alignment was combined to determine the mutation sites. As results, 23 residues with high positive Eij values were identified, and 11 far from the catalytic center were selected for site-directed mutagenesis based on the multiple-sequence alignment. Among them, three residues were mutated to neutral A (D16A, D139A and D216A), five residues to positively charged R or K (E40R, E134R, E190R, D272K and D296K), and three to D, H or G (H58D, D233H, and D235G). All of them were far away from the catalytic center (partial data shown in Fig. 4). On account of the ETSS analysis, when H58, E134 and D296 were mutated to D, R and K, respectively, the Eij values decreased from 2.3 to 1.0, 8.0 to −7.7 and 2.3 to −3.1 kJ/mol, respectively. These modifications converted the enzyme into a more stable state and improved the thermostability accordingly.
Fig. 4.
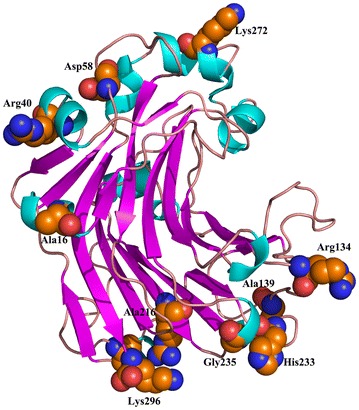
The modeled structure of TlGlu16A viewed from the N-terminal side. The mutated sites are indicated with balls
Generation, expression and purification of wild-type TlGlu16A and its mutants
The 11 site-directed mutants of TlGlu16A were constructed and expressed in P. pastoris, as described herein. Among them, mutants H58D, E134R, D235G and D296K showed greatly increased thermostability and maintained substantial activity (data not shown). These four mutants were then purified. SDS-PAGE analysis revealed that the four mutants had molecular masses of 43–55 kDa. After treatment with Endo H, all enzymes appeared as a single band in agreement with the theoretical mass of 35.5 kDa (Additional file 2).
Comparison of the properties of TlGlu16A and its mutants
Wild-type TlGlu16A and its mutants showed little difference in the pH activity profiles at the pH range of 5.5–7.0 (Fig. 1a). Compared with that of TlGlu16A, the pH adaptability ranges of the mutants shifted to acidic, and the activities of mutants H58D, E134R, D235G and D296K at pH 3.0 showed an increase of 21, 47, 7 and 46 %, respectively. The mutant enzymes were highly stable at pH 2.0–9.0 as TlGlu16A (Fig. 1b). This may benefit the utilization of this protein in the animal feed industry. The four mutants displayed increased activity at higher temperatures (Fig. 1c). The temperature optima of mutants H58D and D296K were both at 70 °C, which was 5 °C higher than that of the original TlGlu16A (65 °C). Although the temperature optima of E134R and D235G were the same as wild-type TlGlu16A, their activity beyond 60 °C decreased slightly more than that of TlGlu16A. At 70 °C, all mutants displayed above 76 % maximal activity, which was higher than that of wild-type TlGlu16A (71 % of maximal activity). When the temperature was increased to 80 °C, all mutants showed above 20 % maximal activity, especially D296K and H58D maintained 62 and 44 % of the activity, respectively, which was much higher than that of wild-type TlGlu16A (6.0 % of maximal activity). This indicates that H58D and D296K are more thermotolerant than the wild-type and other mutants.
T50 determination is a useful method to estimate and directly compare the thermostabilities of enzymes. As demonstrated in Fig. 1d, the T50 values of wild-type TlGlu16A and its mutants were determined at the temperature range of 55–90 °C. The T50 value of wild-type TlGlu16A was confirmed to be 64.5 °C, while that of mutant enzymes H58D and D296K was increased by 16.8 and 0.8 °C. The T50 value of the other two mutants E134R and D235G were 64.2 and 64.0 °C. But the three mutants (E134R, D235G and D296K) had remarkable thermostability improvement at 80 °C (the temperature of feed pelleting) and TlGlu16A almost completely lost the glucanase activity, while E134R, D235G and D296K retained 14, 44 and 52 % of the initial activity. It is strange that these three mutants retained more activity after incubation at 80 °C than at 75 and 70 °C. This phenomenon has been observed in lysozyme [53] and might be ascribed to the protein renaturation at higher temperature. The underlying mechanism might be that the changes of protein surface charge influence the three-dimensional structure of protein folding. GH16 endo-1,3-1,4-β-glucanase has a β-sandwich structure formed by two large anti-parallel β-sheets assembled face to face, which probably more easily renatures after incubation at high temperature, but the detailed mechanism is not clear.
Thermostabilities of recombinant TlGlu16A and its mutants were estimated by their half-lives (t1/2) (Table 1). Compared with wild-type TlGlu16A, all mutants have significant improvements in thermostability as shown in T50. The mutant E134R had a marginally increased t1/2 value (0.6- to 3.5-fold) at 65–75 °C. The D235G was much more thermostable, with an increased t1/2 value up to 49-fold at 80 °C. The mutants H58D manifested the maximal improvement in thermostability, with t1/2 increased up to 2-fold at 65 °C, 17-fold at 70 °C, 36-fold at 75 °C and 61-fold at 80 °C. This result also suggests that renaturation may occur in the mutants.
