Abstract
Primary liver cancer, which includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC) and fibrolamellar HCC, is one of the most common malignancies and the third leading cause of cancer-associated mortality, worldwide. Despite the development of novel therapies, the prognosis of liver cancer patients remains extremely poor. Thus, investigation of the genetic background and molecular mechanisms underlying the development and progression of this disease has gained significant attention. The Notch signaling pathway is a crucial determinant of cell fate during development and disease in several organs. In the liver, Notch signaling is involved in biliary tree development and tubulogenesis, and is also significant in the development of HCC and ICC. These findings suggest that the modulation of Notch pathway activity may have therapeutic relevance. The present review summarizes Notch signaling during HCC and ICC development and discusses the findings of recent studies regarding Notch expression, which reveal novel insights into its function in liver cancer progression.
Keywords: Notch signaling pathway, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, cancer stem cells
1. Introduction
Worldwide primary liver cancer, which includes primary hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC) and fibrolamellar HCC, is the fifth most common cancer and the third leading cause of cancer-associated mortality (1). Currently, surgery and liver transplantation are considered the optimal curative treatments for the disease (2); however, there is a significant shortage of organ donors, and surgical complications, recurrence and metastasis are common (3). Although epidemiological risk factors, including hepatitis B virus (HBV) and hepatitis C virus infection, have been identified, the molecular mechanisms underlying primary liver cancer remain unclear (4). Therefore, elucidation of the molecular pathogenesis of liver cancer and the development of effective targeted therapies is urgently required (5).
The Notch signaling pathway is evolutionarily conserved and controls numerous developmental processes, such as cell fate determination, terminal differentiation and proliferation (6). During embryonic development and adulthood, intracellular Notch signaling is required for cell specification, lineage commitment and maintenance of progenitor cells (7), in particular in the control of endothelial cell differentiation, arteriovenous specification and vascular development (8).
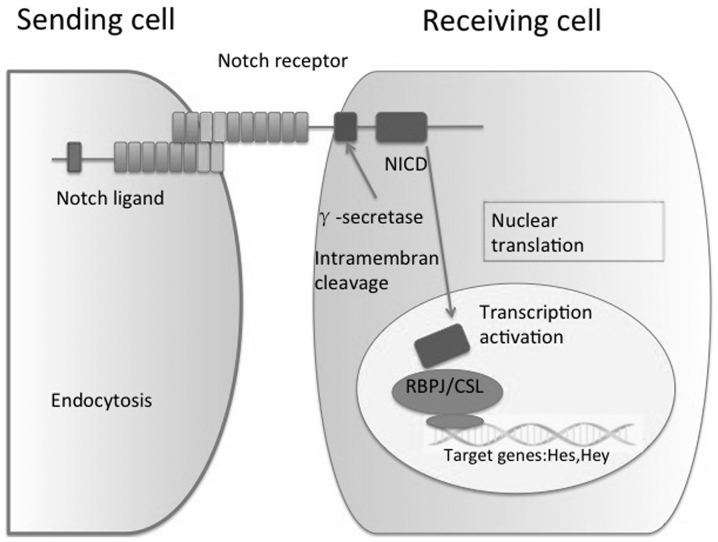
In mammals, the canonical Notch pathway includes four receptors (Notch1, 2, 3 and 4) and two ligand families [Jagged (JAG) 1 and 2 and Delta-like-ligand (Dll) 1, 3 and 4]. The Notch signaling pathway consists of ligand-induced activation of receptors, proteolytic cleavage and subsequent translocation of the notch intracellular domain (NICD) to the nucleus, where it functions as a transcriptional regulator (9). Activation of Notch signaling requires direct or indirect contact between cells expressing Notch ligands or receptors, and transmitting and receiving cells are subsequently modified by the interaction. Initially, cells express Notch receptors and ligands, and as the interaction continues, one cell becomes a transmitting cell by upregulating the expression of ligands and downregulating of receptors, while the receiving cells follow the opposite pattern (10). Prior to the NICD being transported to the nucleus, it is cleaved by the γ-secretase complex. In the nucleus, NICD interacts with C-repeat/DRE binding factor 1, a DNA-binding transcriptional repressor also known as the recombination signal binding protein for immunoglobulin Kappa J region (RBPJ), and converts it into a transcriptional activator that induces the transcription of target genes, including the family of Hes and Hey-associated transcription factors. Furthermore, in the liver, Notch partially controls the expression of Sox9, HNF1 Homeobox B and transforming growth factor-β, which are key regulators in hepatic lineage commitment (11,12) (Fig. 1).
Figure 1.
Model of the Notch pathway in major signaling cascades, including signal initiation through binding of the Notch ligand, cleavage of Notch, nuclear translation and transcriptional activation. NICD, notch intracellular domain; RBPJ, signal binding protein for immunoglobulin Kappa J region.
RBPJ is a DNA-binding protein, also known as CSL, which is a member of the Suppressor of Hairless (Drosophila melanogaster) family of transcription factors that recognizes the consensus sequence C(T)GTGGGAA. RBPJ predominantly acts as a transcriptional repressor of promoters that possess RBPJ binding sites via the recruitment of other co-repressors. The most important function of RBPJ is to mediate signals from Notch receptors. RBPJ is a common downstream transcription factor of Notch receptors and its absence indicates a complete block of the Notch signaling pathway (13).
The present review discusses the findings of recent studies regarding Notch expression and summarizes Notch signaling during HCC and ICC development.
