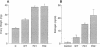Abstract
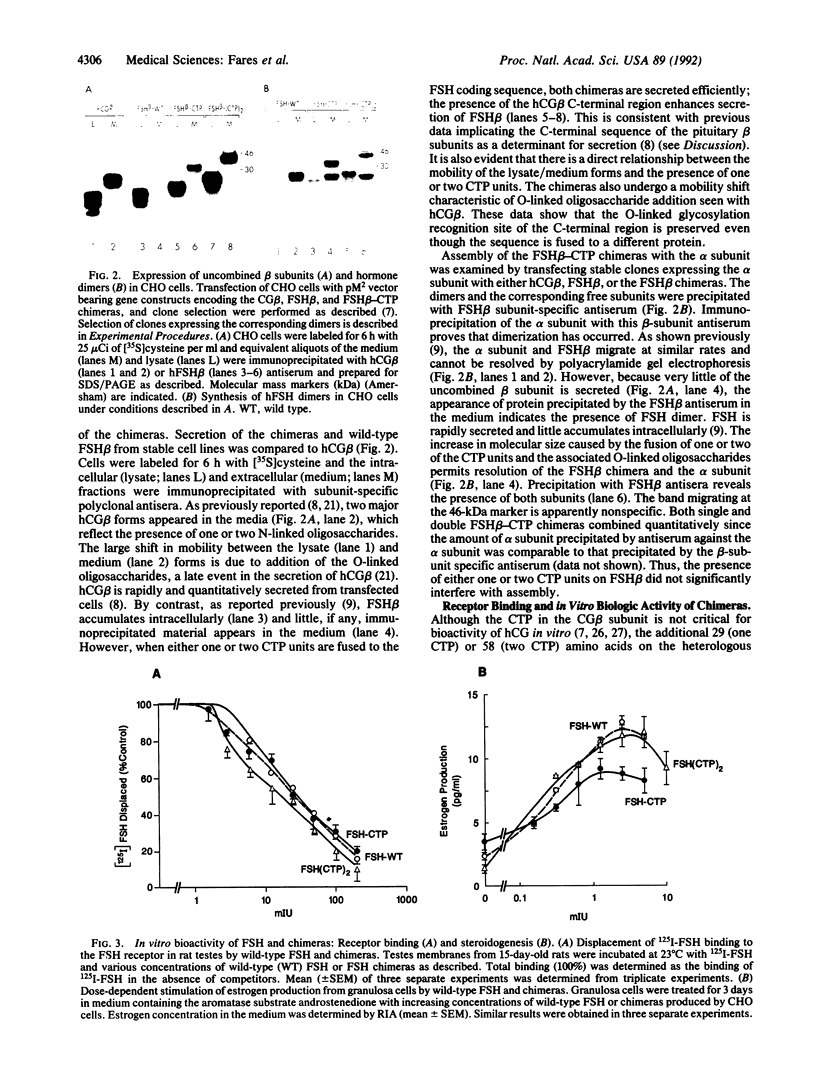
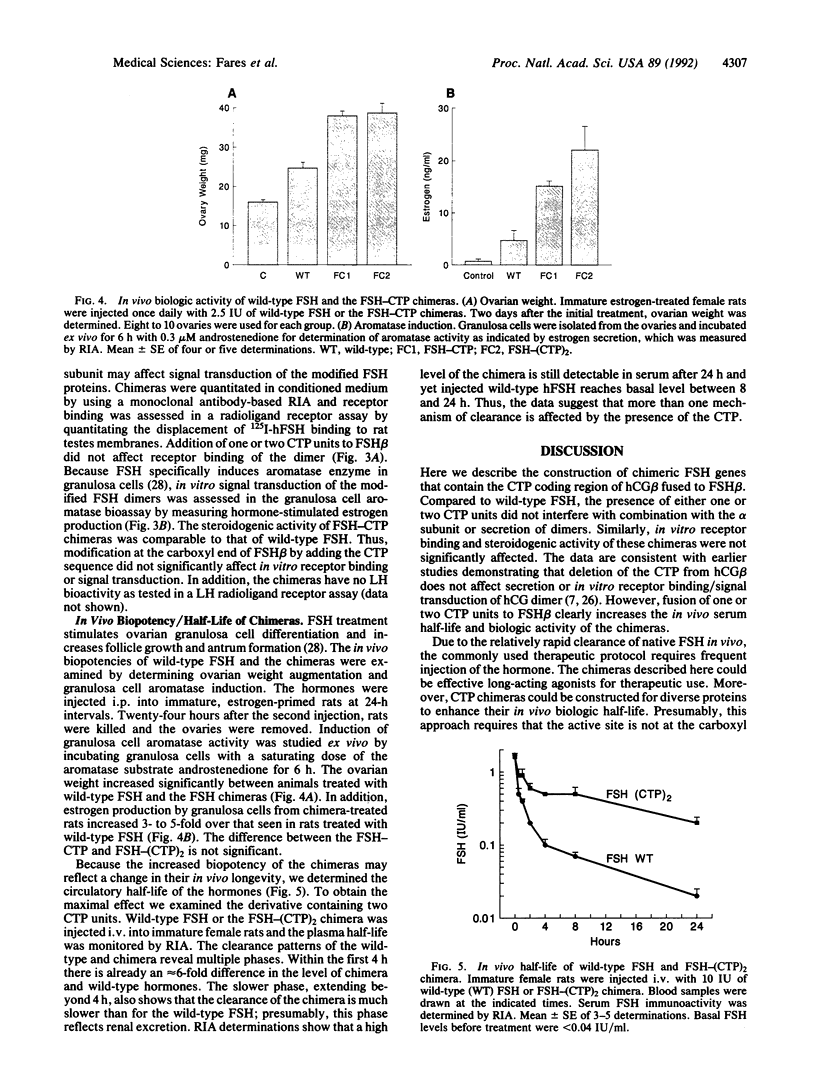
Follitropin (FSH) is a pituitary glycoprotein hormone that is essential for the development of ovarian follicles and testicular seminiferous tubules. FSH is used clinically to stimulate follicular maturation for in vitro fertilization and treatment of anovulatory women. One issue regarding the clinical use of FSH is its short half-life in the circulation. To address this point, we constructed chimeric genes containing the sequence encoding the C-terminal peptide of the chorionic gonadotropin beta subunit (CG beta) fused to the translated sequence of the human FSH beta subunit (FSH beta). This region of CG beta is important for maintaining the prolonged plasma half-life of human CG dimer. The presence of the C-terminal peptide sequence did not significantly affect assembly of FSH beta with the alpha subunit or secretion of the dimer. In vitro receptor binding and steroidogenic activity of dimer bearing the FSH beta-C-terminal peptide chimera were the same as wild-type FSH. However, both the in vivo potency and half-life in circulation of the dimer bearing either one or two C-terminal peptide units were enhanced. Dimers containing FSH beta-CG beta chimeras could serve as potent FSH agonists for clinical use, and the present strategy may have wide applications for enhancing the in vivo half-life of diverse proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y., Hsueh A. J. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulosa cells. J Biol Chem. 1982 Jun 10;257(11):6077–6083. [PubMed] [Google Scholar]
- Albert P. J., Schläfke J., Kaesemann H., Gille J. Pregnancy following induction of ovulation with pure FSH after suppression of endogenous gonadotropins with subcutaneous buserelin. Arch Gynecol Obstet. 1987;241(1):53–56. doi: 10.1007/BF00931442. [DOI] [PubMed] [Google Scholar]
- Amin H. K., Hunter W. M. Human pituitary follicle-stimulating hormone: distribution, plasma clearance and urinary excretion as determined by radioimmunoassay. J Endocrinol. 1970 Nov;48(3):307–317. doi: 10.1677/joe.0.0480307. [DOI] [PubMed] [Google Scholar]
