Abstract
Most infections occur in early life, prompting development of novel adjuvanted vaccines to protect newborns and infants. Several Toll-like receptor (TLR) agonists (TLRAs) are components of licensed vaccine formulations or are in development as candidate adjuvants. However, the type and magnitude of immune responses to TLRAs may vary with the TLR activated as well as age and geographic location. Most notably, in newborns, as compared to adults, the immune response to TLRAs is polarized with lower Th1 cytokine production and robust Th2 and anti-inflammatory cytokine production. The ontogeny of TLR-mediated cytokine responses in international cohorts has been reported, but no study has compared cytokine responses to TLRAs between U.S. neonates and infants at the age of 6 months. Both are critical age groups for the currently pediatric vaccine schedule. In this study, we report quantitative differences in the production of a panel of 14 cytokines and chemokines after in vitro stimulation of newborn cord blood and infant and adult peripheral blood with agonists of TLR4, including monophosphoryl lipid A (MPLA) and glucopyranosyl lipid Adjuvant aqueous formulation (GLA-AF), as well as agonists of TLR7/8 (R848) and TLR9 (CpG). Both TLR4 agonists, MPLA and GLA-AF, induced greater concentrations of Th1 cytokines CXCL10, TNF and Interleukin (IL)-12p70 in infant and adult blood compared to newborn blood. All the tested TLRAs induced greater infant IFN-α2 production compared to newborn and adult blood. In contrast, CpG induced greater IFN-γ, IL-1β, IL-4, IL-12p40, IL-10 and CXCL8 in newborn than infant and adult blood. Overall, to the extent that these in vitro studies mirror responses in vivo, our study demonstrates distinct age-specific effects of TLRAs that may inform their development as candidate adjuvants for early life vaccines.
Keywords: Neonate, newborn, infant, immune ontogeny, adjuvant, toll-like receptor (TLR)
Introduction
As a key feature of its host defense function, the human immune system recognizes a large variety of microbial products. A variety of hematopoietic cells express Toll-like Receptors (TLRs), a family of pattern-recognition receptors (PRRs) that recognize microbial products, to direct them to exert the appropriate effector function. Dendritic cells (DCs), key antigen-presenting cells at the interface of innate and adaptive immunity, employ PRRs to interpret their surroundings and instruct the adaptive immune system to generate a humoral or cellular immune response. Adjuvanted vaccines leverage the ability of APCs to recognize microbial products and subsequently generate an immune response [1]. Many vaccines contain intrinsic components that engage one or more PRRs, such as Bacille Calmette Guérin (BCG; TLR2 and TLR4) [2, 3], oral polio vaccine (OPV;TLR3) [4, 5] and yellow fever vaccine (YFV; TLRs 7,8, and 9) [6]. Subunit vaccines, made from purified microbial products, may require an adjuvant, to instruct and enhance protective immune responses. Such is the case, for example, for, hepatitis B vaccine (HBV), human papilloma vaccine (HPV) and pneumococcal conjugate vaccine (PCV) [7, 8]. Vaccine efficacy is most often evaluated by seroconversion, even though appropriate activation of T-helper (Th)1 or Th17 cells and CD8+ cytotoxic T cells may be important for protection in some cases, especially against intracellular pathogens [9–13].
A growing literature documents the cellular and molecular mechanisms by which PRR agonists can serve as adjuvants [14–19]. Adjuvants such as alum and MF59 enhance the immune response to vaccinal antigens that can involve both Th1 and Th2 cells, whereas adjuvants that activate TLR3, -4, -7, -8 and -9 induce a more Th1-biased T-cell response [7, 20]. The type and magnitude of an immune response to a vaccine or an adjuvant can be age-specific. For instance, children under 2 years of age fail to mount an adequate response to pneumococcal polysaccharide vaccine (PPV), an alum-adjuvanted subunit vaccine that is effective in adults [21]. Instead, children under the age of 2 years can effectively be given a pneumococcal conjugate vaccine (PCV), which is adjuvanted with Alum and CRM197, a tetanus toxoid that enables CD4+ T cells to mediate seroconversion [22]. In vivo and in vitro studies have also demonstrated that the innate immune response to pure TLR agonists (TLRAs) is age-dependent and functionally distinct in cord blood mononuclear cells, as compared to their adult counterparts [23–26]. Newborn monocytes and monocyte-derived DCs (moDCs) produce less of the Th1-polarizing cytokine TNF, in response to stimulation through TLR2, -3 or -4, than adult cells [27, 28]. In contrast, newborn monocytes and moDCs produce more interleukin (IL)-6 and IL-10, potentially leading to less Th1 induction [23, 27, 29]. Of note, newborn monocytes, as well as DCs do produce adult-like levels of TNF and IL-1β in response to TLR7/8 agonists, raising interest in these agonists as potential vaccine adjuvants in early life [23, 30–32].
In this study we employed a whole blood assay (WBA) to study the ontogeny of cytokine responses to four TLRAs: monophosphoryl lipid A (MPLA; TLR4), an aqueous formulation the novel glucopyranosyl lipid antigen (GLA-AF; TLR4) [33, 34], resiquimod (R848; TLR7/8) and CpG oligonucleotide ODN 2395 (TLR9). Although both MPLA and GLA are based on the core lipid A of LPS, there are important differences between these two molecules, including that the latter is synthetic [34]. We measured the production of 14 cytokines [35] and potential reactogenicity biomarker prostaglandin E2 (PGE2) [36] after stimulation of umbilical cord blood, peripheral blood from 6-month old infants and 18–40-year old adults. We noted that the cytokine response varied by age and TLRA. Cytokine production in response to TLR7/8 agonist R848 was comparable between all age groups tested. While the production of Th1 cytokines by newborn cells was impaired, blood from 6-month old infants produced adult-like levels of Th1 cytokines in response to TLR4 agonists. We also observed that CpG induced robust cytokine induction in newborn blood and GLA-AF demonstrated greater potency and efficacy than MPLA in inducing Th-polarizing cytokine production in infant blood.
Materials and Methods
Ethics Statement
Non-identifiable cord blood samples were collected with approval from the Ethics Committee of The Beth Israel Deaconess Medical Center, Boston, MA (protocol number 2011P-000118). All experiments were performed in accordance with relevant institutional and national guidelines, regulations, and local Institutional Review Board (IRB) approval. Adult participants provided de-identified blood samples after written informed consent in accordance with a protocol approved by the IRB of Boston Children’s Hospital (BCH), Boston, MA (protocol number X07- 05-0223). Infant donors were recruited from the Children’s Hospital Primary Care Clinic during routine out-patient visits to receive vaccines as scheduled (diphtheria, tetanus, acellular pertussis (DTaP; 7 of 8 infants), hepatitis B vaccine (HBV; 1 of 8), rotavirus (RV; 7 of 8), haemophilus B conjugate vaccine (PRP-T), pneumococcal conjugate vaccine (PCV13; 7 of 8), inactivated poliovirus (IPV; 3 of 8)). In order to reduce the burden on enrolled infants, and to reduce drop-outs from scheduled immunization, blood draws in this age group occurred immediately after routine 6-month immunizations.. Informed consent was provided by parents for collection of de-identified infant blood samples in accordance with the BCH IRB (protocol number P00010750). Blood was drawn into a syringe with no additives, and then immediately added to 5 ml pyrogen-free polypropylene tubes (Sigma-Aldrich Co. LLC. St Louis, MO, USA) containing pyrogen-free heparin (American Pharmaceutical Partners, Inc.; Schaumberg, IL, USA) in a 1:50 ratio to blood. Blood and heparin were mixed by inversion.
TLR Agonists and Assay Reagents
The following TLRAs were used at the concentrations noted in the figure legends: MPLA (TLR4), R848 (TLR7/8), and ODN 2395 (TLR9) all from from InVivogen (San Diego, CA). GLA-AF (TLR4) was from the Infectious Diseases Research Institute (IDRI; Seattle, WA).
