Abstract
The present study aimed to investigate the regulatory mechanisms underlying sepsis progression in patients with tumor necrosis factor (TNF)-α genetic variations. The GSE5760 expression profile data, which was downloaded from the Gene Expression Omnibus database, contained 30 wild-type (WT) and 28 mutation (MUT) samples. Differentially expressed genes (DEGs) between the two types of samples were identified using the Student's t-test, and the corresponding microRNAs (miRNAs) were screened using WebGestalt software. An integrated miRNA-DEG network was constructed using the Cytoscape software, based on the interactions between the DEGs, as identified using the Search Tool for the Retrieval of Interacting Genes/Proteins database, and the correlation between miRNAs and their target genes. Furthermore, Gene Ontology and pathway enrichment analyses were conducted for the DEGs using the Database for Annotation, Visualization and Integrated Discovery and the KEGG Orthology Based Annotation System, respectively. A total of 390 DEGS between the WT and MUT samples, along with 11 -associated miRNAs, were identified. The integrated miRNA-DEG network consisted of 38 DEGs and 11 miRNAs. Within this network, COPS2 was found to be associated with transcriptional functions, while FUS was found to be involved in mRNA metabolic processes. Other DEGs, including FBXW7 and CUL3, were enriched in the ubiquitin-mediated proteolysis pathway. In addition, miR-15 was predicted to target COPS2 and CUL3. The results of the present study suggested that COPS2, FUS, FBXW7 and CUL3 may be associated with sepsis in patients with TNF-α genetic variations. In the progression of sepsis, FBXW7 and CUL3 may participate in the ubiquitin-mediated proteolysis pathway, whereas COPS2 may regulate the phosphorylation and ubiquitination of the FUS protein. Furthermore, COPS2 and CUL3 may be novel targets of miR-15.
Keywords: tumor necrosis factor-α variation, sepsis, microarray data, ubiquitination, microRNA
Introduction
Multiple trauma, which is commonly associated with severe injuries and multiple organ failure, may lead to various complications, including sepsis and septic shock, which are major healthcare problems worldwide (1–3). There are 400,000–500,000 cases of sepsis in the United States annually (4). Antimicrobial therapy may be applied for the management of sepsis; however, the mortality rate associated with sepsis has increased, and was reported to be as high as 40% in 2003 (5).
Tumor necrosis factor (TNF)-α, a cytokine that is predominantly secreted by macrophages, has been shown to be involved in the regulation of numerous biological processes, including cell proliferation, differentiation, apoptosis, lipid metabolism and coagulation (6–8). However, the role of TNF-α in tumorigenesis remains unclear. This cytokine has been reported to induce tumor necrosis and apoptosis, as well as to promote tumor development (9). However, previous studies investigating the role of TNF-α in clinical sepsis syndrome or septic shock have reported conflicting results (10–12). TNF-α has been established as an effective marker in the diagnosis of neonatal sepsis (13); however, the mechanisms underlying the regulatory role of TNF-α in the development of sepsis syndrome remain undefined. Genetic variations have previously been implicated in the progression of numerous types of cancer (14,15). In addition, the clinical outcomes of sepsis have been associated with genetic polymorphisms in the genes encoding various inflammatory cytokines (16). Menges et al (17) demonstrated that common variants of the TNF-α gene were associated with sepsis syndrome and mortality following severe injury. Reportedly, the common TNF-α gene variant carrying the TNF rs1800629 A allele is correlated with higher TNF-α serum concentrations and alteration of genes strongly associated with proinflammatory and apoptosis (17). Furthermore, TNF rs1800629 A is closely associated with sepsis syndrome and mortality following multiple trauma (18).
The present study re-analyzed the GSE5760 microarray data deposited in the Gene Expression Omnibus (GEO) database by Menges et al (17), in order to detect genes that were differentially expressed between patients with and without TNF-α genetic variations, and to identify their potential functions and pathways. Furthermore, the regulatory associations between differentially expressed genes (DEGs) and microRNAs (miRNAs) were analyzed in order to elucidate the regulatory mechanisms underlying sepsis in patients with TNF-α genetic variations, at the transcriptional and post transcriptional levels.