- Amin H. K., Hunter W. M. Human pituitary follicle-stimulating hormone: distribution, plasma clearance and urinary excretion as determined by radioimmunoassay. J Endocrinol. 1970 Nov;48(3):307–317. doi: 10.1677/joe.0.0480307. [DOI] [PubMed] [Google Scholar]
- Birken S., Canfield R. E. Isolation and amino acid sequence of COOH-terminal fragments from the beta subunit of human choriogonadotropin. J Biol Chem. 1977 Aug 10;252(15):5386–5392. [PubMed] [Google Scholar]
- Birken S., Canfield R., Lauer R., Agosto G., Gabel M. Immunochemical determinants unique to human chorionic gonadotropin: importance of sialic acid for antisera generated to the human chorionic gonadotropin beta-subunit COOH-terminal peptide. Endocrinology. 1980 Jun;106(6):1659–1664. doi: 10.1210/endo-106-6-1659. [DOI] [PubMed] [Google Scholar]
- Bousfield G. R., Liu W. K., Ward D. N. Effects of removal of carboxy-terminal extension from equine luteinizing hormone (LH) beta-subunit on LH and follicle-stimulating hormone receptor-binding activities and LH steroidogenic activity in rat testicular Leydig cells. Endocrinology. 1989 Jan;124(1):379–387. doi: 10.1210/endo-124-1-379. [DOI] [PubMed] [Google Scholar]
- Campbell R. K., Dean-Emig D. M., Moyle W. R. Conversion of human choriogonadotropin into a follitropin by protein engineering. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):760–764. doi: 10.1073/pnas.88.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Wang Y., Puett D. Role of the invariant aspartic acid 99 of human choriogonadotropin beta in receptor binding and biological activity. J Biol Chem. 1991 Oct 15;266(29):19357–19361. [PubMed] [Google Scholar]
- Corless C. L., Matzuk M. M., Ramabhadran T. V., Krichevsky A., Boime I. Gonadotropin beta subunits determine the rate of assembly and the oligosaccharide processing of hormone dimer in transfected cells. J Cell Biol. 1987 May;104(5):1173–1181. doi: 10.1083/jcb.104.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh A. J., Bicsak T. A., Jia X. C., Dahl K. D., Fauser B. C., Galway A. B., Czekala N., Pavlou S. N., Papkoff H., Keene J. Granulosa cells as hormone targets: the role of biologically active follicle-stimulating hormone in reproduction. Recent Prog Horm Res. 1989;45:209–277. doi: 10.1016/b978-0-12-571145-6.50009-1. [DOI] [PubMed] [Google Scholar]