Differential scanning calorimetry (DSC) was executed to measure the Tm values of wild-type TlGlu16A and its mutants over the temperature range of 40–100 °C (Fig. 5). Compared to the Tm of TlGlu16A (50.55 °C), the Tm values of mutants E134R, D235G, H58D and D296K showed an increase of ~0.6 °C (51.14 °C), 1.5 °C (52.02 °C), 3.6 °C (54.19 °C) and 6.0 °C (56.49 °C), respectively. These data support that residue substitutions at sites 58 and 296 make the most outstanding contributions to thermostability improvement.
Fig. 5.
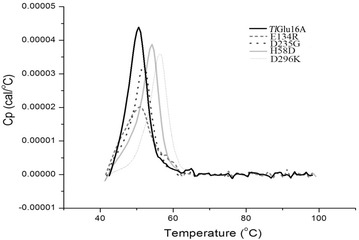
Thermograms of TlGlu16A and its mutants detected using the DSC. The calorimetric recordings were scanned at 1 °C/min in 10 mM PBS (pH 6.8) with the protein concentration of 350 μg/mL, respectively
Under optimum conditions, the specific activities of the four mutants were comparable or higher than that of wild-type TlGlu16A (15,197 U/mg) (Table 3). The catalytic activities and efficiency of the mutants D235G and D296K generated in this work were higher than the wild-type TlGlu16A, and compared to the wild-type TlGlu16A, the specific activities of mutant enzymes D235G and D296K were increased by 58.2 and 22.2 %, respectively, with barley β-glucan as the substrate. The Km and kcat/Km of TlGlu16A are 5.74 mg/mL and 3944 mL/s mg, respectively. Mutant D235G increased the kcat value almost twofold. In contrast, mutant D296K increased the affinity toward β-glucan. As a result, the kcat/Km value of mutants D235G and D296K were onefold to twofold more compared to wild-type TlGlu16A. Enzyme activity had been sacrificed for improved thermostability. In contrast, our main objective in this work is to improve the enzyme catalytic efficiency while improving thermal stability. Compared with the wild-type TlGlu16A, the specific activities of mutant enzymes D235G and D296K were increased by 58.2 and 22.2 %, respectively, when using β-glucan as the substrate. This study reveals that the residue charge–charge interaction plays important roles in stabilizing enzymes; in addition, we managed to improve the enzyme thermostability accompanied by enhanced catalytic efficiency. It was a very rare find in the field of thermostable study. The great care in selection of mutation sites far away from the catalytic core was successful (Fig. 4).
Interest in feed enzymes is high, so thermophilic/thermostable glucanases have been mined from diverse species, cloned and characterized. However, few glucanases are economically viable for feed applications due to limitation in activity and stability under the compulsory processing conditions (70–80 °C for 5 min). Therefore, glucanases with higher stability and activity under the feed processing conditions are of great value. In this study, two mutants H58D and D296K have a remarkable thermostability at 80 °C, and two mutants D235G and D296K exhibit higher specific activity and better thermostability than TlGlu16A.
When the catalytic efficiency of an enzyme increases, the protein structure becomes more flexible. Consequently, the rate of the substrate into the passage and product from the catalytic center will increase, and the thermostability of the protein may be reduced [51]. However, in this study, we not only increased the thermostability of TlGlu16A, but also enhanced its catalytic efficiency and specific activity (Fig. 6) through modifying the charge–charge interaction of the protein surface. It is of importance to reveal that the surface charge of a protein plays key roles in protein thermostability and catalytic performance.
Fig. 6.
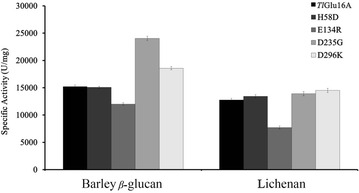
Activity profiles of TlGlu16A and its mutants with barley β-glucan and lichenan as the substrates
Enzymatic hydrolysis of corn stover
Corn stover is composed of cellulose, hemicellulose, and lignin. When incubated at pH 4.8 and 50 °C with agitation, reducing sugars are released from the corn stover under study (Fig. 7). Without enzymatic addition, less than 12 μmol/mL of reducing sugars were released from the corn stover over 60 h. In contrast, the addition of wild-type TlGlu16A and mutant enzyme H58D improved the hydrolysis efficiency, increasing the amounts of reducing sugar to 13.3 and 14.6 μmol/mL, respectively, at 24 h. When lengthening the incubation period to 36 h and longer, no difference was detected between TlGlu16A and H58D. By hydrolyzing the β-1,4 bonds of hemicellulose, TlGlu16A and its mutant H58D may assist in the efficient degradation of corn stover.
Fig. 7.
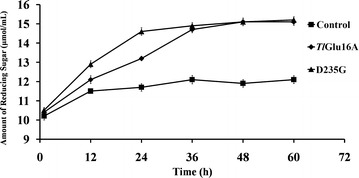
The hydrolysis of pretreated corn stover by wild-type TlGlu16A and mutant D235G (each 0.06 μmol) at 50 °C and pH 4.8
Conclusions
In this study, we mined a thermophilic and highly active endo-1,3-1,4-β-glucanase TlGlu16A from the thermophilic fungus T. leycettanus JCM12802. TlGlu16A has a broad pH stability range, wonderful resistance to most metal ions and chemical reagents, and great enzymatic activity against synthesized and natural substrates. By optimizing the residual charge–charge interactions, the thermostability and catalytic efficiency of TlGlu16A were remarkably and simultaneously improved. This study provides an excellent glucanase candidate in wide industrial applications and indicates the significance of charge–charge interactions in protein stabilization.