2. Mutation of Notch-associated genes
Notch is a highly regulated signaling mechanism with numerous specific features. In humans, mutations in Notch ligands or receptors are associated with various diseases; for example, JAG1 and Notch2 mutations are associated with Alagille syndrome (14), and Notch3 mutations with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (15). Human genetic diseases and mutant mouse models have demonstrated the importance of Notch signaling in the development and remodeling of intrahepatic bile ducts (IHBD). Alagille syndrome (AGS) is a human autosomal dominant disorder that is caused by mutations in the Notch ligand JAG1, and less commonly in the Notch2 receptor (16). The estimated prevalence of AGS is 1 case per 70,000 live births worldwide (17). The disease is a multi-organ disorder most commonly diagnosed by liver abnormalities, which lead to hepatic bile duct paucity and cholestasis at birth (18). Cardiac, skeletal and ophthalmological abnormalities, and less frequently renal or vascular deficiencies, are also observed in patients with AGS (19). Numerous renal abnormalities are observed in patients with AGS, including renovascular disease, renal tubular acidosis, tubulointerstitial nephritis and renal dysplasia/hypoplasia (20). Notably, mice with haploinsufficiency for JAG1 exhibit no significant phenotypic abnormalities, suggesting that additional modifier genes contribute to the AGS phenotype observed in humans (21). Additionally, mutations in Notch3 are associated with inherited vascular diseases, including degenerative vascular disorder, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (22).
3. Notch pathway function in liver disease
The Notch signaling pathway is significantly associated with liver disease (23). In liver cirrhosis, the expression of Notch and Toll-like receptor signaling pathways are disordered (24). Based on the analysis of Notch and its target genes in HCC, the expression of Notch3 and Notch4 is aberrantly increased when compared with para-cancer tissue (25). Notch3 and 4 are rarely observed in normal liver tissue or para-carcinoma chronic hepatitis. In cirrhotic liver tissues, Notch3 mRNA and Hes6 mRNA expression levels are lower compared with those observed in HCC tissues. By contrast, high expression of Notch3 and low expression of Notch4 have been observed in human HCC HepG2 cell lines (26). Compared with HCC tissues of neoplastic cells, non-cancerous tissues adjacent to tumors have a high expression of Notch1 in the cytoplasm and Notch4 in nucleus, and a low expression of Notch2; however there is no significant difference between Notch4 and Notch3 expression. Thus, Notch receptor expression is abnormal in HCC (27). Rodent models with Notch loss or gain of function have demonstrated that Notch is involved in several stages of IHBD morphogenesis (28). Murine knockout (KO) studies for each of the mammalian Notch receptors and ligands have been conducted, and the resulting effects on the liver phenotype are presented in Table I (23–33).
Table I.
Notch pathway phenotypes involved in liver development and regeneration in mouse models.
| Author, year | Disrupted gene | Phenotype | (Ref.) |
|---|---|---|---|
| Croquelois et al, 2005 | Notch1 | Nodular regenerative hyperplasia, disruption of homeostasis of hepatic | (23) |
| and Dill et al, 2012 | sinusoids and stimulation of pre-and postnatal bile duct proliferation | (24) | |
| Geisler et al, 2008 | Notch2 | Impaired intrahepatic bile duct development | (25) |
| Chen et al, 2012 | Notch3 | Regulation of HSC activation. Interruption of Notch3 may be an anti-fibrotic strategy in hepatic fibrosis | (26) |
| Krebs et al, 2000 | Notch4 | Severe defects in angiogenic vascular remodeling | (27) |
| Hofmann et al, 2010 | JAG1 | Exhibition of Alagille syndrome, characterized by a paucity of intrahepatic bile ducts | (28) |
| Jiang et al, 1998 | JAG2 | Perinatal death | (29) |
| Redeker et al, 2013 | Dll1 | Stimulation of neuronal differentiation, lethal at embryonic day 11.5, severe somite patterning defects, hyperplastic CNS | (30) |
| Turnpenny et al, 2003 | Dll3 | Severe abnormalities in somitogenesis and recessive skeletal abnormalities in spondylocostal dysosotosis | (32) |
| Gale et al, 2004 | Dll4 | Arteriovenous shunting, severe vascular remodeling defects | (33) |
| and Djokovic et al, 2010 | (31) |
JAG, Jagged; Dll, Delta-like-ligand; HSC, hematopoietic stem cells; CNS, central nervous system.
Notch receptors
Overview
A number of Notch receptors have been identified in mammals, including Notch1-4. The receptors are transmembrane proteins with three domains: Extracellular Notch domain, a transmembrane domain and NICD. A number of studies have demonstrated that inhibition of the Notch signaling pathway induces the downregulation of Notch receptors (34,35).
Notch1
Notch1 regulates arteriovenous differentiation during embryogenesis and in the hepatic endothelium of adult mice (29). Homozygous disruption of the Notch1 gene is fatal at embryonic day (ED) 10, suggesting that Notch1 is essential for normal embryonic development. After ED10, histological analyses revealed widespread cell death, which was attributed to disorganized and delayed somitogenesis (30,31). Notch1 is required for vascular homeostasis of hepatic sinusoids by inducing quiescence and differentiation of liver sinusoidal endothelial cells. Thus, disruption of the Notch1 pathway leads to intussusceptive angiogenesis and nodular regenerative hyperplasia (36).
