ABSTRACT
The absence of PtsN, the terminal phosphoacceptor of the phosphotransferase system comprising PtsP-PtsO-PtsN, in Escherichia coli confers a potassium-sensitive (Ks) phenotype as the external K+ concentration ([K+]e) is increased above 5 mM. A growth-inhibitory increase in intracellular K+ content, resulting from hyperactivated Trk-mediated K+ uptake, is thought to cause this Ks. We provide evidence that the Ks of the ΔptsN mutant is associated with K+ limitation. Accordingly, the moderate Ks displayed by the ΔptsN mutant was exacerbated in the absence of the Trk and Kup K+ uptake transporters and was associated with reduced cellular K+ content. Conversely, overproduction of multiple K+ uptake proteins suppressed the Ks. Expression of PtsN variants bearing the H73A, H73D, and H73E substitutions of the phosphorylation site histidine of PtsN complemented the Ks. Absence of the predicted inner membrane protein YcgO (also called CvrA) suppressed the Ks, which was correlated with elevated cellular K+ content in the ΔptsN mutant, but the ΔptsN mutation did not alter YcgO levels. Heterologous overexpression of ycgO also led to Ks that was associated with reduced cellular K+ content, exacerbated by the absence of Trk and Kup and alleviated by overproduction of Kup. Our findings are compatible with a model that postulates that Ks in the ΔptsN mutant occurs due to K+ limitation resulting from activation of K+ efflux mediated by YcgO, which may be additionally stimulated by [K+]e, implicating a role for PtsN (possibly its dephosphorylated form) as an inhibitor of YcgO activity.
IMPORTANCE This study examines the physiological link between the phosphotransferase system comprising PtsP-PtsO-PtsN and K+ ion metabolism in E. coli. Studies on the physiological defect that renders an E. coli mutant lacking PtsN to be growth inhibited by external K+ indicate that growth impairment results from cellular K+ limitation that is mediated by YcgO, a predicted inner membrane protein. Additional observations suggest that dephospho-PtsN may inhibit and external K+ may stimulate K+ limitation mediated by YcgO. It is speculated that YcgO-mediated K+ limitation may be an output of a response to certain stresses, which by modulating the phosphotransfer capacity of the PtsP-PtsO-PtsN phosphorelay leads to growth cessation and stress tolerance.
INTRODUCTION
All living cells possess mechanisms to accumulate potassium (K+), and bacteria such as Escherichia coli maintain an intracellular K+ concentration ([K+]i) of 200 to 400 mM that is thought to be required for optimal functioning of several metabolic processes (reviewed in references 1 and 2). In addition, K+ is a major determinant in the maintenance of cell turgor, so that an increase in osmolarity of the medium is associated with increased [K+]i (3–6). K+ has also been proposed to act as a second messenger (2, 7).
The maintenance of cytoplasmic K+ pools in E. coli is achieved through the balance of activities of K+ uptake systems Kdp, Trk, and Kup (formerly known as TrkD) on the one hand and of an as yet unidentified K+ efflux system or systems on the other (1, 2). The well-studied K+ efflux systems KefG/B and KefF/C are known to act as K+/H+ antiporters, with K+ efflux and concomitant H+ influx leading to cytoplasmic acidification, serving to mitigate the detrimental effects of endogenous or exogenous electrophiles (reviewed in reference 8). A residual K+ transport activity, TrkF, present in a kdp kup trk triple K+ transporter-defective mutant is thought to represent a mode of K+ uptake occurring through systems that do not normally transport K+ (9), and an as yet uncharacterized turgor-activated efflux system is also believed to exist (10).
The Kdp transporter, encoded by genes of the kdpFABC operon, is a high-affinity K+ uptake system (11, 12) that is transcriptionally induced when the external K+ concentration ([K+]e) becomes limiting for growth (13, 14). More recent studies have shown that expression and/or activity of the Kdp transporter is also inhibited by [K+]es above 5 mM (15, 16). The Trk and Kup systems, in contrast, are low-affinity K+ uptake systems that are constitutively expressed (1, 2). Of these systems, Kup is a stand-alone K+ transporter, whereas the Trk system is a multicomponent system, and a null mutation in trkA, coding for the regulatory subunit, disables the Trk system (2). The presence of multiple transport systems for K+ allow, within the limits of its osmoregulatory capacity, robust growth of E. coli in media with a wide range of [K+]es.