Methods
Strains, materials and culture medium
Talaromyces leycettanus JCM12802 was purchased from the Japan Collection of Microorganisms RIKEN BioResource Center (Tsukuba, Japan). E. coli Trans1-T1, vector pEASY-T3, the DNA purification kit, LA Taq DNA polymerase and Genome Walking kit were purchased from TaKaRa (Tsu, Japan). The RNA isolation system kit was purchased from Promega (Madison, WI, USA). Restriction endonucleases, T4 DNA ligase and Endo H (endo-β-N-acetylglucosaminidase H) were purchased from New England Biolabs (Ipswich, MA). The DNA isolation kit and pfu DNA polymerase were purchased from Tiangen (Beijing, China). The substrates barley β-glucan, lichenan, laminarin, CMC-Na, birchwood xylan, Avicel, pNPG, pNPC and carob bean gum were purchased from Sigma-Aldrich (St. Louis, MO). The plasmid pPIC9 and P. pastoris GS115 (Invitrogen, Carlsbad, CA) were used as the gene expression vector and expression host strain, respectively. The low-molecular-weight calibration kit was purchased from GE Healthcare (Pittsburgh, PA). Minimal dextrose medium (MD), buffered glycerol-complex medium (BMGY) and buffered methanol-complex medium (BMMY) were prepared as described in the instructions of the Pichia Expression Kit (Invitrogen). All chemicals were of analytical grade and commercially available.
Gene cloning
The total genomic DNA of T. leycettanus JCM12802 was extracted using the Omega Fungal DNA Mini kit (Norcross, GA) after 72 h of shake cultivation in potato dextrose broth at 40 °C and used as template for PCR. A primer set specific for fungal GH16 β-glucanases (GH16F and GH16R) [34] was used to amplify the core region. The final PCR products (~550 bp) were purified, linked with pEASY-T3 vector and then transformed into E. coli Trans1-T1 for sequencing. TAIL-PCR [52] was conducted with the TaKaRa Genome Walking kit and six nested insertion-specific primers (Additional file 4) to obtain the 5′ and 3′ flanking regions of the core region. The flanking regions were sequenced and assembled with the core region sequence to give the full-length gene (Tlglu16A).
To induce β-glucanase production, T. leycettanus JCM12802 was cultured in a medium containing 5 g/L (NH4)2SO4, 1 g/L KH2PO4, 500 mg/L MgSO4·7H2O, 200 mg/L CaCl2, 10 mg/L FeSO4·7H2O and 30 g/L corn straw, pH 4.0 at 40 °C for 3 days. Total RNA was extracted using the Promega SV Total RNA Isolation System, and the cDNA was synthesized with a reverse transcription kit (TOYOBO, Osaka, Japan). The full-length cDNA of Tlglu16A was amplified using the specific primers Tlglu16A-F and Tlglu16A-R (Additional file 4). The PCR products were purified, linked with pEASY-T3 vector and then transformed into E. coli Trans-T1 for sequencing.
Sequence analysis and structure modeling of TlGlu16A
Nucleotide and amino acid sequences were analyzed using the BLASTx and BLASTp programs [53] (http://www.ncbi.nlm.nih.gov/BLAST/) in combination with the homology search. The ORF was identified using the program ORF Finder from the National Center for Biotechnology Information (NCBI). Vector NTI 10.0 software (Invitrogen) was used to forecast the molecular mass of the mature protein. Multiple-sequence alignment was analyzed with ClustalX and rendered by the ESPript3.0 program (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). The SignalP 4.0 server (http://www.cbs.dtu.dk/services/SignalP/) was used to predict signal peptide. The N- and O-glycosylation sites were predicted using online programs NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/) and NetOGlyc 4.0 Server (http://www.cbs.dtu.dk/services/NetOGlyc/), respectively. Based on the known crystal structure of a GH16 β-glucanase from P. thermophila (3WDT), the 3-D structures of TlGlu16A and its mutants were homologically modeled using the Swiss-model program (http://www.swissmodel.expasy.org/).
Protein expression, purification and identification
The gene fragment encoding mature TlGlu16A was amplified by PCR with the primers (Additional file 4) and then cloned into the vector pPIC9 at the EcoRI and NotI restriction sites. Transformed plasmids were electroporated into competent P. pastoris GS115 cells following the manufacturer’s instructions (Invitrogen). Ninety-six individual Pichia clones were selected and screened for β-glucanase activity on 0.5 % barley β-glucan as described by Chen et al. [34]. Those exhibiting the highest β-glucanase activity were subjected to high cell density fermentation in a 15-L fermenter (pH 5.0 and 30 °C) on the basis of the manufacturer’s instructions (Invitrogen). β-Glucanase production in culture supernatant was monitored every 24 h by glucanase activity assay until 144 h. The crude enzyme was loaded onto the HiTrap TM Desalting column and HiTrap TM Q Sepharose XL 5-mL FPLC column (GE Healthcare, Uppsala, Sweden) that were both equilibrated with 20 mM McIlvaine buffer (pH 6.8). Proteins were eluted using a linear gradient of NaCl (0–1.0 M) at a flow rate of 2.5 mL/min. Fractions were collected, assayed for enzyme activity and those having glucanase activities were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli [54]. Liquid chromatography/electrospray ionization tandem time-of-flight mass spectrometry (LC/ESI-TOF-MS) analysis of the trypsin-digested protein was conducted at the Institute of Zoology, Chinese Academy of Sciences.