Materials and methods
Microarray data
The GSE5760 gene expression profile data was downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). Based on the description provided by Menges et al (17), the profile data consisted of 30 wild-type (WT) peripheral blood samples from 12 injured patients without the TNF-α rs1800629 A variant and 28 mutation (MUT) peripheral blood samples from 10 injured patients carrying the TNF-α rs1800629 A variant. The technical replicate numbers for the WT samples were three replicates for 6 patients and two replicates for the other 6 patients. The technical replicate numbers for the MUT samples were three replicates for 8 patients and two replicates for 2 patients. Menges et al (17) had used the GPL4204 platform (GE Healthcare/Amersham Biosciences CodeLink UniSet Human I Bioarray; GE Healthcare, Little Chalfont, UK). The annotation information in the platform was also downloaded.
Data preprocessing and identification of DEGs
The gene expression value was calculated from raw microarray data of the probe value. In the case that multiple probes corresponded to one gene, the average value was calculated as the expression level of this gene, whereas in the case that one probe corresponded to multiple genes, the probe value was removed. Following transformation of the data by log2 and normalization using the median method (19), DEGs between the WT and MUT samples were identified using the Limma package in R software (20) and Student's t-test. The Benjamini-Hochberg procedure (21) was applied, in order to control the false discovery rate (FDR). The threshold criteria for the DEGs were FDR<0.05 and |log2 fold change (FC)| of >0.05.
Selection of miRNAs targeted to DEGs
Following identification of the DEGs, WebGestal software (version 2.0; Vanderbilt University, Nashville, TN, USA; http://bioinfo.vanderbilt.edu/webgestalt/) (22) was used in order to identify miRNAs that were associated with the DEGs. A threshold of adjusted P<0.05 was used.
Construction of an integrated regulatory network between miRNAs and their target DEGs
The DEGs were mapped using the Search Tool for the Retrieval of Interacting Genes/Proteins database (http://string-db.org/) (23), in order to identify potential protein-protein interactions between the DEGs. Interaction pairs were identified using the default parameter of a combined score of ≥0.6. The integrated regulatory miRNA-DEG network was constructed and visualized using the Cytoscape software (24), based on the DEG interaction pairs and the interactions between the 11 miRNAs and their target DEGs.
Function and pathway enrichment analyses for the identified DEGs
The biological functions of the DEGs in the established network were investigated by Gene Ontology (GO) enrichment analysis, using the Database for Annotation, Visualization and Integrated Discovery online software (https://david.ncifcrf.gov/) (25). P<0.05 was considered to indicate a significantly enriched GO term. In addition, pathway enrichment analyses were conducted using the KEGG Orthology Based Annotation System (KOBAS, version 2; http://kobas.cbi.pku.edu.cn/home.do), in order to identify the pathways in which the DEGs were involved. In addition, the statistical method of cumulative hypergeometric distribution was applied and P<0.05 was considered to indicate a significantly enriched pathway.
Results
Identification of DEGs between the WT and MUT samples, and the associated miRNAs
Based on the preset criteria of FDR<0.05 and |log2FC|>0.05, a total of 390 genes were shown to be differentially expressed between the WT and MUT samples, including 238 genes that were upregulated and 152 genes that were downregulated. Based on an established threshold for miRNA searching, 11 miRNAs, including miR-141, miR-374, miR-204, miR-23, miR-182, miR-26, miR-15, miR-30, miR-34, miR-181 and miR-130, were significantly associated with the identified DEGs, and were selected for inclusion in the integrated miRNA-DEG regulatory network (Table I).
Table I.
miRNAs associated with DEGs.
| miRNA | ID | DEG counts | Raw P-value | Adjusted P-value |
|---|---|---|---|---|
| Has_CAGTGTT, miR-141 | DB_ID:690 | 15 | 7.29×10−8 | 1.39×10−6 |
| Has_TATTATA, miR-374 | DB_ID:727 | 14 | 1.72×10−7 | 1.63×10−6 |
| Has_AAAGGGA, miR-204 | DB_ID:682 | 12 | 4.49×10−7 | 2.84×10−6 |
| Has_AATGTGA, miR-23 | DB_ID:683 | 16 | 6.71×10−7 | 3.19×10−6 |
| Has_TTGCCAA, miR-182 | DB_ID:757 | 13 | 4.62×10−6 | 1.76×10−5 |
| Has_TACTTGA, miR-26 | DB_ID:687 | 12 | 9.99×10−6 | 3.16×10−5 |
| Has_TGCTGCT, miR-15 | DB_ID:666 | 17 | 1.48×10−5 | 4.02×10−5 |
| Has_TGTTTAC, miR-30 | DB_ID:667 | 16 | 3.52×10−5 | 7.43×10−5 |
| Has_CACTGCC, miR-34 | DB_ID:673 | 10 | 0.0001 | 0.0002 |
| Has_TGAATGT, miR-181 | DB_ID:669 | 13 | 0.0003 | 0.0005 |
| Has_TTGCACT, miR-130 | DB_ID:676 | 11 | 0.0006 | 0.0009 |
miR/miRNA, microRNA; DEG, differentially expressed gene.