Notch2
Homozygous Notch2-deficient embryos exhibit developmental retardation, widespread cell death and embryonic mortality prior to ED11.5; however, normal somitogenesis is observed compared with a Notch1 KO (37). Alagille syndrome is also associated with Notch2 mutations (38). Jeliazkova et al (39) demonstrated that mice with a perinatal, liver-specific complete elimination of Notch2 exhibited a marked reduction in the number of mature bile ducts, an increased number of disorganized primitive biliary-like structures, portal inflammation, portal tract enlargement and fibrosis and biliary necrosis. Furthermore, neonatal Notch2 KO mice are severely jaundiced, with livers that exhibit no cytokeratin 19 positive ductal structures (40).
Notch3 and Notch4
Young Notch3 KO mice are viable and fertile without any apparent phenotypic abnormalities, whereas adult Notch3 KO mice exhibit arterial defects due to abnormalities in differentiation (41). In the liver, Notch3 regulates the activation of hematopoietic stem cells and may exhibit an anti-fibrogenic effect (42). Notch4 KO mice are viable and fertile, since during development Notch4 expression is restricted to vascular endothelial cells (42). However, Notch1/4 double KO mice exhibit a more severe phenotype, presenting with extensive defects in angiogenic vascular remodeling during embryonic development compared with Notch1 KO mice (43).
Notch ligands
Notch ligands include JAG1, JAG2, Dll1, Dll3 and Dll4. Homozygous disruption of Notch ligands invariably affects the liver. A previous study has demonstrated that deletion of JAG1 in the portal vein mesenchyme lead in jaundice, liver failure and small numbers of IHBDs (28). Furthermore, JAG2 KO mice die perinatally due to craniofacial defects, including fusion of the tongue with the palatal shelves, and syndactyly of the fore and hind limbs (29). Homozygous inactivation of Dll1 causes severe defects in somite patterning and the development of a hyperplastic central nervous system (44). Following ED9, Dll1 KO mice become hemorrhagic and die around ED11.5 (45). Dll3 is expressed in the presomitic mesoderm and is localized to the rostral somatic compartments. Homozygous disruption of Notch1 and Dll3 leads to severe abnormalities in somitogenesis. Mutations in the human Dll3 homolog result in recessive skeletal abnormalities in spondylocostal dysostosis (46). Dll4 is essential for embryonic vascular development and arterial specification and is clearly upregulated in the tumor vessels of humans and mice; Dll4 deficiency leads to severe vascular remodeling defects and embryonic mortality (31).
Notch transcription factor RBPJ
Human and murine RBPJ genes are located on chromosome 4 and 5, respectively (47)Canonical Notch signaling results in the upregulation of transcription via Notch target genes (48). RBPJ is a potent DNA-binding transcription factor that associates with a large number of chromatin regulators, corepressors and coactivators, and mediates Notch signaling (49). In mammals, RBPJ activates transcription by forming a ternary complex with one of the four Notch paralogs (Notch1–4) and the Mastermind (MAM) family of coactivators (MAMl1–3). NICD is released from the membrane and localizes to the nucleus, where it forms a transcriptionally active complex with RBPJ and the coactivator MAM. Assembly of the RBPJ-NICD-MAM ternary complex at a target gene acts as the switch for upregulating transcription (50).
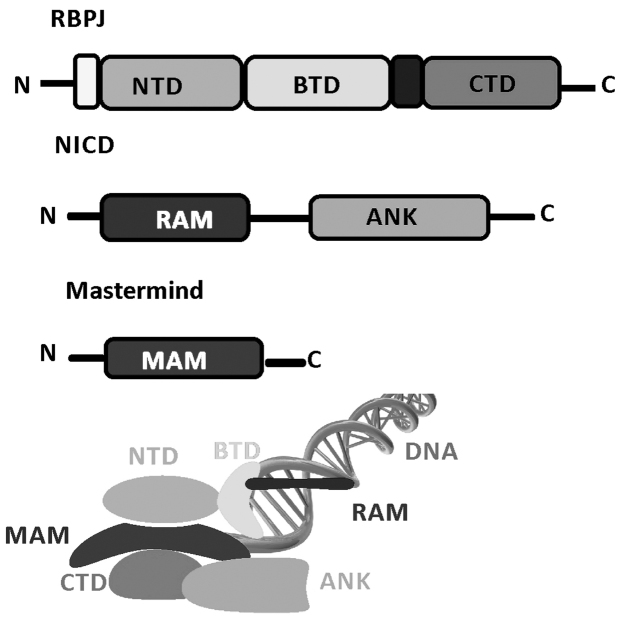
Structural studies of Notch transcription complexes have identified the overall folds, domain organization and interacting regions of RBPJ, NICD and MAM proteins (51–53). RBPJ is composed of three domains: An N-terminal domain (NTD), a β-trefoil domain (BTD) and a C-terminal domain (CTD). The NICD binds RBPJ via a RBPJ-associated molecule and ankyrin (ANK) repeat domains that interact with BTD and CTD. MAM forms a helix with a distinctive bend, in which its N-terminal helical region forms a tripartite complex with ANK and CTD, and its C-terminal helical region binds the NTD of RBPJ. RBPJ binds the consensus DNA sequence, CGTGGGAA with moderate affinity (~200 nm Kd) (54,55), which is a similar site to that of the enhancer and promoter elements of Notch target genes (56). The structures of RBPJ and RBPJ-NICD MAM activator complexes, including assembling at various target genes, have enabled detailed biochemical and cellular studies. These transcriptionally active ternary complexes bind to the promoter and enhancer elements of Notch responsive genes, including hey1 and hes1 promoters (57) (Fig. 2).
Figure 2.
