Abstract
Treatment for cystic fibrosis (CF) has conventionally targeted downstream consequences of the defect such as mucus plugging and infection. More recently, significant advances have been made in treating the root cause of the disease, namely a defective CF transmembrane conductance regulator (CFTR) gene. This review summarizes current pulmonary treatment options and highlights advances in research and development of new therapies.
Keywords: CF transmembrane conductance regulator gene, cystic fibrosis, treatment
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. The most common globally is F508del, however there are over 2000 variations reported, although not all cause disease. The subsequent CFTR protein defect causes abnormalities in both salt and fluid transport across epithelia, which in the lung, leads to dehydration of the airway surface and impaired mucociliary clearance (MCC) [Matsui et al. 1998]. This failure of innate defence and mucus accumulation, possibly in conjunction with impaired bacterial killing, provides an undefended environment for opportunistic pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus and Haemophilus influenzae. The host inflammatory response and subsequent tissue damage contribute to the characteristic decline in lung function as disease progresses [Rowe et al. 2005].
Conventional pulmonary treatment of CF has targeted the downstream consequences of the disease, namely mucus plugging and infection. The last few years have seen the emergence of more upstream therapeutic targets, several of which are in clinical trials, or have progressed to licensed treatments. Here, we discuss pulmonary treatment options based on their mode of action, presenting updated information on those in current clinical use, and highlighting progress in research. Treatments for other organs involved in CF (for example, the pancreas, liver, bones and sinuses) are outside the scope of this article but can be found in the work of Plant and colleagues [Plant et al. 2013]. The importance of optimal nutrition in respiratory health should not be underestimated; evidence in support of this and a review of the field can be found in several articles [Sanders et al. 2015; Munck, 2010]. Ultimately, when medical treatment fails, lung transplantation is the only option; this is a rapidly expanding field, outside the scope of this article, but well reviewed by Lynch and colleagues [Lynch et al. 2015]. The treatment modalities discussed in detail in this article are summarized in Table 1 below.
Table 1.
Summary table of treatment modalities in clinical use and clinical trials.
| Category | Approaches | Current status |
|---|---|---|
| Mucolytic agents | Dornase alfa | Role established in both adults and children |
| N-acetyl-L-cysteine | No consensus of clinical utility | |
| Airway surface rehydration | Hypertonic saline | Role established in adults, less marked results in children with ongoing studies |
| Osmotic agents, mannitol | Adult phase III trials showed meaningful benefit. Phase II trials ongoing in children | |
| Correction of ion transport | P2Y2 receptor agonist, denufosol, failed to show replicable results in phase III trials | |
| ENaC blocker | P-1037 is currently in phase II clinical trials | |
| Anti-infective agents | Prophylaxis | UK guidance for flucloxacillin, USA advises no prophylaxis, suggesting the need for further studies |
| Eradication | Inhaled +/− systemic antibiotics, ongoing large European study comparing IV versus oral antibiotics | |
| Suppression | Nebulized antibiotics including tobramycin, colistin and aztreonam Amikacin currently under investigation |
|
| Acute exacerbations | Oral or IV antibiotics | |
| Other bacterial organisms | AeroVanc in phase II trials for MRSA eradication AZLI recently shown in phase III trials to have no significant improvement in Burkholderia infections |
|
| Other organisms | Current multicentre randomized double-blind control trial investigating Arikace in NTM. ABPA, oral antifungal agents and more recently omalizumab |
|
| Nonantibiotic approaches | Current phase II trials of OligoG to treat chronic pseudomonas infection Multicentre trial ongoing exploring the benefit of IgY antibodies in pseudomonal infections. |
|
| Inflammation | Anti-inflammatory agents | Ibuprofen has been shown to have some benefit in young patients with mild disease in high doses. |
| Inhaled corticosteroids | Inhaled corticosteroids have been shown to have little benefit | |
| LTB4 receptor antagonists | LTB4 phase II trials halted due to significant side effects | |
| Azithromycin | Possible modulation of the inflammatory system. | |
| Antioxidants | No clear benefit but warrants further work | |
| CFTR protein defect | Potentiators | Ivacaftor in clinical use in patients over 6 years of age in Europe (with Asp551Gly mutation) and awaiting regulatory approval in the 2–5 year age bracket |
| Correctors and combination therapy | Lumacaftor/ivacaftor treatment of patients with F508del mutations has finished phase III trials VX-661 is in phase III trials in combination with ivacaftor |
|
| Read-through agents | Ataluren is currently in phase III trials | |
| CFTR gene mutation | Gene therapy (nonviral and viral vectors) | Liposomal CFTR gene therapy has completed phase IIb clinical trials Preclinical work is ongoing on a pseudotyped lentivirus. QR010 is currently in phase Ib trials |
ABPA, allergic bronchopulmonary aspergillosis; AZLI, aztreonam for inhalation solution; CFTR, cystic fibrosis transmembrane conductance regulator; ENaC, epithelial sodium channel; Ig, immunoglobulin; IV, intravenous; LTB4, leukotriene B4; MRSA, methicillin-resistant S. aureus; NTM, nontuberculous mycobacteria.
Mucolytic agents
Dornase alfa
Dornase alfa is a recombinant human deoxyribonuclease (DNase). As part of the inflammatory process in CF there is a significant accumulation and breakdown of neutrophils in the lungs leading to large amounts of extracellular DNA, which significantly increases the viscosity of the sputum. DNase has been show to cleave the extracellular DNA and subsequently aid airway clearance [Konstan and Ratjen, 2012]. Although UK clinical practice historically tended to lean towards reserving DNase for more severe patients, evidence is mounting for earlier intervention. Amin and colleagues showed a significant improvement in lung clearance index (LCI, a sensitive measure of gas mixing inhomogeneity) in children aged between 6–18 years of age who had normal baseline spirometry [Amin et al. 2011]. It has also been shown in younger children to indirectly affect their nutritional status with a 10 percentile increase in body mass index (BMI) in children who commenced DNase at <2 years of age [Konstan and Ratjen, 2012]. The Bronchoalveolar Lavage (BAL) in the Evaluation of Anti-inflammatory Treatment (BEAT) study compared changes in neutrophilic inflammation over time in BAL fluid from patients treated with DNase and controls [Paul et al. 2004]. DNase led to a significant reduction in inflammation and in DNA concentrations, suggesting that treatment should be commenced earlier in the disease course rather than when lung function has already deteriorated.
