Abstract
Dental pain is the most common symptom associated with a wide array of dental problems and significantly impacts the oral health-related quality of life. The epidemiology and prevalence of oral diseases that could lead to dental pain are diverse and indicate regional variations. Several researchers have dwelled into the neurobiology and pathophysiology of dental pain making the pain pathways more clear and deciphering the precise targets for the management of pain. Although a number of pharmacological drugs are available in the market, a significant percentage of the population in India prefers alternative herbal medication for relief from dental pain due to the side effects and interactions of pharmacological treatment. However, there is a void in dental literature pertaining to the use, benefits, and safety of the herbal medicines. Therefore, the present assessment has been penned down, focusing on the current multimodal approaches for treating dental pain, the current unmet need, and the role of herbal medication in India for the management of dental pain, with a discussion on novel herbal dental gel.
Keywords: Alternative medicine, dental pain, herbal gel
Introduction
For there was never yet philosopher; that could endure the toothache patiently
William Shakespeare
Oral health is an integral component of general health. Oral health problems such as dental caries, periodontal diseases, and oral cancers are global concerns restricting and confining the day-to-day errands and chores. The 2005 Liverpool Declaration has reaffirmed that oral health should be considered as a basic human right.[1] Across the world, millions of people experience oral diseases, resulting in unneeded pain and suffering.
The International Association for the study of pain defines pain as an “unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”[2]
Dental pain is a common symptom associated with a variety of dental problems such as dental caries which significantly impacts the oral health-related quality of life.[3]
Epidemiological Data
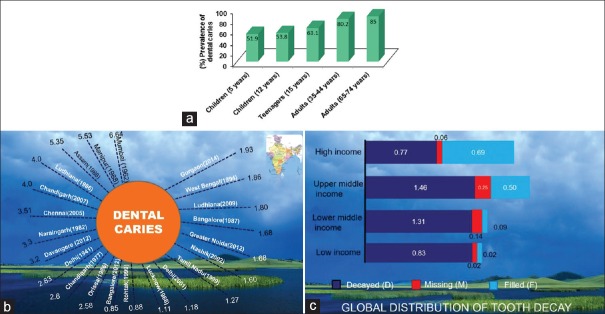
According to the World Health Survey [4] conducted by the World Health Organization (WHO) in India in 2003, 28% respondents had suffered from oral health problem, maximum being from West Bengal, i.e. 42% [Figure 1]. The study conducted by Khan et al.[5] documented that the prevalence of dental caries in some parts of India is as high as 60–65%, whereas the prevalence rate is 35% worldwide.[6] An extensive and comprehensive National Health Survey was conducted in 2004 in India to determine the oral health status and prevalence of dental disease in representative age groups.[7] Percent prevalence of dental caries (coronal and root surfaces) for various age groups, geographical distribution, is shown in Figure 2a–c.
Figure 1.
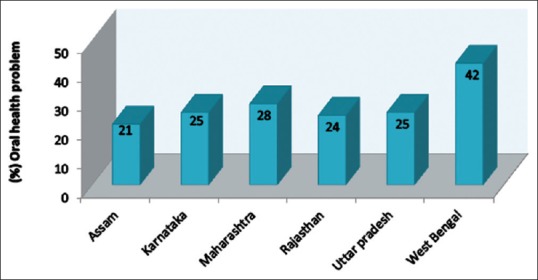
Oral health status in different states of India (World health survey, 2003)
Figure 2.
(a) Occurrence of dental caries in different Socio-economic groups around the Globe. (National Health Survey India, 2004). (b) Occurrence of dental caries in different regions in India. (c) Average number of affected teeth for 12 year old by country income group 2000 or latest available data. High income, upper middle income, lower middle income, low income like India (Oral Health Atlas FDI 2015)
Neurobiology and Pathophysiology of Dental Pain
Dental pain is caused by noxious pain stimuli such as bacterial infections, chemical or mechanical erosion of enamel, and recession of gingiva. Patent dentinal tubules are the first structure to be involved in dentinal pain signal transduction, postdental insult. According to hydrodynamic theory, movement of fluid within the dentinal tubules induces pain via pain fibers located around the odontoblast process and at the pulp-dentine border.[8,9] A dense network of trigeminal sensory axons closely linked to odontoblasts may also be involved in pain transmission.
It has been well documented that odontoblasts express mechano- and/or thermo-sensitive transient receptor potential vanilloid (TRPV) ion channels that are likely to sense heat and/or cold movements of dentinal fluid within the tubules.[10] These receptors are transmembrane receptor-ion channel complex [11] and distributed in peripheral, spinal, and central nervous system.[12] In the TRPV family, TRPV-1 receptors are mainly responsible for the perception of warming, burning, stinging, or itching sensation [Figure 3].
Figure 3.