In E. coli, components of the phosphotransferase system (PTS) mediate uptake of carbohydrates, wherein transport of the incoming sugar is coupled to its phosphorylation (reviewed in references 17, 18, 19, and 20). In each of these systems, a phosphate moiety is transferred from phosphoenolpyruvate (PEP) to the particular sugar via a multiprotein phosphorelay mechanism. E. coli also possesses a PTS comprising PtsP-PtsO-PtsN, with a PEP-dependent phosphorelay operating in the same sequence (21–23). However, the phosphorylation substrate of PtsN is unknown. Given that ptsN and ptsO are member genes of the rpoN operon (21), PtsP, PtsO, and PtsN have also been referred to as EINtr, Npr, and EIIANtr, respectively. A recent study has shown that the phosphorylation state of PtsN can be modulated, depending upon the quality of the nitrogen source in the medium, implicating a role for the PtsP-PtsO-PtsN phosphorelay in sensing cellular nitrogen stress (24).
Previously, an intriguing connection between K+ metabolism and the PtsP-PtsO-PtsN phosphorelay was identified by Lee et al. (25), who showed that (i) a ΔptsN mutant exhibits a K+-sensitive (Ks) phenotype associated with elevated cellular K+ content, and (ii) the PtsN-H73A variant (which lacks the site of phosphorylation on PtsN and hence is constitutively in the dephospho-PtsN form), but not PtsN, forms a complex with TrkA. Lee et al. have proposed that in the absence of dephospho-PtsN, TrkA activity is unfettered, leading to cellular K+ overload due to enhanced Trk-mediated K+ uptake, which causes the Ks (25).
In this study, we found that consistent with earlier reports (25, 26), a strain lacking PtsN was rendered Ks, as [K+]e was raised above 5 mM in a synthetic minimal medium. However, genetic and physiological studies indicated that the Ks of the ΔptsN mutant resulted from K+ limitation and was not due to a K+ overload as proposed previously (25). Absence of the predicted inner membrane protein CvrA (referred to throughout this study as YcgO) (27) suppressed the Ks, and its heterologous overexpression caused a Ks similar in many respects to that displayed by the ΔptsN mutant, implicating YcgO to be the mediator of the Ks of the ΔptsN mutant. An additional implication of this work is that YcgO activity in E. coli is probably rendered cryptic by dephospho-PtsN. A model for the occurrence of K+ limitation in the ΔptsN mutant is proposed, and finally, a probable physiological role for YcgO-mediated K+ limitation is discussed.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
Genotypes of the E. coli K-12 strains used in this study are listed in Table 1. The ΔptsN::Kan, ΔycgO::Kan, ΔkdpA::Kan, and ΔamtB::Kan knockout mutations were obtained from appropriate strains of the Keio collection (28) and introduced into other strains by P1 transduction (29). Wherever required, the gene encoding the kanamycin resistance determinant was excised following treatment of strains with plasmid pCP20 (30). The trkA405 and trkD1 mutations representing loss-of-function alleles of trkA and kup were obtained from strain TL1105A (13) and introduced into other strains by P1 transduction in multiple steps. In this study, the trkA405 and the trkD1 alleles are referred to as trkA and kup, respectively. The routinely used rich growth media were LB and KML media. KML is a modified LB medium in which NaCl is substituted for with KCl (31), and it was used for the propagation of strains triply defective for K+ transport (uptake) systems. The antibiotics ampicillin (Amp), chloramphenicol (Cm), kanamycin (Kan), and tetracycline (Tet) and the lac inducer isopropyl-β-d-thiogalactoside (IPTG) were used at appropriate concentrations, and the growth temperature was 37°C.
TABLE 1.
E. coli strains and plasmids used in this studya
| Strain or plasmid | Genotype or description |
|---|---|
| Strains | |
| MC4100 | Δ(argF-lac)U169 rpsL150 relA1 spoT1 araD139 flbB5301 deoC1 ptsF25 |
| JD17 | MC4100 ΔptsN::Kan |
| JD466 | MC4100 ΔycgO::Kan |
| JD509 | MC4100 ΔptsN ΔycgO::Kan |
| JD624 | MC4100 kup trkA |
| JD637 | JD624 ΔkdpA |
| JD656 | MC4100 ΔkdpA |
| JD657 | JD637 zha-203::Tn10 |
| JD658 | JD657 ΔptsN::Kan |
| JD660 | JD624 ΔptsN::Kan |
| JD662 | JD656 ΔptsN::Kan |
| JD693 | JD637 ΔycgO::Kan |
| JD694 | JD656 ΔycgO::Kan |
| JD696 | JD624 ΔycgO |
| JD710 | JD624 ΔycgO ΔptsN::Kan |
| JD714 | JD637 ΔycgO ΔptsN::Kan zha-203::Tn10 |
| JD723 | JD624 ycgOFL ΔKan |
| JD724 | JD723 ΔptsN::Kan |
| JD725 | JD656 ΔycgO ΔptsN::Kan |
| JD726 | MC4100 attλ::(Ptrc99A bla) |
| JD727 | MC4100 attλ::(PtrcycgO bla) |
