Abstract
Objective:
To assess and compare the salivary flow rate (SFR), pH and taste perception among oral submucous fibrosis (OSMF) and apparently healthy subjects.
Materials and Methods:
Ninety subjects (45 OSMF + 45 controls) were enrolled in the study for estimating and analogizing the SFR, pH, and taste perception executing modified Schirmer, pH, and taste strips. The SFR, pH, and taste perception were evaluated and compared between 14 Stage I and 31 Stage II OSMF subjects. The entered data were analyzed using SPSS 21.0 software.
Results:
A statistically significant decrease in SFR among OSMF group (23.4 mm at 3rd min) and hypogeusia to salty (62.2%), and dysgeusia to sour taste (40%) when compared to apparently healthy subjects (30.7 mm at 3rd min) was noted. Statistical significance (P < 0.05%) inferring hyposalivation in Stage II OSMF (24.1 mm at 3rd min) juxtaposing with Stage I OSMF (31.4 mm at 3rd min). Statistically significant hypogeusia to salty (n = 23) and sweet (n = 16) and dysgeusia (n = 14) to sour among Stage II OSMF when differentiated with Stage I OSMF. The mean pH among the OSMF and control groups demonstrated no statistical significance.
Conclusion:
The findings from the study demonstrated marked decrease of SFR and taste perception to salty and sour among Stage II OSMF when compared to Stage I OSMF subjects.
Keywords: Modified Schirmer strips, oral submucous fibrosis, pH paper, salivary flow rate, salivary pH, taste perception, taste strips
Introduction
Oral submucous fibrosis (OSMF) is a chronic fibrotic potentially malignant disease characterized by an inflammatory reaction of epithelium along with fibrosis of submucosal tissues which was described by Schwartz in early 1950s as an idiopathic disorder.[1] Surveys conducted since then reflected on multiple etiological variables predominating areca nut to be the main causative agent.[2] Experimental studies estimated the higher concentration of areca nut in areca nut products such as pan masala, gutka, and mawa when compared to areca nut alone and thereby increasing the incidence of OSMF.[3] Hypothesis suggests increased copper content in areca nut interfere with the synthesis of extracellular matrix molecules such as collagen leading to either upgradation in collagen production or decreased collagen degradation, subsequently leading to increase collagen production causing fibrosis of subepithelial tissues, which has been demonstrated on oral biopsies.[4]
Areca nut is composed of several components such as carbohydrates, fats, proteins, crude fiber, polyphenols (flavonols and tannins), alkaloids, and mineral matter which have several biological and stimulating effects.[5] Arecoline in areca nut have parasympathomimetic effect, and eventually, the leaching of the chemicals of areca nut in oral mucosa and saliva alters the saliva and taste parameters.[6]
Saliva is a critical fluid in the oral cavity preserving the oral cavity. It also serves a primary role in dissolving the taste stimulus to taste buds. Alteration in salivary flow due to any reason can in turn cause changes in its pH important in buffering action and consequently the taste perception.[7] Several studies and cases have reported with the manifestation of altered salivary flow and taste perception among the OSMF affected individuals,[8] yet corroboration correlating the intensity of changes in saliva and taste in OSMF is sparse in these parameters. This contemplated the intention of the present study.
Materials and Methods
A comparative study was conducted to assess and compare the salivary flow rate (SFR), salivary pH, and taste perception among 90 outpatients (45 OSMF + 45 controls) between the age group of 18–45 years attending. The Oxford Dental College and Research Hospital in Department of Oral medicine and maxillofacial Radiology. The Ethical clearance was obtained from the institutional committee for the study design.
A specially designed pro forma was constructed for interviewing the patient, and informed consent was obtained for the patient participation in the study. The subjects were clinically examined and diagnosed with OSMF based on the classification of Pindborg's.[9] Control group comprised apparently healthy subjects with no history of habits and systemic illnesses and medications.
The study group constituted patients with a history of areca nut chewing for more than 6 months and those with clinically proven OSMF (Stage I and Stage II) [Figures 1 and 2] (burning sensation, restricted mouth opening, and palpable fibrous bands). Exclusion criteria were those above the age of 45 years, the presence of associated habits such as smoking, drinking, or tobacco chewing; patients undergoing treatment for OSMF and subjects with known systemic illness or those taking medications which may cause alteration in perception of taste.