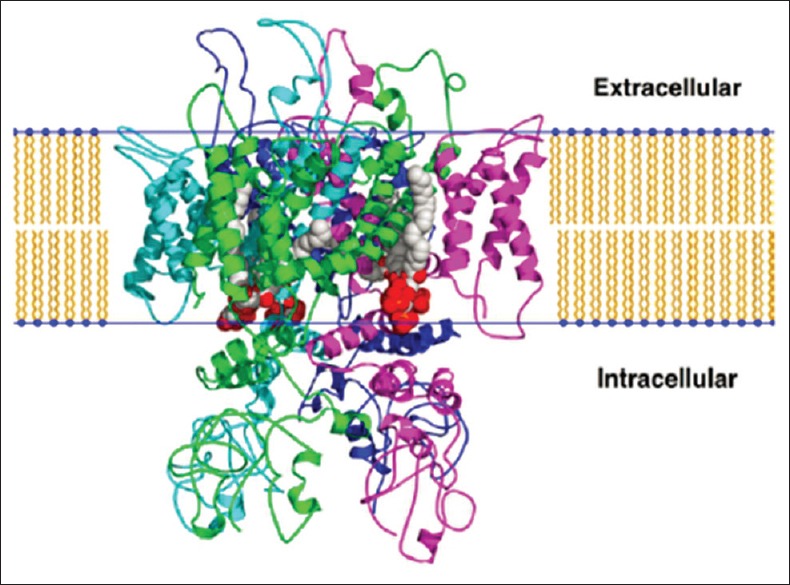
The transient receptor potential vanilloid 1 ion channel
The nerve supply of the dentin-odontoblast-pulp complex is mainly made up of mechanosensitive nociceptors, namely, A fibers (both δ and β) and C fibers [Figure 4] which selectively express TRPV-1 receptors. The A fibers transmit pain directly to the thalamus, generating a fast, sharp pain that can be easily localized. The C fibers get influenced by many modulating interneurons before reaching the thalamus, thus resulting in a slow pain which is generally characterized as dull and aching. Because of their location and arrangement, the C fibers are responsible for referred pain. The excitement of A-δ fibers seems to have a negligible effect, whereas activation of C fibers increases pulpal blood flow. This increase induced by C fibers is caused by neurokinins, especially substance P (SP), which is released from C fibers nerve terminals and is involved both in inflammation and in pain.[13] Neurogenic inflammation due to peripheral release of neuropeptides causes changes in vascular permeability of the dental pulp.[14]
Figure 4.
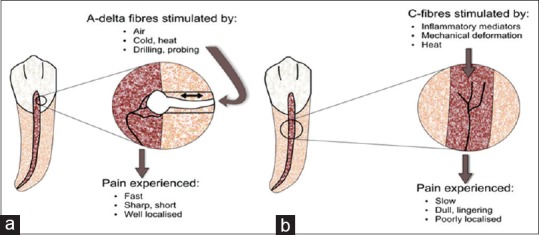
Diagram illustrating the characteristics of A-delta (a) and C-nerve (b) fibers within the dental pulp
Through the A and C nociceptors, pain (action potential) reaches to the dorsal horn of the spinal cord. From dorsal horns, pain signals travel to thalamus via the spinothalamic tract. Thalamus acts as a relay station for processing the pain information. Pain signals are then transmitted to somatosensory cortex to localize and characterize the pain. Cortex sends signals to descending pathway to modulate (change or inhibit) the pain impulse [Figure 5]. These descending fibers release substances (endogenous opioids, serotonin, and norepinephrine) that bind to the opioid receptors and prevent the release of the neurotransmitters such as glutamate or SP, thereby obstructing the pain signal from being transmitted.
Figure 5.
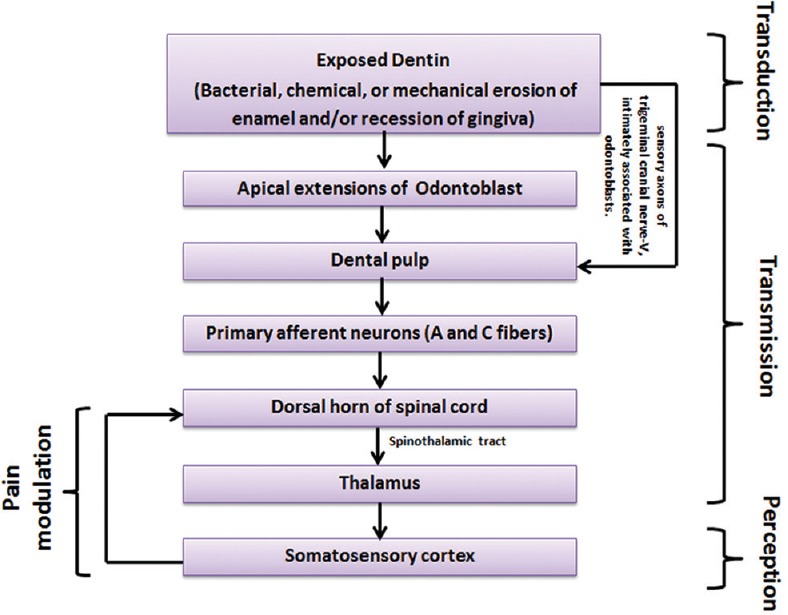
Neurological dental pain pathway from site of injury to brain
Some people have well-defined modulation pathways while others have less ability to modulate same amplitude of pain, which is one of the reasons why pain is a very subjective feeling.