| JD728 | JD624 attλ::(Ptrc99A bla) |
| JD729 | JD624 attλ::(PtrcycgO bla) |
| JD732 | JD624 attλ::(PtrcycgO bla::Kan) |
| JD734 | JD637 attλ::(Ptrc99A bla) |
| JD735 | JD637 attλ::(PtrcycgO bla) |
| GJ14949 | JD656 attλ::(Ptrc99A bla) |
| GJ14950 | JD656 attλ::(PtrcycgO bla) |
| Plasmids | |
| pHYD3025 | Derivative of plasmid pTrc99A, described in reference 41 |
| pHYD724 | pTrc99A in which kdpA′, which encodes N-terminal 135 amino acids of KdpA, is placed under expression control of Ptrc promoter, described in reference 43 |
| pHYD1852 | pHYD3025 bearing kup expressed from Ptrc promoter, present within EcoRI and HindIII sites |
| pHYD1858 | pTrc99A containing ycgO, present within NcoI and HindIII sites, and expressed from Ptrc promoter |
| pHYD5006 | pHYD3025 containing ptsN appended at its 3′ end with DNA sequence encoding hexahistidine, placed under expression control of Ptrc promoter, present within NdeI and HindIII sites |
| pHYD5007 | Derivative of pHYD5006 encoding PtsN bearing H73A amino acid substitution |
| pHYD5008 | Derivative of pHYD5006 encoding PtsN bearing H73D amino acid substitution |
| pHYD5009 | Derivative of pHYD5006 encoding PtsN bearing H73E amino acid substitution |
All strains listed are E. coli K-12 strains, and MC4100 was from our laboratory collection. The ΔptsN::Kan, ΔycgO::Kan, and ΔkdpA::Kan deletion insertion mutations were obtained from strains JW3171, JW5184, and JW0686, respectively, of the Keio collection (28) and introduced into appropriate strains by P1 transduction. Removal of the antibiotic cassette was performed as described previously (30), and such mutations are indicated in the table by “Δ.” trkA and kup represent the trkA405 and trkD1 alleles, respectively, which were obtained from strain TL1105A (13) and introduced into MC4100 by P1 transduction in multiple steps. zha-203::Tn10 was obtained from CAG12072 (33) and introduced into appropriate strains by P1 transduction. ycgOFL encodes YcgO bearing a 3× FLAG epitope attached to the C terminus of YcgO, and the attλ::(PtrcycgO bla), attλ::(Ptrc99A bla), and attλ::(PtrcycgO bla::Kan) chromosomal constructs were obtained by the plasmid-to-chromosome shuttling system (see the “Methods” section in the supplemental material for details). The ancestral plasmid pTrc99A is described in reference 34.
For tests of the K+-sensitive (Ks) phenotype, the medium of Epstein and Kim (32) was used, which is made with reciprocally varied concentrations of Na+ and K+ by mixing together suitable proportions of 115 mM K+ phosphate-buffered medium and 115 mM Na+ phosphate-buffered medium, each of pH 7.2. In this study, K1 medium with a [K+]e of 1 mM refers to a 115 mM Na+ phosphate medium containing 1 mM KCl. In K10, K20, and K40 media, the K+ concentrations are 10, 20, and 40 mM, respectively, and the Na+ concentrations are 105, 95, and 75 mM, respectively. K115 medium contains 115 mM K+. The minimal media mentioned above were supplemented with glucose (0.2%), MgSO4 (1 mM), and B1 (0.0001%). Phosphate-buffered media of reciprocally varied Na+ and K+ concentrations at pHs of 6.0 and 7.8 were made after appropriate adjustments of the ratios of monobasic and dibasic phosphates. Glucose and other medium supplements were used as described above.
ΔptsN::Kan derivatives of MC4100 or of MC4100 lacking the Kdp transporter were obtained by isolating Kan-resistant (Kanr) transductants on LB agar plates containing Kan using an appropriate donor P1 lysate. A ΔptsN::Kan derivative of a strain doubly defective for TrkA and Kup was obtained by isolating Kanr transductants on Kan-supplemented glucose K1 agar plates containing 0.2% Casamino Acids, and the strain was maintained on the aforementioned medium. Plasmids were introduced into this strain (and its PtsN+ ancestor) by isolating transformants on antibiotic-supplemented glucose K1 agar plates containing 0.2% Casamino Acids, and the transformants were maintained on the same medium. To generate the ΔptsN::Kan derivative of the triple K+ transporter-defective strain, the ΔptsN::Kan allele was introduced via cotransduction with the zha-203::Tn10 insertion derived from strain CAG12072 (33), with a primary selection for Tet-resistant transductants using a P1 lysate prepared on strain JD650 (MC4100 ΔptsN::Kan zha-203::Tn10) on KML agar plates. zha-203::Tn10 derivatives of MC4100 or of the triple K+ transporter-defective strain JD637 grew at rates identical to those of their respective parental strains on media of various [K+]es. Furthermore, the zha-203::Tn10 marker did not affect the Ks of the ΔptsN::Kan mutant.