Schematic of the RBPJ structure and domains demonstrating the domain organization of RBPJ, NICD and MAM. The transcriptionally active RBPJ-NICD-MAM ternary complex binds to DNA. MAM is red and DNA is green. NTD, N-terminal domain; BTD, β-trefoil domain; CTD, C-terminal domain; RAM, RBPJ-associated module; ANK, ankyrin; MAM, Mastermind; RBPJ, signal binding protein for immunoglobulin Kappa J region; NICD, notch intracellular domain.
4. Role of Notch in HCC and ICC development
Liver cirrhosis is commonly observed during the early stages of HCC and ICC (58). Previously, alterations in the Notch pathway have been identified in various solid tumors, such as breast, ovarian, pancreatic and liver cancer, melanoma and glioblastoma (59). The Notch signaling pathway exhibits a carcinogenic role in HCC. However, various members of the Notch signaling pathway may function as suppressors or enhancers of HCC. For example, the suppressive effect of Runt-related transcription factor 3 in HCC may be a result of Notch signaling inhibition (60). The Notch signaling pathway is involved in stem cell self-renewal and differentiation, and Notch signaling has been reported to promote the self-renewal of cancer stem-like cell niches in primary and metastatic tumors. Notch activation triggers epithelial-mesenchymal transformation (61). Several studies have revealed that a loss of the epithelial phenotype via epithelial-mesenchymal-transition promotes the acquisition of a stem-like phenotype and drug resistance (62–64). In addition, in patients with HCC an epithelial gene signature has been associated with sensitivity to the epidermal growth factor receptor inhibitors gefitinib and cetuximab (65). Deregulated expression of Notch, including the overexpression and activation of Notch proteins, ligands and targets, has been identified in numerous solid tumors, including cervical, endometrial, renal, lung and gastric carcinomas (66). These findings suggest that additional signaling pathways may affect whether Notch functions as a tumor suppressor or oncogene in a particular tissue (67).
Gain or loss of function mutations in the Notch pathway have been identified in several types of cancers, including neural stem cell tumors, lung carcinomas and prostate cancer (68). Although Notch does not directly lead to unregulated cell proliferation or genetic alterations that are associated with tumor progression, it alters the developmental state of cells and consequently maintains cells in a proliferative or undifferentiated state (69). In chronic HBV infection (CHB), repression of Notch receptors was demonstrated to lead to immune dysfunction (70). The contribution that a decreased expression of Notch receptors makes to ongoing fibrosis, cirrhosis and HCC is unclear; however, repression of Notch receptors in CHB has been suggested to repress immune regulation, resulting in the inhibition of differentiation and proliferation of effector cells leading to additional pathogenesis of CHB (71). Notably, the pro-mitogenic function of Notch was demonstrated in a model of partial hepatectomy (72). The pro-oncogenic function of Notch was also investigated by genome wide analysis of HCC samples, which revealed that the Notch coactivator MAML2 is a target of genetic alterations (73). Additionally, Notch signaling is crucial for the differentiation of hepatocytes into biliary lineage cells during the early stages of ICC developments. Gain and loss of function studies have demonstrated that ICC develops via the Notch-mediated differentiation of hepatocytes into biliary lineage cells, and that the malignancy and progression of the tumor are dependent on the intensity of Notch signaling in hepatocytes (74). Notably, hepatitis-infected hepatocytes may be converted into biliary lineage cells via Notch signal activation and thus become the point of origin for ICC (75). Therefore, suppression of Notch signaling may present a novel strategy for the treatment of ICC, since it may inhibit the conversion of hepatocytes into biliary lineage cells during the early stages of ICC development (76). Mice with liver-specific constitutive activation of Notch1 intracellular domain (N1ICD) may develop HCC when they reach adult age (77). Genomic profiling technology has revealed that a Notch-specific gene expression signature reported in mice overexpressing NICD was also present in a cluster of patients with HCC (78). Histological analyses of the mouse liver tissue exhibited similar features compared with that of the HCC patients, which included the presence of proliferating K-19 positive cells. Constitutive Notch2 overexpression causes HPCs to spontaneously develop into dedifferentiated HCC cells (79). In addition, Notch-induced malignant hepatocyte transformation is associated with downregulation of hepatocyte-associated genes and Sox9 expression (80). Fate-mapping studies have demonstrated that clear-cell adenocarcinoma (CCA) cells derived from hepatocytes may be converted to a biliary K-19 positive phenotype (81). Accordingly, in CCA development, N1ICD association with protein kinase B signaling in hepatocytes stimulated their malignant dedifferentiation (82). The stimulation of N1ICD expression by inflammatory mediators has also been reported in human CCA, which additionally supports the role of Notch in liver cancer (83) (Table II) (9,12,24,31,84–87).
Table II.
Various liver cancer phenotypes associated with the Notch pathway.
| Author, year | Mouse model | Liver defects | Liver tumorigenesis | (Ref.) |
|---|---|---|---|---|
| Dou et al, 2008 | Dll4 KO | Inhibition of hepatic vascular alterations | Inhibition of tumor growth | (9) |
| Zong et al, 2009 | AFP, AlbCre/RBPJ, NICD | Reduction in bile duct number | Association with ICC | (12) |
| Dill et al, 2012 | Mx1cre/RBPJflox | Disruption of vascular homeostasis | Spontaneous angioma | (24) |
| Djokovic et al, 2010 | Dll4 KO | Inhibition of hepatic vascular alterations | Inhibition of tumor growth | (31) |
| Fan B et al, 2012 | AlbCre/Hes1, Notch1flox | ICC | ICC arises through Notch-mediated hepatocytes | (85) |
| Villanueva et al, 2012 | AFP Cre/NICD KO | Dysplasia | Differentiated HCC | (84) |
| Viatour et al, 2011 | AdCre/TKO | Stem/progenitor cell expansion | HCC development | (86) |
| Vincent et al, 2009 | Hu-AGT-TG | Reduced liver volume, blood flow velocities and arterial vessel density | Antagonized tumor angiogenesis and delayed tumor growth | (87) |
Dll, Delta-like-ligand; KO, knockout; AFP, α-fetoprotein; RBPJ, signal binding protein for immunoglobulin Kappa J region; NICD, notch intracellular domain; ICC, intrahepatic cholangiocarcinoma; TKO, technical knockout; HCC, hepatocellular carcinoma.
