Abstract
Chrysin is a major active ingredient of flavonoids, known to exhibit protective effects against various types of cancer. However, the anticancer role of chrysin against hepatocellular carcinoma (HCC) and the underlying molecular mechanisms remain unclear. In order to evaluate the effects of chrysin on cell viability and apoptosis in human HCC, HepG2 and QGY7701 cells were used in the present study. Cell viability was monitored using an MTT assay. In addition, an Annexin V-fluorescein isothiocyanate/propidium iodide kit was used for the labeling of the apoptotic cells, which were then measured using flow cytometry. Western blotting was used to examine the protein expression of p53, B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X (Bax), Bcl-2-associated death promoter (Bad), Bcl-2 homologous antagonist/killer (Bak), caspases-3 and −9, and cleaved-caspases-3 and −9. The results of the present study revealed that chrysin suppressed the cell viability of HepG2 and QGY7701 cells in a concentration-dependent manner. In addition, chrysin induced significant apoptosis in HepG2 and QGY7701 cells. Furthermore, it was demonstrated that chrysin treatment increased the expression of proapoptotic proteins, including p53, Bax, Bad and Bak, while it decreased the protein level of antiapoptotic protein Bcl-2. It was also demonstrated that chrysin induced apoptosis in the HCC cells by regulating the p53/Bcl-2/caspase-9 signaling pathway. In conclusion, the results of the present study suggested that chrysin may be a potential candidate agent for the induction of cell apoptosis in human HCC.
Keywords: p53/B-cell lymphoma-2/caspase-9 pathway, chrysin, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) has become a global health problem, as it is the third most common cause of cancer-associated mortalities worldwide, with >800,000 mortalities per year (1,2); however, systemic treatment options for HCC are limited. Surgery remains the most efficacious and mainstream solution for the eradication of cancer nodules; however, relapse and distant organ invasion are common following tumor dissection (3). Anti-tumor agents have demonstrated strong cytotoxicity against cancer cells and oral administration of chemotherapeutics is recommended for the clearance of tumor cells that survive tumor dissection (4). Therefore, choosing appropriate anti-tumor agents and planning their administration is an indispensable part of systemic treatment HCC.
Natural products have been widely used in the development of various anticancer drugs. Paclitaxel, which is extracted from bark of the Pacific yew (Taxus brevifolia), has been successfully used to treat breast, lung and ovarian cancer (5). Chrysin, a natural flavonoid that is commonly found in honey and bee propolis (Fig. 1), is known for its various biological activities (6,7). Previous studies have suggested that chrysin possesses antioxidant (8), antihypertensive (9), antidiabetogenic (10) and anxiolytic properties (11). In addition, chrysin exerts a strong anticancer effect in multiple types of human cancer, since it induces cell cycle arrest and cell apoptosis (12); however, whether chrysin may be used as a novel antitumor drug against HCC has not been extensively investigated.
Figure 1.
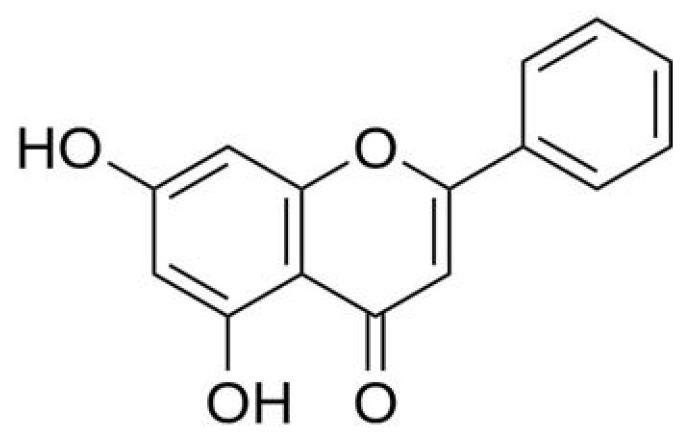
Molecular structure of chrysin.
Therefore, the aim of the present study was to examine the anticancer effect of chrysin on HCC cells, and determine the underlying mechanism of chrysin-induced apoptosis in HepG2 and QGY7701 human HCC cells.
Materials and methods
Cell culture
HepG2 and QGY7701 cells were provided by The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum plus 100 U/ml penicillin and 100 µg/ml streptomycin (all Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C, in a humidified atmosphere of 5% CO2. Chrysin was diluted to various concentrations with cell culture media. After the cells had reached 60% confluence, various concentrations of chrysin were added (0, 10, 15, 20, 25, 30, 40 and 50 µg/ml).