Figure 1.
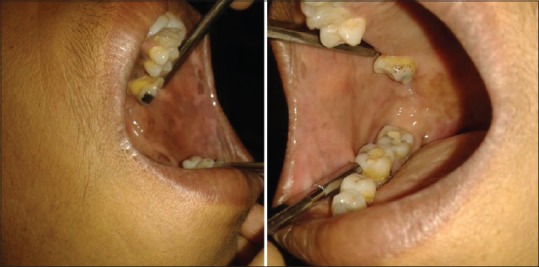
Clinical photograph showing Stage I oral submucous fibrosis
Figure 2.
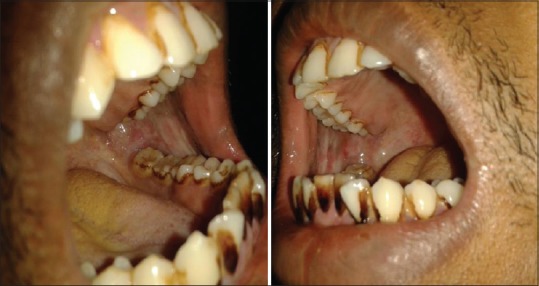
Clinical photograph showing Stage II oral submucous fibrosis
Subjective symptoms of xerostomia and variation in taste were recorded using Fox et al. questionnaire [10] and Questionnaire by University of Connecticut Health Centre,[11] respectively. The study was carried in the morning hour (09.00–11.00 am) for preventing the bias in results caused due to circadian variation, and subjects were refrained from eating and drinking 2 h prior the appointment.
This study implemented Schimer paper (Tear touch from Madhu Instruments Pvt. Ltd., Medline), pH indicator strips (Colormetric paper strips from Carolina Biologicals [pH 5–10], www.carolina.com), and taste papers (Sweet [Arrow India Limited, Mumbai], salt paper (sodium benzoate-17-4020), sour paper (PTC-17-4010), bitter paper (thiourea-17-4030) [Carolina genetics-Blades biological Ltd., Cowden, The United Kingdom]) were utilized in the present study.
Salivary flow rate assessment
The subject was asked not to eat or drink 2 h before the modified Schirmer test (MST) procedure.[12] After a period of 3–5 min rest, the patient is asked to swallow all the saliva in the mouth before the test, and not to swallow anymore during the test. In addition, the patient is asked to rest the tongue on the hard palate, so that the test strip would not touch the tongue during the test. The MST strip is held vertically with a cotton plier, and the rounded end of the strip is positioned at the floor of the mouth [Figure 3]. When the round end of the strip contacts moisture, the saliva travels up the strip and its distance is read at 1, 2, and 3 min and recorded immediately.
Figure 3.
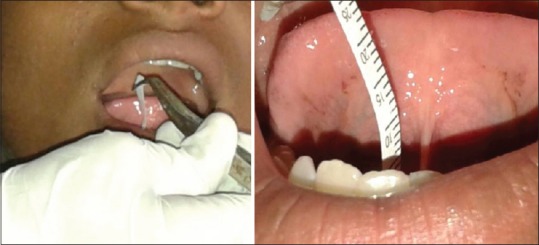
Salivary flow rate determination
The normal SFR ranges between 25 mm and 30 mm. It is considered dry mouth when SFR drops between 10 mm and 15 mm, mildly dry when value ranges from 06 mm to 10 mm, moderately dry with SFR 02–05 mm, and considered severely dry with SFR 00–01 mm.[12] The test was carried out in the morning hours between 09.00 am and 11.00 am considering the circadian variation.[12,13,14]
Determination of pH
Salivary pH is measured immediately after measuring SFR using the pH strips.[12] Stimulated saliva is collected from the floor of the mouth through the submandibular ducts [Figure 4]. The change in color of the strip is noted and matched with the color coding based on the pH strip and noted accordingly.[13]
Figure 4.