Blood Sample Processing and in vitro Stimulation
For assessment of TLRA activity in whole blood, we used an adaptation of the method of Kollmann et al [37]. Neonatal cord blood, infant peripheral blood, or adult peripheral blood was diluted 5x with sterile pre-warmed (37°C) RPMI 1640 medium (Invitrogen, Carlsbad, CA) and 135 μL of the suspension was added to each well of a 96 well round-bottom plate (Becton Dickinson, Franklin Lakes, NJ, USA) containing 15 μl freshly prepared specific TLRAs at 10x the final concentration. Suspensions containing 150 μl/well were gently mixed by pipetting and incubated for 18 h at 37°C in a humidified incubator at 5% CO2. After culture, plates were centrifuged at 500 × g and 3 aliquots of 35 μl of supernatant were carefully removed by pipetting without disturbing the cell pellet. Supernatants derived from human leukocyte stimulations were assayed by ELISA for TNF (BD Biosciences, San Jose, CA, USA) and PGE2 (Cayman Chemicals; Ann Arbor, MI) and by multiplexing bead array (Millipore).
Cytokine measurement by multi-analyte fluorescent bead-based array
Cytokine concentrations in supernatants derived from whole blood assays was measured by multi-analyte bead array (Milliplex) using a custom-designed Cytokine Human Magnetic Panel from Invitrogen – Life Technologies (Carlsbad, CA, USA), including IFN-alpha2, IFN-γ, IL-10, IL-12p40, IL-12p70, IL-1β, IL-6, and TNF. Results were obtained with a MAGPIX system with Luminex xPONENT software (both from Luminex Corp., Austin, TX, USA). Cytokine concentrations were determined using Milliplex Analyst (version 3.5.5.0, Millipore) software.
Statistical Analyses and Graphics
Data were analyzed using Prism for MacIntosh v. 5.0b (GraphPad Software Inc., San Diego, CA). Data in figures represent means ± SEM. Differences between conditions at a single concentration were evaluated using a Student’s t-test, and differences across a range of different concentrations were evaluated via repeated measured analysis of variance (RM-ANOVA), and p values <0.05 were considered significant.
Results
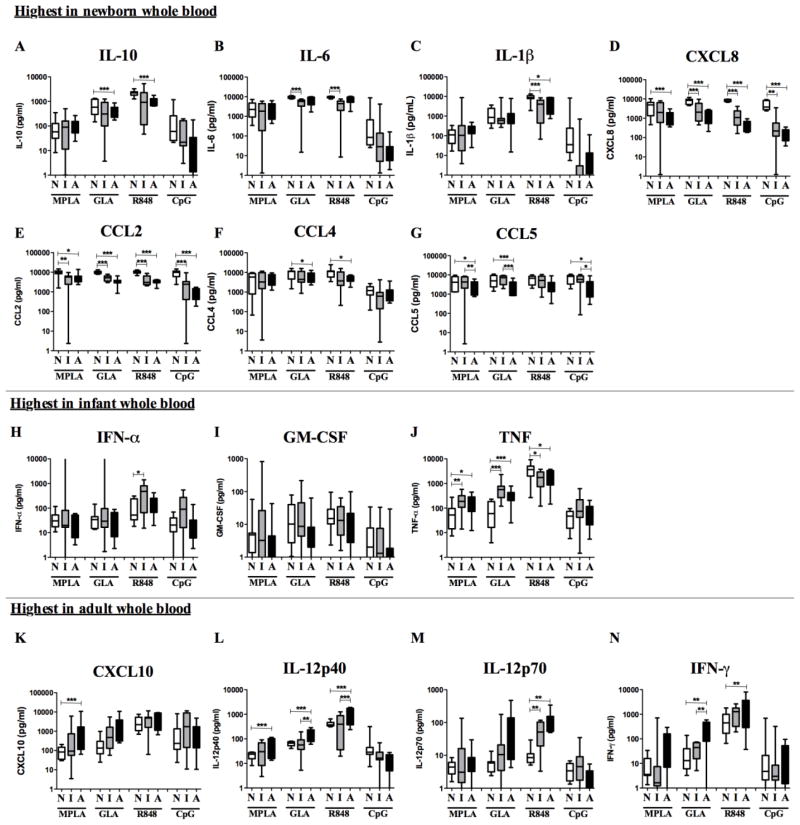
The age specific effects of four different TLRAs were evaluated in vitro using whole blood from three cohorts: (a) cord blood from full term newborns (mean gestational age of 39 weeks), (b) infant peripheral blood donors (mean age of 6 months) and (c) adult peripheral blood donors (mean age of 35 years; n = 8 per cohort). Concentration-dependent cytokine production in response to MPLA, GLA-AF, R848 and CpG was quantified after stimulation of RPMI-diluted blood. The reduced ability of newborn blood to produce Th1 cytokines in response to many TLRAs is well described [24, 29, 37]. To investigate the production of 14 cytokines and PGE2, we tested 12-point concentration-response curves for each of these analytes to determine the maximal response to TLRAs by each age group and for each analyte. These maximal responses were evaluated for statistically significant differences between age groups in Fig. 1 (for a complete overview of dose-response curves, see also Supplementary Fig. 1 and Table 1). Consistent with prior reports [23, 24, 29, 38, 39], the production of IL-10, IL-6 and CXCL8 was higher in newborn whole blood than in adult whole blood after stimulation with TLR4 or TLR7/8 agonists. Newborn blood produced greater concentrations of these cytokines than infant blood, and were highest in newborns after stimulation with CpG (Fig. 1). However, differences in maximal response between all age groups in CpG-stimulated blood as well as between newborns and adults in GLA- or R848-stimulated blood were not statistically significant. The differences between the 12-point dose-response curves under all these conditions were statistically significant when measured by RM-ANOVA (Supplementary Fig. 1, see also Fig. 4). As previously reported for moDCs [32], a higher production of IL-1β by newborns after stimulation with R848 was also observed. Panels E–F indicate that the production of chemokines CCL2, CCL3 and CCL5 in response to TLRAs is more distinct in newborns as well. Of note, TLR4-mediated production of the pro-inflammatory cytokines IFN-α, GM-CSF and TNF in response to MPLA and GLA-AF was highest in the infant age group. Although the higher production of IFN-α in infants after stimulation with MPLA, GLA-AF or CpG was not statistically significant from that of other age groups, these TLRAs were more potent in infant than in newborn or adult blood (MPLA infant 62.5 ng/ml vs newborn 1000 ng/ml, adult 500 ng/ml; GLA infant 250 ng/ml vs. newborn 1000 ng/ml, adult 500 ng/ml; CpG infant 6.25 μg/ml vs. newborn and adult 12.5 μg/ml; see also Supplementary Figure 1). TLR4 and TLR7/8-mediated production of the Th1 cytokines CXCL10, IL12 and IFN-γ demonstrated age-dependency, from least production in newborns, intermediate in infants to most in adults. In contrast, newborn whole blood demonstrated adult-level CpG-induced Th1 cytokine production. CpG results, as well as CXCL10 production in response to GLA were statistically significant when comparing the dose-response curves using RM-ANOVA.
Fig. 1. Age-dependent TLR agonist-induced cytokine production in whole blood.
Newborn, infant or adult peripheral blood was diluted 5x and stimulated with MPLA (TLR4; 0.98–1000 ng/ml), GLA-AF (TLR4; 0.98–1000 ng/ml), R848 (TLR7/8; 0.098–100 μM) or CpG (TLR9; 0.049–50 μg/ml). Maximal response was determined for each age group (N=Newborn, I=Infant and A=Adult). Comparison of TLRA-induced cytokine production between newborns, infants and adults is shown. Stars indicate statistical significance between age groups indicated as measured by Student’s t-test. (*p<0.05, **p<0.01, ***p<0.001)
Table 1. Age-dependent TLRA-induced cytokine production.