Construction of the integrated miRNA-DEG regulatory network
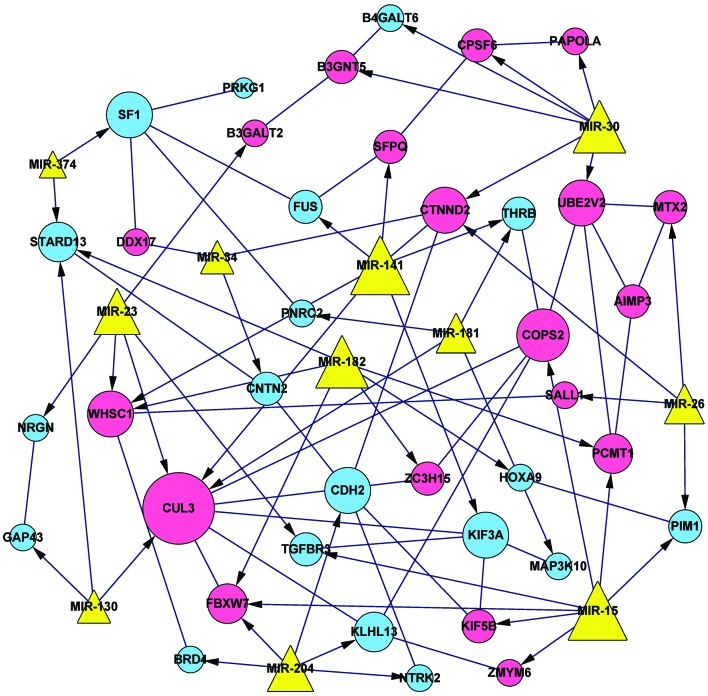
A total of 36 DEG interaction pairs were identified, according to their predicted protein-protein interactions. Combined with the identified DEGs targeted by miRNAs, the integrated miRNA-DEG regulatory network was constructed. This network comprised 49 nodes and 88 edges, involving 11 miRNAs and 38 DEGs, including 19 upregulated and 19 downregulated genes (Fig. 1).
Figure 1.
Integrated microRNA (miRNA)-differentially expressed gene (DEG) network. Circular nodes represent DEGs (pink circles, upregulated genes; blue circles, downregulated genes). Yellow triangles represent the miRNAs, with edges with arrowheads depicting associations between miRNAs and DEGs, and edges with lines depicting protein-protein interactions between the DEGs.
Biological function and pathway annotation of the DEGs
In order to investigate the functions of the DEGs associated with TNF-α genetic variations, the identified DEGs were subjected to GO analysis. As presented in Table II, the DEGs were predominantly enriched in seven GO terms associated with transcriptional events, phosphorylation and RNA functions. DEGs associated with transcriptional regulation were as follows: COPS2, THRB, SFPQ, SALL1, PNRC2, CTNND2, PIM1, SF1, MAP3K10, TGFBR3, HOXA9 and WHSC1. DEGs associated with transcription were as follows: COPS2, PAPOLA, THRB, SFPQ, SALL1, PNRC2, CTNND2, SF1, HOXA9 and WHSC1. In addition, DEGs associated with GO terms such as phosphorylation and protein amino acid phosphorylation were NTRK2, PIM1, MAP3K10, TGFBR3, BRD4 and PRKG1. The six DEGs associated with RNA processing were FUS, DDX17, PAPOLA, SFPQ, SF1 and CPSF6. Those involved in mRNA metabolic processes, which was the most significantly enriched GO term (P=0.001632), included FUS, PAPOLA, SFPQ, PNRC2, SF1 and CPSF6. DEGs, including NTRK2, PIM1, MAP3K10, TGFBR3, BRD4 and GAP43, were significantly associated with positive regulation of molecular function.
Table II.