N-acetyl-L-cysteine
N-acetyl-L-cysteine (NAC) has previously been shown to be mucolytic in CF and may also increase levels of the intracellular antioxidant glutathione (GSH), thereby protecting against the neutrophil-driven tissue damage in the lungs. Clinical trials have however resulted in somewhat conflicting results, [Conrad et al. 2015; Dauletbaev et al. 2009] so consensus is lacking on the clinical utility of NAC for CF.
Airway clearance
Physiotherapy and exercise
Physiotherapy to aid clearance of airway secretions has been one of the mainstays of treatment for CF and, in our opinion, is likely to be responsible for much of the improved prognosis over the last few decades. However, multiple different techniques are used, and the optimal approach is not clear. A recent multicentre randomized controlled study found no evidence in support of high-frequency chest wall oscillating devices (vests) when compared to positive expiratory pressure (the most commonly used method of airway clearance) and actually found a higher level of pulmonary exacerbations (PEs) requiring antibiotics associated with their use [McIlwaine et al. 2013]. Multiple interventions to promote health and exercise have been trialled although there is no unanimous verdict over which technique has been the most successful [Cox et al. 2013]. A current trial is examining the effect of a 12-month partially supervised exercise intervention with regular motivation on forced expiratory volume in 1 second (FEV1) with secondary end points including levels of depression, anxiety, quality of life and blood sugar levels [ClinicalTrials.gov identifier: NCT01744561].
Airway surface rehydration strategies
Hypertonic saline
Hypertonic saline (HS) aids MCC by increasing hydration of the airway surface in the short term [Donaldson et al. 2006]. As it may cause bronchoconstriction, it is commonly used with an associated bronchodilator. When compared with a placebo, HS was safe, inexpensive and effective in reducing the number of PEs requiring intravenous (IV) antibiotics. However, these were secondary outcome measures and the primary measure of improvements in FEV1 was not achieved [Elkins et al. 2006]. Effects in children were less marked, possibly relating to the preponderance of viral exacerbations in this age group [Rosenfeld et al. 2012]. However, in this age group, a beneficial effect could be detected on LCI, and HS is currently being studied in more detail using such sensitive physiology and computerized tomography (CT) scans in this age group [ClinicalTrials.gov identifier: NCT02378467]. A small number of studies [Buonpensiero et al. 2010; Furnari et al. 2012; Ros et al. 2014] have concluded that the addition of low concentration hyaluronic acid to HS improves tolerability and acceptability to patients, although the long term outcomes of this combination have not been explored.
Mannitol
Mannitol is a nonabsorbable sugar alcohol which provides an osmotic gradient on the airway surface leading to rehydration, and an increase in volume surface liquid, which aids in the clearance of mucus. An international phase III trial showed a sustained and clinically meaningful benefit (increase in FEV1 and a decrease in the number of PEs) even with concomitant DNase use in patients 18 years of age and above. However it also showed an increased number of adverse events such as haemoptysis and cough. Current evidence suggests mannitol is safe to use in patients who are able to tolerate it [Bilton et al. 2011]. Two large-scale randomized controlled trials showed a mean improvement in FEV1 and a decrease in PEs [Aitken et al. 2012; Bilton et al. 2013]. Both studies showed a slightly high percentage of adverse events including cough and haemoptysis in the actively treated group. The benefits in children have not yet been clearly shown although the results of a recent phase II trial are eagerly awaited.
Denufosol
Denufosol tetrasodium, a P2Y2 purinergic receptor agonist, stimulates chloride secretion and ciliary beat frequency independent of CFTR. Phase III study results were initially promising, showing a small but statistically significant increase in lung function compared with the placebo [Accurso et al. 2011]. However these findings were not replicated in a subsequent study [Ratjen et al. 2012] and there are no current clinical studies of this agent.
P-1037
In addition to its function as a chloride ion channel, CFTR interacts with a number of neighbouring proteins, the best recognized being the epithelial sodium channel, ENaC. The loss of normal inhibitory function is thought to lead to ENaC over-activity and sodium hyperabsorption which contributes to airway surface and mucus dehydration. Blocking of ENaC with amiloride led to disappointing clinical results, likely to have been related to a very short half-life on the airway surface [Pons et al. 2000; Graham et al. 1993]. More recently, Parion has developed P-1037, an inhaled ENaC blocker which is currently progressing to phase II clinical trials [ClinicalTrials.gov identifier: NCT02343445]. One potential drawback of this approach generally is hyperkalaemia as a side effect of renal exposure, so low dose or poorly absorbed formulations are required.
Anti-infective agents
Conventional antibacterial agents
Antibiotics are used in four different contexts in CF: prophylaxis, eradication of early infection, ‘suppression’ of chronic infection and in the treatment of exacerbations. The pathogens found in CF lungs vary with age. In infancy the most common bacteria cultured is S. aureus, with H. influenzae increasing during childhood; by adolescence and young adulthood by far the commonest pathogen cultured is P. aeruginosa (see www.cysticfibrosis.org.uk/media/82010/CD_Antibiotic_treatment_for_CF_May_09.pdf). However, the advent of culture-independent molecular tools to identify bacterial species, has revealed the polymicrobial nature of the CF lower airway. The relevance of the microbiome to health and disease progression is currently the focus of much research [Rogers et al. 2015].
Prophylaxis
Current guidelines in the UK and much of Europe recommend the use of anti-staphylococcal antibiotics (such as flucloxacillin) from the point of diagnosis until ~3 years of age. This regimen has been shown to reduce the incidence of methicillin-susceptible S. aureus (MSSA) although improvement in clinical outcomes has not been confirmed [Mogayzal et al. 2013] https://www.cysticfibrosis.org.uk/the-work-we-do/clinical-care/consensus-documents (Antibiotic Treatment for Cystic Fibrosis. Third edition. May 2009).
However current recommendations in the USA are against the use of prophylactic anti-staphylococcal antibiotics [Mogayzal et al. 2013], in part based on one trial of a cephalosporin reporting an increased rate of pseudomonas infection [Stutman et al. 2002]. Good quality clinical trial data are needed to resolve this issue.
Eradication of early infection
Whilst clinicians will often treat an early bacterial infection with a view to both managing symptoms and decreasing the likelihood of chronic infection, the organism for which evidence supports the latter most strongly is P. aeruginosa [Langton Hewer and Smyth, 2014]. If not detected and treated aggressively, this gram-negative, opportunistic bacterium will become chronic; the resulting inflammatory response is closely linked to decline in lung function. Eradication strategies vary between countries and even between sites but comprise inhaled +/− systemic antibiotics. In North America, inhaled tobramycin is the first line treatment [Mogayzel et al. 2014], whereas in Europe, a multicentre trial is currently assessing whether IV or oral antibiotics are superior, when administered with nebulized colistin (see http://www.torpedo-cf.org.uk/).