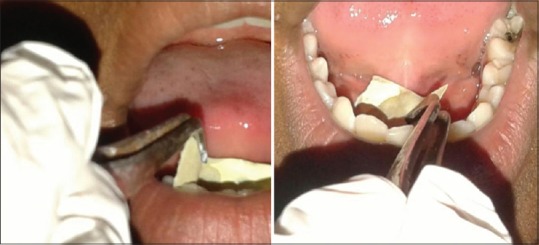
Salivary pH determination
The normal salivary pH ranges from 6.5 to 7.5. A pH value below 6.5 indicates acidic pH, and it is considered alkaline when pH increases above 7.5.[13,14]
Taste determination
Before assessing the taste in the individuals, the subjects were asked not to eat or drink 1 h prior procedure and asked to rinse the mouth in tap water. The taste strips [Figure 5][15] were four in number with the four basic tastants, i.e., sweet, salty, sour, and bitter. Each taste strip with specific taste was placed over the dorsum portion of the tongue, and the subject was asked to identify the taste of the strip. The strip gradually dissolved within 2 min.[15]
Figure 5.
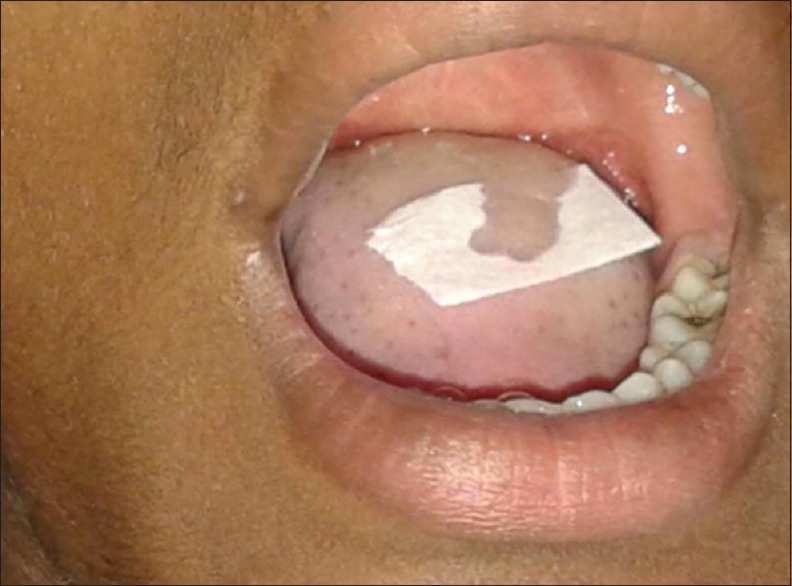
Taste perception determination using taste strips
The taste perceived by the subjects were recorded as hypogeusia, hypergeusia, dysgeusia, and ageusia.[16,17]
Statistical analysis
All the findings were entered in Microsoft Excel using SPSS 21.0 software and were expressed as mean ± standard deviation and calculated using 1-way analysis (ANOVA). The groups were compared using Chi-square test. The degree of freedom between variables was also observed. Multiple comparison of the parameter was done by Neumans–Keuls post hoc test whenever significant difference had been revealed. A P< 0.05 indicated significant association at 5% level of significance.
Results
A total control of 45 control subjects were matched according to gender and age with the 45 OSMF group. The study demonstrated 39 males with a mean age of 28.7 years and 6 females with a mean age of 29.6 years. Of 45 OSMF subjects 14 (31%) belonged to Stage I and 31 (68.9%) belonged to Stage II OSMF, respectively.
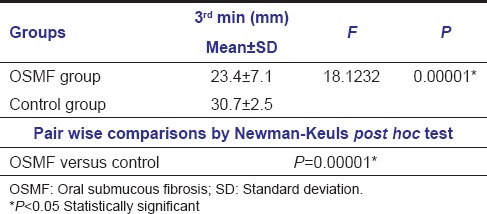
Comparison of the SFR between study subjects at 3rd min revealed an overall mean of 23.4 mm for OSMF and 30.7 mm for control groups showing statistical significance (P = 0.00001) suggestive of marked reduction of SFR among OSMF group [Table 1 and Graph 1].
Table 1.
Comparison of salivary flow rate among the study groups at 3rd min by one-way analysis of variance and pair-wise comparison
Graph 1.
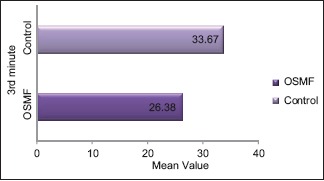
Comparison of salivary flow rate among oral submucous fibrosis and control group
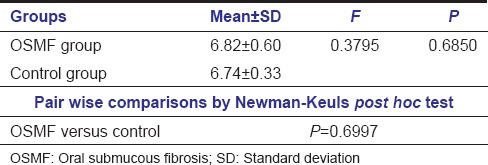
Comparison of the study groups with respect to salivary pH corroborated a mean among of 6.82 in OSMF and 6.74 among control subjects. Pairwise comparison showed no statistical significance (P = 0.6997) [Table 2].