GO enrichment analysis of the DEGs in the integrated regulatory network.
| Term | DEGs | P-value |
|---|---|---|
| GO:0016071 - mRNA metabolic process | FUS, PAPOLA, SFPQ, PNRC2, SF1, CPSF6 | 0.001632 |
| GO:0006396 - RNA processing | FUS, DDX17, PAPOLA, SFPQ, SF1, CPSF6 | 0.008649 |
| GO:0044093 - positive regulation of molecular function | NTRK2, PIM1, MAP3K10, TGFBR3, BRD4, GAP43 | 0.011448 |
| GO:0006468 - protein amino acid phosphorylation | NTRK2, PIM1, MAP3K10, TGFBR3, BRD4, PRKG1 | 0.019140 |
| GO:0045449 - regulation of transcription | COPS2, THRB, SFPQ, SALL1, PNRC2, CTNND2, PIM1, SF1, MAP3K10, TGFBR3, HOXA9, WHSC1 | 0.031480 |
| GO:0016310 - phosphorylation | NTRK2, PIM1, MAP3K10, TGFBR3, BRD4, PRKG1 | 0.038131 |
| GO:0006350 - transcription | COPS2, PAPOLA, THRB, SFPQ, SALL1, PNRC2, CTNND2, SF1, HOXA9, WHSC1 | 0.049845 |
GO, gene ontology; DEG, differentially expressed gene.
KOBAS analysis was also conducted in order to identify significantly enriched pathways. Two significantly enriched pathways were identified, including the glycosphingolipid biosynthesis (including B3GNT5 and B3GALT2) and ubiquitin-mediated proteolysis, which was the most significantly enriched pathway with three DEGs (including CUL3, FBXW7 and KLHL13) (Table III). Based on the information from the integrated regulatory network (Fig. 1), both COPS2 and CUL3 were found to be targets of miR-15.
Table III.
Enriched pathways of the differentially expressed genes in the integrated regulatory network.
| ID | Pathway | P-value | Genes |
|---|---|---|---|
| hsa04120 | Ubiquitin mediated proteolysis | 0.02628 | CUL3, FBXW7, KLHL13 |
| hsa00601 | Glycosphingolipid biosynthesis | 0.04213 | B3GNT5, B3GALT2 |
Discussion
The present study identified 390 genes that were differentially expressed between the WT and MUT (carrying TNF-α rs1800629 A variant) samples, and established a regulatory network containing 38 DEGs and 11 miRNAs. This network suggested that COPS2 was predominantly associated with transcriptional functions, whereas FUS was primarily involved in mRNA metabolic processes. Other DEGs, including FBXW7 and CUL3, were enriched in the ubiquitin-mediated proteolysis pathway. Furthermore, miR-15 was predicted to target both COPS2 and CUL3.
COPS2, also known as COP9 signalosome subunit 2 or CSN2, is an essential component of the COP9 signalosome complex, which is involved in diverse cellular processes and acts as a critical regulator of the ubiquitin conjugation pathway (26). In a previous study it was demonstrated that TNF-α was able to promote the stabilization of Snail (the most important transcriptional repressor of E-cadherin) and β-catenin, by inhibiting GSK-3β-mediated phosphorylation of these proteins via the nuclear factor-κB and Akt signalling pathways, which in turn promoted tumor cell invasion. Notably, COPS2 had a crucial role in this process since it was able to inhibit the phosphorylation and ubiquitination of Snail by preventing the binding of Snail to its receptors (27).
The protein encoded by FUS is a multifunctional component of the heterogeneous nuclear ribonucleoprotein complex, which is involved in pre-mRNA splicing and the export of fully processed mRNA to the cytoplasm (28). FUS, which contains multiple RNA binding domains and an N-terminal glutamine-rich domain, acts as a mediator of RNA binding and as a potent transcriptional activator (29). In addition, FUS has previously been associated with familial amyotrophic lateral sclerosis (30) and the pathogenesis of myxoid liposarcoma (31); however, to the best of our knowledge, no previous studies have investigated the association between FUS and sepsis. In the present study, COPS2 was shown to be upregulated in the MUT samples, and was strongly associated with transcriptional functions, whereas FUS was downregulated in the TNF-α rs1800629 A allele variant samples, and was associated with mRNA metabolic processes. Furthermore, COPS2 and FUS were shown to interact in the integrated regulatory network. These results suggested that COPS2 may play a vital role in the pathogenesis of sepsis in patients with TNF-α rs1800629 A allele variation by regulating the phosphorylation and ubiquitination of the protein encoded by FUS at the transcriptional level.