Suppression of chronic infection
Once bacterial infection has become chronic, emphasis switches from eradication to chronic suppression in the hope of reducing the inflammatory response. Systemically-delivered antibiotics carry with them the potential for side effects (such as renal or hearing impairment with aminoglycosides) and may lead to suboptimal sputum concentrations [Waters et al. 2015]. Therefore much research has focused on inhalation as a route of antimicrobial delivery, which by delivering of the drug directly to the site of infection and in high concentrations, can enhance bacterial killing whilst limiting side effects [Hewer, 2012]. Disadvantages include, in some cases, an unpleasant taste, the potential for bronchospasm, particularly with the dry powder formulations, and the time required both to administer the drug and care for the equipment, which may impact adherence. The most commonly used nebulized antibiotics against P. aeruginosa are tobramycin, colistin and more recently, aztreonam; in many cases, a cycling approach is used, administering on a month-on, month-off basis or alternating drugs.
In an attempt to reduce the time needed to deliver the drug to the airway and the preparation and cleaning required, dry powder formulations have been developed for both colistin and tobramycin. Colistin [Schuster et al. 2013] and tobramycin [Konstan et al. 2011] were delivered using a dry powder inhaler (DPI) and short term results showed they were noninferior to the nebulized versions of tobramycin. There was no evidence of increased adherence, although this is a difficult outcome to measure. It appeared there was an increase in the reporting of cough in the DPI group [Uttley et al. 2013]. Further research needs to be carried out looking at the long term outcomes of using a DPI rather than the more traditional nebulized medicines [Tappenden et al. 2013]. However, Harrison and colleagues did show improved adherence to inhaled tobramycin when compared with the nebulized version in adults with CF [Harrison et al. 2014].
In an open-label 18-month safety and efficacy trial, patients with chronic P. aeruginosa were treated with aztreonam for inhalation solution (AZLI). On alternating monthly treatments a dose response improvement in FEV1 and reduction in bacterial burden was seen. Interestingly, significant weight gain was also noted and sustained during the 18 months. The best results were achieved using a three-times-daily regimen which may be challenging for patients [Oermann et al. 2010]. A modest improvement in FEV1 and reduction in bacterial burden was also noted in patients who only had mild lung impairment, possibly suggesting a role of earlier intervention and treatment in relatively well patients [Wainwright et al. 2011]. A recent open-label, parallel group international trial compared the use of tobramycin (nebulized) with AZLI. The results showed a significant improvement in lung function in the AZLI group when compared with the tobramycin group and a reduction in PEs over three treatment courses. AZLI was well tolerated and showed an equal decrease in P. aeruginosa density when compared to tobramycin. It should be noted that only a small number of their study group were between 6–11 years of age, therefore care needs to be taken extrapolating their results to children with chronic P. aeruginosa infection [Assael et al. 2013].
Several newer agents are currently under investigation. A liposomal formulation of amikacin is being trialled as a new once-a-day alternative. An attractive feature of the liposome is its breakdown by bacterial rhamnolipids, effectively meaning the drug becomes activated at the site of need. In both phase II and III trials similar increases in FEV1 were seen as for tobramycin [Clancy et al. 2013; Ehsan et al. 2014]. Levofloxacin inhalation solution (MP-376) has recently been shown to be well tolerated and noninferior to tobramycin [Stuart Elborn et al. 2015]. Finally, a double-blind, placebo-controlled multicentre trial compared a combination antibiotic fosfomycin/tobramycin with a placebo after a 28-day open-label run-in course of aztreonam and showed maintenance of the substantial improvement in FEV1 in patients after the aztreonam course [Trapnell et al. 2012]. This suggests that continuous antibiotic treatment using alternating antibiotics may be an appealing future to the treatment of P. aeruginosa infection, although debate remains regarding which combination of antibiotics that should include.
Acute exacerbations
PEs are constellations of symptoms and worsening lung function; their mechanism is poorly understood. In childhood, it is common for viruses to be detected in airway secretions at the time of PE and on occasions, a new bacterial organism might be isolated, but much more commonly, neither the type of organism isolated nor its number appear to have changed significantly. This raises the possibility that it is the bacterial behaviour, for example the production of virulence factors, or the host response which has changed. PEs are treated with oral or IV antibiotics depending on severity. Traditionally patients receive a 14-day course of IV antibiotics; however one study has shown that after 10 days of IV treatment, maximal lung function is achieved and no further benefit is obtained thereafter [Waters and Ratjen, 2014]. This is the focus of a large, multicentre research programme led by the CF Foundation in the USA [ClinicalTrials.gov identifier: NCT02109822].
Other organisms
Methicillin-resistant S. aureus (MRSA) is on the increase in patients with CF, being isolated in up to 25% of patients in the USA. MRSA has been associated with reduced lung function and higher mortality. A recent study into the Staphylococcal Cassette Chromosome mec (SCCmec) types in children with CF showed that the SCCmec type isolated in the sputum correlated with exacerbation rates and the number of courses of antibiotics received by each patient. Those chronically infected with SCCmec II MRSA isolates had higher exacerbation rates. The authors suggest that this raises the question about whether we should routinely type isolated MRSA to allow for direction of the eradication technique [Heltshe et al. 2015]. STAR-TOO is a current, randomized, controlled, intervention study comparing an early eradication protocol for MRSA with the current standard of only treating PEs [ClinicalTrials.gov identifier: NCT01349192]. The use of AeroVanc, Savara USA to reduce MRSA colony-forming units is also being examined in a phase II randomized, double-blind, placebo-controlled trial [ClinicalTrials.gov identifier: NTC01746095]. Burkholderia cepacia is associated with higher rates of morbidity and mortality and patients with known B. cepacia infections are routinely excluded from clinical trials looking at efficacy of inhaled antibiotics. A recent phase III, double-blind, placebo-controlled, two-part multisite study compared AZLI with a placebo and concluded there was no significant improvement in lung function. The authors did postulate that nonstudy antibiotic use may have confounded their results [Tullis et al. 2014].