The purified recombinant TlGlu16A was deglycosylated by Endo H at 37 °C for 2 h according to the manufacturer’s specifications. The deglycosylated enzyme was also analyzed by SDS-PAGE.
Glucanase activity assay
The glucanase activity was analyzed by measuring the release of reducing sugar from barley β-glucan and lichenan with 3,5-dinitrosalicylic acid (DNS) [55]. The standard reaction mixture system consisting of 100 μL of appropriately diluted enzyme and 900 μL of substrate solution (0.5 % [wt/vol] barley β-glucan or lichenan in McIlvaine buffer [200 mM Na2HPO4, 100 mM citric acid, pH4.0]) was incubated at 60 °C for 10 min. The reaction was terminated with 1.5 mL of DNS reagent. One unit of glucanase activity was defined as the amount of enzyme that produced 1 μmol of reducing sugar per minute. Each reaction and its controls were run in triplicate.
Biochemical characterization of recombinant TlGlu16A
The pH profile of TlGlu16A was determined by measuring the glucanase activity after incubation at 60 °C in McIlvaine buffer (pH 2.2–8.0) containing 0.5 % barley β-glucan for 10 min. To determine the temperature for maximal activity, enzyme activity was measured at optimal pH and temperatures between 30 and 90 °C in McIlvaine buffer (pH 4.0) for 10 min. To verify pH stability, the enzyme was preincubated in buffers of various pH values (pH 1.0–12.0) at 37 °C for 1 h, and the residual enzyme activity was detected under standard conditions (pH 4.5, 65 °C and 10 min). The buffers used were 100 mM glycine–HCl (pH 1.0–3.0), McIlvaine buffer (pH 2.5–8.0), 100 mM Tris–HCl (pH 8.0–9.0) and 100 mM glycine–NaOH (pH 9.0–12.0).
Thermal stability was determined by measuring the residual enzyme activity after incubation at 55–90 °C for 30 min and the half-life of enzyme inactivation (t1/2) at 65–80 °C and optimal pH. The residual enzyme activities were measured under standard conditions.
To evaluate the effect of metal ions and chemical reagents on the glucanase activity of purified recombinant TlGlu16A, different salts (KCl, NaCl, CaCl2, CoCl2, MgSO4, NiSO4, CrCl3, CuSO4, FeCl3, MnSO4, AgNO3, Pb(CH3COO)2, ZnSO4 and HgCl2) (5 and 10 mM) and chemical reagents (EDTA, SDS and β-mercaptoethanol) (5 and 10 mM) were added to the assay system individually. The relative activities were determined against the activity of the control sample without any addition [39].
Substrate specificity of TlGlu16A was assayed at 65 °C for 10 min in McIlvaine buffer (pH 4.5) containing 1.0 % (w/v) barley β-glucan, lichenan, laminarin, birch wood, xylan, Avicel, CMC-Na, pNPG or pNPC. The kinetic parameters Km and Vmax were determined in McIlvaine buffer (pH 4.5) containing 0.25–5.0 mg/mL of barley β-glucan and lichenan for 5 min. The kinetic values were determined by fitting the Lineweaver–Burk plot [56].
The hydrolysis products of barley β-glucan and lichenan by TlGlu16A were determined using the TLC. Each enzyme (50 U) was added into 1 mL of 1 % (w/v) of each substrate in 50 mM Na2HPO4–citric acid (pH 4.5) and incubated at 55 °C for 1 h. Aliquots were withdrawn at different time intervals and boiled for 5 min to terminate the reactions. The hydrolysis products were spotted on a silica gel plate (model 60F254; Merck, Darmstadt, Germany). The plate was developed with two runs in a solvent system of butanol:acetic acid:water (2:1:1, v/v/v). After spraying with methanol:sulfuric acid (95:5, v/v) solvent, the sugars on the plate were visualized by heating for a few minutes at 130 °C. A cellooligosaccharide mixture consisting of glucose (G1), cellobiose (G2), cellotriose (G3), cellotetraose (G4) and cellopentaose (G5) was used as the standard.
ETSS calculation and site-directed mutagenesis
The total interaction energy (Eij) between charged amino acids of TlGlu16A was calculated using the ETSS [57, 58]. Any mutation that changes the Eij value from positive to negative values may improve the structural stability of TlGlu16A. According to the combined strategy of potential mutation site selection [33], multiple-sequence alignment and structure modeling (PyMOL; Delano Scientific, Portland, OR), charged residues were modified with oppositely charged ones and otherwise to neutral Ala.
Site-directed mutagenesis was conducted by overlap extension PCR [33] with the gene fragment of Tlglu16A as the DNA template and the primers listed in additional files 1, 2, 3 and 4. The PCR products were ligated to the vector pEASY-T3 for sequencing. The mutants harboring the correct mutations were digested by restriction endonucleases, expressed in P. pastoris and characterized as described above.