5. Notch-based therapeutic strategies in liver cancer
Persistent activation of Notch signaling may lead to oncogenesis depending on modifier factors, including the inflammatory environment or the presence of other carcinogenic conditions that may cause HCC or CCA (88). A Notch signature has been reported in a subset of HCC patients and an overexpression of Notch receptors has been reported in human CCAs, which is fundamentally required for the development of targeted therapies (89). Silencing of the Notch pathway may potentially inhibit Notch-driven tumor progression and interfere with tumor aggressiveness, since Notch activation has been associated with a more malignant phenotype (84). However, the identification of a reliable tissue-specific biomarker of Notch inhibition is critical for the application of Notch-targeted therapy. Notably, the hepatic Notch target gene Sox9 is associated with a poor prognosis in liver cancers (90). Therefore, the role of Sox9 as a potential biomarker of Notch involvement and indication for Notch-targeted treatment requires additional study.
Roma et al (91) reported that pharmacodynamically active drugs, including emtricitabine/rilpivirine/tenofovir or ‘GSI’, inhibits Notch signaling, which prevents metastasis and recurrence of tumors and increases the disease-free survival time of patients. However, gastrointestinal toxicity is a primary side-effect of GSI use and these drugs are more effective against tumors that exhibit upregulated Notch signaling. Therefore, other potential modulating factors remain to be identified (92).
6. Conclusion
An increased understanding of the mechanisms involved in liver cancer proliferation and differentiation may aid the development of therapeutic strategies for liver cancer. The Notch pathway is emerging as a critical signaling pathway, which regulates cell proliferation, differentiation and necrosis associated with normal development, as well as stem cell renewal and differentiation. In conclusion, loss or disruption of Notch signaling may be a key contributing factor in bile ductular disorders, sinusoidal capillarization and the neovascularization of portal regions in the liver. To develop effective therapeutic approaches for the treatment of liver cancer, additional studies that investigate the Notch pathway are urgently required.
Acknowledgements
The present study was supported by the National Science Foundation of China (grant nos. 81300340 and 81270515), Shanghai Municipal Health Bureau Project (grant nos. 20124107 and 2011287) and Chinese Foundation for Prevention and Control of Hepatitis (grant nos. WBN20100021 and CFHPC20131011).
References
- 1.Tinkle CL, Haas-Kogan D. Hepatocellular carcinoma: natural history, current management, and emerging tools. Biologics. 2012;6:207–219. doi: 10.2147/BTT.S23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jie L, Fan W, Weiqi D, Yingqun Z, Ling X, Miao S, Ping C, Chuanyong G. The hippo-yes association protein pathway in liver cancer. Gastroenterol Res Pract. 2013;2013:187070. doi: 10.1155/2013/187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackl C, Schlitt HJ, Kirchner GI, Knoppke B, Loss M. Liver transplantation for malignancy: Current treatment strategies and future perspectives. World J Gastroenterol. 2014;20:5331–5344. doi: 10.3748/wjg.v20.i18.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam PH, Obirieze AC, Ortega G, Nwokeabia I, Onyewu S, Purnell SD, Samimi MM, Weeks CB, Lee EL, Shokrani B, et al. Characterization of hepatitis B and C among liver transplant recipients with hepatocellular carcinoma: An analysis of the Nationwide Inpatient Sample Database. Transplant Proc. 2016;48:123–127. doi: 10.1016/j.transproceed.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL, Wu SM, Cheng P, Zhang Y, Shen M, et al. Salinomycin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells in vitro and in vivo. PLoS One. 2012;7:e50638. doi: 10.1371/journal.pone.0050638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai W, Wang F, He L, Lin C, Wu S, Chen P, Zhang Y, Shen M, Wu D, Wang C, et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: Partial mediation by the transcription factor NFAT1. Mol Carcinog. 2015;54:301–311. doi: 10.1002/mc.22100. [DOI] [PubMed] [Google Scholar]
- 8.Roca C, Adams RH. Regulation of vascular morphogenesis by notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 9.Dou GR, Wang YC, Hu XB, Hou LH, Wang CM, Xu JF, Wang YS, Liang YM, Yao LB, Yang AG, Han H. RBP-J, the transcription factor downstream of Notch receptors, is essential for the maintenance of vascular homeostasis in adult mice. FASEB J. 2008;22:1606–1617. doi: 10.1096/fj.07-9998com. [DOI] [PubMed] [Google Scholar]