Other challenging infections
Nontuberculous mycobacteria
Nontuberculous mycobacteria (NTM) are commonly found in the environment and frequently cultured from the respiratory tract of CF patients. Some of them, most notably, Mycobacterium abscessus, carry a poorer prognosis (Park and Olivier, 2015) and concerns have been raised recently about patient-to-patient spread (Bryant et al. 2013). A recent Cochrane review reported a lack of randomized controlled trials addressing the treatment of NTM pulmonary infections [Waters and Ratjen, 2014]. Patients tend to be treated by local or national protocols of multiple agents for long periods of time. There is a current multicentre randomized double-blind control trial investigating Arikace, INSMED USA in the treatment of NTM [Olivier et al. 2014].
Fungal disease and allergic bronchopulmonary aspergillosis
Infection can occur with a number of fungal organisms, most commonly, Aspergillus fumigatus (Af). This can lead to chronic infection or an allergic response – allergic bronchopulmonary aspergillosis (ABPA) – manifested by wheezing, infiltrates on chest radiography, eosinophilia and raised total and specific immunoglobulin (Ig)E. Recently, a new classification system has been proposed: nondiseased group, serologic ABPA, Aspergillus-sensitized and Aspergillus bronchitis, based on the integration of two new markers (galactomannan and real-time PCR) to standard serological and culture-based tests [Baxter et al. 2013].
Antifungal agents include itraconazole, voriconazole, posaconazole, ketoconazole, nystatin and amphotericin b. In a systematic review, oral azoles were associated with improvements in symptoms and a decrease in the frequency of exacerbations, however adverse effects were also common [Moreira et al. 2014]. Optimal treatment of fungal bronchitis is unclear, and systemic antifungals of the azole class have significant side effects including hepatotoxicity and photosensitive skin reactions [Sheu et al. 2015]. A recent Cochrane review highlighted the lack of evidence for treatments of ABPA [Elphick and Southern, 2014]; therapy is usually based around systemic corticosteroids in combination with antifungal agents; the former may be administered as pulses of IV methylprednisolone, which appears to confer fewer side effects [Cohen-Cymberknoh et al. 2009].
More recently, since the recombinant anti-IgE monoclonal antibody, omalizumab, has become established for the treatment of severe asthma [Lehmann et al. 2014], there have been several reported case studies in CF patients, some reporting success, although there is a lack of good quality clinical trial data [Lehmann et al. 2014; Tanou et al. 2014; Zicari et al. 2014].
Nonantibiotic approaches to bacterial infection
OligoG
Chronic P. aeruginosa grows in the CF airways in a biofilm. This consists of the bacterium itself embedded in a complex matrix of neutrophil DNA, exopolysaccharide and airway mucins, which makes it highly resistant to antibiotic therapy. OligoG, a dry powder formulation of seaweed-derived alginate oligosaccharide, appears to possess both antibiofilm and mucolytic properties and is currently in phase II trials [ClinicalTrials.gov identifier: NCT02157922].
IgY antibodies
A small clinical trial has previously suggested that IgY derived from immunized hen eggs could offer protection from P. aeruginosa infection [Kollberg et al. 2003]. A multicentre trial is currently exploring the benefits of this antibody administered as a gargle solution [ClinicalTrials.gov identifier: NCT01455675].
Anti-inflammatory agents and antioxidants
As mentioned earlier, inflammation plays a significant role in the progression of CF-related lung disease.
Early trials of systemic corticosteroids showed some benefit, but at the expense of significant side effects [Auerbach et al. 1985].
Nonsteroidal anti-inflammatory agents
This led to trials of the nonsteroidal anti-inflammatory agent, ibuprofen, which showed some benefit particularly in younger patients with milder disease [Konstan et al. 1995]. There are issues however related to dosing. Low dose ibuprofen has been shown to be proinflammatory and high dose has associated side effects [Lands and Stanojevic, 2013] although the study by Lahiri and colleagues [Lahiri et al. 2014] showed no significant correlation between high dose ibuprofen and biomarkers of kidney injury. A recent Cochrane review concluded that high dose ibuprofen slowed the decline in lung function and decreased the number of days spent in hospital. However, long-term side effects have not been examined and care should be taken when treating with IV aminoglycosides and concomitant gastric cover should be prescribed [Lands and Stanojevic, 2013]. These drugs are not in widespread use in the majority of European countries.
Inhaled corticosteroids
A topical, rather than systemic, approach could reduce side effects, whilst directly targeting the organ of interest. However, in a multicentre randomized double-blind control withdrawal trial, inhaled corticosteroids were shown to have little effect [Balfour-Lynn et al. 2006].
Leukotriene B4 receptor antagonists
Leukotriene B4 (LTB4) is produced by both macrophages and polymorphonuclear neutrophils (PMNs) in response to infection and plays a significant role in the CF inflammatory response. It was therefore postulated that a LTB4 receptor antagonist could be a beneficial treatment. However, a phase II clinical trial was halted prematurely based on a significant increase in side effects including PEs in the actively treated group [Konstan et al. 2014]. This highlights the potential protective effects of inflammation, perhaps by localizing infection in the lungs, and suggests that for an anti-inflammatory agent to be well tolerated and effective, a balance needs to be achievable. There are currently a small number of clinical trials exploring anti-inflammatory agents in the CF Foundation Therapeutic Development Network drug pipeline (https://tools.cff.org/research/drugdevelopmentpipeline/).
Azithromycin
The macrolide antibiotic, azithromycin has shown benefits in CF patients with and without chronic P. aeruginosa [Southern et al. 2012]. Its mechanism is incompletely understood, although it appears to possess antibiofilm properties and may also be modulating the inflammatory system. It is used widely in patients, although recent data describing apparent antagonism with the nebulized aminoglycoside, tobramycin, have raised concern [Nick et al. 2014] and require further study. There have also been some concerns raised about the emergence of NTM, although reports are inconsistent [Renna et al. 2011; Coolen et al. 2015].
Antioxidants
Given the high levels of oxidative stress markers in the CF airway, treatment with antioxidants is a rational avenue to explore. Several, mostly small trials have been undertaken with oral or inhaled antioxidants, summarized in a recent Cochrane review [Ciofu and Lykkesfeldt, 2014]. Although some studies reported benefits, including improvements in lung function, there was no clear pattern and the authors considered that further work was needed before a conclusion on efficacy can be reached.