Thermal properties of the wild-type TlGlu16A and its mutants
Thermostability was determined by measuring the half-life of enzyme inactivation (t1/2) at 65–80 °C and each optimum pH. All purified enzymes were diluted to 100 mg/mL in McIlvaine buffer and incubated for different durations (TlGlu16A (pH 4.5), H58D (pH 4.5), E134R (pH 4.0), D235G (pH 4.0) and D296K (pH 4.0)). The residual enzyme activities were measured under optimal conditions of each enzyme (TlGlu16A (pH 4.5 and 65 °C), H58D (pH 4.5 and 70 °C), E134R (pH 4.0 and 65 °C), D235G (pH 4.0 and 70 °C) and D296K (pH 4.0 and 70 °C)). To determine the thermal tolerance (temperature at which 50 % of activity is lost [T50]), wild-type TlGlu16A and mutants were diluted to 50 μg/mL in McIlvaine buffer (TlGlu16A (pH 4.5), H58D (pH 4.5), E134R (pH 4.0), D235G (pH 4.0) and D296K (pH 4.0)) and then heated for 30 min at temperatures ranging from 55 to 90 °C at intervals of 5 °C. After heating, the enzymes were immediately placed on ice for 10 min, followed by the residual glucanase activity assay as described above. All reactions were performed in triplicate.
DSC analysis
The melting temperature, Tm, was determined by a Nano-DSC (TA Instruments, New Castle, DE). Each protein sample, 350 μg, was dissolved in 1 mL of 10 mM PBS (pH 6.8). The same amount of buffer was used as the blank control. The degassed protein samples and control were loaded into the calorimeter cells and treated with a heating rate of 1 °C/min over the temperature range of 25–100 °C. The transition state was scanned at the rate of 1 °C/min. Each experiment was repeated at least three times.
Enzymatic hydrolysis of corn stover
Corn stover was treated with 15 % (w/w) ammonia solution for 24 h as previously reported [9]. Wild-type TlGlu16A and mutant H58D (each at 0.06 μmol) were each incubated in 100 mM sodium citrate (pH 4.8) containing 5 % (w/v) corn stover at 50 °C and 165 rpm for 60 h. The reducing sugars released were determined every 12 h using the DNS method. The experiment was performed in triplicate.
Nucleotide sequence accession number
The nucleotide sequence of the endoβ-1,3-1,4-glucanase gene (Tlglu16A) from T. leycettanus JCM12802 was deposited in the GenBank database under accession no. KU194473.
Authors’ contributions
SY designed the study, performed the major experiments containing the gene cloning, site-directed mutagenesis and enzyme production, and wrote the manuscript. TT performed the ETSS calculation and key residue identification. LZ wrote the ETSS calculation program. YW participated in the discussion. HH carried out fermentation and discussion. RM participated in the discussion and revised the manuscript. PS performed the HPAEC analysis. YB performed the HPAEC and DSC analysis. XS performed the DSC analysis. ZL performed the CD experiment and analyzed the data. HL supervised the work and the writing of the manuscript. YB supervised the work and the writing of the manuscript. HL and YB were the corresponding authors. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors provide their consent for publication of their manuscript in Biotechnology for Biofuels.
Funding
Funding was provided by the National High Technology Research and Development Program of China (2013AA102803), the Special Fund for Agro-Scientific Research in the Public Interest (201403047), the National Science Fund for Distinguished Young Scholars (31225026) and the China Modern Agriculture Research System (CARS-42).
Abbreviations
- CMC-Na
carboxymethyl cellulose-sodium
- TAIL-PCR
thermal asymmetric interlaced-PCR
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- DNS
3,5-dinitrosalicylic acid
- RT-PCR
reverse-transcription PCR
- ORF
open reading frame
- LC/ESI-TOF-MS
liquid chromatography/electrospray ionization tandem time-of-flight mass spectrometry
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight
- ETSS
enzyme thermal stability system
- MD
minimal dextrose medium
- BMGY
buffered glycerol-complex medium
- BMMY
buffered methanol-complex medium
- TLC
thin layer chromatography
- Endo H
endo-β-N-acetylglucosaminidase H
- pNPG
4-nitrophenyl α-d-galactopyranoside
- pNPC
4-nitrophenyl β-d-cellobioside
- DSC
differential scanning calorimetry
Additional files
10.1186/s13068-016-0544-8 Multiple sequence alignment of deduced TlGlu16A and five other fungal counterparts of GH16.
10.1186/s13068-016-0544-8 SDS-PAGE analysis of purified recombinant TlGlu16A and its mutants. Lanes: M, the standard protein molecular weight markers; G1, the crude enzyme of wild-type TlGlu16A; G2, 2, 4, 6 and 8, the purified TlGlu16A and mutants H58D, E134R, D235G and D296K, respectively; G3, 1, 3, 5 and 7, the deglycosylated TlGlu16A and mutants H58D, E134R, D235G and D296K, respectively.
10.1186/s13068-016-0544-8 Total interaction energies of TlGlu16A determined by ETSS.
10.1186/s13068-016-0544-8 Oligonucleotide primers used in this study.
Contributor Information
Shuai You, Email: ytyoushuai@163.com.
Tao Tu, Email: tut715@sina.com.
Lujia Zhang, Email: ljzhang@ecust.edu.cn.
Yuan Wang, Email: wangyuan08@caas.cn.
Huoqing Huang, Email: huoqinghuang@caas.cn.
Rui Ma, Email: marui@caas.cn.
Pengjun Shi, Email: shipengjun@caas.cn.
Yingguo Bai, Email: baiyingguo@caas.cn.
Xiaoyun Su, Email: suxiaoyun@caas.cn.
Zhemin Lin, Email: Lzmin01@126.com.
Huiying Luo, Phone: +86 10 82106053, Email: luohuiying@caas.cn.
Bin Yao, Phone: +86 10 82106053, Email: binyao@caas.cn.