The plasmids used in this study are listed in Table 1 (also see Table S2 in the supplemental material) and are derivatives of plasmids pTrc99A (34) and pACYC184 (35). Additional details regarding their construction are provided in the supplemental material. The oligonucleotide primers used in this study are listed in Table S1 in the supplemental material. Procedures for PCR, cloning, and overlap extension PCR-based site-directed mutagenesis were followed as described in reference 36, and the integrity of cloned genes was confirmed by DNA sequencing.
Testing for the Ks phenotype.
For scoring of potassium-sensitivity (Ks), strains were streaked onto K1, K20, K40, and K115 glucose agar plates, and their growth proficiency was gauged. To assess the influence of the ΔptsN::Kan mutation on growth rates in different K+ transporter-defective backgrounds, pairs of ptsN+ and ΔptsN::Kan strains were grown in K1 medium to an A600 of 0.2 and were transferred to appropriate media at a starting A600 of 0.05, and the growth rates were determined in the exponential phase of growth. The aforementioned growth regimen was employed for pairs that contained an intact Kdp transporter. Pairs bearing a deficiency for the Kdp transporter were grown in K10 medium to early exponential phase, washed with K1 medium, and subcultured in media of the indicated [K+]e at an initial A600 of 0.05. A triple K+ transporter-defective strain pair lacking the Kdp, Trk, and Kup K+ uptake systems was initially grown in KML broth to A600 of 0.2, washed with K1 medium, and inoculated as described above. For measurements of the effect of Ks caused by overexpression of ycgO on the growth rate in different K+ transporter backgrounds, pairs of isogenic strains bearing either ycgO (expressed from the Ptrc promoter, integrated at attB) or the vector equivalent (at attB) were grown in the absence of the inducer (IPTG) in K1 medium (for Fig. 5A to C) and KML medium (for the strain pair in Fig. 5D) until early log phase. Cultures were then washed in K1 medium and inoculated at an A600 of 0.05 in the media of the indicated K+ concentration, and all media contained 0.1 mM IPTG. Values reported for growth rates are means ± standard errors (SE) of values obtained from three independent measurements.
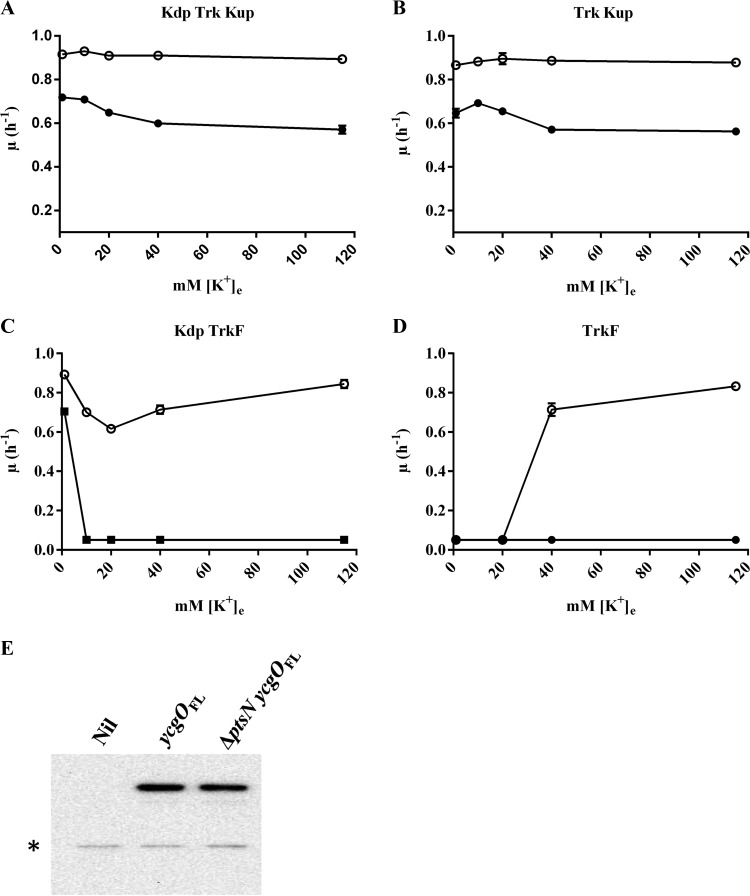
FIG 5.