Therapies targeting the basic defect
CFTR modulators
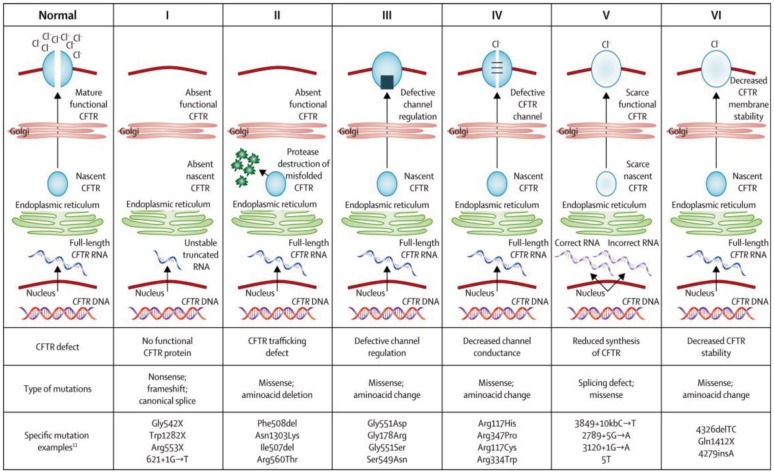
There are six main classes of CFTR mutations based on their consequences on function (Figure 1). Class I mutations cause a total or partial lack of production of a functional CFTR, most commonly as a result of a premature termination codon (PTC). Class II mutations lead to misfolding of the protein and failure of trafficking to the cell surface. Class III are mainly gating mutations, which fail to open in response to intracellular signals and Class IV mutations demonstrate reduced ion conductance. Class V are splicing mutations resulting in reduced amounts of CFTR protein. Also described are Class VI mutations which possess a shortened half-life. These are summarized in Figure 1. Novel therapies are being developed which target these various classes of mutation [Sawczak et al. 2015].
Figure 1.
Classification of CF mutations based on CFTR structure and function [reproduced with permission from Boyle and De Boeck, 2013].
Current research and treatment is focussed on three groups of drugs: potentiators, correctors or read-through agents. Potentiators enhance the activity of the CFTR channel if it is correctly located. Correctors aim to correct defects such as protein misfolding in F508del allowing trafficking to the cell surface. Read-through agents allow the ribosome to ‘ignore’ a premature termination codon and produce a full length protein. Over the last few years, drugs in all of these groups have progressed into, and in some cases through, clinical trials.
Potentiators
The most significant advance in the treatment of CF over the last few years has been the development of ivacaftor (Kalydeco, Vertex USA). Trials have confirmed efficacy in Asp551Gly (G551D) the commonest mutation in this class [Ramsey et al. 2011; Davies et al. 2013] and also more recently in rarer gating mutations [De Boeck et al. 2014]. Significant improvements in FEV1 (~10% absolute improvement), exacerbation rate, weight and health-related quality of life led to ivacaftor being licensed for use in these patients aged 6 years and above. A clinical trial has been conducted in children aged 2–5 years in which the drug was found to be safe and led to similar improvements in the CFTR biomarker, sweat chloride; regulatory approval has been granted by the US Food and Drug Administration and is currently being sought in Europe. A trial in patients with the class IV conductance defect, arg 117His (R117H) failed to confirm efficacy overall but did show an effect in adult patients [Moss et al. 2015]; the drug is currently approved for patients with this mutation in the US.
Correctors and combination therapy
F508del is the commonest CF mutation globally. Lumacaftor (VX-809) restored CFTR function to around 15% of wild-type CFTR levels in vitro, but did not lead to significant clinical changes in F508del patients [Clancy et al. 2012]. Similarly, single-agent ivacaftor had shown little effect in this patient group, likely due to insufficient CFTR available at the cell surface for potentiation [Flume et al. 2012]. Therefore the benefit of combined lumicaftor/ivacaftor treatment has been investigated. The phase III TRAFFIC and TRANSPORT trials showed significant improvements in FEV1, although this was of a lower magnitude (3–4%) than seen in class III patients with ivacaftor [Wainwright et al. 2015] and there was a substantial decrease in the number of PEs, in particular those leading to the requirement of IV antibiotics. Some recent work has demonstrated an adverse impact of ivacaftor on the stability of corrected CFTR [Cholon et al. 2014] which may explain the limited efficacy. The newer corrector, VX-661 is currently undergoing large, phase III clinical trial testing in combination with ivacaftor in patients with a range of mutations [ClinicalTrials.gov identifiers: NCT02565914, NCT02392234, NCT02516410], and several other pharmaceutical companies are also active in this space, providing encouragement that wider coverage may be available in the near future. There is however, a significant issue of cost; whilst insurance-based health care systems may be able to support the relatively small number of patients with CF suitable for these drugs, this is likely to pose a significant problem for nationalized health care systems. Strategies to tackle this, and the inevitable global inequalities which will arise, are urgently needed.
Read-through agents
Ataluren promotes ribosomal read-through of premature termination codons resulting in the production of a full length CFTR. No statistically significant difference was seen in the ataluren treatment group when compared to the placebo group in a large phase III trial, although intriguingly, significant benefits were seen in patients not receiving inhaled aminoglycoside antibiotics; these drugs also act on the ribosome, so inhibition is highly plausible [Kerem et al. 2014]. A second phase III trial is currently underway in patients 6 years and older not receiving these drugs [ClinicalTrials.gov identifier: NCT02139306]. Read-through agents also have therapeutic potential for a range of other inherited diseases caused by stop mutations; conditional approval has recently been granted for certain types of Duchenne muscular dystrophy.
CFTR gene therapy
Since the CFTR gene was first discovered, over 20 clinical trials in gene therapy have been conducted but these have largely been single dose and focussed on correction of molecular or electrophysiological defects [Armstrong et al. 2014]. The UK CF Gene Therapy Consortium (GTC) has recently conducted a large, phase IIb, clinical trial of liposomal CFTR gene therapy [Alton et al. 2015]. Patients received monthly nebulized doses for a year, or placebo. The primary outcome was met, in that treated patients had stabilization of FEV1 whereas the placebo group declined, with a statistically significant difference of 3.7%. Greater effects were seen in patients with more severe baseline lung disease, which may reflect more proximal deposition. Trials are planned to explore whether improvements can be further amplified by higher or more frequent dosing. In parallel, the GTC has developed a pseudotyped lentivirus (modified to bind to respiratory epithelial cells), which is showing promise as a repeatable, long-duration vector [Griesenbach et al. 2012]. Further preclinical work is underway to explore safety prior to a proposed first-in-man trial in the near future.
Alternative approaches to genetic medicine involve correcting rather than replacing the gene. A phase Ib clinical trial of the mRNA repair molecule QR010 delivered by nebulization is currently underway [ClinicalTrials.gov identifier: NCT02532764].