References
- 1.Buliga GS, Brant DA, Fincher GB. The sequence statistics and solution conformation of a barley (1 → 3, 1 → 4)-β-D-glucan. Carbohydr Res. 1986;157:139–156. doi: 10.1016/0008-6215(86)85065-0. [DOI] [PubMed] [Google Scholar]
- 2.Stone BA, Clarke AE. Chemistry and biology of 1, 3-β-glucans. Melbourne: La Trobe University Press; 1992. [Google Scholar]
- 3.Planas A. Bacterial 1,3-1,4-β-glucanases: structure, function and protein engineering. Biochim Biophys Acta: Protein Struct Mol Enzymol. 2000;1543:361–382. doi: 10.1016/S0167-4838(00)00231-4. [DOI] [PubMed] [Google Scholar]
- 4.Dashtban M, Schraft H, Qin W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int J Biol Sci. 2009;5:578. doi: 10.7150/ijbs.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy T, Hanniffy O, Savage AV, Tuohy MG. Catalytic properties and mode of action of three endo-β-glucanases from Talaromyces emersonii on soluble β-1,4-and β-1,3-1,4-linked glucans. Int J Biol Macromol. 2003;33:141–148. doi: 10.1016/S0141-8130(03)00080-1. [DOI] [PubMed] [Google Scholar]
- 6.Celestino KR, Cunha RB, Felix CR. Characterization of a β-glucanase produced by Rhizopus microsporus var. microsporus, and its potential for application in the brewing industry. BMC Biochem. 2006;7:23. doi: 10.1186/1471-2091-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckmann L, Simon O, Vahjen W. Isolation and identification of mixed linked β-glucan degrading bacteria in the intestine of broiler chickens and partial characterization of respective 1,3-1,4-β-glucanase activities. J Basic Microbiol. 2006;46:175–185. doi: 10.1002/jobm.200510107. [DOI] [PubMed] [Google Scholar]
- 8.Bamforth C, Martin HL. The degradation of β-glucan during malting and mashing: the role of β-glucanase. J Inst Brew. 1983;89:303–307. doi: 10.1002/j.2050-0416.1983.tb04190.x. [DOI] [Google Scholar]
- 9.Li X, Kim TH, Nghiem NP. Bioethanol production from corn stover using aqueous ammonia pretreatment and two-phase simultaneous saccharification and fermentation (TPSSF) Bioresour Technol. 2010;101:5910–5916. doi: 10.1016/j.biortech.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Berka RM, Grigoriev IV, Otillar R, Salamov A, Grimwood J, Reid I, et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat Biotechnol. 2011;29:922–927. doi: 10.1038/nbt.1976. [DOI] [PubMed] [Google Scholar]
- 11.Chan L, Cross HF, She JK, Cavalli G, Martins HF, Neylon C. Covalent attachment of proteins to solid supports and surfaces via Sortase-mediated ligation. PLoS One. 2007;2:e1164. doi: 10.1371/journal.pone.0001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teather RM, Erfle JD. DNA sequence of a Fibrobacter succinogenes mixed-linkage β-glucanase (1,3-1,4-β-D-glucan 4-glucanohydrolase) gene. J Bacteriol. 1990;172:3837–3841. doi: 10.1128/jb.172.7.3837-3841.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi P, Yao G, Yang P, Li N, Luo H, Bai Y, et al. Cloning, characterization, and antifungal activity of an endo-1,3-β-D-glucanase from Streptomyces sp. S27. Appl Microbiol Biotechnol. 2010;85:1483–1490. doi: 10.1007/s00253-009-2187-1. [DOI] [PubMed] [Google Scholar]
- 14.Qiao J, Dong B, Li Y, Zhang B, Cao Y. Cloning of a β-1,3-1,4-glucanase gene from Bacillussubtilis MA139 and its functional expression in Escherichia coli. Appl Biochem Biotechnol. 2009;152:334–342. doi: 10.1007/s12010-008-8193-4. [DOI] [PubMed] [Google Scholar]
- 15.Teng D, Fan Y, Yang Y, Tian Z, Luo J, Wang J. Codon optimization of Bacillus licheniformis β-1,3-1,4-glucanase gene and its expression in Pichia pastoris. Appl Microbiol Biotechnol. 2007;74:1074–1083. doi: 10.1007/s00253-006-0765-z. [DOI] [PubMed] [Google Scholar]
- 16.Bai Y, Wang J, Zhang Z, Shi P, Luo H, Huang H, et al. A novel family 9 β-1,3(4)-glucanase from thermoacidophilic Alicyclobacillus sp. A4 with potential applications in the brewing industry. Appl Microbiol Biotechnol. 2010;87:251–259. doi: 10.1007/s00253-010-2452-3. [DOI] [PubMed] [Google Scholar]
- 17.Hua C, Yan Q, Jiang Z, Li Y, Katrolia P. High-level expression of a specific β-1,3-1,4-glucanase from the thermophilic fungus Paecilomyces thermophila in Pichia pastoris. Appl Microbiol Biotechnol. 2010;88:509–518. doi: 10.1007/s00253-010-2759-0. [DOI] [PubMed] [Google Scholar]
- 18.Niture S. Comparative biochemical and structural characterizations of fungal polygalacturonases. Biologia. 2008;63:1–19. doi: 10.2478/s11756-008-0018-y. [DOI] [Google Scholar]