Averaged baseline (vehicle control condition) and maximal cytokine levels for each study age group and agonist, as well as the agonist concentration (ng/ml for MPLA and GLA; μM for R848 and μg/ml for CpG) required for the maximal cytokine response. p-Values of 2-way ANOVA comparing the age groups are indicated.
| Agonist | Cytokine | Newborn Baseline |
Newborn Max resp. |
Newborn [Agonist] |
Infant Baseline |
Infant Max resp. |
Infant [Agonist] |
Adult Baseline |
Adult Max resp. |
Adult [Agonist] |
RM-ANOVA NB vs IN |
RM-ANOVA IN vs AD |
RM-ANOVA NB vs AD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPLA | CXCL10 | 23.40 | 94.84 | 1000.00 | 30.60 | 947.78 | 1000.00 | 14.39 | 1900.63 | 1000.00 | *0.0188 | 0.4403 | *0.0152 |
| CCL4 | 30.51 | 5091.51 | 1000.00 | 43.433 | 4767.39 | 1000.00 | 83.35 | 5084.25 | 1000.00 | ***0.0006 | 0.7342 | ***0.0009 | |
| CCL5 | 2521.03 | 4649.00 | 1000.00 | 2861.61 | 4514.48 | 1000.00 | 1951.22 | 2517.60 | 1000.00 | 0.1259 | ***<0.0001 | ***<0.0001 | |
| IFN-γ | 1.83 | 8.85 | 125.00 | 13.93 | 103.76 | 3.91 | 4.73 | 78.23 | 1000.00 | *0.0130 | *0.2260 | **0.0019 | |
| TNF | 20.44 | 73.82 | 1000.00 | 23.71 | 225.35 | 1000.00 | 40.73 | 201.57 | 1000.00 | ***0.0002 | 0.6236 | **0.0053 | |
| IL-12p70 | 1.94 | 4.56 | 1000.00 | 3.82 | 21.28 | 1000.00 | 1.68 | 7.85 | 1000.00 | **0.0033 | **0.0033 | 0.9055 | |
| IFN-α | 25.92 | 40.98 | 1000.00 | 21.43 | 1315.04 | 31.25 | 13.35 | 28.82 | 500.00 | **0.0056 | **0.0047 | ***<0.0001 | |
| IL-1β | 1.46 | 127.82 | 1000.00 | 1.89 | 1189.93 | 250.00 | 1.22 | 217.29 | 1000.00 | *0.0177 | 0.0770 | 0.0279 | |
| GM-CSF | 2.74 | 10.29 | 1000.00 | 3.44 | 108.49 | 125.00 | 3.30 | 12.27 | 500.00 | 0.1149 | 0.0935 | 0.4930 | |
| IL-6 | 12.59 | 2847.25 | 1000.00 | 8.23 | 2353.76 | 1000.00 | 13.17 | 2514.88 | 1000.00 | 0.0658 | *0.0176 | 0.4060 | |
| IL-12p40 | 5.13 | 21.22 | 1000.00 | 16.51 | 36.75 | 1000.00 | 4.21 | 56.18 | 1000.00 | **0.0023 | *0.0457 | 0.1138 | |
| IL-10 | 11.54 | 96.39 | 1000.00 | 5.84 | 128.96 | 1000.00 | 9.18 | 112.81 | 1000.00 | *0.0144 | 0.8903 | *0.0148 | |
| CCL2 | 114.51 | 9550.86 | 1000.00 | 301.78 | 5878.50 | 500.00 | 219.85 | 5652.82 | 500.00 | ***0.0002 | 0.6236 | **0.0053 | |
| CXCL8 | 63.98 | 4628.43 | 1000.00 | 34.15 | 3806.51 | 500.00 | 63.73 | 1299.08 | 1000.00 | 0.1669 | ***<0.0001 | ***<0.0001 | |
| PGE2 | 174.41 | 294.51 | 1000.00 | 74.34 | 117.64 | 250.00 | 16.65 | 57.48 | 62.50 | ***<0.0001 | *0.0236 | ***<0.0001 | |
| GLA | CXCL10 | 25.02 | 245.67 | 1000.00 | 12.89 | 2481.00 | 250.00 | 14.66 | 3397.00 | 250.00 | ***<0.0001 | ***<0.0001 | 0.0703 |
| CCL4 | 36.20 | 9338.89 | 1000.00 | 52.00 | 6828.87 | 1000.00 | 40.33 | 6420.38 | 1000.00 | 0.3209 | ***<0.0001 | ***<0.0001 | |
| CCL5 | 2579.37 | 5716.69 | 1000.00 | 1513.69 | 6242.68 | 1000.00 | 918.29 | 2854.60 | 1000.00 | 0.1839 | ***<0.0001 | ***<0.0001 | |
| IFN-γ | 1.83 | 31.31 | 1000.00 | 13.93 | 46.25 | 31.25 | 4.73 | 275.62 | 1000.00 | 0.0515 | 0.5833 | ***<0.0001 | |
| TNF | 41.20 | 90.59 | 1000.00 | 101.64 | 700.63 | 1000.00 | 22.15 | 351.05 | 1000.00 | ***<0.0001 | ***0.0005 | ***<0.0001 | |
| IL-12p70 | 1.80 | 6.00 | 1000.00 | 2.08 | 30.42 | 31.25 | 1.21 | 89.54 | 250.00 | **0.0062 | *0.0284 | *0.0237 | |
| IFN-α | 21.06 | 42.69 | 1000.00 | 21.06 | 1322.14 | 250.00 | 11.84 | 34.85 | 500.00 | **0.0091 | **0.0080 | **0.0076 | |
| IL-1β | 2.41 | 1425.75 | 1000.00 | 8.91 | 1562.25 | 250.00 | 3.63 | 1667.05 | 1000.00 | 0.2228 | 0.0611 | **0.0079 | |
| GM-CSF | 1.48 | 22.10 | 1000.00 | 3.34 | 39.55 | 125.00 | 3.07 | 11.50 | 1000.00 | 0.0784 | *0.0134 | 0.1961 | |
| IL-6 | 18.13 | 9245.33 | 1000.00 | 9.31 | 4924.44 | 1000.00 | 13.75 | 6221.11 | 1000.00 | ***0.0001 | **0.0022 | ***<0.0001 | |
| IL-12p40 | 5.63 | 63.49 | 1000.00 | 6.20 | 70.13 | 1000.00 | 4.75 | 160.11 | 1000.00 | *0.0153 | 0.2148 | ***0.0005 | |
| IL-10 | 9.68 | 705.00 | 1000.00 | 8.52 | 475.31 | 1000.00 | 18.84 | 438.13 | 1000.00 | 0.2141 | **0.0017 | **0.0026 | |
| CCL2 | 177.07 | 9909.23 | 1000.00 | 575.10 | 5726.38 | 125.00 | 147.12 | 4275.25 | 125.00 | ***<0.0001 | ***0.0005 | ***<0.0001 | |
| CXCL8 | 66.86 | 8013.28 | 1000.00 | 19.59 | 3634.63 | 125.00 | 30.16 | 1604.63 | 1000.00 | *0.0316 | ***<0.0001 | ***<0.0001 | |