Summary
The prognosis for patients with CF has improved dramatically over the last few decades. Multiple therapies are currently available targeting the downstream complications of the disease and we now have the first drugs in the new classes of CFTR modulators. The research field is currently extremely active, providing encouragement that further novel therapies, with perhaps genuinely transformative potential, will become available for larger numbers of patients in the near future. However, there are a number of challenges recognized: to continue development and testing of new therapies targeting the basic defect in CFTR once drugs with this mode of action become standards of care; to optimize new, sensitive outcome measures for clinical trials in a population with ever improving pulmonary health; to ensure equity of access to expensive mutation-specific medicines in the current economic climate. Continued close working relationships between CF patients and their families, clinical research teams, national and international organizations, trial networks, and the pharmaceutical industry is paramount if we are to continue to make such encouraging progress in further improving therapies and, ultimately quality of life for people with this disease.
Acknowledgments
Work conducted by J.C. Davies is supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, UK.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Claire Edmondson has no interests to declare. J.C. Davies has served on advisory boards, undertaken educational activities and lead clinical trials for which Imperial College London, UK has received fees, for the following companies relevant to this review: Vertex, PTC, AlgiPharma, Novartis and Chiesi; she is also part of the UK CF Gene Therapy Consortium which has received funding from the Cystic Fibrosis Trust and the NIHR.
Contributor Information
Claire Edmondson, Royal Brompton & Harefield NHS Foundation Trust, Paediatric Respiratory Medicine, London, UK.
Jane C. Davies, Imperial College London, Paediatric Respirology and Experimental Medicine, London SW7 2AZ, UK.
References
- Accurso F., Moss R., Wilmott R., Anbar R., Schaberg A., Durham T., et al. (2011) Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am J Respir Crit Care Med 183: 627–634. [DOI] [PubMed] [Google Scholar]
- Aitken M., Bellon G., De Boeck K., Flume P., Fox H., Geller D., et al. (2012) Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med 185: 645–652. [DOI] [PubMed] [Google Scholar]
- Alton E., Armstrong D., Ashby D., Bayfield K., Bilton D., Bloomfield E., et al. (2015) Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomized, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 3: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R., Subbarao P., Lou W., Jabar A., Balkovec S., Jensen R., et al. (2011) The effect of Dornase Alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J 37: 806–812. [DOI] [PubMed] [Google Scholar]
- Armstrong D., Cunningham S., Davies J., Alton E. (2014) Gene therapy in cystic fibrosis. Arch Dis Child 99: 465–468. [DOI] [PubMed] [Google Scholar]
- Assael B., Pressler T., Bilton D., Fayon M., Fischer R., Chiron R., et al. (2013) Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: a comparative efficacy trial. J Cyst Fibros 12: 130–140. [DOI] [PubMed] [Google Scholar]
- Auerbach H., Williams M., Kirkpatrick J., Colten H. (1985) Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. Lancet 28: 686–688. [DOI] [PubMed] [Google Scholar]
- Balfour-Lynn I, Lees B., Hall P., Phillips G., Khan M., Flather M., et al. (2006) Multicenter randomized controlled trial of withdrawal of inhaled corticosteroids in cystic fibrosis. Am J Respir Crit Care Med 173: 1356–1362. [DOI] [PubMed] [Google Scholar]
- Baxter C., Dunn G., Jones A., Webb K., Gore R., Richardson M., et al. (2013) Novel immunologic classification of aspergillosis in adult cystic fibrosis.J Allergy Clin Immunol 132: 560–566. [DOI] [PubMed] [Google Scholar]
- Bilton D., Daviskas E., Anderson S., Kolbe J., King G., Stirling R., et al. (2013) Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non-cystic fibrosis bronchiectasis. Chest 144: 215–225. [DOI] [PubMed] [Google Scholar]
- Bilton D., Robinson P., Cooper P., Gallagher C., Kolbe J., Fox H., et al. (2011) Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. Eur Respir J 38: 1071–1080. [DOI] [PubMed] [Google Scholar]
- Boyle M., De Boeck K. (2013) A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med 1: 158–163. [DOI] [PubMed] [Google Scholar]
- Bryant J., Grogono D., Greaves D., Foweraker J., Roddick I., Inns T., et al. (2013) Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 4: 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonpensiero P., De Gregorio F., Sepe A., Di Pasqua A., Ferri P., Siano M., et al. (2010) Hyaluronic acid improves “pleasantness” and tolerability of nebulized hypertonic saline in a cohort of patients with cystic fibrosis. Adv Ther 27: 870–878. [DOI] [PubMed] [Google Scholar]
- Cholon D., Quinney N., Fulcher M., Esther C., Das J., Dokholyan N., et al. (2014) Potentiator ivacaftor abrogates pharmacological correction of δf508 CFTR in cystic fibrosis. Sci Transl Med 6: 246ra296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofu O., Lykkesfeldt J. (2014) Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database Syst Rev 7: CD007020. [DOI] [PubMed] [Google Scholar]
- Clancy J., Dupont L., Konstan M., Billings J., Fustik S., Goss C., et al. (2013) Phase II studies of nebulised arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 68: 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy J., Rowe S., Accurso F., Aitken M., Amin R., Ashlock M., et al. (2012) Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cymberknoh M., Blau H., Shoseyov D., Mei-Zahav M., Efrati O., Armoni S., et al. (2009) Intravenous monthly pulse methylprednisolone treatment for ABPA in patients with cystic fibrosis.J Cyst Fibros 8: 253–257. [DOI] [PubMed] [Google Scholar]
- Conrad C., Lymp J., Thompson V., Dunn C., Davies Z., Chatfield B., et al. (2015) Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J Cyst Fibros 14:219–227. [DOI] [PubMed] [Google Scholar]
- Coolen N., Morand P., Martin C., Hubert D., Kanaan R., Chapron J., et al. (2015) Reduced risk of nontuberculous mycobacteria in cystic fibrosis adults receiving long-term azithromycin. J Cyst Fibros 14: 594–599. [DOI] [PubMed] [Google Scholar]