- 19.Wang C, Luo H, Niu C, Shi P, Huang H, Meng K, et al. Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity. Appl Microbiol Biotechnol. 2015;99:1217–1228. doi: 10.1007/s00253-014-5979-x. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Wang H, Ma R, Shi P, Niu C, Luo H, et al. Biochemical characterization of a novel thermophilic α-galactosidase from Talaromyces leycettanus JCM12802 with significant transglycosylation activity. J Biosci Bioeng. 2015;S1389–1723:00197–00198. doi: 10.1016/j.jbiosc.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Szilágyi A, Závodszky P. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure. 2000;8:493–504. doi: 10.1016/S0969-2126(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 22.Chakravarty S, Varadarajan R. Elucidation of factors responsible for enhanced thermal stability of proteins: a structural genomics based study. Biochemistry. 2002;41:8152–8161. doi: 10.1021/bi025523t. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Zhou X, Lu P. Structural features of thermozymes. Biotechnol Adv. 2005;23:271–281. doi: 10.1016/j.biotechadv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Luo H, Tian J, Turunen O, Huang H, Shi P, et al. Thermostability improvement of a Streptomyces xylanase by introducing proline and glutamic acid residues. Appl Environ Microbiol. 2014;80:2158–2165. doi: 10.1128/AEM.03458-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki K, Wintrode PL, Grayling RA, Rubingh DN, Arnold FH. Directed evolution study of temperature adaptation in a psychrophilic enzyme. J Mol Biol. 2000;297:1015–1026. doi: 10.1006/jmbi.2000.3612. [DOI] [PubMed] [Google Scholar]
- 26.Strickler SS, Gribenko AV, Gribenko AV, Keiffer TR, Tomlinson J, Reihle T, et al. Protein stability and surface electrostatics: a charged relationship. Biochemistry. 2006;45:2761–2766. doi: 10.1021/bi0600143. [DOI] [PubMed] [Google Scholar]
- 27.Schweiker KL, Zarrine-Afsar A, Davidson AR, Makhatadze GI. Computational design of the Fyn SH3 domain with increased stability through optimization of surface charge–charge interactions. Protein Sci. 2007;16:2694–2702. doi: 10.1110/ps.073091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanford C, Kirkwood JG. Theory of protein titration curves. I. General equations for impenetrable spheres. J Am Chem Soc. 1957;79:5333–5339. doi: 10.1021/ja01577a001. [DOI] [Google Scholar]
- 29.Richmond TJ. Solvent accessible surface area and excluded volume in proteins: analytical equations for overlapping spheres and implications for the hydrophobic effect. J Mol Biol. 1984;178:63–89. doi: 10.1016/0022-2836(84)90231-6. [DOI] [PubMed] [Google Scholar]
- 30.Bashford D, Karplus M. Multiple-site titration curves of proteins: an analysis of exact and approximate methods for their calculation. J Phys Chem. 1991;95:9556–9561. doi: 10.1021/j100176a093. [DOI] [Google Scholar]
- 31.Havranek JJ, Harbury PB. Tanford–Kirkwood electrostatics for protein modeling. Proc Natl Acad Sci USA. 1999;96:11145–11150. doi: 10.1073/pnas.96.20.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Tang X, Cui D, Yao Z, Gao B, Jiang S, et al. A method to rationally increase protein stability based on the charge–charge interaction, with application to lipase LipK107. Protein Sci. 2014;23:110–116. doi: 10.1002/pro.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu T, Luo H, Meng K, Cheng Y, Ma R, Shi P, et al. Improvement in thermostability of an Achaetomium sp. strain Xz8 endopolygalacturonase via the optimization of charge–charge interactions. Appl Environ Microbiol. 2015;81:6938–6944. doi: 10.1128/AEM.01363-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Meng K, Shi P, Bai Y, Luo H, Huang H, et al. High-level expression of a novel Penicillium endo-1,3(4)-β-D-glucanase with high specific activity in Pichia pastoris. J Ind Microbiol Biotechnol. 2012;39:869–876. doi: 10.1007/s10295-012-1087-z. [DOI] [PubMed] [Google Scholar]
- 35.Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev. 2000;24:45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 36.Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22:249–270. doi: 10.1002/yea.1208. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Yang P, Luo H, Tang H, Shao N, Yuan T, et al. High-level expression of a truncated 1,3-1,4-β-D-glucanase from Fibrobacter succinogenes in Pichia pastoris by optimization of codons and fermentation. Appl Microbiol Bbiotechnol. 2008;78:95–103. doi: 10.1007/s00253-007-1290-4. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Tang C, Shi H, Wu M. Cloning and optimized expression of a neutral endoglucanase gene (ncel5A) from Volvariella volvacea WX32 in Pichia pastoris. J Biosci Bioeng. 2011;111:537–540. doi: 10.1016/j.jbiosc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Luo H, Yang J, Li J, Shi P, Huang H, Bai Y, et al. Molecular cloning and characterization of the novel acidic xylanase XYLD from Bispora sp. MEY-1 that is homologous to family 30 glycosyl hydrolases. Appl Microbiol Biotechnol. 2010;86:1829–1839. doi: 10.1007/s00253-009-2410-0. [DOI] [PubMed] [Google Scholar]