Dentist–patient Interaction
Dentist–patient communication is a major factor in pain management and patient satisfaction. The line of communication may be direct (face-to-face) or indirect (telephonic/email/chat). In both cases, it has been observed that patients prefer dentists who are interactive and nondominating in nature.[15] Some of the positive and negative behavioral aspects are listed in Table 1. A simple “perceived lack” of caring and/or collaboration by the dentist may be associated with nonresponsiveness of the patient to the intervention. Positive communication is a key to building patient's confidence and belief toward further interventions. Without such faith and trust, there may be a poor “fit” between the intended messages by dentist and what is understood by the patient. A study by Lahti et al.[16] showed that patient expectations were met on the most dentist characteristics, except fair support and mutual communication, indicating that more attention needs to be paid to the communication skills of dentists.
Table 1.
Behavioral guidance to dentists while treating patient in dental pain

Nature of Pain
Common types of acute dental pain which are likely to cause a patient to seek emergency care are categorized in Figure 6. The nature of pain can help the clinician achieve a proper diagnosis.
Figure 6.
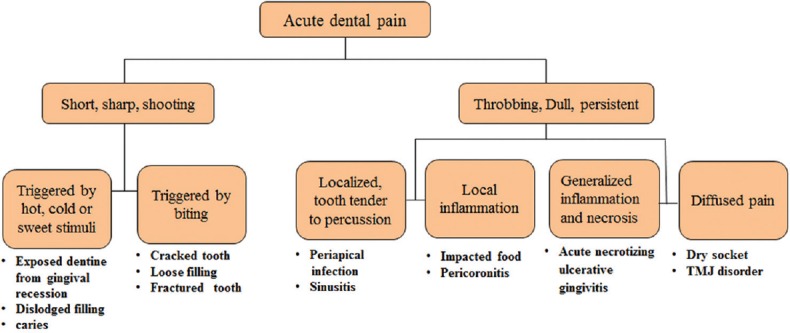
Probable causes of common types of acute dental pain
Short, sharp, shooting pain
This type of pain can be generalized due to tooth sensitivity/dentin hypersensitivity which is a short, sharp pain caused due to exposed dentin in response to external stimuli. The pain is localized to the affected tooth which can be attributed to fractured dental restorations, cracked cusps, or pulp exposure. Intermittent, shooting, and sharp pains are also symptomatic of trigeminal neuralgia, so good care must be taken not to mistakenly label toothache as neuralgia.[17]
Persistent, dull, throbbing pain
This type of pain may have several causes and the most common one is dental caries. It is commonly seen with recurrent or secondary caries associated with an existing restoration. In case of irreversible pulpitis, necrosis of pulp may follow leading to the development of a periapical infection. Soft-tissue problems may also cause dull, throbbing, persistent pain with local inflammation (e.g., gingivitis associated with food impaction), or pericoronitis. Other causes may include dry socket, temporomandibular disorders, and maxillary sinusitis.[17]
Management of Dental Pain in Clinical Practice
Three-dimensional approach
The “3-D” principle [Figure 7] is used in the following order to manage pain in dental practice:[18]
Figure 7.
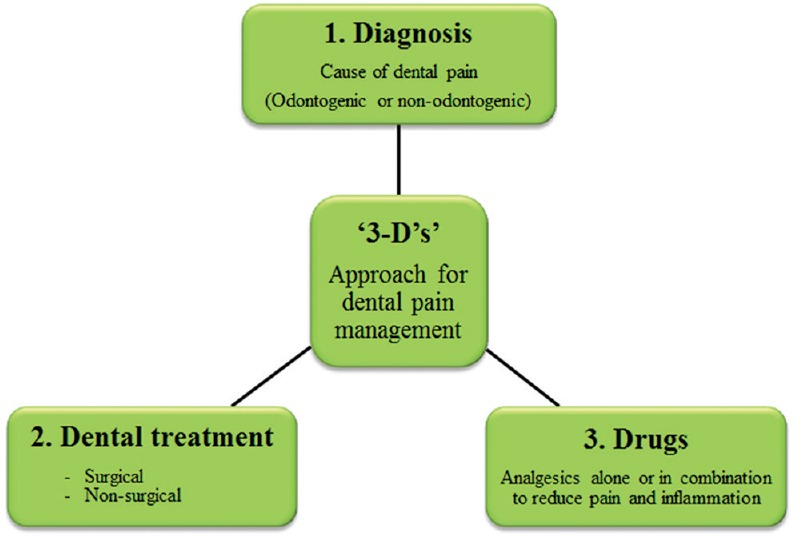
Principle of ”3-D”s’ in dental pain management
Diagnosis
Dental treatment
Drugs.