FBXW7 and CUL3 are two critical DEGs, which were found to be upregulated in the TNF-α rs1800629 A allele variant samples. CUL3 (also known as cullin 3) encodes a member of the cullin protein family, and is the core component and scaffold protein of an E3 ubiquitin-protein ligase complex, which mediates the ubiquitiation and subsequent proteasomal degradation of target proteins (32). As major subunits of the cullin-RING ligase (CRL) family, cullins are active components of ~50% of human E3 ubiquitin ligases and are responsible for one fifth of all cellular proteasome-dependent protein degradation (33). FBXW7 (also known as F-box and WD repeat domain-containing 7) is a substrate receptor for CRL1, and facilitates the ubiquitination and degradation of numerous proteins (34). Cyclin E, a member of the cyclin family, is a positive regulator of proliferation in mammalian fibroblasts. CUL1 and CUL3 have previously been implicated in the degradation of cyclin E via two distinct pathways (35). In comparison with the CUL1/FBXW7-based E3 ligase-dependent pathway, cyclin E does not require phosphorylation at threonine 380, and may be recognized as a substrate by CUL3 in the CUL3-based E3 ligase pathway (36), which may be essential for the maintenance of quiescence in mammalian cells (35). Therefore, consistent with the findings of the present study, previous reports have suggested that FBXW7 and CUL3 may be involved in the ubiquitin-mediated proteolysis pathway (37,38).
Based on a novel mechanism of autophagy, CaMKIV (also known as calcium/calmodulin-dependent protein kinase IV) may inhibit ubiquitin-mediated mTOR degradation through the inhibition of FBXW7 recruitment. However, in sepsis, mTOR expression is required for autophagy (39), indicating that FBXW7 may regulate mTOR. Although Qiao et al (40) did not identify CUL3 as a DEG in sepsis, it is associated with DEGs in the PPI network, suggesting their potential involvement in sepsis. However, no study has previously reported a direct interaction between FBXW7 and CUL3. The aforementioned findings collectively suggest that FBXW7 and CUL3 may exert proteolytic functions via ubiquitination in sepsis progression in patients with TNF-α genetic variations. In addition, there may be regulators between FBXW7 and CUL3 that facilitate the functions of the two genes.
miRNAs have a critical role in the regulation of gene expression at the post-transcriptional level (41). miR-15 has been demonstrated to be associated with apoptosis in various types of cancer. For instance, a previous study identified that miR-15 was closely associated with apoptosis in chronic lymphocytic leukemia, and it was demonstrated to induce apoptosis by negatively regulating the expression of B-cell lymphoma 2 (42). Furthermore, miR-15(a/b) was reported to have inhibited the expression of cyclin E and was identified as a novel transcriptional target of E2F1, which is a vital transcription factor that induces proliferation and cell death (43). Thus, miR-15 and CUL3 may co-regulate the expression of cyclin E. Up-regulation of miR-15 has been found in the serum of neonatal sepsis patients, and this miRNA is proposed as a potential biomarker for neonatal sepsis prognosis (44). Notably, the present study identified CUL3 as a potential target of miR-15, which is consistent with this hypothesis. In addition, although previous investigations have been unable to uncover a regulatory association between miR-15 and COPS2, the present study predicted that COPS2 was a target of miR-15, thus suggesting that COPS2 may be a novel target gene for miR-15 in the progression of sepsis.
In conclusion, in the present study, COPS2, FUS, FBXW7 and CUL3 were found to be associated with the progression of sepsis in patients with the TNF-α rs1800629 A variant. Of these genes, FBXW7 and CUL3 may exert regulatory roles in the ubiquitin-mediated proteolysis pathway, whereas COPS2 may affect the development of sepsis by regulating the phosphorylation and ubiquitination of the FUS protein. In addition, COPS2 and CUL3 may be novel targets of miR-15. The aforementioned genes and miRNAs may be used as therapeutic biomarkers for sepsis diagnosis in patients with the TNF-α rs1800629 A variant.
Acknowledgements
The present study was supported by the Shanghai Medical Key Subject Construction Project (grant no. ZK2012A28), the Shanghai Special Project for Advanced and Suitable Technology (grant no. 2013SY039) and the National Clinical Key Specialty Construction Project.