- 40.Yang P, Shi P, Wang Y, Bai Y, Meng K, Luo H, et al. Cloning and overexpression of a Paenibacillus β-glucanase in Pichia pastoris: purification and characterization of the recombinant enzyme. J Microbiol Biotechnol. 2007;17:58–66. [PubMed] [Google Scholar]
- 41.Görlach JM, Van Der Knaap E, Walton JD. Cloning and targeted disruption of MLG1, a gene encoding two of three extracellular mixed-linked glucanases of Cochliobolus carbonum. Appl Environ Microbiol. 1998;64:385–391. doi: 10.1128/aem.64.2.385-391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, Li X, Ljungdahl LG. Sequencing of a 1,3-1,4-β-D-glucanase (lichenase) from the anaerobic fungus Orpinomyces strain PC-2: properties of the enzyme expressed in Escherichia coli and evidence that the gene has a bacterial origin. J Bacteriol. 1997;179:6028–6034. doi: 10.1128/jb.179.19.6028-6034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Qiaojuan Y, Jiang Z, Fan G, Wang L. Biochemical characterization of a novel thermostable β-1,3-1,4-glucanase (lichenase) from Paecilomyces thermophila. J Agric Food Chem. 2008;56:5345–5351. doi: 10.1021/jf800303b. [DOI] [PubMed] [Google Scholar]
- 44.Hong MR, Kim YS, Joo AR, Lee JK, Kim YS, Oh DK. Purification and characterization of a thermostable beta-1,3-1,4-glucanase from Laetiporus sulphureus var. miniatus. J Microbiol Biotechnol. 2009;8:818–822. [PubMed] [Google Scholar]
- 45.Murray PG, Grassick A, Laffey CD, Cuffe MM, Higgins T, Savage AV, et al. Isolation and characterization of a thermostable endo-β-glucanase active on 1,3-1,4-β-D-glucans from the aerobic fungus Talaromyces emersonii CBS 814.70. Enzyme Microb Technol. 2001;29:90–98. doi: 10.1016/S0141-0229(01)00354-4. [DOI] [PubMed] [Google Scholar]
- 46.Hua C, Yi H, Jiao L. Cloning and expression of the endo-1,3(4)-β-glucanase gene from Paecilomyces sp. FLH30 and characterization of the recombinant enzyme. Biosci Biotechnol Biochem. 2011;75:1807–1812. doi: 10.1271/bbb.110354. [DOI] [PubMed] [Google Scholar]
- 47.Tang Y, Yang S, Yan Q, Zhou P, Cui J, Jiang Z. Purification and characterization of a novel β-1,3–1,4-glucanase (lichenase) from thermophilic Rhizomucor miehei with high specific activity and its gene sequence. J Agric Food Chem. 2012;60:2354–2361. doi: 10.1021/jf2049799. [DOI] [PubMed] [Google Scholar]
- 48.Grishutin SG, Gusakov AV, Dzedzyulya EI, Sinitsyn AP. A lichenase-like family 12 endo-(1 → 4)-β-glucanase from Aspergillus japonicus: study of the substrate specificity and mode of action on β-glucans in comparison with other glycoside hydrolases. Carbohydr Res. 2006;341:218–229. doi: 10.1016/j.carres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Kaur J, Chadha BS, Kumar BA, Saini HS. Purification and characterization of two endoglucanases from Melanocarpus sp. MTCC 3922. Bioresour Technol. 2007;98:74–81. doi: 10.1016/j.biortech.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Chaari F, Bhiri F, Blibech M, Maktouf S, Ellouz-Chaabouni S, Ellouz-Ghorbel R. Potential application of two thermostable lichenases from a newly isolated Bacillus licheniformis UEB CF: purification and characterization. Process Biochem. 2012;47:509–516. doi: 10.1016/j.procbio.2011.12.010. [DOI] [Google Scholar]
- 51.Ertan H, Siddiqui KS, Muenchhoff J, Charlton T, Cavicchioli R. Kinetic and thermodynamic characterization of the functional properties of a hybrid versatile peroxidase using isothermal titration calorimetry: insight into manganese peroxidase activation and lignin peroxidase inhibition. Biochimie. 2012;94:1221–1231. doi: 10.1016/j.biochi.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Mitsukawa N, Oosumi T, Whittier RF. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995;8:457–463. doi: 10.1046/j.1365-313X.1995.08030457.x. [DOI] [PubMed] [Google Scholar]
- 53.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 55.Breuil C, Saddler J. Comparison of the 3,5-dinitrosalicylic acid and Nelson-Somogyi methods of assaying for reducing sugars and determining cellulase activity. Enzyme Microb Technol. 1985;7:327–332. doi: 10.1016/0141-0229(85)90111-5. [DOI] [Google Scholar]
- 56.Thomson AB. A theoretical discussion of the use of the Lineweaver–Burk plot to estimate kinetic parameters of intestinal transport in the presence of unstirred water layers. Can J Physiol Pharmacol. 2011;59:932–948. doi: 10.1139/y81-144. [DOI] [PubMed] [Google Scholar]
- 57.Strickler SS, Gribenko AV, Gribenko AV, Keiffer TR, Tomlinson J, Reihle T, Loladze VV, Makhatadze GI. Protein stability and surface electrostatics: a charged relationship. Biochemistry. 2006;45:2761–2766. doi: 10.1021/bi0600143. [DOI] [PubMed] [Google Scholar]
- 58.Schweiker KL, Zarrine-Afsar A, Davidson AR, Makhatadze GI. Computational design of the Fyn SH3 domain with increased stability through optimization of surface charge charge interactions. Protein Sci. 2007;16:2694–2702. doi: 10.1110/ps.073091607. [DOI] [PMC free article] [PubMed] [Google Scholar]