- Cox N., Alison J., Holland A. (2013) Interventions for promoting physical activity in people with cystic fibrosis. Cochrane Database Syst Rev 12: CD009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauletbaev N., Fischer P., Aulbach B., Gross J., Kusche W., Thyroff-Friesinger U., et al. (2009) A phase II study on safety and efficacy of high-dose N-acetylcysteine in patients with cystic fibrosis. Eur J Med Res 14: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Wainwright C., Canny G., Chilvers M., Howenstine M., Munck A., et al. (2013) Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551d mutation. Am J Respir Crit Care Med 187: 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boeck K., Munck A., Walker S., Faro A., Hiatt P., Gilmartin G., et al. (2014) Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros 13: 674–680. [DOI] [PubMed] [Google Scholar]
- Donaldson S., Bennett W., Zeman K., Knowles M., Tarran R., Boucher R. (2006) Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 354: 241–250. [DOI] [PubMed] [Google Scholar]
- Ehsan Z., Wetzel J., Clancy J. (2014) Nebulized liposomal amikacin for the treatment of Pseudomonas aeruginosa infection in cystic fibrosis patients. Expert Opin Investig Drugs 23: 743–749. [DOI] [PubMed] [Google Scholar]
- Elkins M., Robinson M., Rose B., Harbour C., Moriarty C., Marks G., et al. (2006) A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 354: 229–240. [DOI] [PubMed] [Google Scholar]
- Elphick H., Southern K. (2014) Antifungal therapies for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev 11: CD002204. [DOI] [PubMed] [Google Scholar]
- Flume P., Liou T., Borowitz D., Li H., Yen K., Ordoñez C., et al. (2012) VX 08–770–104 Study Group. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest 142: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari M., Termini L., Traverso G., Barrale S., Bonaccorso M., Damiani G., et al. (2012) Nebulized hypertonic saline containing hyaluronic acid improves tolerability in patients with cystic fibrosis and lung disease compared with nebulized hypertonic saline alone: a prospective, randomized, double-blind, controlled study. Ther Adv Respir Dis 6: 315–322. [DOI] [PubMed] [Google Scholar]
- Graham A., Hasani A., Alton E., Martin G., Marriott C., Hodson M., et al. (1993) No added benefit from nebulized amiloride in patients with cystic fibrosis. Eur Respir J 6: 1243–1248. [PubMed] [Google Scholar]
- Griesenbach U., Inoue M., Meng C., Farley R., Chan M., Newman N., et al. (2012) Assessment of F/HN-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am J Respir Crit Care Med 186: 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M., McCarthy M., Fleming C., Hickey C., Shortt C., Eustace J. (2014) Inhaled versus nebulised tobramycin: a real world comparison in adult cystic fibrosis (CF). J Cyst Fibros 13: 692–698. [DOI] [PubMed] [Google Scholar]
- Heltshe S., Saiman L., Popowitch E., Miller M., Kloster M., Thompson V., et al. (2015) Outcomes and treatment of chronic methicillin-resistant staphylococcus aureus differs by staphylococcal cassette chromosome mec (SCCmec) type in children with cystic fibrosis. J Pediatric Infect Dis Soc 4: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewer S. (2012) Inhaled antibiotics in cystic fibrosis: what’s new? J R Soc Med 105: S19–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem E., Konstan M., De Boeck K., Accurso F., Sermet-Gaudelus I., Wilschanski M., et al. (2014) Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomized, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med 2: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollberg H., Carlander D., Olesen H., Wejåker P., Johannesson M., Larsson A. (2003) Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol 35: 433–440. [DOI] [PubMed] [Google Scholar]
- Konstan M., Byard P., Hoppel C., Davis P. (1995) Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 332: 848–854. [DOI] [PubMed] [Google Scholar]
- Konstan M., Doring G., Heltshe S., Lands L., Hilliard K., Koker P., et al. (2014) A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J Cyst Fibros 13: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan M., Flume P., Kappler M., Chiron R., Higgins M., Brockhaus F., et al. (2011) Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: the EAGER trial. J Cyst Fibros 10: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan M., Ratjen F. (2012) Effect of Dornase Alfa on inflammation and lung function: potential role in the early treatment of cystic fibrosis. J Cyst Fibros 11: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri T., Guillet A., Diehl S., Ferguson M. (2014) High-dose ibuprofen is not associated with increased biomarkers of kidney injury in patients with cystic fibrosis. Pediatr Pulmonol 49: 148–153. [DOI] [PubMed] [Google Scholar]
- Lands L., Stanojevic S. (2013) Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst Rev 6: CD001505. [DOI] [PubMed] [Google Scholar]
- Langton Hewer S., Smyth A. (2014) Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev 11: CD004197. [DOI] [PubMed] [Google Scholar]
- Lehmann S., Pfannenstiel C., Friedrichs F., Kroger K., Wagner N., Tenbrock K. (2014) Omalizumab: a new treatment option for allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Ther Adv Respir Dis 8: 141–149. [DOI] [PubMed] [Google Scholar]
- Lynch J., Sayah D., Belperio J., Weigt S. (2015) Lung transplantation for cystic fibrosis: results, indications, complications, and controversies. Semin Respir Crit Care Med 36: 299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwaine M., Alarie N., Davidson G., Lands L., Ratjen F., Milner R., et al. (2013) Long-term multicentre randomized controlled study of high frequency chest wall oscillation versus positive expiratory pressure mask in cystic fibrosis. Thorax 68: 746–751. [DOI] [PubMed] [Google Scholar]
- Matsui H., Grubb B., Tarran R., Randell S., Gatzy J., Davis C., et al. (1998) Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015. [DOI] [PubMed] [Google Scholar]
- Mogayzel P., Jr., Naureckas E., Robinson K., Mueller G., Hadjiliadis D., Hoag J., et al. (2013) Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 187: 680–689. [DOI] [PubMed] [Google Scholar]
- Mogayzel P., Naureckas E., Robinson K., Brady C., Guill M., Lahiri T., et al. (2014) Cystic Fibrosis Foundation pulmonary guideline. Pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc 11: 1640–1650. [DOI] [PubMed] [Google Scholar]
- Moreira A., Silva D., Ferreira A., Delgado L. (2014) Antifungal treatment in allergic bronchopulmonary aspergillosis with and without cystic fibrosis: a systematic review. Clin Exp Allergy 44: 1210–1227. [DOI] [PubMed] [Google Scholar]
- Moss R., Flume P., Elborn J., Cooke J., Rowe S., McColley S., et al. (2015) VX11–770–110 (KONDUCT) Study Group. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomized controlled trial. Lancet Respir Med 3: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A. (2010) Nutritional considerations in patients with cystic fibrosis. Expert Rev Respir Med 4: 47–56. [DOI] [PubMed] [Google Scholar]