MTT assay
Cells were seeded in 96-well plates at a density of 5,000–10,000 cells/well. Different concentrations of chrysin (0, 10, 15, 20, 25, 30, 40 and 50 µg/ml; 5 replicates per concentration group) were added to the cell cultures for 24 h, followed by 20 µl MTT solution (5 mg/ml; Beyotime Institute of Biotechnology, Shanghai, China). After incubation for 4 h at 37°C in an atmosphere of 5% CO2, supernatants were removed and 150 µl dimethyl sulfoxide (Beyotime Institute of Biotechnology) was added. The plates were then placed on an orbital shaker for 10 min. Subsequently, the absorbance at 490 nm was measured using a spectrophotometer (EnSpire 2300 Multilabel reader; PerkinElmer Inc., Waltham, MA, USA). The inhibitory rate was calculated according to the following formula: (A490control-A490treated)/(A490control-A490blank) ×100%.
Apoptosis assay
Apoptotic cells were detected using an Annexin V-FITC/PI kit (BioVision Inc., Milpitas, CA, USA). HepG2 and QGY7701 cells were seeded in 6-well plates at a density of 1×105 cells/well. According to the results of the MTT assay, the half maximal inhibitory concentration (IC50) concentration of chrysin was acquired for each cell line using GraFit-Erithacus IC50 software (Erithacus Software Ltd., Horley, UK). Lower (20 µg/ml) and higher dosages (40 µg/ml) were selected for the cell apoptosis assay. Cells were incubated in different concentrations of chrysin (0, 20 and 40 µg/ml) for 24 h. The samples were then washed twice with cold D-Hank's buffer solution and resuspended in binding buffer (1×106 cells/ml). Subsequently, 100 µl cell supernatants were transferred to a tube with 5 µl Annexin V-FITC (BD Biosciences, Franklin Lakes, NJ, USA) and 5 µl PI (Beyotime Institute of Biotechnology). Following incubation for 15 min at room temperature in the dark, the apoptotic cells were detected using flow cytometry (FACS Canto II; BD Biosciences), and analyzed using Modfit and CellQuest 5.1 software (BD Biosciences). Cells located in the Q4 zone were deemed to be in the early apoptosis stage, whereas cells in the Q2 zone were in the late apoptosis stage.
Western blot analysis
Drug-induced cell apoptosis is due to mitochondrial dysfunction and apoptotic signaling pathway activation (13). p53, B-cell lymphoma (Bcl)-2, Bcl-2-associated death promoter (Bad), Bcl-2-associated X (Bax), Bcl-2 homologous antagonist/killer (Bak), caspase-9 and caspase-3 are associated with apoptosis-induced mitochondrial damage (14); therefore the expression levels of these proteins were investigated. Cultured cells were collected and total protein was extracted using radioimmunoprecipitation assay lysis buffer [50 mmol/l Tris (pH 7.4), 150 mmol/l NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM EDTA and 40 µg.ml leupeptin], containing 1 mM phenylmethylsulfonyl fluoride (both Beyotime Institute of Biotechnology). After laying the extraction on ice for 30 min, cell lysate was centrifuged for 12,000 × g for 10 min at 4°C and the supernatants were collected. The total protein concentration in each supernatant sample was measured using a BCA protein assay kit (P0012; Beyotime Institute of Biotechnology). Subsequently, proteins (20 µg/lane) were separated by 8% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Following blocking with 5% non-fat milk, the membrane was incubated with specific primary antibodies at a dilution of 1:1,000 for 2 h at room temperature. The following primary antibodies were used: p53 (2527), Bcl-2 (2870), Bax (5023), Bak (6947), Bad (9239), caspase-3 (9665), caspase-9 (9505) and GAPDH (2118; all Cell Signaling Technology, Beverly, CA, USA). The membrane was then washed three times in Tris-buffered saline-Tween 20 (TBST) for 5 min, and incubated with a goat anti-rabbit horseradish peroxidase-labeled secondary antibody (E030120-01; EarthOx Life Sciences, Millbrae, CA, USA) at a dilution of 1:5,000 for 1 h at room temperature. Following three washes in TBST (10 min each), the membranes were exposed to an enhanced chemiluminescence buffer (EMD Millipore, Billerica, MA, USA) and measured on an Sally Sue western blot imaging system (ProteinSimple, San Jose, CA, USA). Gel images were then analyzed using ImageJ (National Institutes of Health, Bethesda, MA, USA) to calculate the gray value of each band.