Ks associated with overexpression of ycgO and its modulation by K+ uptake systems and levels of YcgO in the parent strain and ΔptsN mutant. Shown are the growth rates (μ) of pairs of isogenic strains bearing single-copy integrations of the vector (open circles) and ycgO under the expression control of the Ptrc promoter (solid circles) in attB with respect to [K+]e. The predominant K+ uptake systems present in the pairs of strains are indicated (A to D). Growth rates of all strains were determined following their growth in K1, K20, K40, and K115 media containing 0.1 mM IPTG. The strain pairs employed are represented by open and solid symbols, respectively: JD726 and JD727 (A), GJ14949 and GJ14950 (B), JD728 and JD729 (C), and JD734 and JD735 (D). (E) Expression levels of a 3× FLAG-tagged YcgO encoded by ycgOFL in JD723 (ycgOFL) and JD724 (ΔptsN ycgOFL). The lane labeled “Nil” contains a whole-cell extract of strain JD624. Cultures of JD624, JD723, and JD724 obtained after growth in K1 medium to mid-exponential phase were centrifuged and suspended in SDS sample buffer at an A600 of 0.002/μl. Equal volumes were loaded onto a 12% SDS-PAGE gel that was processed and immunoblotted with anti-FLAG M2 monoclonal antibody. A nonspecific anti-FLAG immunoreactive material indicative of equal loading is marked with an asterisk.
Isolation of a transposon insertion in ycgO.
Following transposon Tn10dCm mutagenesis (37) of the hns trxA double mutant GJ1495, which is a derivative of MC4100 and also exhibits a Ks similar to that displayed by the ΔptsN mutant, Cm-resistant (Cmr) suppressors of the Ks of GJ1495 were selected on K115 glucose Cm medium (i.e., double selection). P1 lysates prepared on the isolated suppressors were introduced into GJ1495 to establish that the Cmr and the suppressor phenotypes were 100% linked. The site of Tn10dCm insertion in one of the suppressors wherein the Tn10dCm insertion caused the suppressor phenotype was determined by cloning the Tn10dCm element with the aid of the mini-Mu cloning procedure (38). The transposon junction site was sequenced with the primer 5′-TCCCTCCTGTTCAGCTACTGA-3′, which reads out from Tn10dCm, and the transposition event was located after nucleotide 1287 of ycgO.
Determination of cellular K+ content.
K+ content of bacterial strains was determined by the method of Papp-Wallace and Maguire (39), with modifications. Cultures for K+ content determination were grown until they reached an A600 of 0.2 in K1 medium. The cells were pelleted and inoculated in K1, K40, and K115 media at an initial A600 of 0.05 for the parent. For the strain bearing the ΔptsN::Kan mutation, the inoculum was adjusted to A600s of 0.2 in K40 and K115 media and 0.05 in K1 medium. A similar inoculum adjustment was performed for the strain in which the effect of overexpression of ycgO on the cellular K+ pool was to be determined. Following growth for 2 h, 1 ml of culture was centrifuged at room temperature for 1 min at 13,000 rpm through a 0.3-ml layer of a 2:1 (vol/vol) mixture of dibutyl phthalate and dioctyl pthalate. The upper aqueous layer was discarded, and residual K+ present over the organic phase was removed by repeated cycles of washing with 1 ml of Milli-Q water. After the organic layer had been thoroughly aspirated, the cell pellet was digested overnight in 0.1 ml of 1 N nitric acid, diluted with Milli-Q water to 5 ml, and clarified by centrifugation. K+ in the nitric acid extract was quantified by inductively coupled plasma mass spectrometry (ICP-MS) (model ELAN DRC-e). 39K was measured in conjunction with appropriate standards. K+ content was expressed as nanomoles of K+ per A600 of the culture at time of harvest. Results are reported as means ± standard deviations (SD) of single measurements conducted on three independent cultures. For cultures of a strain bearing the ΔptsN mutation or cultures in which ycgO was overexpressed, a control experiment was included to verify that the proportion of spontaneous suppressors was <10−5. Where the effect of overexpression of ycgO on cellular K+ content was assessed, the growth medium at the time of the 2-h exposure contained 0.1 mM IPTG and the appropriate plasmid-specific antibiotic selection as required.
Other procedures.
Procedures for transfer of ycgO from plasmid into the chromosomal attB site, construction of a strain encoding a C-terminal 3× FLAG epitope-tagged version of YcgO, transfer of ilvG+ into E. coli K-12, and immunoblotting are described in the supplemental material.
RESULTS
Absence of PtsN yields a Ks that is exacerbated by the absence of constitutive K+ uptake systems and is associated with reduced cellular K+ content.