| PGE2 | 174.41 | 1817.03 | 1000.00 | 74.34 | 310.54 | 1000.00 | 20.29 | 322.76 | 1000.00 | ***<0.0001 | 0.1184 | ***<0.0001 | |
| R848 | CXCL10 | 27.01 | 6207.86 | 0.20 | 13.12 | 6982.85 | 3.13 | 16.80 | 4629.75 | 1000.00 | 0.0079 | 0.1352 | 0.2075 |
| CCL4 | 64.83 | 11133.67 | 100.00 | 1303.32 | 7509.34 | 6.25 | 57.45 | 4931.45 | 1000.00 | **0.0018 | 0.0838 | ***<0.0001 | |
| CCL5 | 2503.74 | 6087.67 | 100.00 | 2812.48 | 5796.27 | 3.13 | 808.00 | 3403.80 | 12.50 | 0.6063 | ***<0.0001 | ***<0.0001 | |
| IFN-γ | 1.83 | 649.88 | 12.50 | 13.93 | 1205.75 | 25.00 | 4.73 | 2304.30 | 25.00 | *0.0448 | ***<0.0001 | ***<0.0001 | |
| TNF | 22.12 | 3880.15 | 25.00 | 106.54 | 2027.31 | 12.50 | 22.13 | 1967.20 | 25.00 | ***0.0002 | 0.4638 | ***<0.0001 | |
| IL-12p70 | 2.00 | 10.68 | 50.00 | 2.43 | 30.77 | 100.00 | 1.67 | 128.82 | 12.50 | ***<0.0001 | ***<0.0001 | ***<0.0001 | |
| IFN-α | 22.99 | 116.98 | 1.56 | 28.69 | 589.38 | 1.56 | 13.86 | 167.18 | 6.25 | ***<0.0001 | ***<0.0001 | **0.0078 | |
| IL-1β | 5.12 | 8790.84 | 50.00 | 20.44 | 3803.85 | 12.50 | 5.29 | 5472.60 | 12.50 | ***<0.0001 | 0.123 | ***<0.0001 | |
| GM-CSF | 2.07 | 29.91 | 50.00 | 4.20 | 34.58 | 12.50 | 4.42 | 32.05 | 50.00 | 0.5519 | 0.7408 | 0.4248 | |
| IL-6 | 30.93 | 10156.18 | 50.00 | 193.22 | 6085.32 | 6.25 | 14.45 | 8518.30 | 12.50 | ***<0.0001 | **0.0033 | ***<0.0001 | |
| IL-12p40 | 7.70 | 407.00 | 12.50 | 9.47 | 478.94 | 12.50 | 4.25 | 1150.25 | 6.25 | 0.8557 | ***<0.0001 | ***<0.0001 | |
| IL-10 | 10.67 | 2195.38 | 12.50 | 70.89 | 1652.82 | 6.25 | 7.67 | 1381.13 | 6.25 | ***0.0005 | 0.3275 | ***<0.0001 | |
| CCL2 | 268.44 | 10275.11 | 50.00 | 1313.88 | 6216.66 | 1.56 | 248.37 | 5212.38 | 1.56 | ***0.0002 | 0.4638 | ***<0.0001 | |
| CXCL8 | 96.57 | 9207.12 | 50.00 | 70.79 | 1495.50 | 12.50 | 36.51 | 467.75 | 12.50 | ***<0.0001 | ***<0.0001 | ***<0.0001 | |
| PGE2 | 174.41 | 5132.93 | 12.50 | 74.34 | 116.93 | 100.00 | 25.26 | 74.42 | 6.25 | ***0.0002 | 0.8489 | ***0.0002 | |
| ODN | CXCL10 | 24.19 | 1698.78 | 0.10 | 10.60 | 3783.92 | 6.25 | 46.41 | 1116.45 | 12.50 | 0.2249 | ***0.0001 | *0.0148 |
| CCL4 | 39.13 | 1287.25 | 50.00 | 28.26 | 1229.00 | 12.50 | 346.63 | 1401.43 | 12.50 | 0.0450 | 0.2523 | 0.5270 | |
| CCL5 | 3437.82 | 6827.82 | 12.50 | 1567.06 | 6071.13 | 25.00 | 993.43 | 2618.82 | 6.25 | 0.7905 | ***0.0001 | ***0.0001 | |
| IFN-γ | 1.83 | 92.21 | 50.00 | 13.93 | 65.93 | 0.10 | 4.73 | 26.52 | 25.00 | 0.7653 | **0.0057 | *0.0277 | |
| TNF | 39.93 | 44.79 | 0.10 | 61.21 | 159.79 | 0.39 | 27.06 | 77.77 | 1.56 | **0.0015 | *0.0231 | **0.0075 | |
| IL-12p70 | 2.13 | 3.63 | 50.00 | 1.74 | 8.33 | 12.50 | 1.67 | 2.34 | 12.50 | *0.0120 | ***0.0001 | ***0.0007 | |
| IFN-α | 30.54 | 31.15 | 3.13 | 30.55 | 160.14 | 6.25 | 9.85 | 33.29 | 12.50 | ***0.0007 | ***<0.0001 | ***0.0004 | |
| IL-1β | 1.86 | 1728.29 | 25.00 | 8.71 | 115.53 | 0.20 | 5.24 | 17.80 | 50.00 | *0.0331 | **0.0058 | *0.0156 | |
| GM-CSF | 2.91 | 7.32 | 25.00 | 1.83 | 7.28 | 12.50 | 3.17 | 6.11 | 12.50 | 0.4665 | 0.1486 | 0.3776 | |
| IL-6 | 12.14 | 1692.84 | 25.00 | 75.18 | 787.49 | 0.20 | 28.60 | 41.03 | 50.00 | *0.0408 | ***0.0010 | **0.0018 | |
| IL-12p40 | 6.87 | 98.65 | 6.25 | 4.90 | 31.82 | 50.00 | 26.01 | 26.01 | 0.00 | *0.0115 | **0.0021 | *0.0156 | |
| IL-10 | 9.78 | 287.52 | 6.25 | 27.89 | 63.21 | 12.50 | 7.37 | 29.69 | 12.50 | **0.0029 | ***0.0009 | ***0.0001 | |
| CCL2 | 121.53 | 8958.76 | 50.00 | 850.22 | 3622.37 | 12.50 | 231.66 | 944.98 | 25.00 | ***0.0001 | ***0.0001 | ***0.0001 | |
| CXCL8 | 79.18 | 5059.90 | 50.00 | 54.04 | 659.78 | 50.00 | 27.36 | 154.74 | 50.00 | ***<0.0001 | ***<0.0001 | *0.0282 | |
| PGE2 | 174.41 | 286.31 | 50.00 | 74.34 | 157.53 | 50.00 | 25.03 | 41.16 | 6.25 | ***<0.0001 | 0.0597 | ***<0.0001 |
p<0.05,
p<0.01,
p<0.001
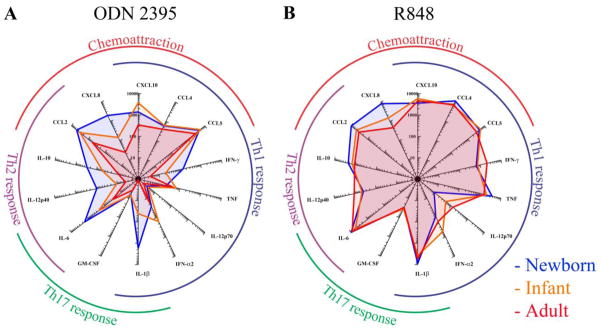
Fig. 4. Robust R848- and CpG-induced cytokine production in human newborn cord blood.
Newborn, infant or adult blood was diluted 5x and stimulated with A) CpG (TLR9 agonist; 6.25 μg/ml) or B) R848 (TLR7/8 agonist; 12.5 μM). Radar graph compares the magnitude of production of 14 cytokines between newborns, infants and adults as measured by multiplex assay.