- 10.Fortini ME. Notch signaling: The core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- 12.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morell CM, Fiorotto R, Fabris L, Strazzabosco M. Notch signalling beyond liver development: emerging concepts in liver repair and oncogenesis. Clin Res Hepatol Gastroenterol. 2013;37:447–454. doi: 10.1016/j.clinre.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Ahn KJ, Yoon JK, Kim GB, Kwon BS, Go JM, Moon JS, Bae EJ, Noh CI. Alagille syndrome and a JAG1 mutation: 41 Cases of experience at a single center. Korean J Pediatr. 2015;58:392–397. doi: 10.3345/kjp.2015.58.10.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Zuo Y, Sun W, Zhang W, Lv H, Huang Y, Xiao J, Yuan Y, Wang Z. The genetic spectrum and the evaluation of CADASIL screening scale in Chinese patients with NOTCH3 mutations. J Neurol Sci. 2015;354:63–69. doi: 10.1016/j.jns.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 17.Brooks AS, Dooijes D. From gene to disease: Arteriohepatic dysplasia or Alagille syndrome. Ned Tijdschr Geneeskd. 2003;147:1213–1215. [PubMed] [Google Scholar]
- 18.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habib R, Dommergues JP, Gubler MC, Hadchouel M, Gautier M, Odievre M, Alagille D. Glomerular mesangiolipidosis in alagille syndrome (arteriohepatic dysplasia) Pediatr Nephrol. 1987;1:455–464. doi: 10.1007/BF00849254. [DOI] [PubMed] [Google Scholar]
- 20.Nyfeler Y, Kirch RD, Mantei N, Leone DP, Radtke F, Suter U, Taylor V. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 2005;24:3504–3515. doi: 10.1038/sj.emboj.7600816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P, Dai W, Wang F, Lu J, Shen M, Chen K, Li J, Zhang Y, Wang C, Yang J, et al. Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1-RAGE and AKT pathways. Biochem Biophys Res Commun. 2014;443:1162–1168. doi: 10.1016/j.bbrc.2013.12.064. [DOI] [PubMed] [Google Scholar]
- 23.Croquelois A, Blindenbacher A, Terracciano L, Wang X, Langer I, Radtke F, Heim MH. Inducible inactivation of Notch1 causes nodular regenerative hyperplasia in mice. Hepatology. 2005;41:487–496. doi: 10.1002/hep.20571. [DOI] [PubMed] [Google Scholar]
- 24.Dill MT, Rothweiler S, Djonov V, Hlushchuk R, Tornillo L, Terracciano L, Meili-Butz S, Radtke F, Heim MH, Semela D. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis and angiosarcomas in livers of mice. Gastroenterolog. 2012;142:967–977. doi: 10.1053/j.gastro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 25.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM, Siveke JT. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 26.Chen JY, Feng L, Zhang HL, Li JC, Yang XW, Cao XL, Liu L, Qin HY, Liang YM, Han H. Differential regulation of bone marrow-derived endothelial progenitor cells and endothelial outgrowth cells by the notch signaling pathway. PLoS One. 2012;7:e43643. doi: 10.1371/journal.pone.0043643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: Insights into alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–1057. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redeker C, Schuster-Gossler K, Kremmer E, Gossler A. Normal development in mice over-expressing the intracellular domain of DLL1 argues against reverse signaling by DLL1 in vivo. PLoS One. 2013;8:e79050. doi: 10.1371/journal.pone.0079050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djokovic D, Trindade A, Gigante J, Badenes M, Silva L, Liu R, Li X, Gong M, Krasnoperov V, Gill PS, Duarte A. Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer. 2010;10:641. doi: 10.1186/1471-2407-10-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnpenny PD, Whittock N, Duncan J, Dunwoodie S, Kusumi K, Ellard S. Novel mutations in DLL3, a somitogenesis gene encoding a ligand for the Notch signalling pathway, cause a consistent pattern of abnormal vertebral segmentation in spondylocostal dysostosis. J Med Genet. 2003;40:333–339. doi: 10.1136/jmg.40.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traustadóttir GÁ, Jensen CH, Thomassen M, Beck HC, Mortensen SB, Laborda J, Baladrón V, Sheikh SP, Andersen DC. Evidence of non-canonical NOTCH signaling: Delta-like 1 homolog (DLK1) directly interacts with the NOTCH1 receptor in mammals. Cell Signal. 2016;28:246–254. doi: 10.1016/j.cellsig.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T, Gust KM, Wyatt AW, Goriki A, Jäger W, Awrey S, Li N, Oo HZ, Altamirano-Dimas M, Buttyan R, et al. Not all NOTCH Is Created Equal: The Oncogenic Role of NOTCH2 in Bladder Cancer and Its Implications for Targeted Therapy. Clin Cancer Res. 2016 Jan 14; doi: 10.1158/1078-0432.CCR-15-2360. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 36.Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 37.Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 38.Kamath BM, Bauer RC, Loomes KM, Chao G, Gerfen J, Hutchinson A, Hardikar W, Hirschfield G, Jara P, Krantz ID, et al. NOTCH2 mutations in Alagille syndrome. J Med Genet. 2012;49:138–144. doi: 10.1136/jmedgenet-2011-100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeliazkova P1, Jörs S, Lee M, Zimber-Strobl U, Ferrer J, Schmid RM, Siveke JT, Geisler F. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013;57:2469–2479. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- 40.Falix FA, Aronson DC, Lamers WH, Gaemers IC. Possible roles of DLK1 in the notch pathway during development and disease. Biochim Biophys Acta. 2012;1822:988–995. doi: 10.1016/j.bbadis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Pippucci T, Maresca A, Magini P, Cenacchi G, Donadio V, Palombo F, Papa V, Incensi A, Gasparre G, Valentino ML, et al. Homozygous NOTCH3 null mutation and impaired NOTCH3 signaling in recessive early-onset arteriopathy and cavitating leukoencephalopathy. EMBO Mol Med. 2015;7:848–858. doi: 10.15252/emmm.201404399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen YX, Weng ZH, Zhang SL. Notch3 regulates the activation of hepatic stellate cells. World J Gastroenterol. 