Drugs in Management of Dental Pain
Pharmacological approach
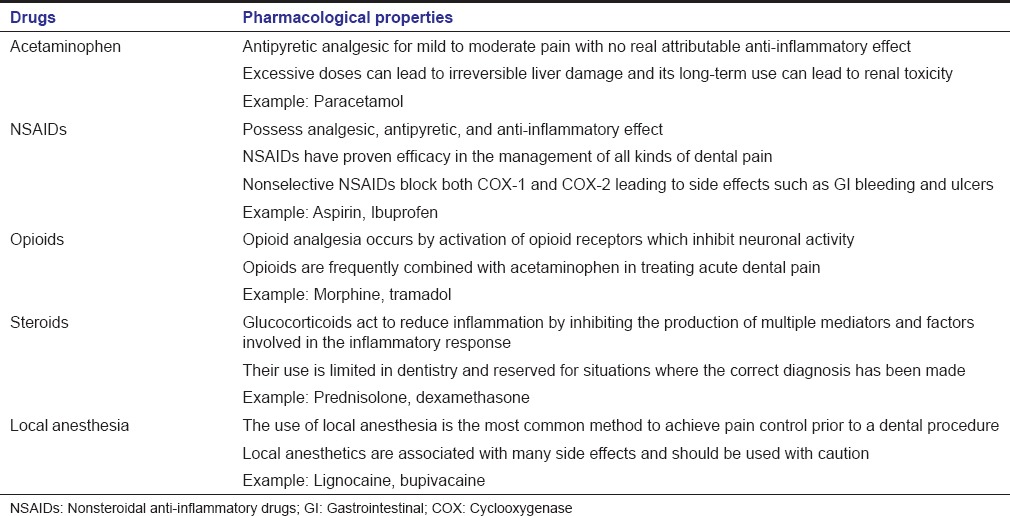
Several analgesics with different mechanisms of actions and acting at different sites in the nervous system are routinely prescribed in the current clinical practice [Table 2].[19,20,21,22,23,24]
Table 2.
Commonly prescribed drugs in management of dental pain
Limitations of pharmacological methods
Inflammation is not always the cause of pain.[25] Even the strongest of the anti-inflammatory medications do not render substantial therapeutic benefits to the patient if the underlying cause of pain persists. Despite widespread use in dental pain management, pharmacological interventions are associated with plenty of adverse events and other limitations such as those listed below:
Delayed onset of action in case of orally administered medications [26]
Selective and nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated in patients allergic to aspirin [27]
Nonselective NSAIDs may cause gastrointestinal toxicity, depression of renal function, increased risk of heart failure, and inhibition of platelet aggregation due to cyclooxygenase (COX-1) inhibition. Selective COX-2 antagonists may cause myocardial infarction [28]
Commonly used NSAIDs have a “ceiling effect” which limits their efficacy and dose [29]
Opioid analgesics are controlled substances and have greater abuse potential [30]
Use of opioids is associated with variability in patient response and several adverse reactions including nausea, emesis, and respiratory depression [31,32,33]
Corticosteroids are immunosuppressants, therefore not suitable for pain management in dental problems/dental infections [34]
Side effects with injectable local anesthesia: Paresthesia, hematoma, tachycardia, and inferior alveolar nerve damage [35]
Drug–drug interactions are a common problem with all pharmacologic agents discussed above.[36]
Nonpharmacological Methods
Role of alternative/herbal medications in dental pain management
The major drawback of conventional drug therapies is the associated side effects. This has led to renewed interest in the use of complimentary herbal medicines such as clove oil, neem leaves, and turmeric, which have been popular household remedies for centuries.
According to Lavigne and Sessle,[37] there may be both “push” and “pull” reasons behind the use of these alternative medications. Push reasons comprise dissatisfaction with conventional medicine due to side effects, long waiting lists in clinics, ineffective treatments, and lack of time. Pull reasons include a belief in the safety and effectiveness of natural, holistic, noninvasive options that are in sync with their personal philosophy. Treatments range from traditional herbal or Chinese medicine, meditation, biofeedback, physical therapy, massage, chiropractic therapy, acupuncture, and electric fields, to name a few.
Keeping the scope of this article in mind, all further discussion will be restricted to the use of herbal medicines for dental pain management. Common herbal options used for management of dental pain are listed in Table 3.[38,39,40,41,42,43,44,45,46,47,48]
Table 3.