Statistical analysis
Experiments were repeated ≥3 times and all data were analyzed using Graphpad Prism 5.0 software. Between-group differences were analyzed using Student's t-test. Data are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference (*P<0.05; **P<0.01; ***P<0.001).
Results
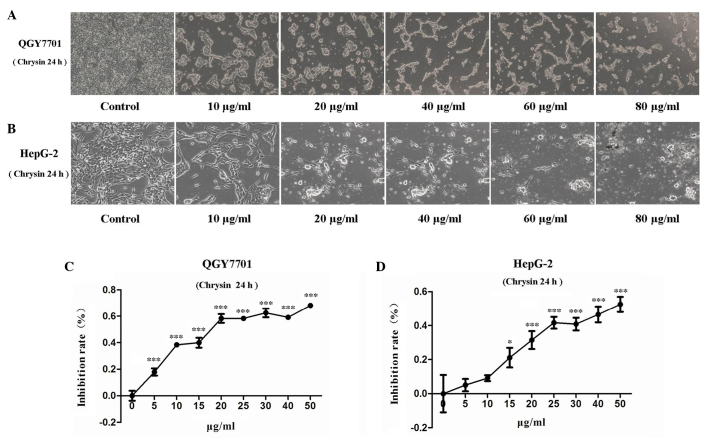
Chrysin inhibits the viability of HCC cells
Following treatment of the QGY7701 and HepG2 cells with chrysin, the cells were found to slow growing, distorted, round in shape and detached from the bottom of the plate. Furthermore, the numbers of detached cells increased with increasing drug concentration (Figs. 2A and B). MTT assay was used to evaluate the viability of QGY7701 and HepG2 cells treated with chrysin. The present results revealed that, after 24 h of treatment, chrysin significantly inhibited cell viability in the HCC cell lines in a dose-dependent manner (P<0.001; Figs. 2C and D). IC50 is an index for the evaluation of drug sensitivity. The IC50 values of chrysin in QGY7701 and HepG2 cells were measured using GraFit-Erithacus IC50 software and were found to be 18 and 25 µg/ml, respectively.
Figure 2.
Various concentrations of 24-h chrysin treatment inhibited HepG2 and QGY7701 cell viability. Microscopic analysis of (A) QGY7701 and (B) HepG2 cells (magnification, ×100). Quantification of MTT assay data for (C) QGY7701 and (D) HepG2 cells. Data are presented as the mean ± standard deviation (n=5). *P<0.05 and ***P<0.001 vs. 0 µg/ml chrysin.
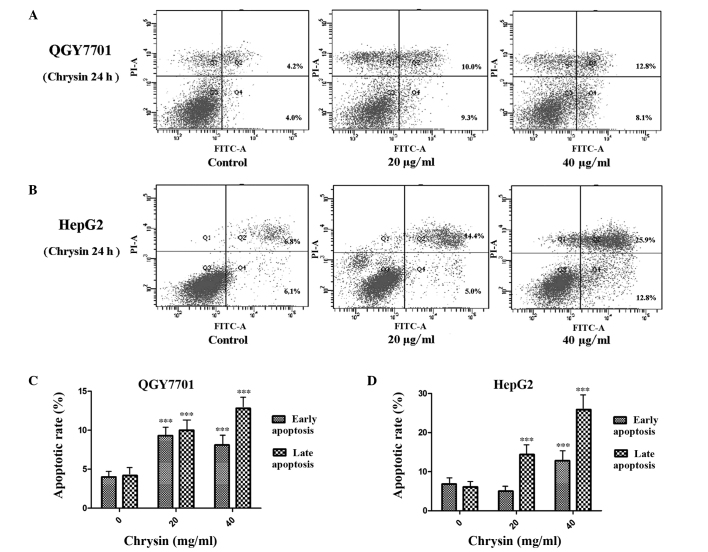
Chrysin promotes HCC cell apoptosis
The apoptosis of the QGY7701 and HepG2 cells was detected using Annexin V/PI double staining and flow cytometric analysis. The results indicated that chrysin induced HCC cell apoptosis. Chrysin induced-apoptosis in HepG2 and QGY7701 cells was found to significantly increase in a concentration-dependent manner (P<0.001; Fig. 3). QGY7701 cells were found to be more sensitive to chrysin treatment, with higher levels of apoptosis observed compared with the HepG2 cells.
Figure 3.