In the background of the widely used laboratory strain of E. coli K-12 MC4100, we found that absence of PtsN led to a modest K+-sensitive (Ks) phenotype. Whereas MC4100 and its ΔptsN::Kan derivative, JD17, grew at comparable rates in K1 medium, the growth rate of JD17 decreased as the medium [K+]e was increased above 5 mM, and beyond a [K+]e of 40 mM, JD17 displayed a constant but a lower growth rate than MC4100 (Fig. 1A). In a strain lacking the Kdp transporter, the absence of PtsN also led to Ks, and the pattern of Ks with respect to the [K+]e of strain JD662 (Δkdp ΔptsN::Kan) was similar to that displayed by JD17 in K20, K40, and K115 media. In the lower [K+]e range (10 mM and below), growth of JD662 was more retarded than that of its parent, JD656 (Fig. 1B). Absence of the constitutive K+ uptake systems Trk and Kup increased the severity of Ks. In a strain that bears Kdp as the only active K+ uptake system, JD624, the absence of PtsN (strain JD660) led to severely diminished growth in K20 and K40 media in comparison to JD624, and in K115 medium, the growth rate of JD660 increased further but remained below that of JD624 (Fig. 1C).
FIG 1.
K+-sensitive growth of the ΔptsN mutant and its modulation by K+ uptake systems. Shown are growth rates (μ) of pairs of isogenic ptsN+ (open circles) and ΔptsN (solid circles) strains bearing combinations of K+ uptake systems with respect to [K+]e. The predominant K+ transport systems present in each pair are indicated. Growth rates of all strain pairs were determined following their growth in K1, K20, K40, and K115 media. The ptsN+ and ΔptsN strain pairs, respectively, are MC4100 and JD17 (A), JD656 and JD662 (B), JD624 and JD660 (C), and JD657 and JD658 (D).
Herein, a feature of the growth pattern exhibited by the kdp+ kup trkA strain JD624 is worth noting (Fig. 1C). JD624 grew at lower rates in media of intermediate [K+]e, that is, in K20 and K40 media, with the reduced growth rate being more marked in K20 medium than in K1 or K115 medium. This growth pattern can be accounted for based upon the previously reported and apparently anomalous growth pattern displayed by a kdp+ kup trkA strain that is known to be growth inhibited in media of intermediate (10 to 40 mM) but not low (1 mM) or high (115 mM) [K+]es (15, 16, 40). The inhibition of the expression and/or activity of the Kdp system by [K+]e is thought to cause the reduction in growth rate, which is very prominent when the medium pH is 6.0 (15, 16) (Fig. 2A). Growth inhibition by intermediate [K+]es of the aforementioned strain correlates with K+ limitation, and activities of the inducible Kdp transporter and the low-affinity TrkF K+ uptake (9) are thought to satisfy the K+ needs of this strain in media of low (1 mM) and high (>40 mM) [K+]es, respectively (15, 16). In the studies described above, we employed the trkA and the kup mutations, which are not precise deletions but represent loss-of-function alleles (13). We found that a kdp+ strain bearing clean deletions of TrkA and Kup also displayed an exacerbation of the Ks in the absence of PtsN (data not shown).
FIG 2.
Modulation of the K+-sensitive growth of the ΔptsN mutant by alterations in external pH and its alleviation by elevated [K+]e. Shown are the growth rates (μ) of JD624 (open circles) and its ΔptsN::Kan derivative, JD660 (solid circles), with respect to [K+]e. The K+ transporters present in JD624 and JD660 are indicated. Growth rates were determined for JD624 and JD660 following their growth in K1, K20, K40, and K115 media whose pH was maintained at 6.0 (A) and 7.8 (B). (C) Growth rates of JD624 (open circles) and JD660 (solid circles) with respect to [K+]e in K115 medium and in K115 medium containing 100, 200, and 400 mM KCl. All growth media in panel C also contained 1 mM betaine, and their pH was 7.2.
Ks imparted by the ΔptsN::Kan mutation persisted in the absence of the three active K+ uptake systems. Both JD657, which is a triple K+ transporter-defective strain, and its ΔptsN::Kan derivative, JD658, did not grow in K1 or K20 medium. However, while the growth rate of JD657 increased as the [K+]e was raised above 20 mM, JD658 displayed a moderate increase in growth rate only in K115 medium, which was lower than that displayed by JD657 (Fig. 1D) and was also well below that displayed by MC4100, which contains all active K+ uptake systems. Overall, these observations indicated that to some extent the constitutive K+ uptake systems and the low-affinity TrkF K+ uptake aid in mitigating the Ks caused by the ΔptsN mutation.
PtsN is the terminal phosphoacceptor protein of the PTS pathway comprising PtsP-PtsO-PtsN (18, 19, 20, 21), and the Ks of the ΔptsN mutant was unaffected by the absence of PtsP (data not shown). In MG1655, we found as seen for MC4100 absence of PtsN caused a modest Ks that was exacerbated by the absence of TrkA and Kup, and the Ks of the ΔptsN mutant also persisted in a derivative of MG1655 lacking all K+ uptake systems (data not shown).