Alternative (herbal) medications in management of dental pain

Limitations of Herbal Medications
Use of alternative and complementary medicine is on the rise. In India, more than 70% of the population uses herbal drugs and this constitutes mostly the rural population who depends solely upon herbal-based products.[49,50] Eugenol is usually used in dentistry with few reported side effects which are mainly in individuals sensitive to eugenol. It can cause local irritation, some cytotoxic effects, and hypersensitivity reactions. It is considered safe when used correctly in small amounts; however, it can cause liver and respiratory problems when ingested in large quantities.[51,52] Eugenol-containing materials, therefore, need to be used in appropriate amounts and manufacturer's instruction should be followed. Turmeric is generally considered safe; however, at higher doses, it may cause gastric irritation, nausea, diarrhea, allergic skin reaction, and antithrombotic events.[11]
The Unmet Need for Herbal Medications in Dentistry
Herbs have always been a very popular self-medication option for centuries due to accessibility, trusted efficacy and safety in relieving oral/dental problems. With this perspective, herbal medications have always been an area of exploration for researchers to overcome shortcomings of current preparations. Stated below are a few reasons that support the need for new herbal dental formulations:
Conventional pharmacological medicines may be associated with several limitations and side effects with routine use
Patient demand and an unprecedented need for safe medications that provide immediate relief for the entire family are fast acting, without side effects/interactions, effective and last till the time they visit the dental clinic.
Recommendations on Clinical Applications of Herbal Dental Gel
Regardless of etiology, dental pain is a common symptom routinely seen in clinical practice. Patient with dental pain often have a sense of anxiety with the use of pharmacological agents and tend to prefer use of home-based natural remedies due to its trusted efficacy and safety for all age groups. Another reason for a home-based remedy on priority is the time lag between pain initiation and visit to a dental clinic. To bridge this gap, patients consume these easily available remedies. Keeping patient's perspective, troubles, and concern's as a point of focus, there is an unmet need for an alternative medication which contains reliable natural ingredients with sustained activity in oral cavity.
A novel herbal formulation in the form of a dental gel was developed for dental pain management keeping in mind both the patient's and dentist's perspective of dental pain as most of the patients in India approach dentist when they experience moderate to severe pain.
For this purpose, a selective combination of herbal extracts was used to formulate the product. Herbal extracts are effective because they interact with specific chemical receptors within the body and many side effects, which are generally associated with conventional medicines, can be averted.[38] In fact, many of these natural compounds also work by inhibiting the inflammatory pathways in a similar manner as NSAIDs.[53]
Formulation Optimization
An orally acceptable analgesic “leave-on” gel formulation was developed to provide sustained delivery of pain relieving herbal extracts to the affected tooth. The novel formulation contains a combination of three essential oils – clove oil (primarily eugenol), menthol, and camphor – dispersed in a unique polymer matrix which is retained on the tooth and released gradually over a period of time.
Characteristics of the Herbal Dental Gel
It is a thick, translucent gel, and the constituents impart good spreadability, evenness, instant numbing, and noticeable cooling sensation of menthol when applied orally.
Clove oil (primarily eugenol)
Clove oil being the major component exerts majority of its therapeutic actions, and literature suggests that eugenol and acetyl eugenol are the major constituents of clove oil that provide an anti-inflammatory action and analgesic benefit. An article by Bley [54] reported that eugenol exerts analgesic activity due to its anti-nociceptive capacity via TRPV1 receptors. Upon continuous activation, TRPV1 receptors not only increase permeability of Ca++ ions but also increase its release from the endoplasmic reticulum and other intracellular organelles. These multiple sources of calcium result higher levels of intracellular calcium and can induce the depolarization of cytoskeletal components such as microtubules. Therefore, according to these widely recognized effects, constant exposure of TRPV1 to eugenol leads to impaired local nociceptor function for extended periods.[55,56] Concentration alteration may lead to a difference in effects such as having either an analgesic or an anesthetic effect.[54]
Camphor
Camphor has a counter-irritant and mild local anesthetic action, which is effective in relieving pain due to dental caries and sensitivity.[57] Camphor also has cooling and soothing effect which aids in increasing patient comfort. Studies have revealed that camphor at higher concentration exhibits significant antibacterial activity against several pathogenic Gram-positive bacteria.[39]
Menthol
Menthol is primarily used as cooling and flavoring agent. A study conducted by Alvarado et al.[40] revealed that menthol produces a cooling sensation by activating transient receptor potential melastatin 8, a nonselective cation receptor.
Polymer matrix
The polymer helps form an emulsion of the analgesic oils and also provides a bioadhesive property to the gel when applied to the affected tooth surface.
Clinical experience with the herbal dental gel
The herbal dental gel is very effective in managing acute dental pain due to a variety of dental problems such as caries, pulpitis (reversible and irreversible), erosion, abrasion, and even in cases such as cracked tooth syndrome. However, the herbal gel should be considered as a symptomatic treatment only and patients need to visit a dentist for proper diagnosis and definitive treatment. In addition, efforts should be made to explore other therapeutic areas or clinical conditions to further extend its scope, for example, in dry socket, etc.
Algorithm for dental pain management
An algorithm on dental pain management establishing the role of herbal dental gel can be used as a ready reference for dental practitioners to follow [Figure 8]. Treatment ideally should start with the initial phase of building rapport to ease and comfort patients in pain. It is essential to elucidate the source of dental pain with thorough review of patient's medical and dental history and clinical/diagnostic examination. The gel or other relevant intervention should be recommended once odontogenic cause of dental pain is confirmed by preliminary tests or radiographic examination.
Figure 8.