Table 2.
Comparison of salivary pH between study groups and pair-wise comparison
A comparison of SFR between Stage I and Stage II OSMF group at 3rd min is highlighted in Table 3 and Graph 2 with statistical significance (P < 0.0007) representing increased hyposalivation among Stage II OSMF when compared to Stage I OSMF. The overall mean of salivary pH showed no statistically significant changes between the two stages of OSMF.
Table 3.
Comparison of salivary flow rate and pH between Stages I and II oral submucous fibrosis subjects
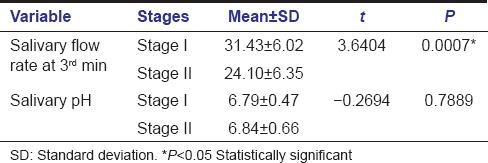
Graph 2.
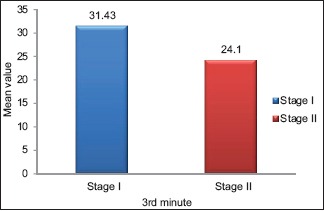
Comparison of salivary flow rate between Stages I and II oral submucous fibrosis groups
Comparisons of taste perception among OSMF and control subjects were demonstrated in Table 4. Of the 45 OSMF subjects; 22 (48.9%) normal, 17 (37.8%) hypogeusia, 5 (11.1%) hypergeusia, 1 (2.2%) showed dysgeusia with no subjects showing ageusia to sweet taste; 28 (62.2%) hypogeusia, 10 (22.2%) normal, 4 (8.9%) dysgeusia, 2 (4.4%) ageusia, and 1 (2.2%) hypergeusia to salty taste; 18 (40%) dysgeusia, 15 (33.3%) hypogeusia, 7 (15.6%) normal, 4 (8.9%) ageusia, and 1 (2.2%) hypergeusia to sour taste; 25 (55.6%) normal, 16 (35.6%) hypogeusia, 4 (8.9%) hypergeusia with no subjects showing ageusia and dysgeusia to bitter taste perception were observed.
Table 4.
Comparison of taste perception among oral submucous fibrosis and control groups
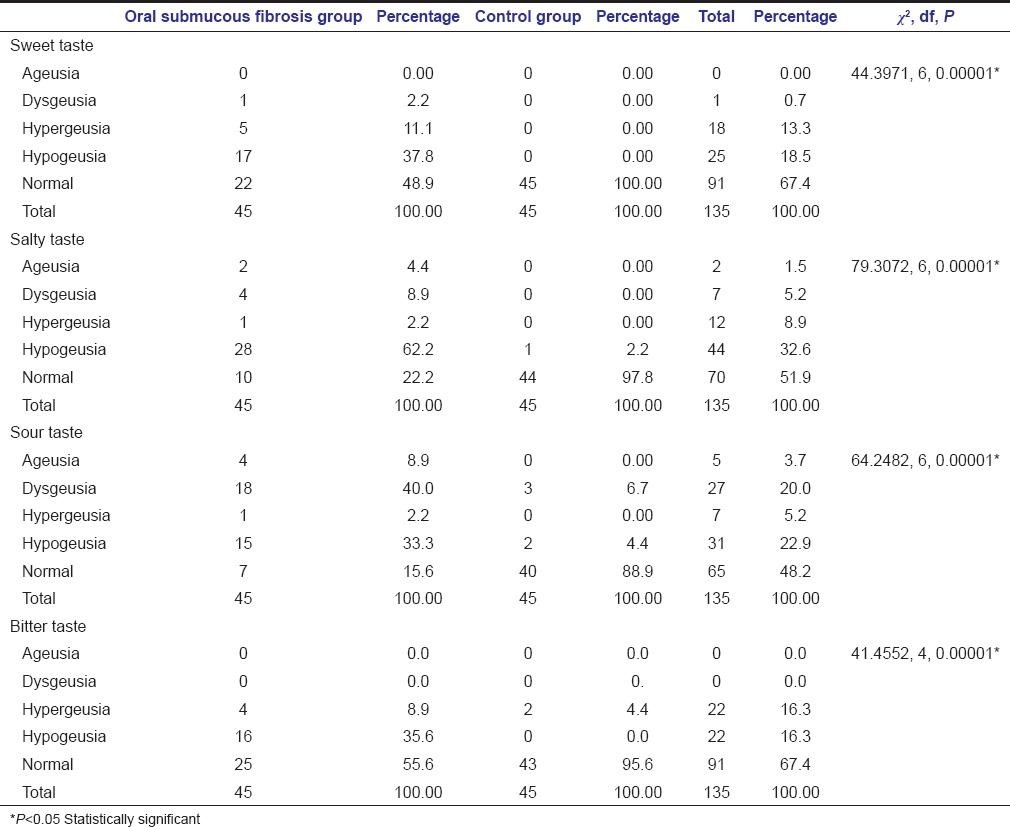
Out of the 45 control subjects, 1 (2.2%) hypogeusia to salty taste, 3 (6.7%) dysgeusia and 2 (4.4%) hypogeusia to sour taste, and 2 (4.4%) hypergeusia to bitter taste were observed. The remaining subjects had the normal perception to the different tastants. Statistically significant result (P = 0.00001) was observed among the two groups; suggestive of hypogeusia among OSMF group, and normal taste perception among control group.