We measured cellular K+ content in the parent JD624 and its ΔptsN::Kan derivative, JD660, after their growth in K1 medium followed by a shift for 2 h into K1, K40, and K115 media (Table 2). Compared to JD624, JD660 displayed an approximately 30 to 40% reduction in cellular K+ content in K40 and K115 media, whereas in K1 medium, the K+ contents of JD624 and JD660 remained comparable. These observations indicate that the Ks of JD660 in K40 and K115 media was associated with a decrease in the cellular K+ pool.
TABLE 2.
Reduced cellular K+ content in the ΔptsN mutant and its restoration by the ΔycgO mutation and by overexpression of kup
| Strain | Relevant genotype | Relative K+ content after growth in mediuma: |
||
|---|---|---|---|---|
| K1 | K40 | K115 | ||
| JD624 | kdp+ kup trkA | 1 (0.10) | 1 (0.04) | 1 (0.15) |
| JD660 | kdp+ kup trkA ΔptsN | 0.98 (0.02) | 0.58 (0.12) | 0.63 (0.37) |
| JD696 | kdp+ kup trkA ΔycgO | 1.07 (0.07) | 1.07 (0.29) | 0.99 (0.02) |
| JD710 | kdp+ kup trkA ΔycgO ΔptsN | 0.92 (0.05) | 0.91 (0.01) | 1.12 (0.25) |
| JD660/pHYD3025b,c | kdp+ kup trkA ΔptsN | 0.99 (0.07) | 0.6 (0.17) | 0.68 (0.06) |
| JD660/pHYD1852c,d | kdp+ kup trkA ΔptsN | 1.07 (0.02) | 1.08 (0.05) | 1 (0.03) |
Shown is the cellular K+ content of strains JD660 (ΔptsN), JD696 (ΔycgO), and JD710 (ΔycgO ΔptsN) relative to parent strain JD624 following 2 h of growth in K1, K40, and K115 media. The K+ content obtained for JD624 in K1, K40, and K115 media was set at 100% = 1, and the relative K+ contents for the other strains are indicated. The relative standard deviations (SD) for the measurements are indicated in parentheses. The K+ contents (mean ± SD) for JD624 following its growth in K1, K40, and K115 media were 165 ± 17.3, 169 ± 7, and 241 ± 38.1 nmol K+/A600, respectively.
Vector.
K+ content was obtained following growth in K1, K40, and K115 media containing 0.1 mM IPTG.
Plasmid bearing kup under the expression control of the Ptrc promoter.
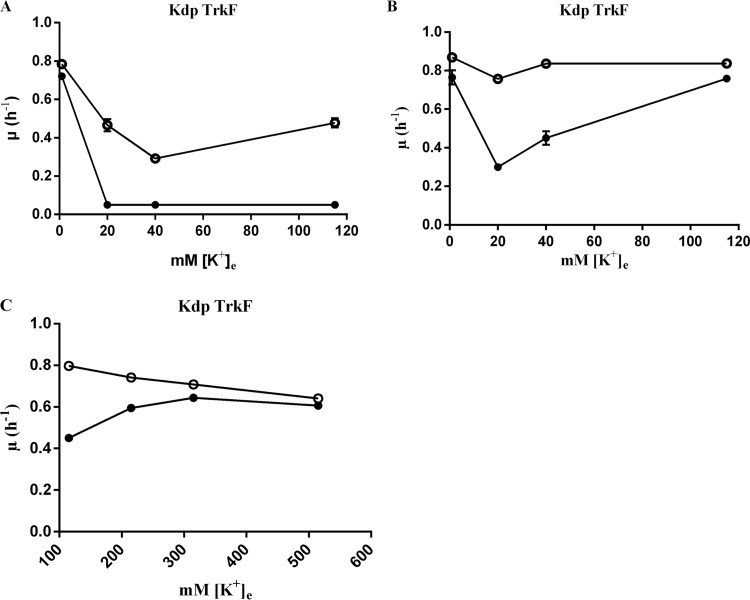
Modulation of Ks by alterations in external pH and its rescue by elevated [K+]e.