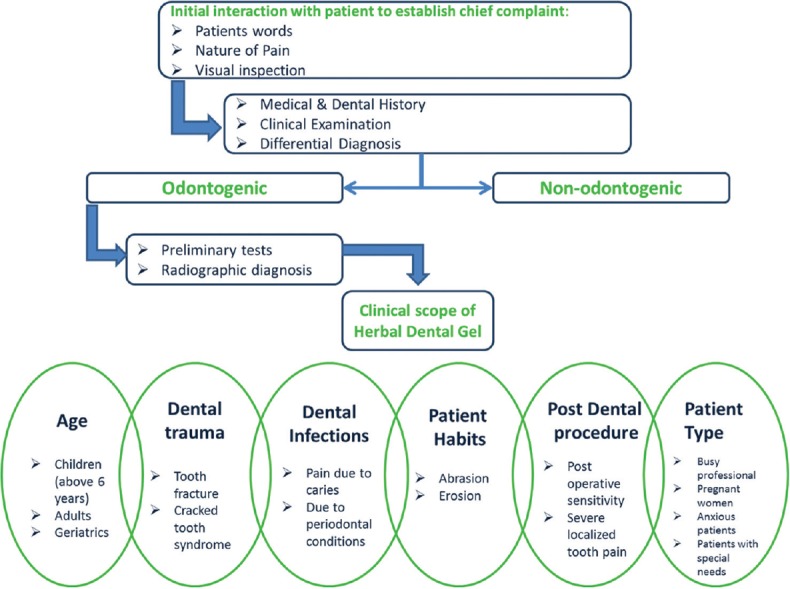
Algorithm establishing role of the novel herbal gel in dental pain management
“Traffic Light approach” model for dental pain management
In 1986, the WHO presented the analgesic ladder approach as a framework that physicians could use when developing treatment plans for cancer pain. A similar but more visual “traffic light” approach can be adopted in the management of dental pain, taking significant analgesic activity of this herbal gel into account. Based on this, a model was created for the usage of the herbal dental gel considering intensity/severity, duration, site, and frequency of dental pain [Figure 9].
Figure 9.
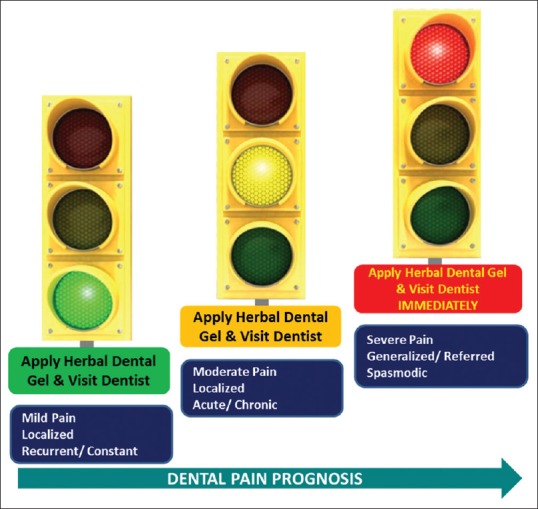
“Traffic Light” approach for addressing various nature of dental pain
Conclusion
Present targets for pain management are associated with multiple limitations, and therefore, the exploration of newer pathways/alternatives (TRPV1-related) is of utmost priority. The herbal dental gel is a unique formulation of three essential oils, namely, clove oil, camphor, and menthol which renders it effective in dental pain management when applied locally.
A favorable clinical experience with the novel herbal dental gel implied it:
Can be a “home remedy” or as a “ first aid” for symptomatic relief of dental pain
Can be useful in managing dental pain in certain group of patients such as geriatrics, busy professionals, and patient's with special needs
Should be considered as a symptomatic treatment only and the patient needs to visit a dentist for proper diagnosis and definitive treatment.
However, need exists for more studies and investigations to discuss other clinical/therapeutic indications for this herbal gel in dental practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge the support of Jeevan Scientific Technology Limited, Hyderabad, India, in the development of this manuscript.