Comparison of taste perception among the various tastants between the two stages of OSMF inferred 5 hypergeusia, 1 hypogeusia, 8 normal to sweet; 1 hypergeusia and 5 hypogeusia, 8 normal to salty taste; 1 ageusia, 4 dysgeusia, 1 hypergeusia, 3 hypogeusia and 5 normal to sour taste and 4 hypeuergeuesia, 10 normal to bitter taste among 14 Stage I OSMF subjects. 31 subjects of Stage II OSMF displayed 16 hypogeusia, 1 ageusia and 14 normal to sweet, 23 hypogeusia, 4 dysgeusia, 2 ageusia and normal to salty taste, 14 dysgeusia, 12 hypogeusia, 3 ageusia and 2 normal to sour, and 16 hypogeusia and 15 normal to bitter taste. Statistical significance (P < 0.05) suggestive of hypogeusia to sweet, salty, and bitter taste among Stage II OSMF is noted [Table 5 and Graph 3].
Table 5.
Comparison of taste perception between stage I and stage II OSMF
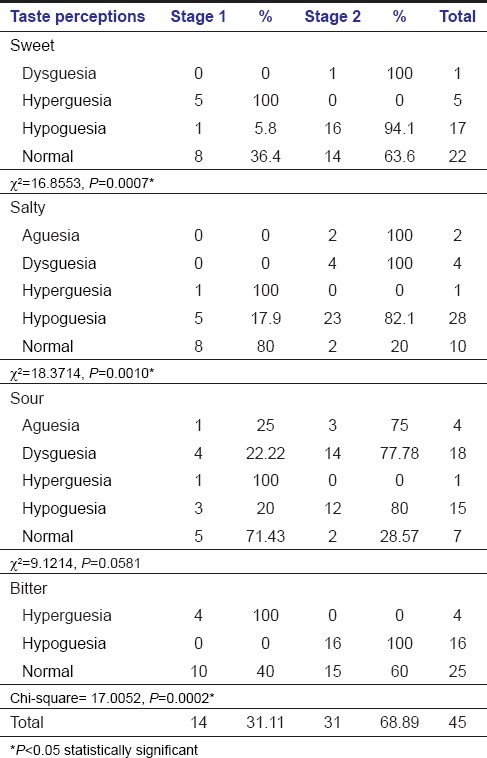
Graph 3.
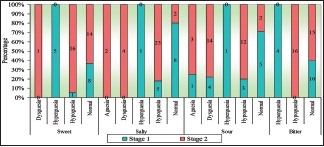
Comparison of taste perception between Stages I and II oral submucous fibrosis subjects
Discussion
OSMF is a potentially malignant disease predominantly seen in people of Asian descent. OSMF is a cause for concern because it is one of the preventable, prevalent, premalignant conditions of oral mucosa in India. Based on the clinical and epidemiological studies it has been noted that OSMF is increasingly associated with areca nut chewing habit.[1,2,18]
Most of the dental physicians tend to overlook the changes in SFR and taste changes by focusing more toward the clinical presentation of OSMF and the management of the condition.