- Nick J., Moskowitz S., Chmiel J., Forssén A., Kim S., Saavedra M., et al. (2014) Azithromycin May antagonize inhaled tobramycin when targeting Pseudomonas aeruginosa in cystic fibrosis. Ann Am Thorac Soc 11: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oermann C., Retsch-Bogart G., Quittner A., Gibson R., McCoy K., Montgomery A., et al. (2010) An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. Pediatr Pulmonol 45: 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier K., Shaw P., Glaser T., Bhattacharyya D., Fleshner M., Brewer C., et al. (2014) Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc 11: 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Olivier K. (2015) Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med 36: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K., Rietschel E., Ballmann M., Griese M., Worlitzsch D., Shute J., et al. (2004) Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. Am J Respir Crit Care Med 169: 719–725. [DOI] [PubMed] [Google Scholar]
- Plant B., Goss C., Plant W., Bell S. (2013) Management of comorbidities in older patients with cystic fibrosis. Lancet Respir Med 1: 164–174. [DOI] [PubMed] [Google Scholar]
- Pons G., Marchand M., d’Athis P., Sauvage E., Foucard C., Chaumet-Riffaud P., et al. (2000) French multicenter randomized double-blind placebo-controlled trial on nebulized amiloride in cystic fibrosis patients. The Amiloride-AFLM Collaborative Study Group. Pediatr Pulmonol 30: 25–31. [DOI] [PubMed] [Google Scholar]
- Ramsey B., Davies J., Mcelvaney N., Tullis E., Bell S., Drevinek P., et al. (2011) A CFTR potentiator in patients with cystic fibrosis and the G551d mutation. N Engl J Med 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratjen F., Durham T., Navratil T., Schaberg A., Accurso F., Wainwright C., et al. (2012) Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J Cyst Fibros 11: 539–549. [DOI] [PubMed] [Google Scholar]
- Renna M., Schaffner C., Brown K., Shang S., Tamayo M., Hegyi K., et al. (2011) Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest 121: 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G., Shaw D., Marsh R., Carroll M., Serisier D., Bruce K. (2015) Respiratory microbiota: addressing clinical questions, informing clinical practice. Thorax 70: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros M., Casciaro R., Lucca F., Troiani P., Salonini E., Favilli F., et al. (2014) Hyaluronic acid improves the tolerability of hypertonic saline in the chronic treatment of cystic fibrosis patients: a multicenter, randomized, controlled clinical trial. J Aerosol Med Pulm Drug Deliv 27: 133–137. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M., Ratjen F., Brumback L., Daniel S., Rowbotham R., Mcnamara S., et al. (2012) Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial. JAMA 307: 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe S., Stacey Miller M., Sorscher M. (2005) Cystic fibrosis. N Engl J Med 352: 1992–2001. [DOI] [PubMed] [Google Scholar]
- Sanders D., Fink A., Mayer-Hamblett N., Schechter M., Sawicki G., Rosenfeld M., et al. (2015) Early life growth trajectories in cystic fibrosis are associated with pulmonary function at age 6 years. J Pediatr 167: 1081.e1–1088.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawczak V., Getsy P., Zaidi A., Sun F., Zaman K., Gaston B. (2015) Novel approaches for potential therapy of cystic fibrosis. Curr Drug Targets 16: 923–936. [DOI] [PubMed] [Google Scholar]
- Schuster A., Haliburn C., Döring G., Goldman M. Freedom Study Group (2013) Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomized study. Thorax 68: 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu J., Hawryluk E., Guo D., London W., Huang J. (2015) Voriconazole phototoxicity in children: a retrospective review. J Am Acad Dermatol 72: 314–320. [DOI] [PubMed] [Google Scholar]
- Southern K., Barker P., Solis-Moya A., Patel L. (2012) Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev 11: CD002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart Elborn J., Geller D., Conrad D., Aaron S., Smyth A., Fischer R., et al. (2015) A phase 3, open-label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT-1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. J Cyst Fibros 14: 507–514. [DOI] [PubMed] [Google Scholar]
- Stutman H., Lieberman J., Nussbaum E., Marks M. (2002) Antibiotic prophylaxis in infants and young children with cystic fibrosis: a randomized controlled trial. J Pediatr 140: 299–305. [DOI] [PubMed] [Google Scholar]
- Tanou K., Zintzaras E., Kaditis A. (2014) Omalizumab therapy for allergic bronchopulmonary aspergillosis in children with cystic fibrosis: a synthesis of published evidence. Pediatr Pulmonol 49: 503–507. [DOI] [PubMed] [Google Scholar]
- Tappenden P., Harnan S., Uttley L., Mildred M., Carroll C., Cantrell A. (2013) Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis: systematic review and economic model. Health Technol Assess 17: v–xvii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell B., Mccolley S., Kissner D., Rolfe M., Rosen J., Mckevitt M., et al. (2012) Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. Am J Respir Crit Care Med 185: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis D., Burns J., Retsch-Bogart G., Bresnik M., Henig N., Lewis S., et al. (2014) Inhaled aztreonam for chronic Burkholderia infection in cystic fibrosis: a placebo-controlled trial. J Cyst Fibros 13: 296–305. [DOI] [PubMed] [Google Scholar]
- Uttley L., Harnan S., Cantrell A., Taylor C., Walshaw M., Brownlee K., et al. (2013) Systematic review of the dry powder inhalers colistimethate sodium and tobramycin in cystic fibrosis. Eur Respir Rev 22: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright C., Elborn J., Ramsey B., Marigowda G., Huang X., Cipolli M., et al. (2015) Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 373: 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright C., Quittner A., Geller D., Nakamura C., Wooldridge J., Gibson R., et al. (2011) Aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis, mild lung impairment, and P. Aeruginosa. J Cyst Fibros 10: 234–242. [DOI] [PubMed] [Google Scholar]
- Waters V., Ratjen F. (2014) Antibiotic treatment for nontuberculous mycobacteria lung infection in people with cystic fibrosis. Cochrane Database Syst Rev 12: CD010004. [DOI] [PubMed] [Google Scholar]
- Waters V., Ratjen F. (2015) Is there a role for antimicrobial stewardship in cystic fibrosis? Ann Am Thorac Soc 11: 1116–1119. [DOI] [PubMed] [Google Scholar]
- Zicari A., Celani C., De Castro G., Valerio De, Biase R., Duse M. (2014) Anti IgE antibody as treatment of allergic bronchopulmonary aspergillosis in a patient with cystic fibrosis. Eur Rev Med Pharmacol Sci 18: 1839–1841. [PubMed] [Google Scholar]