References
- 1.Dandi KK, Rao EV, Margabandhu S. Dental pain as a determinant of expressed need for dental care among 12-year-old school children in India. Indian J Dent Res. 2011;22:611. doi: 10.4103/0970-9290.90320. [DOI] [PubMed] [Google Scholar]
- 2.Merskey H, Bogduk N. Classification of Chronic Pain, IASP Task Force on Taxonomy. Seattle, WA: International Association for the Study of Pain Press; 1994. [Google Scholar]
- 3.Clementino MA, Gomes MC, Pinto-Sarmento TC, Martins CC, Granville-Garcia AF, Paiva SM. Perceived impact of dental pain on the quality of life of preschool children and their families. PLoS One. 2015;10:e0130602. doi: 10.1371/journal.pone.0130602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health System Performance Assessment: World Health Survey 2003 India. Mumbai: IIPS; International Institute for Population Sciences (IIPS), World Health Organization (WHO), and World Health Organization (WHO) – India – WR Office. 2006. [Google Scholar]
- 5.Khan AA, Jain SK, Shrivastav A. Prevalence of dental caries among the population of Gwalior (India) in relation of different associated factors. Eur J Dent. 2008;2:81–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Marcenes W, Kassebaum NJ, Bernabé E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990-2010: A systematic analysis. J Dent Res. 2013;92:592–7. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bali RK, Mathur VB, Talwar PP, Chanana HB. Dental Council of India. New Delhi: National Oral health Survey and fluoride mapping; 2004. [Google Scholar]
- 8.Brännström M, Garberoglio R. The dentinal tubules and the odontoblast processes. A scanning electron microscopic study. Acta Odontol Scand. 1972;30:291–311. doi: 10.3109/00016357209004598. [DOI] [PubMed] [Google Scholar]
- 9.Figdor D. Aspects of dentinal and pulpal pain. Pain of dentinal and pulpal origin – A review for the clinician. Ann R Australas Coll Dent Surg. 1994;12:131–42. [PubMed] [Google Scholar]
- 10.Magloire H, Maurin JC, Couble ML, Shibukawa Y, Tsumura M, Thivichon-Prince B, et al. Topical review. Dental pain and odontoblasts: Facts and hypotheses. J Orofac Pain. 2010;24:335–49. [PubMed] [Google Scholar]
- 11.Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125:181–95. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Nagy I, Sántha P, Jancsó G, Urbán L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–69. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Eilers H, Schumacher MA. Mechanosensitivity of primary afferent nociceptors in the pain pathway. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005. [PubMed] [Google Scholar]
- 14.Natanael G. Dental Pulp Sensory Function. Pain. E. J. E. R. Electronic Journal of Endodontics Rosario. 2011;2:540–52. [Google Scholar]
- 15.Mataki S. Patient-dentist relationship. J Med Dent Sci. 2000;47:209–14. [PubMed] [Google Scholar]
- 16.Lahti S, Tuutti H, Hausen H, Käärlänen R. Patients' expectations of an ideal dentist and their views concerning the dentist they visited: Do the views conform to the expectations and what determines how well they conform? Community Dent Oral Epidemiol. 1996;24:240–4. doi: 10.1111/j.1600-0528.1996.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 17.Wetherell J, Richards L, Sambrook P, Townsend G. Management of acute dental pain: A practical approach for primary healthcare providers. Aust Prescr. 2001;24:144–8. [Google Scholar]
- 18.Hargreaves KM, Keiser K, Byrne E. Endodontic pharmacology. In: Cohen S, Hargreaves KM, editors. Pathways of the Pulp. St. Louis: Mosby; 2005. [Google Scholar]
- 19.Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: Lessons from acute postoperative pain. J Pain Symptom Manage. 1999;18:427–37. doi: 10.1016/s0885-3924(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 20.Haas DA. An update on analgesics for the management of acute postoperative dental pain. J Can Dent Assoc. 2002;68:476–82. [PubMed] [Google Scholar]
- 21.Malmstrom K, Kotey P, Coughlin H, Desjardins PJ. A randomized, double-blind, parallel-group study comparing the analgesic effect of etoricoxib to placebo, naproxen sodium, and acetaminophen with codeine using the dental impaction pain model. Clin J Pain. 2004;20:147–55. doi: 10.1097/00002508-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JG. Consideration of steroids for endodontic pain. Endod Topics. 2002;3:41–51. [Google Scholar]
- 23.Bahl R. Local anesthesia in dentistry. Anesth Prog. 2004;51:138–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Scarlett MI. Local anesthesia in today's dental practice. Continuing education course. American Dental Assistants Association. 2010:3–4. [Google Scholar]
- 25.Acute Pain Management in Adults: Operative Procedures. Rockville, MD: Department of Health and Human Services, Agency for Health Care Policy and Research, AHCPR Publication No. 92-0019; 1992. Agency for Health Care Policy and Research. [Google Scholar]
- 26.Noble SL, King DS, Olutade JI. Cyclooxygenase-2 enzyme inhibitors: Place in therapy. Am Fam Physician. 2000;61:3669–76. [PubMed] [Google Scholar]
- 27.Gierse JK, Hauser SD, Creely DP, Koboldt C, Rangwala SH, Isakson PC, et al. Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem J. 1995;305(Pt 2):479–84. doi: 10.1042/bj3050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis. Meta-analysis of randomised trials? BMJ. 2006;332:1302–8. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motov SM, Tamar D. Is there a limit to the analgesic effect of pain medications. Medscape Emergency Medicine. 2008 [Google Scholar]