During areca nut, chewing lot of chemicals and metals such as copper, iron are leached out into saliva, which in turn alter the property and composition of saliva. In betel quid chewer's variations in the saliva flow rate, pH has been reported. Production of reactive oxygen species is enhanced by the presence of alkaline pH of saliva.[19] Taste stimuli play a major role in salivation, by affecting output quantitatively and qualitatively.
Certain studies reported the changes in SFR and pH. Considerably, fewer studies have been reported on changes in SFR and taste perception among OSMF and its various stages. Therefore, this study was contemplated for assessing and comparing the SFr, pH, and taste perception among the OSMF affected individuals.
This study group was divided into OSMF and control group who were matched by gender and age as of the OSMF group. Of 45 OSMF 14 (31.1%) were among Stage I and 31 (68.9%) were Stage II OSMF, which was in conjunction with the findings observed by Wahab et al.[20]
This study showed an overall mean of the SFR among OSMF group were as 12.8 mm, 19.5 mm and 26.4 mm; and among control group were 14.9 mm, 24.2 mm, and 33.7 mm suggestive of decrease in SFR among OSMF groups was observed when compared to control group which was similar to reports by Rooban et al.[21]
A decrease in SFR among OSMF subjects could be due to conversion by lime from arecoline to arecadine or due to an atrophy of the acinar cells as disease progresses. Nyachhon et al.[12] conducted a study among OSMF patients showing various stages of acinar degeneration that manifested as small clear cytoplasmic vacuoles with pyknotic nuclei. Areas of mucin pooling can be seen within the connective tissue stroma was also noted.
Contradictory findings of an increase in SFR among ghutka chewers and OSMF were noted by Sangeeth et al. using spitting method. The variation of results could be due to utilization of MST and use of areca nut habit without tobacco.[19]
Our study showed a statistically significant increase (P < 0.05) in salivary flow Stage I OSMF, which could be due to the parasympathomimetic activity of arecoline and arecadine present in areca nut.[21]
The mean pH in this study among OSMF and control groups was 6.82 and 6.74, respectively with no statistical significance studies which were similar to studies conducted by Rooban et al.[21] and Sangeeth et al.[19] A mild increase in pH among the OSMF group may be due to an increase in the bicarbonate secretion.[21]
OSMF group showed hypogeusia 28 (62.2%) to salty taste followed by dysgeusia (40%) to sour, and normal (55.6% and 48.9%) to bitter and sweet taste in our study. Among OSMF group, decreased or altered perception may be due to atrophy of the papillae and decrease in SFR. In our study, the salty taste and sour are mainly affected followed by bitter and sweet when compared to a study conducted by Deeplaxmi et al.[22] in solutions showed decreased taste perception to sweet taste followed by salt, bitter, and sour.
Our study has also demonstrated hypogeusia to sweet, salty, and bitter taste among Stage II OSMF when compared to Stage I OSMF subjects which have showed hypergeusia to sweet and salty taste. As no study is so far conducted comparing the different stages we could not compare our results.
This study has entailed the changes in saliva and taste parameters among the Stage I and Stage II OSMF subjects. However, due to a limited number of Stage III OSMF subjects, we could not encorporate it in our study protocol. Therefore, future studies can be conducted including all three stages of OSMF.