- Jia X. C., Hsueh A. J. Granulosa cell aromatase bioassay for follicle-stimulating hormone: validation and application of the method. Endocrinology. 1986 Oct;119(4):1570–1577. doi: 10.1210/endo-119-4-1570. [DOI] [PubMed] [Google Scholar]
- Jones G. S., Acosta A. A., Garcia J. E., Bernardus R. E., Rosenwaks Z. The effect of follicle-stimulating hormone without additional luteinizing hormone on follicular stimulation and oocyte development in normal ovulatory women. Fertil Steril. 1985 May;43(5):696–702. doi: 10.1016/s0015-0282(16)48550-x. [DOI] [PubMed] [Google Scholar]
- Kaetzel D. M., Virgin J. B., Clay C. M., Nilson J. H. Disruption of N-linked glycosylation of bovine luteinizing hormone beta-subunit by site-directed mutagenesis dramatically increases its intracellular stability but does not affect biological activity of the secreted heterodimer. Mol Endocrinol. 1989 Nov;3(11):1765–1774. doi: 10.1210/mend-3-11-1765. [DOI] [PubMed] [Google Scholar]
- Kalyan N. K., Bahl O. P. Role of carbohydrate in human chorionic gonadotropin. Effect of deglycosylation on the subunit interaction and on its in vitro and in vivo biological properties. J Biol Chem. 1983 Jan 10;258(1):67–74. [PubMed] [Google Scholar]
- Keene J. L., Matzuk M. M., Otani T., Fauser B. C., Galway A. B., Hsueh A. J., Boime I. Expression of biologically active human follitropin in Chinese hamster ovary cells. J Biol Chem. 1989 Mar 25;264(9):4769–4775. [PubMed] [Google Scholar]
- Kessel B., Liu Y. X., Jia X. C., Hsueh A. J. Autocrine role of estrogens in the augmentation of luteinizing hormone receptor formation in cultured rat granulosa cells. Biol Reprod. 1985 Jun;32(5):1038–1050. doi: 10.1095/biolreprod32.5.1038. [DOI] [PubMed] [Google Scholar]
- Kessler M. J., Mise T., Ghai R. D., Bahl O. P. Structure and location of the O-glycosidic carbohydrate units of human chorionic gonadotropin. J Biol Chem. 1979 Aug 25;254(16):7909–7914. [PubMed] [Google Scholar]
- Keutmann H. T., Williams R. M. Human chorionic gonadotropin. Amino acid sequence of the hormone-specific COOH-terminal region. J Biol Chem. 1977 Aug 10;252(15):5393–5397. [PubMed] [Google Scholar]