2012;18:1397–1403. doi: 10.3748/wjg.v18.i12.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, Messina LM, Capobianco AJ, Werb Z, Wang R. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci USA. 2005;102:9884–9889. doi: 10.1073/pnas.0504391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocha SF, Lopes SS, Gossler A, Henrique D. Dll1 and Dll4 function sequentially in the retina and pV2 domain of the spinal cord to regulate neurogenesis and create cell diversity. Dev Biol. 2009;328:54–65. doi: 10.1016/j.ydbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 45.de Hrabĕ Angelis M, McIntyre J, II, Gossler A. Maintenance of somite borders in mice requires the delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- 46.Maemura K, Yoshikawa H, Yokoyama K, Ueno T, Kurose H, Uchiyama K, Otsuki Y. Delta-like 3 is silenced by methylation and induces apoptosis in human hepatocellular carcinoma. Int J Oncol. 2013;42:817–822. doi: 10.3892/ijo.2013.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by notch signaling status. Genes Dev. 2013;27:1059–1071. doi: 10.1101/gad.211912.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan Z, Friedmann DR, VanderWielen BD, Collins KJ, Kovall RA. Characterization of CSL (CBF-1, Su (H), Lag-1) mutants reveals differences in signaling mediated by Notch1 and Notch2. J Biol Chem. 2012;287:34904–34916. doi: 10.1074/jbc.M112.403287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopan R, Ilagan MX. The canonical notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borggrefe T, Oswald F. The notch signaling pathway: Transcriptional regulation at notch target genes. Cell Mol Life Sci. 2009;66:1631–1646. doi: 10.1007/s00018-009-8668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovall RA, Blacklow SC. Mechanistic insights into notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol. 2010;92:31–71. doi: 10.1016/S0070-2153(10)92002-4. [DOI] [PubMed] [Google Scholar]
- 52.Clayton T, Poe MM, Rallapalli S, Biawat P, Savić MM, Rowlett JK, Gallos G, Emala CW, Kaczorowski CC, Stafford DC, Arnold LA, Cook JM. A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model. Int J Med Chem. 2015;2015:430248. doi: 10.1155/2015/430248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou JH, Xue TC, Sun C, Li Y, Liu BB, Sun RX, Chen J, Ren ZG, Ye SL. Prognostic significance of Hes-1, a downstream target of notch signaling in hepatocellular carcinoma Asian Pac. J Cancer Prev. 2015;16:3811–3816. doi: 10.7314/apjcp.2015.16.9.3811. [DOI] [PubMed] [Google Scholar]
- 54.Friedmann DR, Kovall RA. Thermodynamic and structural insights into CSL-DNA complexes. Protein Sci. 2010;19:34–46. doi: 10.1002/pro.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai W, Wang C, Wang F, Wang Y, Shen M, Chen K, Cheng P, Zhang Y, Yang J, Zhu R, et al. Anti-miR-197 inhibits migration in HCC cells by targeting KAI 1/CD82. Biochem Biophys Res Commun. 2014;446:541–548. doi: 10.1016/j.bbrc.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Zou J, Zhao B, Johannsen E, Ashworth T, Wong H, Pear WS, Schug J, Blacklow SC, Arnett KL, et al. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc Natl Acad Sci USA. 2011;108:14908–14913. doi: 10.1073/pnas.1109023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 58.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 59.Wolfe MS, Esler WP, Das C. Continuing strategies for inhibiting alzheimer's gamma-secretase. J Mol Neurosci. 2002;19:83–87. doi: 10.1007/s12031-002-0015-5. [DOI] [PubMed] [Google Scholar]
- 60.Tchorz JS, Kinter J, Müller M, Tornillo L, Heim MH, Bettler B. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology. 2009;50:871–879. doi: 10.1002/hep.23048. [DOI] [PubMed] [Google Scholar]
- 61.Zhang QD, Xu MY, Cai XB, Qu Y, Li ZH, Lu LG. Myofibroblastic transformation of rat hepatic stellate cells: The role of Notch signaling and epithelial-mesenchymal transition regulation. Eur Rev Med Pharmacol Sci. 2015;19:4130–4138. [PubMed] [Google Scholar]
- 62.Wen L, Liang C, Chen E, Chen W, Liang F, Zhi X, Wei T, Xue F, Li G, Yang Q, Gong W, Feng X, Bai X, Liang T. Regulation of Multi-drug Resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci Rep. 2016;6:23269. doi: 10.1038/srep23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen QY, Jiao DM, Wang J, Hu H, Tang X, Chen J, Mou H, Lu W. miR-206 regulates cisplatin resistance and EMT in human lung adenocarcinoma cells partly by targeting MET. Oncotarget. 2016 Mar 21; doi: 10.18632/oncotarget.8229. (Epub ahead of print). doi: 10.18632/oncotarget.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lombardo Y, Faronato M, Filipovic A, Vircillo V, Magnani L, Coombes RC. Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells. Breast Cancer Res. 2014;16:R62. doi: 10.1186/bcr3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Espinoza I, Pochampally R, Xing F, Watabe K, Miele L. Notch signaling: Targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther. 2013;6:1249–1259. doi: 10.2147/OTT.S36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang H, An HJ, Song JY, Kim TH, Heo JH, Ahn DH, Kim G. Notch3 and Jagged2 contribute to gastric cancer development and to glandular differentiation associated with MUC2 and MUC5AC expression. Histopathology. 2012;61:576–586. doi: 10.1111/j.1365-2559.2012.04274.x. [DOI] [PubMed] [Google Scholar]