Conclusion
OSMF is a debilitating condition which manifests with several clinical features whose manifestation worsens with the severity of the condition. Several literatures have surveyed the clinical features which changed with the progression of the disease but with little or no emphasis on changes in saliva and taste parameters. The present has therefore showed relative changes in saliva and taste between the various stages of OSMF.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rajendran R. Oral submucous fibrosis: Etiology, pathogenesis, and future research. Bull World Health Organ. 1994;72:985–96. [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz SR. Oral submucous fibrosis: An unusual disease. J N J Dent Assoc. 1997;68:17–9. [PubMed] [Google Scholar]
- 3.Ranganathan K, Devi MU, Joshua E, Kirankumar K, Saraswathi TR. Oral submucous fibrosis: A case-control study in Chennai, South India. J Oral Pathol Med. 2004;33:274–7. doi: 10.1111/j.0904-2512.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 4.Dyavanagoudar SN. Oral submucous fibrosis: Review on etiopathogenesis. J Cancer Sci Ther. 2009;1:72–7. [Google Scholar]
- 5.Mathew P, Austin RD, Varghese SS, Kumar AD. Role of areca nut and its commercial products in oral submucous fibrosis – A review. J Adv Med Dent Sci Res. 2014;2:192–200. [Google Scholar]
- 6.Sabharwal R, Gupta S, Kapoor K, Puri A, Rajpal K. Oral submucous fibrosis – A review. J Adv Med Dent Sci Res. 2013;1:29–37. [Google Scholar]
- 7.Ganapathy KS, Gurudath S, Balikai B, Ballal S, Sujatha D. Role of iron deficiency in oral submucous fibrosis: An initiating or accelerating factor. J Indian Acad Oral Med Radiol. 2011;23:25–8. [Google Scholar]
- 8.Ali FM, Aher V, Prasant MC, Bhushan P, Mudhol A, Suryavanshi H. Oral submucous fibrosis: Comparing clinical grading with duration and frequency of habit among areca nut and its products chewers. J Cancer Res Ther. 2013;9:471–6. doi: 10.4103/0973-1482.119353. [DOI] [PubMed] [Google Scholar]
- 9.More CB, Gupta S, Joshi J, Varma SN. Classification system for oral submucous fibrosis. J Indian Acad Oral Med Radiol. 2012;24:24–9. [Google Scholar]
- 10.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc. 1987;115:581–4. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 11.University of Connecticut Health. Facts about taste and smell. Taste and Smell Center. Connecticut chemosensory clinical research center. 1984 copyright;HCH-1543, Eff. 11/2006:1-2. [Google Scholar]
- 12.Nyachhyon R, Boaz K, Sumanth KN. Minor salivary gland changes in oral submucous fibrosis (OSMF): Retrospective pilot study. J Nepal Dent Assoc. 2011;12:26–8. [Google Scholar]
- 13.Timothy SM, Birgitte N, Svensson P. Clinical Oral Physiology. Copenhagen: Quintessence Publishers; 2004. pp. 1–109. [Google Scholar]
- 14.Edgar M, Dawes C, Mullane DO. Saliva and Oral Health. 4th ed. London: British Dental Association; 2012. pp. 1–36. [Google Scholar]
- 15.Smutzer G, Lam S, Hastings L, Desai H, Abarintos RA, Sobel M, et al. Atest for measuring gustatory function. Laryngoscope. 2008;118:1411–6. doi: 10.1097/MLG.0b013e31817709a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health and Nutrition Examination Survey (NHANES) Taste and Smell Examination Component Manual. CDC. 2013-2014:1–90. [Google Scholar]
- 17.Stillman JA, Morton RP, Hay KD, Ahmad Z, Goldsmith D. Electrogustometry: Strengths, weaknesses, and clinical evidence of stimulus boundaries. Clin Otolaryngol Allied Sci. 2003;28:406–10. doi: 10.1046/j.1365-2273.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad MS, Ali SA, Ali AS, Chaubey KK. Epidemiological and etiological study of oral submucous fibrosis among gutkha chewers of Patna, Bihar, India. J Indian Soc Pedod Prev Dent. 2006;24:84–9. doi: 10.4103/0970-4388.26022. [DOI] [PubMed] [Google Scholar]
- 19.Sangeeth S, Ashok L, Sujatha P. Estimation of unstimulated salivary flow rate, pH, copper and iron in ghutkachewers with and without oral submucous fibrosis- a preliminary study. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2014;5:300–6. [Google Scholar]
- 20.Wahab N, Ali AS, Khan M, Khan S, Mehdi H, Sawani A. Frequency and clinical presentation of oral submucous fibrosis. Pak J Med Dent. 2014;3:1–4. [Google Scholar]
- 21.Rooban T, Mishra G, Elizabeth J, Ranganathan K, Saraswathi TR. Effect of habitual arecanut chewing on resting whole mouth salivary flow rate and pH. Indian J Med Sci. 2006;60:95–105. [PubMed] [Google Scholar]
- 22.Deeplaxmi R, Sakarde SK, Sur J, Singh AP, Jain S, Mujoo S, et al. Altered taste perception in oral submucous fibrosis: A research. J Indian Acad Oral Med Radiol. 2012;24:288–91. [Google Scholar]