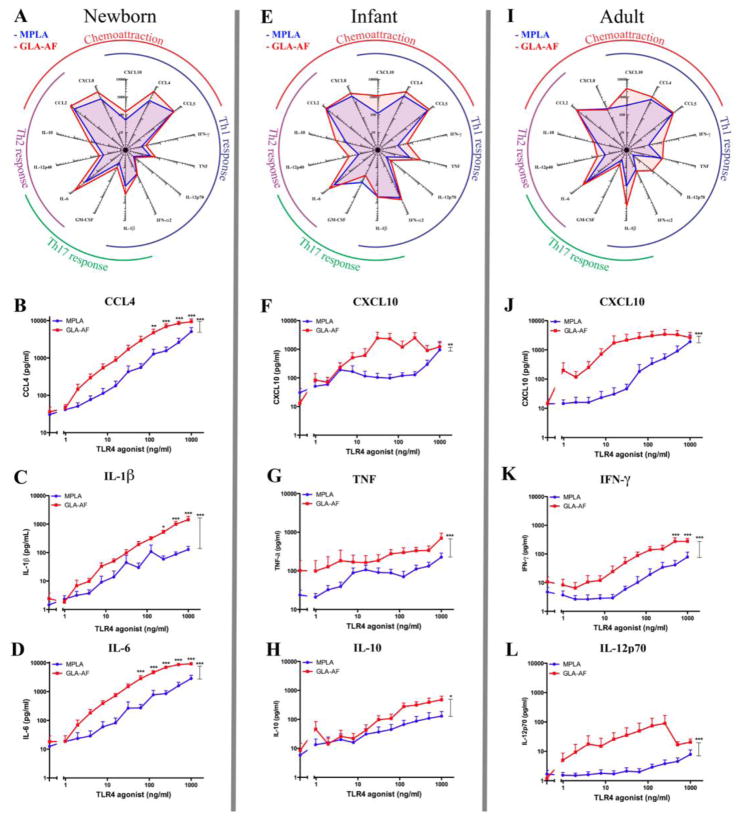
A direct comparison of the cytokine profiles induced by the TLR4 agonists, MPLA and GLA-AF, (Fig. 2) shows that both GLA-AF and MPLA induce a similar cytokine profile that changes per age group (Fig. 2A, E, I), with a number of exceptions, which are highlighted in the sub-panels. For the radar graphs a fixed concentration of TLR4 agonist is displayed (62.5 ng/ml). At this concentration near-maximal responses were consistently observed in both GLA- and MPLA- stimulated samples for most cytokines. In general, GLA-AF was significantly more potent and more effective than MPLA at inducing Th-polarizing cytokine production in vitro, including (a) greater neonatal IL-1β, IL-6 and CCL4 production (Fig. 2B–D), (b) greater CXCL10 production in both infants and in adults (Fig. 2F,J), greater TNF and IL-10 in infants (G–H), and greater IFN-γ and IL12p70 in adults (K–L).
Fig. 2. GLA-AF is more potent and effective than MPLA in inducing cytokines in all age groups.
Newborn, infant or adult blood was diluted 5x and stimulated with MPLA or GLA-AF at concentrations indicated. A,E,I) Radar graphs demonstrate the magnitude of production of 14 cytokines at a fixed concentration of GLA-AF and MPLA, both 62.5 ng/ml. B–L) Concentration-response graphs compare the production of CCL4 (B), CXCL10 (F,J), IL-1β (C), TNF (G), IFN-γ (K), IL-6 (D), IL-10 (H) and IL-12p70 (L) between MPLA and GLA-AF at concentrations as indicated. Stars adjacent to a curve indicate statistical significance at that concentration as measured by Student’s t-test. Stars above the connector-lines indicate statistical significance between the connected curves, as measured by 2-way ANOVA. (*p<0.05, **p<0.01, ***p<0.001)
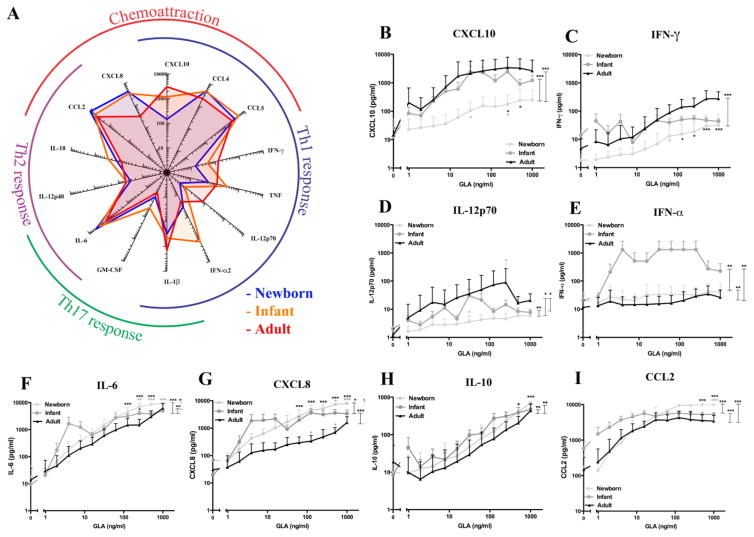
In response to the TLR4 agonist GLA-AF, newborns demonstrated impaired production of Th1-associated cytokines typical of their responses to other TLRAs [27, 29, 37, 40, 41] (Fig. 3). Age-dependent differences were noted in the production of all cytokines measured at a fixed concentration (62.5 ng/ml; Fig. 3A). While the production of Th1 cytokines CXCL10, IFN-γ, and IL12p70 was approximately one log lower in newborns as compared to adults as well as infants (Fig. 3 B–D), the production of Th2 cytokine IL-6 and chemokines CCL2 and CXCL8 (formerly IL-8) and the anti-inflammatory cytokine IL-10 were similar to or greater than adult levels in newborns as well as infants (F–I). Of note, GLA-induced production of IFN-α was greater in infants as compared to newborns and adults (E). Indeed, newborns and adults were significantly different at multiple agonist concentrations (Student’s t-test), and across the entire concentration spectrum (repeated-measures ANOVA; Fig. 3B–I).
Fig. 3. GLA-AF induces robust Th2 cytokine production in newborns and infants as well as age-dependent maturation Th1 cytokine production.
Newborn, infant or adult blood was diluted 5x and stimulated with GLA-AF at the concentrations indicated. A) Radar plot demonstrates the magnitude of production of 14 cytokines at a fixed concentration of GLA-AF, 62.5 ng/ml. B–I) Concentration-response graphs compare the production of CXCL10 (B), IFN-γ (C), IL-12p70 (D), IFN-α (E), IL-6 (F), CXCL8 (IL-8) (G), IL-10 (H) and CCL2 (I) between newborns, infants and adults at concentrations of GLA-AF indicated. Stars adjacent to a curve indicate statistical significance at that concentration as measured by Student’s t-test. Stars above the connector-lines indicate statistical significance between the curves connected by the connector-line, as measured by 2-way ANOVA. (*p<0.05, **p<0.01, ***p<0.001)
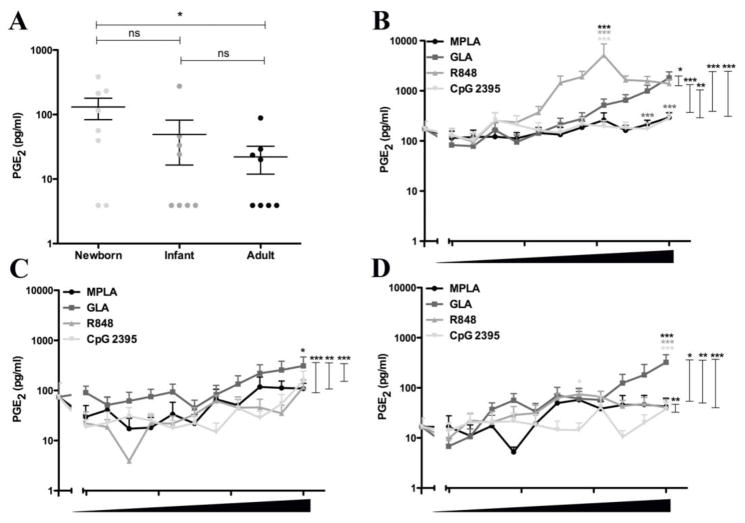
In contrast to the pattern with other TLRAs, CpG induced the greatest cytokine response in newborns as compared to infants or adults (Fig. 4A). Neonatal CpG-induced production of IFN-γ, IL-1β, IL-6, IL-12p40, IL-10, CCL2 and CXCL8 was greater than that of infants and adults. As has been noted in other cohorts [31, 32, 40], R848 induced the most robust cytokine production of all TLRAs tested in all three age groups- i.e., newborns, infants and adults (Fig. 4B; Supplementary Fig. 1). Prostaglandin E2 (PGE2) exerts multiple effects on the immune system [42, 43], and has been suggested based on studies of monoMac cells as a potential in vitro biomarker for adjuvant reactogenicity [36]. Interestingly, newborn blood demonstrated the greatest basal and TLRA-induced levels of PGE2 (Fig. 5). PGE2 secretion in response to TLRAs was most pronounced in response to R848 (Fig. 5B–D).