Chrysin promotes cell apoptosis in HepG2 and QGY7701 cells. Apoptotic rate in (A) HepG2 and (B) QGY7701 cells following treatment with various concentrations of chrysin for 24 h. Apoptotic cells were detected using flow cytometry. Quantification of the flow cytometry data for (C) QGY7701 and (D) HepG2 cells identified the rates of early and late chrysin-induced apoptosis. Data are presented as the mean ± standard deviation (n=3). ***P<0.001 vs. 0 µg/ml chrysin.
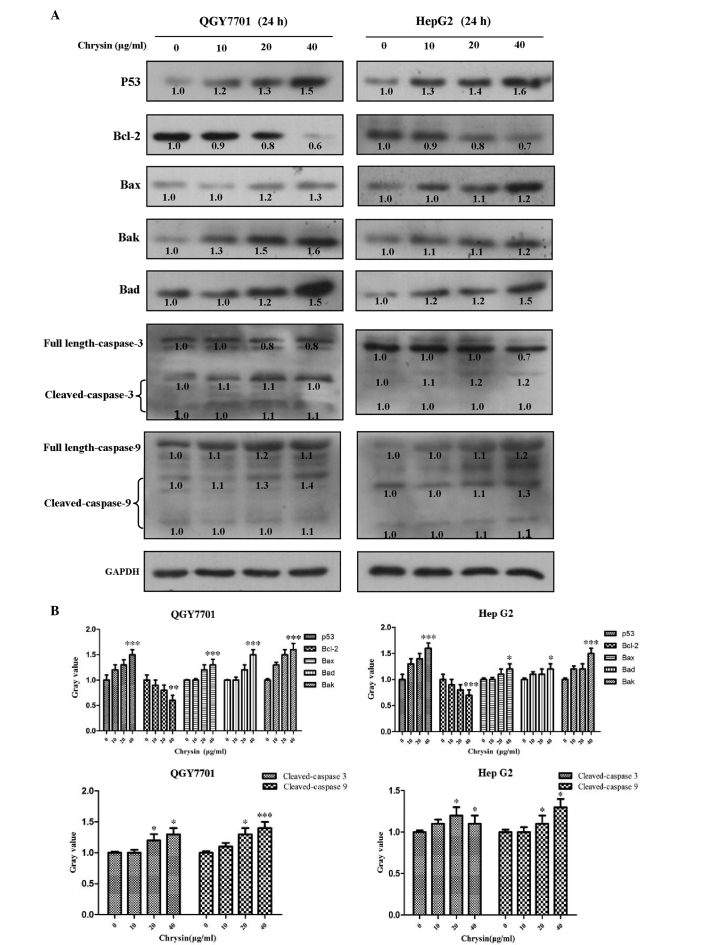
p53/Bcl-2/caspase-9 signaling pathway activation following treatment with chrysin in HCC cells
HCC cells were treated with various concentrations of chrysin for 24 h. Apoptosis protein expression levels of cleaved caspase-3 and caspase-9 were found to significantly increase in a concentration-dependent manner following chrysin treatment in the QGY7701 and HepG2 cell lines (P<0.001; Fig. 4). These results indicated that the caspase-9/caspase-3-associated apoptosis pathway was activated. In addition, the possible activation of the p53/Bcl-2 signaling pathway was also investigated based on the protein expression levels of p53, Bcl-2, Bax, Bad and Bak. The results demonstrated that this intrinsic apoptosis pathway was also activated following chrysin treatment in the two HCC cell lines (P<0.001; Fig. 4).
Figure 4.
Chrysin regulates the p53/Bcl-2/caspase-9 signaling pathway. Chrysin regulates the content of apoptotic-related proteins in HepG2 and QGY7701 cells following various concentrations of chrysin treatment for 24 h. (A) Western blotting was performed in order to analyze the protein content of p53, Bcl-2, Bax, Bak, Bad, caspases-3 and −9, and cleaved-caspases-3 and −9 (B) and this data was subsequently quantified. *P<0.05, **P<0.01 and ***P<0.001 vs. 0 µg/ml chrysin. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X; Bad, Bcl-2-associated death promoter; Bak, Bcl-2 homologous antagonist/killer.