- 30.Titsas A, Ferguson MM. Impact of opioid use on dentistry. Aust Dent J. 2002;47:94–8. doi: 10.1111/j.1834-7819.2002.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 31.McQuay H, Edwards J. Meta-analysis of single dose oral tramadol plus acetaminophen in acute postoperative pain. Eur J Anaesthesiol Suppl. 2003;28:19–22. [PubMed] [Google Scholar]
- 32.Kumara R, Zacharias M. Effectiveness of tramadol as an analgesic in oral surgery. N Z Dent J. 2002;98:9–11. [PubMed] [Google Scholar]
- 33.Joshi A, Parara E, Macfarlane TV. A double-blind randomised controlled clinical trial of the effect of preoperative ibuprofen, diclofenac, paracetamol with codeine and placebo tablets for relief of postoperative pain after removal of impacted third molars. Br J Oral Maxillofac Surg. 2004;42:299–306. doi: 10.1016/j.bjoms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Seymour RA, Meechan JG, Yates MS. Pharmacology and Dental Therapeutics. 3rd ed. Oxford: Oxford University Press; 1999. pp. 56–8. [Google Scholar]
- 35.McCaughey W. Adverse effects of local anaesthetics. Drug Saf. 1992;7:178–89. doi: 10.2165/00002018-199207030-00003. [DOI] [PubMed] [Google Scholar]
- 36.Ament PW, Bertolino JG, Liszewski JL. Clinically significant drug interactions. Am Fam Physician. 2000;61:1745–54. [PubMed] [Google Scholar]
- 37.Lavigne GJ, Sessle BJ. Canadian Orofacial Pain Team workshop report on the global year against orofacial pain. Pain Res Manag. 2015;20:7–14. doi: 10.1155/2015/785692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taheri JB, Azimi S, Rafieian N, Zanjani HA. Herbs in dentistry. Int Dent J. 2011;61:287–96. doi: 10.1111/j.1875-595X.2011.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahboubi M, Kazempour N. The antimicrobial activity of essential oil from Perovskia abrotanoides Karel and its main components. Indian J Pharm Sci. 2009;71:343–7. doi: 10.4103/0250-474X.56016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarado LT, Perry GM, Hargreaves KM, Henry MA. TRPM8 Axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod. 2007;33:1167–71. doi: 10.1016/j.joen.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon SE, Kim HY, Cha JD. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch Oral Biol. 2011;56:907–16. doi: 10.1016/j.archoralbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Chaturvedi TP. Uses of turmeric in dentistry: An update. Indian J Dent Res. 2009;20:107–9. doi: 10.4103/0970-9290.49065. [DOI] [PubMed] [Google Scholar]
- 43.Cikrikci S, Mozioglu E, Yılmaz H. Biological activity of curcuminoids isolated from Curcuma longa. Rec Nat Prod. 2008;2:19–24. [Google Scholar]
- 44.Nagpal M, Sood S. Role of curcumin in systemic and oral health: An overview. J Nat Sci Biol Med. 2013;4:3–7. doi: 10.4103/0976-9668.107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anand P, Bley K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107:490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epstein JB, Marcoe JH. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg Oral Med Oral Pathol. 1994;77:135–40. doi: 10.1016/0030-4220(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 47.Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–5. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 48.Nivetha R, Murthykumar K, Ashwin KS, Kumar N, Priyadarshini R. Effects of natural products on oral health: A review. Asian J Pharm Clin Res. 2014;7:279–82. [Google Scholar]
- 49.Vaidya AD, Devasagayam TP. Current status of herbal drugs in India: An overview. J Clin Biochem Nutr. 2007;41:1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahoo N, Manchikanti P. Herbal drug regulation and commercialization: An Indian industry perspective. J Altern Complement Med. 2013;19:957–63. doi: 10.1089/acm.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarrami N, Pemberton MN, Thornhill MH, Theaker ED. Adverse reactions associated with the use of eugenol in dentistry. Br Dent J. 2002;193:257–9. doi: 10.1038/sj.bdj.4801539. [DOI] [PubMed] [Google Scholar]
- 52.Hensten-Pettersen A, Jacobsen N. Perceived side effects of biomaterials in prosthetic dentistry. J Prosthet Dent. 1991;65:138–44. doi: 10.1016/0022-3913(91)90066-6. [DOI] [PubMed] [Google Scholar]
- 53.Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surg Neurol Int. 2010;1:80. doi: 10.4103/2152-7806.73804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bley KR. TRPV1 agonist approaches for pain management. In: Gomtsyan A, Faltynek CR, editors. Vanilloid Receptor TRPV1 in Drug Discovery: Targeting Pain and Other Pathological Disorders. New York: Wiley; 2010. pp. 325–47. [Google Scholar]
- 55.Mandadi S, Roufogalis BD. ThermoTRP channels in nociceptors: Taking a lead from capsaicin receptor TRPV1. Curr Neuropharmacol. 2008;6:21–38. doi: 10.2174/157015908783769680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamatou GP, Vermaak I, Viljoen AM. Eugenol – From the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules. 2012;17:6953–81. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.George J, Hegde S, Rajesh KS, Kumar A. The efficacy of a herbal-based toothpaste in the control of plaque and gingivitis: A clinico-biochemical study. Indian J Dent Res. 2009;20:480–2. doi: 10.4103/0970-9290.59460. [DOI] [PubMed] [Google Scholar]