Fig. 5. Newborn cord blood demonstrates higher basal and TLR agonist-induced Prostaglandin E2 concentrations than infant and adult blood.
Newborn, infant and adult blood was diluted five-fold with RPMI, then stimulated with buffer control, MPLA (highest concentration 1 μg/ml), GLA-AF (highest concentration 1 μg/ml), R848 (highest concentration 100 μM) or CpG (highest concentration 50 μg/ml) as indicated and ten 2-fold dilutions thereof. A) Basal levels of PGE2 in blood stimulated with vehicle only shows higher levels of basal PGE2 in newborns. B–D). After stimulation with MPLA, GLA-AF, R848 or CpG at concentrations indicated, the production of PGE2 was greater in newborn blood as compared to infant and adult blood. Stars adjacent to a curve indicate statistical significance at that concentration as measured by Student’s t-test. Stars above the connector-lines indicate statistical significance between the curves connected by the connector-line, as measured by 2-way ANOVA. (*p<0.05, **p<0.01, ***p<0.001)
Discussion
In vitro experiments using whole blood from healthy donors is a practical approach to assess the immunological activity of pathogens or (potential) vaccine adjuvants [40, 44–47], and has revealed age-specific differences in the immune response to TLRAs [27, 31, 37, 48–51]. Of all three age groups compared in the present study, the infant age group (age 2–6 months) receives the most vaccines within the vaccination schedules of most industrialized/wealthy countries, including in the U.S. and E. U. [52]. Paradoxically, this is also the age group in which the immune response is the least studied in vitro, likely due to limitations in accessibility and restrictions in blood volume. In a prior study, the production of 9 cytokines was measured in a whole blood assay with newborns, adults and infants of age 3, 6, 9 and 12 months [39] after stimulation with LPS or CpG. Both agonists were used at a single concentration only. In this study, newborn, infant and adult peripheral blood was used to investigate the innate immune response to candidate vaccine adjuvants MPLA, GLA-AF, R848 and CpG. We used these agonists at a range of 12 different concentrations, allowing not only for comparison of maximal cytokine responses, but also for comparison of potency of these adjuvants between the age groups. The most commonly measured cytokines in a whole-blood assay are TNF and IL-6. In this study we characterized production of 14 cytokines representing different immune functions, such as innate immune cell activation, T-cell polarization and chemoattraction.
As compared to adults, the immune system of newborns is characterized by skewed TLR-mediated cytokine production, with impaired production of Th1-polarizing cytokines such as TNF and IL-12p70, but robust production of Th2/Th17-polarizing IL-6. [23, 37]. Newborn lymphocytes include predominantly immature B cells [53], and naïve CD4+ T lymphocytes are skewed to develop into T-helper 2 cells [26]. Limited information is available about the extent to which some of the unique features of the newborn immune system extend into early childhood [39, 40]. Studying the innate immune system of 2–6 month old infants in response to vaccine adjuvants is relevant, as this is the age group that receives the most vaccines in developed countries.
The functionally distinct impairment in secretion of Th1 cytokines in response to TLRAs in newborns is not observed in infants, which may explain why many vaccines are effective in infants but not in newborns (Figure 1). Interestingly, infants (mean age 6 months) were the only age group that produces significant amounts of IFN-α and TNF in response to TLR4 stimulation (Fig. 1 H–J). In order to reduce the burden on enrolled infants, and to reduce drop-outs from scheduled immunization, blood draws in this age group occurred immediately after routine 2-month immunizations, which may have modulated the cytokine response in the whole-blood assay. This is unlikely, however, as peripheral blood collection took place within an hour of immunization and baseline levels of any cytokine were not significantly different in any age group as compared to the other age groups. In addition, a peak IFN-α response at 6 months has been previously described [49].
To our knowledge, ours is the first study characterizing the activity of GLA-AF across three age groups, including newborns and infants. Prior to our current study all published (Pubmed as of March 29, 2016) studies reporting cytokine production of newborn cells in response to TLR4 stimulation employed LPS or MPLA. Although structurally similar to MPLA, GLA is a synthetic product with molecular and immunological properties that are distinct from MPLA [34, 54]. GLA-AF was more potent and effective than MPLA in inducing multiple cytokines (Figure 2). In newborns, the production of CCL4, IL-1β and IL-6 is ~10-fold more after stimulation with GLA-AF than MPLA. Impairment in Th1 cytokine production was much less profound in infants (Figures 2 and 3). Specific differences between the TLR4 agonists in infants include greater production of TNF, IL-10 and CXCL10 after stimulation with GLA-AF than MPLA. Most strikingly, GLA-AF induced a significant increase in Th1-polarizing cytokines including IFN-γ, IL-12p70 and CXCL10.
Compared to adults, newborns produced less TNF and more IL-6 in response to a TLR4 agonist (Fig. 2–4), as has been noted previously [23, 27]. We also noted impaired TLR4-mediated production of additional Th1-cytokines, including CXCL10, IFN-γ and IL-12p70. In contrast, neonatal production of non-Th1 cytokines, such as IL-6, IL-10 and CXCL8, was equal or greater than in adults. In contrast to the pattern with other TLRAs, CpG, a TLR9 agonist, induced the greatest cytokine response in newborns as compared to infants or adults (Figure 4), with the exception of CXCL10, TNF, IL-12p40 and IFN-α. Prior studies have reported impaired responses of human newborn plasmacytoid DCs to CpG, but robust IFN-α production by neonatal plasmacytoid DCs (pDCs) in response to viruses that engage TLR7 and TLR9 [55–57]. The discrepancy between our results demonstrating robust CpG-induced cytokines in newborns and cited literature is currently unclear though we note our study employed 5x diluted blood instead of whole blood or purified pDCs. Neonatal plasma is rich in immunomodulatory molecules and dilution may account for modulation of cytokine responses to TLRAs. Of note, it is uncertain what the relevant concentration (e.g., volume/volume) of plasma components may be at local tissues or sites of immunization, though it is unlikely to be 100%.
Moreover, the absolute number of pDCs per volume of blood is highest at birth, and declines with age [58, 59], providing an alternative explanation for the observed robust response to CpG in newborn blood, despite the above-mentioned functional impairment of these cells. Speculation as to the contribution of cell type composition to the observed cytokine responses in each age group is complicated by the fact that absolute quantity of several myeloid and lymphoid subsets is higher early in life, but the function and phenotype of these cells is more immature in nature [53, 60].
Of all the adjuvant TLRAs evaluated in our study, the TLR7/8 agonist R848 induced the most robust cytokine production at all age groups- newborns, infants and adults (Figure 4B).
In addition to cytokine production, we also measured production of PGE 2, an immunologically active mediator that modulates immunity [43, 61]. PGE2 plays a key role in the modulation of innate [29, 43, 61] as well as adaptive immune responses [62–64] and provides additional characterization of age- and adjuvant-specific differences in leukocyte activation. Of note, adjuvant-induced PGE2 induction by the monomac6 monocytic cell-line tested in vitro has been linked to reactogenicity in vivo [36], though it has yet to be established that PGE2 serves as a reactogenicity biomarker in other in vitro model systems, such as whole blood [65]. We noted that basal levels of PGE2 were elevated in newborns and TLRAs induced robust neonatal PGE2 induction (Fig. 5). This age-specific effect was apparently diminished by 6 months of age, as infant plasma PGE2 levels were comparable to those of adults.