References
- 1.Wafaisade A, Lefering R, Bouillon B, Sakka SG, Thamm OC, Paffrath T, Neugebauer E, Maegele M. Trauma Registry of the German Society for Trauma Surgery: Epidemiology and risk factors of sepsis after multiple trauma: An analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011;39:621–628. doi: 10.1097/CCM.0b013e318206d3df. [DOI] [PubMed] [Google Scholar]
- 2.Coimbra R. From the trauma surgeon's viewpoint: Multiple injuries - which cavity to open first? J Trauma Nurs. 2005;12:7–9. doi: 10.1097/00043860-200512010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balk RA. Severe sepsis and septic shock: Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000;16:179–192. doi: 10.1016/S0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- 5.Hanna NF. Sepsis and septic shock. Adv Emerg Nurs J. 2003;25:158–165. [Google Scholar]
- 6.Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor α triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemaître N, Sebbane F, Long D, Hinnebusch BJ. Yersinia pestis YopJ suppresses tumor necrosis factor alpha induction and contributes to apoptosis of immune cells in the lymph node but is not required for virulence in a rat model of bubonic plague. Infect Immun. 2006;74:5126–5131. doi: 10.1128/IAI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg JD, Kremer JM, Curtis JR, Hochberg MC, Reed G, Tsao P, Farkouh ME, Nasir A, Setoguchi S, Solomon DH. CORRONA Investigators: Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576–582. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 9.Yu M, Zhou X, Niu L, Lin G, Huang J, Zhou W, Gan H, Wang J, Jiang X, Yin B, Li Z. Targeting transmembrane TNF-α suppresses breast cancer growth. Cancer Res. 2013;73:4061–4074. doi: 10.1158/0008-5472.CAN-12-3946. [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Li X, Zhang X, Li Z, Wang L, Sun Y, Liu Z, Ma X. Elevated levels of plasma TNF-α are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock. 2014;41:275–281. doi: 10.1097/SHK.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 11.Rice TW, Bernard GR. Therapeutic intervention and targets for sepsis. Annu Rev Med. 2005;56:225–248. doi: 10.1146/annurev.med.56.082103.104356. [DOI] [PubMed] [Google Scholar]
- 12.Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52:447–456. doi: 10.1016/j.neuint.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocabaş E, Sarikçioğlu A, Aksaray N, Seydaoğlu G, Seyhun Y, Yaman A. Role of procalcitonin, C-reactive protein, interleukin-6, interleukin-8 and tumor necrosis factor-alpha in the diagnosis of neonatal sepsis. Turk J Pediatr. 2007;49:7–20. [PubMed] [Google Scholar]
- 14.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 15.Lièvre A, Bachet J-B, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland AM, Walley KR. Bench-to-bedside review: Association of genetic variation with sepsis. Crit Care. 2009;13:210. doi: 10.1186/cc7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menges T, König IR, Hossain H, Little S, Tchatalbachev S, Thierer F, Hackstein H, Franjkovic I, Colaris T, Martens F, et al. Sepsis syndrome and death in trauma patients are associated with variation in the gene encoding tumor necrosis factor. Crit Care Med. 2008;36:1456–1462. doi: 10.1097/CCM.0B013E318170ABB6. e1–e6. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Han N, Li G, Li X, Li G, Liu Y, Wu W, Wang Y, Chen Y, Sun G, et al. Prediction of feature genes in trauma patients with the TNF rs1800629 A allele using support vector machine. Comput Biol Med. 2015;64:24–29. doi: 10.1016/j.compbiomed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Fujita A, Sato JR, Rodrigues LO, Ferreira CE, Sogayar MC. Evaluating different methods of microarray data normalization. BMC Bioinformatics. 2006;7:469. doi: 10.1186/1471-2105-7-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth GK. Limma: Linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [DOI] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 22.Duncan D, Prodduturi N, Zhang B. WebGestalt2: An updated and expanded version of the Web-based Gene Set Analysis Toolkit. BMC Bioinformatics. 2010;11(Suppl 4):10. doi: 10.1186/1471-2105-11-S4-P10. [DOI] [Google Scholar]