- Matzuk M. M., Hsueh A. J., Lapolt P., Tsafriri A., Keene J. L., Boime I. The biological role of the carboxyl-terminal extension of human chorionic gonadotropin [corrected] beta-subunit. Endocrinology. 1990 Jan;126(1):376–383. doi: 10.1210/endo-126-1-376. [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Krieger M., Corless C. L., Boime I. Effects of preventing O-glycosylation on the secretion of human chorionic gonadotropin in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6354–6358. doi: 10.1073/pnas.84.18.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Spangler M. M., Camel M., Suganuma N., Boime I. Mutagenesis and chimeric genes define determinants in the beta subunits of human chorionic gonadotropin and lutropin for secretion and assembly. J Cell Biol. 1989 Oct;109(4 Pt 1):1429–1438. doi: 10.1083/jcb.109.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Sowers J. R., Pekary A. E., Hershman J. M., Kanter M., DiStefano J. J., 3rd Metabolism of exogenous human chorionic gonadotrophin in men. J Endocrinol. 1979 Jan;80(1):83–89. doi: 10.1677/joe.0.0800083. [DOI] [PubMed] [Google Scholar]
- Sowers J. R., Pekary A. E., Hershman J. M., Kanter M., DiStefano J. J., 3rd Metabolism of exogenous human chorionic gonadotrophin in men. J Endocrinol. 1979 Jan;80(1):83–89. doi: 10.1677/joe.0.0800083. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Vamvakopoulos N. C., Fiddes J. C. Evolution of the genes for the beta subunits of human chorionic gonadotropin and luteinizing hormone. Nature. 1984 Jan 5;307(5946):37–40. doi: 10.1038/307037a0. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Wide L. The regulation of metabolic clearance rate of human FSH in mice by variation of the molecular structure of the hormone. Acta Endocrinol (Copenh) 1986 Jul;112(3):336–344. doi: 10.1530/acta.0.1120336. [DOI] [PubMed] [Google Scholar]
- el-Deiry S., Kaetzel D., Kennedy G., Nilson J., Puett D. Site-directed mutagenesis of the human chorionic gonadotropin beta-subunit: bioactivity of a heterologous hormone, bovine alpha-human des-(122-145)beta. Mol Endocrinol. 1989 Oct;3(10):1523–1528. doi: 10.1210/mend-3-10-1523. [DOI] [PubMed] [Google Scholar]