Discussion
HCC is a highly malignant tumor that affects individuals worldwide. Efficacious chemotherapeutic is critical for the eradication of tumors that cannot be completely removed by surgery. Although there are several therapeutic agents for the management of HCC, drug resistance and adverse effects remain pivotal issues (3). Natural products are a large source of novel therapeutic agents and natural products have been widely investigated as anti-cancer drugs, however the exact mechanism of their anti-cancer action requires further elucidation. In the present study, chrysin was demonstrated to significantly inhibit the viability of two HCC cell lines, suggesting that chrysin may comprise a novel candidate agent for the treatment of HCC.
Cell apoptosis is a complicated biological process that is associated with complex signaling pathway responses. The activation of cysteine proteases, in particular caspases, is a key intracellular regulator of cell apoptosis (15,16). Caspase-3 is an important mediator of apoptosis (17) that is activated by a variety of activators classified into two main signaling pathways: The death receptor-mediated pathway, involving caspase-8 and caspase-10, and the mitochondria-mediated pathway, involving caspase-9 (18,19). Caspase-3 is an executioner caspase that is activated by death ligands and mitochondrial dysfunction-induced cell apoptosis (20). In the present study, chrysin treatment was not found to have a significant effect on the expression of caspase-8, but promoted the accumulation of cleaved caspase-9, suggesting that chrysin selectively induces apoptosis in HCC cells via the mitochondria-mediated apoptosis pathway. Due to the role of caspase-3 as an executioner of cell apoptosis, caspase-3 expression levels were also detected to confirm cell apoptosis. The results demonstrated that caspase-3 was cleaved significantly, indicating that the cells were undergoing apoptosis. The tumor suppressor protein, p53, is a positive regulator of the Bax, Bad and Bak proapoptotic proteins to prevent Bcl-2 capture. Free Bax, Bad and Bak subsequently bind to the mitochondrial membrane to induce mitochondrial damage and cell apoptosis (21–23). Previous studies have demonstrated that p53 promotes the transcription of Bax and Bak, which regulate the release of cytochrome c from the mitochondria, and result in cell apoptosis by activating the cleaving of caspase-3 and caspase-9 (22,24). In the present study, p53, Bax, Bad and Bak were found to be significantly upregulated, whereas Bcl-2 was found to be downregulated in the HepG2 and QGY7701 cells following chrysin treatment. These results suggested that chrysin upregulates p53 and activates its downstream target gene, which induces mitochondrial dysfunction by releasing cytochrome c and inducing the apoptosis of cancer cells. Cell apoptosis is a process of programmed cell death which includes early membrane extroversion in the early stage and cell membrane disruption and cell death during late apoptosis. In the present study, it was demonstrated that the early and late stages of apoptosis were increased in QGY7701 cells; whereas only late apoptosis was increased in HepG2 cells. Notably, significantly more HepG2 cells were killed, as compared with QGY7701 cells, which suggests that chrysin may have a stronger effect on HepG2 cells by promptly inducing late cell apoptosis. These results demonstrated that the anti-cancer effect of chrysin anticancer effect is associated with the genetic background of the cancer cells rather than a general cytotoxic effect.
In conclusion, the results of the present study suggested that chrysin effectively inhibits cell viability and induces cell apoptosis in HCC cells. It was confirmed that chrysin promoted HCC cell apoptosis via the activation of the p53/Bcl2/caspase-9 apoptotic signaling pathway. Based on the aforementioned findings, the present study suggests that chrysin may be a candidate agent for the treatment of human HCC.