- 67.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loomes KM, Taichman DB, Glover CL, Williams PT, Markowitz JE, Piccoli DA, Baldwin HS, Oakey RJ. Characterization of notch receptor expression in the developing mammalian heart and liver. Am J Med Genet. 2002;112:181–189. doi: 10.1002/ajmg.10592. [DOI] [PubMed] [Google Scholar]
- 70.Pei J, Tang Z, Zang G, Yu Y. Blockage of Notch1 signaling modulates the T-helper (Th)1/Th2 cell balance in chronic hepatitis B patients. Hepatol Res. 2010;40:799–805. doi: 10.1111/j.1872-034X.2010.00680.x. [DOI] [PubMed] [Google Scholar]
- 71.Nijjar SS, Crosby HA, Wallace L, Hubscher SG, Strain AJ. Notch receptor expression in adult human liver: A possible role in bile duct formation and hepatic neovascularization. Hepatology. 2001;34:1184–1192. doi: 10.1053/jhep.2001.29399. [DOI] [PubMed] [Google Scholar]
- 72.Alvaro D, Bragazzi MC, Benedetti A, Fabris L, Fava G, Invernizzi P, Marzioni M, Nuzzo G, Strazzabosco M, Stroffolini T. Cholangiocarcinoma in Italy: A national survey on clinical characteristics, diagnostic modalities and treatment. Results from the ‘Cholangiocarcinoma’ committee of the Italian association for the study of liver disease. Dig Liver Dis. 2011;43:60–65. doi: 10.1016/j.dld.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 74.Gao J, Chen Y, Wu KC, Liu J, Zhao YQ, Pan YL, Du R, Zheng GR, Xiong YM, Xu HL, Fan DM. RUNX3 directly interacts with intracellular domain of Notch1 and suppresses notch signaling in hepatocellular carcinoma cells. Exp Cell Res. 2010;316:149–157. doi: 10.1016/j.yexcr.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 75.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weng AP, Aster JC. Multiple niches for notch in cancer: Context is everything. Curr Opin Genet De. 2004;14:48–54. doi: 10.1016/j.gde.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Lu J, Zhou Y, Hu T, Zhang H, Shen M, Cheng P, Dai W, Wang F, Chen K, Zhang Y, et al. Notch Signaling Coordinates Progenitor Cell-Mediated Biliary Regeneration Following Partial Hepatectomy. Sci Rep. 2016;6:22754. doi: 10.1038/srep22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther. 2013;139:95–110. doi: 10.1016/j.pharmthera.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayashi Y, Osanai M, Lee GH. NOTCH2 signaling confers immature morphology and aggressiveness in human hepatocellular carcinoma cells. Oncol Rep. 2015;34:1650–1658. doi: 10.3892/or.2015.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kohn A, Rutkowski TP, Liu Z, Mirando AJ, Zuscik MJ, O'Keefe RJ, Hilton MJ. Notch signaling controls chondrocyte hypertrophy via indirect regulation of Sox9. Bone Res. 2015;3:15021. doi: 10.1038/boneres.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fowlkes BJ, Robey EA. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J Immunol. 2002;169:1817–1821. doi: 10.4049/jimmunol.169.4.1817. [DOI] [PubMed] [Google Scholar]
- 82.Zhu R, Yang J, Xu L, Dai W, Wang F, Shen M, Zhang Y, Zhang H, Chen K, Cheng P, et al. Diagnostic performance of des-γ-carboxy prothrombin for hepatocellular carcinoma: A meta-analysis. Gastroenterol Res Pract. 2014;2014:529314. doi: 10.1155/2014/529314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nalesnik MA, Tseng G, Ding Y, Xiang GS, Zheng ZL, Yu Y, Marsh JW, Michalopoulos GK, Luo JH. Gene deletions and amplifications in human hepatocellular carcinomas: Correlation with hepatocyte growth regulation. Am J Pathol. 2012;180:1495–1508. doi: 10.1016/j.ajpath.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S, Stanger BZ, Llovet JM. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–1669. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viatour P, Ehmer U, Saddic LA, Dorrell C, Andersen JB, Lin C, Zmoos AF, Mazur PK, Schaffer BE, Ostermeier A, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–1976. doi: 10.1084/jem.20110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vincent F, Bonnin P, Clemessy M, Contrerès JO, Lamandé N, Gasc JM, Vilar J, Hainaud P, Tobelem G, Corvol P, Dupuy E. Angiotensinogen delays angiogenesis and tumor growth of hepatocarcinoma in transgenic mice. Cancer Res. 2009;69:2853–2860. doi: 10.1158/0008-5472.CAN-08-2484. [DOI] [PubMed] [Google Scholar]
- 88.Wang F, Dai W, Wang Y, Shen M, Chen K, Cheng P, Zhang Y, Wang C, Li J, Zheng Y, et al. The synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma. PLoS One. 2014;9:e97414. doi: 10.1371/journal.pone.0097414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates notch-1 in mouse cholangiocytes: Implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 91.Roma J, Masià A, Reventós J, de Sánchez Toledo J, Gallego S. Notch Pathway Inhibition Significantly Reduces Rhabdomyosarcoma Invasiveness and Mobility. Vitro Clin Cancer Res. 2011;17:505–513. doi: 10.1158/1078-0432.CCR-10-0166. [DOI] [PubMed] [Google Scholar]
- 92.Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of notch signaling. Onco Targets Ther. 2013;6:943–955. doi: 10.2147/OTT.S33766. [DOI] [PMC free article] [PubMed] [Google Scholar]