Our study features a number of important strengths. To our knowledge, ours is the first study that: evaluated the ability of TLR-activating adjuvants to induce cytokine and chemokine production in U.S.-based infants, compared commonly used TLRAs to MPLA and to GLA-AF, a novel and promising adjuvant currently being evaluated in human clinical trials [33, 66], and that included measurement of PGE2, a potential biomarker of reactogenicity [36]. Our study also has some limitations. It was an in vitro study, and although recent studies suggest that TLRAs active in vitro are also active in humans in vivo [31, 32, 67, 68], the extent of correlation with responses in vivo remains uncertain. In addition, our study size was limited, and infant blood was drawn after routine immunization, reflecting the well described challenges of enrolling U.S. infant cohorts [69, 70].
Overall, our study reveals significant differences in TLRA-induced cytokine production in vitro, varying with both TLRA and age group. To the extent that our in vitro data reflect the bioactivity of these TLRAs in vivo, our results suggest the importance of accounting for age-and adjuvant-specific responses in informing future pediatric vaccine development. In particular, CpG induced robust production of an array of Th-polarizing cytokines and chemokines in newborn blood and the novel adjuvant GLA-AF was more potent and effective than MPLA in inducing Th polarizing cytokine production in all age groups. Further in vitro and in vivo studies, including those in appropriate animal models, are needed to assess the potential of CpG, GLA-AF and other TLRAs as candidate pediatric vaccine adjuvants.
Supplementary Material
Newborn, infant or adult blood was diluted 5x and stimulated with MPLA (0.98–1000 ng/ml), GLA-AF (0.98–1000 ng/ml), R848 (0.098–100 μM) or CpG (0.049–50 μg/ml). Stars adjacent to a curve indicate statistical significance at that concentration as measured by Student’s t-test. Stars above the connector-lines indicate statistical significance between the curves connected by the connector-line, as measured by 2-way ANOVA. (*p<0.05, **p<0.01, ***p<0.001)
Highlights.
Cytokine responses to TLR ligands were compared in blood from U.S. neonates, infants and adults.
Age-dependent levels of cytokine secretion occur after stimulation of blood with TLR ligands.
TLR4 ligands induced greater Th1 cytokine production in infant and adult blood than in newborn blood.
TLR ligands induced greater IFN-α2 production in infant blood compared to newborn and adult blood.
A TLR9 ligand induced greater cytokine production in newborn blood than in infant or adult blood.
Acknowledgments
OL’s laboratory is supported by a Boston Children’s Hospital Department of Medicine award to the Precision Vaccines Program as well as Global Health (OPPGH5284) and Grand Challenges Explorations (OPP1035192) awards from the Bill & Melinda Gates Foundation and by NIH grants 1R01AI100135-01 and 3R01AI067353- 05S1 and National Institute of Allergy & Infectious Diseases Adjuvant Discovery Program, Contract No. HHSN272201400052C. SvH was supported by an Early Career Award from the Thrasher Research Fund. We thank the Labor and Delivery staff at The Brigham & Women’s Hospital, Boston, MA and Beth Israel Deaconess Medical Center Boston, MA for their kind assistance with sample acquisition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–96. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldwein KA, Liang MD, Andresen TK, Thomas KE, Marty AM, Cuesta N, et al. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J Leukoc Biol. 2003;74:277–86. doi: 10.1189/jlb.0103026. [DOI] [PubMed] [Google Scholar]
- 3.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–75. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris KG, Coyne CB. Enter at your own risk: how enteroviruses navigate the dangerous world of pattern recognition receptor signaling. Cytokine. 2013;63:230–6. doi: 10.1016/j.cyto.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oshiumi H, Okamoto M, Fujii K, Kawanishi T, Matsumoto M, Koike S, et al. The TLR3/TICAM-1 pathway is mandatory for innate immune responses to poliovirus infection. J Immunol. 2011;187:5320–7. doi: 10.4049/jimmunol.1101503. [DOI] [PubMed] [Google Scholar]
- 6.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–24. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy O, Goriely S, Kollmann TR. Immune response to vaccine adjuvants during the first year of life. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg R, Latimer L, Gerdts V, Potter A, van Drunen Littel-van den Hurk S. Vaccination with the RSV fusion protein formulated with a combination adjuvant induces long-lasting protective immunity. J Gen Virol. 2014;95:1043–54. doi: 10.1099/vir.0.062570-0. [DOI] [PubMed] [Google Scholar]
- 10.Milicic A, Kaur R, Reyes-Sandoval A, Tang CK, Honeycutt J, Perrie Y, et al. Small cationic DDA:TDB liposomes as protein vaccine adjuvants obviate the need for TLR agonists in inducing cellular and humoral responses. PLoS One. 2012;7:e34255. doi: 10.1371/journal.pone.0034255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holt PG. Functionally mature virus-specific CD8(+) T memory cells in congenitally infected newborns: proof of principle for neonatal vaccination? J Clin Invest. 2003;111:1645–7. doi: 10.1172/JCI18805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumsden JM, Pichyangkul S, Srichairatanakul U, Yongvanitchit K, Limsalakpetch A, Nurmukhambetova S, et al. Evaluation of the safety and immunogenicity in rhesus monkeys of a recombinant malaria vaccine for Plasmodium vivax with a synthetic Toll-like receptor 4 agonist formulated in an emulsion. Infect Immun. 2011;79:3492–500. doi: 10.1128/IAI.05257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen AC, Mills KH. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev Vaccines. 2014;13:1253–64. doi: 10.1586/14760584.2014.936391. [DOI] [PubMed] [Google Scholar]
- 14.Caproni E, Tritto E, Cortese M, Muzzi A, Mosca F, Monaci E, et al. MF59 and Pam3CSK4 Boost Adaptive Responses to Influenza Subunit Vaccine through an IFN Type I-Independent Mechanism of Action. J Immunol. 2012 doi: 10.4049/jimmunol.1101764. [DOI] [PubMed] [Google Scholar]
- 15.Ghimire TR, Benson RA, Garside P, Brewer JM. Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol Lett. 2012 doi: 10.1016/j.imlet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert SL, Yang CF, Liu Z, Sweetwood R, Zhao J, Cheng L, et al. Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant Glucopyranosyl Lipid A. PLoS One. 2012;7:e51618. doi: 10.1371/journal.pone.0051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, et al. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc Natl Acad Sci U S A. 2011;108:7914–9. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori A, Oleszycka E, Sharp FA, Coleman M, Ozasa Y, Singh M, et al. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur J Immunol. 2012 doi: 10.1002/eji.201242372. [DOI] [PubMed] [Google Scholar]
- 19.Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008;105:10501–6. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastelic Gavillet B, Eberhardt CS, Auderset F, Castellino F, Seubert A, Tregoning JS, et al. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J Immunol. 2015;194:4836–45. doi: 10.4049/jimmunol.1402071. [DOI] [PubMed] [Google Scholar]
- 21.Balmer P, Borrow R, Arkwright PD. The 23-valent pneumococcal polysaccharide vaccine does not provide additional serotype antibody protection in children who have been primed with two doses of heptavalent pneumococcal conjugate vaccine. Vaccine. 2007;25:6321–5. doi: 10.1016/j.vaccine.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Shinefield HR, Black S, Ray P, Chang I, Lewis N, Fireman B, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999;18:757–63. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–9. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 24.Levy O. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res. 2005;11:113–6. doi: 10.1179/096805105X37376. [DOI] [PubMed] [Google Scholar]