Acknowledgements
The present study was supported by grants from the Special Funds from Education Department of Guangdong Province (grant no. JB1212), the Chinese NSFC grants (grant no. 31370824), the Yangfan Plan of Talents Recruitment Grant, Guangdong, China (grant nos. YueRenCaiBan[2014]1 and YueRenCaiBan[2016]1), the University Talents Recruitment Grant of Guangdong, China (grant no. YueCaiJiao[2012]328), The Excellent Postgraduate Essay Development Project of Guangdong Medical College (grant no. 2014-18) and the Key Laboratory of Zhanjiang project (grant no. 2013A402-4).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Au JS, Frenette CT. Management of hepatocellular carcinoma: Current status and future directions. Gut Liver. 2015;9:437–448. doi: 10.5009/gnl15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzmorris P, Shoreibah M, Anand BS, Singal AK. Management of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2015;141:861–876. doi: 10.1007/s00432-014-1806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manfredi JJ, Horwitz SB. Taxol: An antimitotic agent with a new mechanism of action. Pharmacol Ther. 1984;25:83–125. doi: 10.1016/0163-7258(84)90025-1. [DOI] [PubMed] [Google Scholar]
- 6.Pichichero E, Cicconi R, Mattei M, Muzi MG, Canini A. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. Int J Oncol. 2010;37:973–981. doi: 10.3892/ijo_00000748. [DOI] [PubMed] [Google Scholar]
- 7.Barbarić M, Mišković K, Bojić M, Lončar MB, Smolčić-Bubalo A, Debeljak Z, Medić-Šarić M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J Ethnopharmacol. 2011;135:772–778. doi: 10.1016/j.jep.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Khan R, Khan AQ, Qamar W, Lateef A, Tahir M, Rehman MU, Ali F, Sultana S. Chrysin protects against cisplatin-induced colon toxicity via amelioration of oxidative stress and apoptosis: Probable role of p38MAPK and p53. Toxicol Appl Pharmacol. 2012;258:315–329. doi: 10.1016/j.taap.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Cherkaoui-Tangi K, Lachkar M, Wibo M, Morel N, Gilani AH, Lyoussi B. Pharmacological studies on hypotensive, diuretic and vasodilator activities of chrysin glucoside from Calycotome villosa in rats. Phytother Res. 2008;22:356–361. doi: 10.1002/ptr.2322. [DOI] [PubMed] [Google Scholar]
- 10.Song MY, Jeong GS, Kwon KB, Ka SO, Jang HY, Park JW, Kim YC, Park BH. Sulfuretin protects against cytokine-induced beta-cell damage and prevents streptozotocin-induced diabetes. Exp Mol Med. 2010;42:628–638. doi: 10.3858/emm.2010.42.9.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahir M, Sultana S. Chrysin modulates ethanol metabolism in Wistar rats: A promising role against organ toxicities. Alcohol Alcohol. 2011;46:383–392. doi: 10.1093/alcalc/agr038. [DOI] [PubMed] [Google Scholar]
- 12.Yang F, Jin H, Pi J, Jiang JH, Liu L, Bai HH, Yang PH, Cai JY. Anti-tumor activity evaluation of novel chrysin-organogermanium (IV) complex in MCF-7 cell. Bioorg Med Chem Lett. 2013;23:5544–5551. doi: 10.1016/j.bmcl.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 13.Wen S, Zhu D, Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med Chem. 2013;5:53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mu R, Lu N, Wang J, Yin Y, Ding Y, Zhang X, Gui H, Sun Q, et al. An oxidative analogue of gambogic acid-induced apoptosis of human hepatocellular carcinoma cell line HepG2 is involved in its anticancer activity in vitro. Eur J Cancer Prev. 2010;19:61–67. doi: 10.1097/CEJ.0b013e328333fb22. [DOI] [PubMed] [Google Scholar]
- 16.Alenzi FQ, Alenazi BQ, Al-Anazy FH, Mubaraki AM, Salem ML, Al-Jabri AA, Lotfy M, Bamaga MS, Alrabia MW, Wyse RK. The role of caspase activation and mitochondrial depolarisation in cultured human apoptotic eosinophils. Saudi J Biol Sci. 2010;17:29–36. doi: 10.1016/j.sjbs.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf BB, Schuler M, Echeverri F, Green DR. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem. 1999;274:30651–30656. doi: 10.1074/jbc.274.43.30651. [DOI] [PubMed] [Google Scholar]
- 18.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 19.Ling Y, Lu N, Gao Y, Chen Y, Wang S, Yang Y, Guo Q. Endostar induces apoptotic effects in HUVECs through activation of caspase-3 and decrease of Bcl-2. Anticancer Res. 2009;29:411–417. [PubMed] [Google Scholar]
- 20.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, −6, and −7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 21.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. Bcl-2, Bcl-X(L) sequester BH3 domain-only molecules preventing BAX and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/S1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 22.Degenhardt K, Chen G, Lindsten T, White E. BAX and BAK mediate p53 independent suppression of tumorigenesis. Cancer Cell. 2002;2:193–203. doi: 10.1016/S1535-6108(02)00126-5. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Shu Y, Zhang Q, Liu B, Xia J, Qiu M, Miao H, Li M, Zhu R. Dihydromyricetin induces apoptosis and inhibits proliferation in hepatocellular carcinoma cells. Oncol Lett. 2014;8:1645–1651. doi: 10.3892/ol.2014.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry H, Thomas A, Shen Y, White E. Regulation of the mitochondrial checkpoint in p53 mediated apoptosis confers resistance to cell death. Oncogene. 2002;21:748–760. doi: 10.1038/sj.onc.1205125. [DOI] [PubMed] [Google Scholar]