- 23.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smoot ME, Ono K, Ruscheinski J, Wang P-L, Ideker T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 26.Wei N, Deng XW. The COP9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–286. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz JC, Podell ER, Han SS, Berry JD, Eggan KC, Cech TR. FUS is sequestered in nuclear aggregates in ALS patient fibroblasts. Mol Biol Cell. 2014;25:2571–2578. doi: 10.1091/mbc.E14-05-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentmann E, Neumann M, Tahirovic S, Rodde R, Dormann D, Haass C. Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43) J Biol Chem. 2012;287:23079–23094. doi: 10.1074/jbc.M111.328757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 31.Riggi N, Cironi L, Provero P, Suvà ML, Stehle JC, Baumer K, Guillou L, Stamenkovic I. Expression of the FUS-CHOP fusion protein in primary mesenchymal progenitor cells gives rise to a model of myxoid liposarcoma. Cancer Res. 2006;66:7016–7023. doi: 10.1158/0008-5472.CAN-05-3979. [DOI] [PubMed] [Google Scholar]
- 32.Pintard L, Willis JH, Willems A, Johnson JL, Srayko M, Kurz T, Glaser S, Mains PE, Tyers M, Bowerman B, Peter M. The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature. 2003;425:311–316. doi: 10.1038/nature01959. [DOI] [PubMed] [Google Scholar]
- 33.Saito N, Sakakibara K, Sato T, Friedman JM, Kufe DW, VonHoff DD, Kawabe T. CBS9106-induced CRM1 degradation is mediated by cullin ring ligase activity and the neddylation pathway. Mol Cancer Ther. 2014;13:3013–3023. doi: 10.1158/1535-7163.MCT-14-0064. [DOI] [PubMed] [Google Scholar]
- 34.Tron AE, Arai T, Duda DM, Kuwabara H, Olszewski JL, Fujiwara Y, Bahamon BN, Signoretti S, Schulman BA, DeCaprio JA. The glomuvenous malformation protein Glomulin binds Rbx1 and regulates cullin RING ligase-mediated turnover of Fbw7. Mol Cell. 2012;46:67–78. doi: 10.1016/j.molcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEvoy JD, Kossatz U, Malek N, Singer JD. Constitutive turnover of cyclin E by Cul3 maintains quiescence. Mol Cell Biol. 2007;27:3651–3666. doi: 10.1128/MCB.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer JD, Gurian-West M, Clurman B, Roberts JM. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 1999;13:2375–2387. doi: 10.1101/gad.13.18.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eide PW, Cekaite L, Danielsen SA, Eilertsen IA, Kjenseth A, Fykerud TA, Ågesen TH, Bruun J, Rivedal E, Lothe RA, Leithe E. NEDD4 is overexpressed in colorectal cancer and promotes colonic cell growth independently of the PI3K/PTEN/AKT pathway. Cell Signal. 2013;25:12–18. doi: 10.1016/j.cellsig.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Yu S, Yi H, Wang Z, Dong J. Screening key genes associated with congenital heart defects in Down syndrome based on differential expression network. Int J Clin Exp Pathol. 2015;8:8385–8393. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Howell GM, Guo L, Collage RD, Loughran PA, Zuckerbraun BS, Rosengart MR. CaMKIV-dependent preservation of mTOR expression is required for autophagy during lipopolysaccharide-induced inflammation and acute kidney injury. J Immunol. 2014;193:2405–2415. doi: 10.4049/jimmunol.1302798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao FS, Wei C, Yun J, Qian LX. Insights into the molecular mechanisms in sepsis with microarray technology. Eur Rev Med Pharmacol Sci. 2014;18:2405–2412. [PubMed] [Google Scholar]
- 41.Geng J, Luo H, Pu Y, Zhou Z, Wu X, Xu W, Yang Z. Methylation mediated silencing of miR-23b expression and its role in glioma stem cells. Neurosci Lett. 2012;528:185–189. doi: 10.1016/j.neulet.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 42.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ofir M, Hacohen D, Ginsberg D. MiR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol Cancer Res. 2011;9:440–447. doi: 10.1158/1541-7786.MCR-10-0344. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Wang X, Liu X, Wang X, Xu J, Hou S, Zhang X, Ding Y. miR-15a/16 are upregulated in the serum of neonatal sepsis patients and inhibit the LPS-induced inflammatory pathway. Int J Clin Exp Pathol. 2015;8:5683–5690. [PMC free article] [PubMed] [Google Scholar]