- 25.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 26.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–91. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 28.Lemoine S, Jaron B, Tabka S, Ettreiki C, Deriaud E, Zhivaki D, et al. Dectin-1 activation unlocks IL12A expression and reveals the T1 potency of neonatal dendritic cells. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Belderbos ME, Levy O, Stalpers F, Kimpen JL, Meyaard L, Bont L. Neonatal plasma polarizes TLR4-mediated cytokine responses towards low IL-12p70 and high IL-10 production via distinct factors. PLoS One. 2012;7:e33419. doi: 10.1371/journal.pone.0033419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowling DJ, Tan Z, Prokopowicz ZM, Palmer CD, Matthews MA, Dietsch GN, et al. The Ultra-Potent and Selective TLR8 Agonist VTX-294 Activates Human Newborn and Adult Leukocytes. PLoS One. 2013;8:e58164. doi: 10.1371/journal.pone.0058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–90. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philbin VJ, Dowling DJ, Gallington LC, Cortes G, Tan Z, Suter EE, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130:195–204. e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clegg CH, Roque R, Perrone LA, Rininger JA, Bowen R, Reed SG. GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza. PLoS One. 2014;9:e88979. doi: 10.1371/journal.pone.0088979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–21. e1–70. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 36.Zaitseva M, Romantseva T, Blinova K, Beren J, Sirota L, Drane D, et al. 159-Use of human MonoMac6 cells for development of in vitro assay predictive of adjuvant safety in vivo. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dembinski J, Behrendt D, Reinsberg J, Bartmann P. Endotoxin-stimulated production of IL-6 and IL-8 is increased in short-term cultures of whole blood from healthy term neonates. Cytokine. 2002;18:116–9. doi: 10.1006/cyto.2002.0880. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, et al. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, et al. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One. 2011;6:e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimabukuro-Vornhagen A, Liebig TM, Koslowsky T, Theurich S, von Bergwelt-Baildon MS. The ratio between dendritic cells and T cells determines whether prostaglandin E has a stimulatory or inhibitory effect. Cell Immunol. 2013;281:62–7. doi: 10.1016/j.cellimm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez M, Domingo E, Municio C, Alvarez Y, Hugo E, Fernandez N, et al. Polarization of the Innate Immune Response by Prostaglandin E2: a Puzzle of Receptors and Signals. Mol Pharmacol. 2013 doi: 10.1124/mol.113.089573. [DOI] [PubMed] [Google Scholar]
- 44.Blankley S, Graham CM, Howes A, Bloom CI, Berry MP, Chaussabel D, et al. Identification of the key differential transcriptional responses of human whole blood following TLR2 or TLR4 ligation in-vitro. PLoS One. 2014;9:e97702. doi: 10.1371/journal.pone.0097702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coch C, Luck C, Schwickart A, Putschli B, Renn M, Holler T, et al. A Human In Vitro Whole Blood Assay to Predict the Systemic Cytokine Response to Therapeutic Oligonucleotides Including siRNA. PLoS One. 2013;8:e71057. doi: 10.1371/journal.pone.0071057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strunk T, Power Coombs MR, Currie AJ, Richmond P, Golenbock DT, Stoler-Barak L, et al. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One. 2010;5:e10111. doi: 10.1371/journal.pone.0010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchant EA, Kan B, Sharma AA, van Zanten A, Kollmann TR, Brant R, et al. Attenuated innate immune defenses in very premature neonates during the neonatal period. Pediatr Res. 2015;78:492–7. doi: 10.1038/pr.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity. 2012;37:771–83. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reikie BA, Adams RC, Ruck CE, Ho K, Leligdowicz A, Pillay S, et al. Ontogeny of Toll-Like Receptor Mediated Cytokine Responses of South African Infants throughout the First Year of Life. PLoS One. 2012;7:e44763. doi: 10.1371/journal.pone.0044763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shey MS, Nemes E, Whatney W, de Kock M, Africa H, Barnard C, et al. Maturation of innate responses to mycobacteria over the first nine months of life. J Immunol. 2014;192:4833–43. doi: 10.4049/jimmunol.1400062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganapathi L, Van Haren S, Dowling DJ, Bergelson I, Shukla NM, Malladi SS, et al. The Imidazoquinoline Toll-Like Receptor-7/8 Agonist Hybrid-2 Potently Induces Cytokine Production by Human Newborn and Adult Leukocytes. PLoS One. 2015;10:e0134640. doi: 10.1371/journal.pone.0134640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3:90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morbach H, Eichhorn EM, Liese JG, Girschick HJ. Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol. 2010;162:271–9. doi: 10.1111/j.1365-2249.2010.04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orr MT, Duthie MS, Windish HP, Lucas EA, Guderian JA, Hudson TE, et al. MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant. Eur J Immunol. 2013;43:2398–408. doi: 10.1002/eji.201243124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Lepelley A, Azria E, Lebon P, Roguet G, Schwartz O, et al. Neonatal Plasmacytoid Dendritic Cells (pDCs) Display Subset Variation but Can Elicit Potent Anti-Viral Innate Responses. PLoS One. 2013;8:e52003. doi: 10.1371/journal.pone.0052003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danis B, George TC, Goriely S, Dutta B, Renneson J, Gatto L, et al. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol. 2008;38:507–17. doi: 10.1002/eji.200737760. [DOI] [PubMed] [Google Scholar]
- 57.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–2. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 58.Orsini G, Legitimo A, Failli A, Massei F, Biver P, Consolini R. Enumeration of human peripheral blood dendritic cells throughout the life. Int Immunol. 2012;24:347–56. doi: 10.1093/intimm/dxs006. [DOI] [PubMed] [Google Scholar]
- 59.Heinze A, Elze MC, Kloess S, Ciocarlie O, Konigs C, Betz S, et al. Age-matched dendritic cell subpopulations reference values in childhood. Scand J Immunol. 2013;77:213–20. doi: 10.1111/sji.12024. [DOI] [PubMed] [Google Scholar]
- 60.Peoples JD, Cheung S, Nesin M, Lin H, Tatad AM, Hoang D, et al. Neonatal cord blood subsets and cytokine response to bacterial antigens. Am J Perinatol. 2009;26:647–57. doi: 10.1055/s-0029-1220788. [DOI] [PubMed] [Google Scholar]
- 61.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Landi A, Babiuk LA, van Drunen Littel-van den Hurk S. Dendritic cells matured by a prostaglandin E2-containing cocktail can produce high levels of IL-12p70 and are more mature and Th1-biased than dendritic cells treated with TNF-alpha or LPS. Immunobiology. 2011;216:649–62. doi: 10.1016/j.imbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Muthuswamy R, Mueller-Berghaus J, Haberkorn U, Reinhart TA, Schadendorf D, Kalinski P. PGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cells. Blood. 2010;116:1454–9. doi: 10.1182/blood-2009-12-258038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 65.Mastelic B, Garcon N, Del Giudice G, Golding H, Gruber M, Neels P, et al. Predictive markers of safety and immunogenicity of adjuvanted vaccines. Biologicals. 2013 doi: 10.1016/j.biologicals.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Fox CB, Moutaftsi M, Vergara J, Desbien AL, Nana GI, Vedvick TS, et al. TLR4 ligand formulation causes distinct effects on antigen-specific cell-mediated and humoral immune responses. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 67.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102:15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bavdekar SB. Pediatric clinical trials. Perspect Clin Res. 2013;4:89–99. doi: 10.4103/2229-3485.106403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKechnie L, Gill AB. Consent for neonatal research. Arch Dis Child Fetal Neonatal Ed. 2006;91:F374–6. doi: 10.1136/adc.2005.075036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Newborn, infant or adult blood was diluted 5x and stimulated with MPLA (0.98–1000 ng/ml), GLA-AF (0.98–1000 ng/ml), R848 (0.098–100 μM) or CpG (0.049–50 μg/ml). Stars adjacent to a curve indicate statistical significance at that concentration as measured by Student’s t-test. Stars above the connector-lines indicate statistical significance between the curves connected by the connector-line, as measured by 2-way ANOVA. (*p<0.05, **p<0.01, ***p<0.001)