In JD624, which bears Kdp as the sole active K+ uptake system, the Ks imparted by the ΔptsN::Kan mutation could be modulated by alterations in the pH of the medium. Growth of the ΔptsN::Kan derivative of JD624, JD660, was severely restricted in K20, K40, and K115 media of pH 6.0 (Fig. 2A), whereas considerable improvement of its growth in K40 and K115 media occurred when the medium pH was raised to 7.8 (Fig. 2B). In K1 medium under the two different external pH conditions, JD660 grew at rates comparable to those of JD624. It may be noted that alteration in medium pH itself affected the growth of the ptsN+ parent of JD660, JD624. The growth rate of JD624 decreased quite sharply as the [K+]e was raised above 1 mM with the external pH held at 6.0 and increased marginally in pH 6.0 media with [K+]es of >20 mM (Fig. 2A). In media of pH 7.8, the growth rate of JD624 decreased marginally as the [K+]e was raised above 1 mM, and the growth rates of JD624 in media with [K+]es of >20 mM were comparable to its growth rate in K1 medium (Fig. 2B). In JD624, the inhibition by external K+ of Kdp expression and/or activity renders its growth dependent on K+ uptake via TrkF in media with [K+]es of >20 mM (15, 16). K+ uptake via TrkF is known to be modulated by alterations in medium pH, with acidification leading to reduction and alkalinization to enhancement of its K+ uptake activity (9, 16). Thus, the modulation of the growth of JD624 by alterations in the pH of media with [K+]es of >20 mM represents the modulation of the K+ uptake via the TrkF pathway. It may be noted that medium alkalinization led to marked improvement in the growth rate of the ΔptsN::Kan mutant JD660 in K40 and K115 media but not in K20 medium (Fig. 2B). A probable explanation for this and also for the Ks in K20 medium imparted by the ΔptsN mutation in a strain that bears Kdp as the only active K+ transporter is provided in the section describing the effect of the ΔycgO mutation on Ks.
Increasing the [K+]e of the K115 medium by supplementation with KCl led to a noticeable alleviation of Ks (Fig. 2C). To minimize osmotic effects arising due to high external concentrations of K+, 1 mM betaine, an osmoprotectant (1), was added to all media. As the [K+]e was raised to 515 mM, the growth rate of the parent strain, JD624, decreased. The growth rate of the ΔptsN::Kan derivative of JD624, JD660, increased as the [K+]e was raised above 115 mM until it reached a concentration of 215 mM (Fig. 2C). Further elevation of the medium [K+]e led to a modest decrease in the growth rate of JD660. Addition of equimolar amounts of NaCl to K115 medium did not alleviate the Ks of JD660 (data not shown), indicating that the enhancement in the growth rate of JD660 was mediated by excess K+ in the medium.
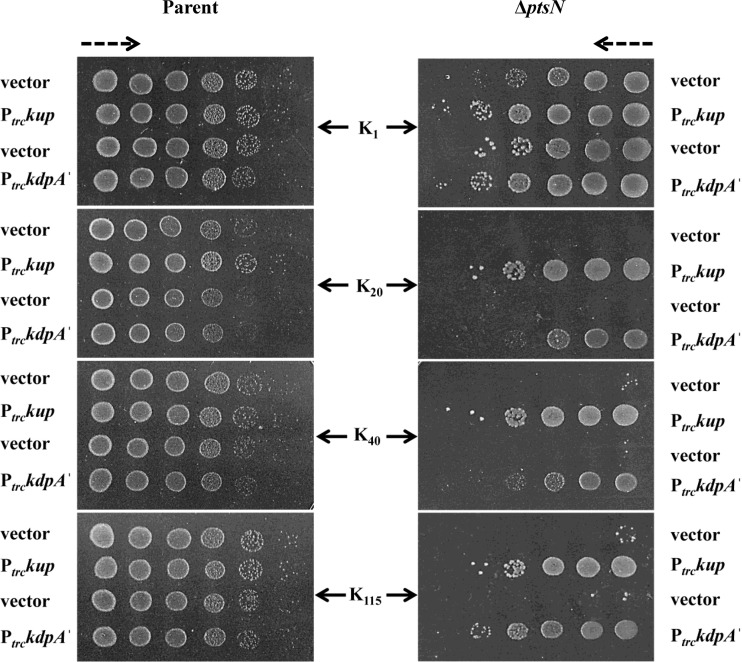
Overproduction of multiple K+ uptake proteins alleviates the Ks.
Since the severity of the Ks was found to increase with the absence of the Trk and the Kup K+ transporters, we tested the effects of overproduction of K+ uptake proteins on Ks. Ptrc-directed overproduction of Kup from plasmid pHYD1852 with 0.1 mM IPTG suppressed the Ks of JD660, whereas JD660 bearing plasmid pHYD3025 (vector) (41) displayed Ks (Fig. 3). Suppression of Ks by overproduction of Kup was also associated with elevation in K+ content of JD660 bearing plasmid pHYD1852 in comparison to its vector-bearing counterpart in K40 and K115 media (Table 2).
FIG 3.
Alleviation of the Ks of the ΔptsN mutant by overproduction of K+ uptake proteins Kup and KdpA′. Tenfold serial dilutions of cultures of the parent strain (JD624 [kdp+ trkA kup]) and its ΔptsN::Kan derivative, JD660 (ΔptsN), bearing the plasmids pHYD3025 (vector), pHYD1852 (Ptrckup), pTrc99A (vector), and pHYD724 (PtrckdpA′), were spotted on the surface of K1, K20, K40, and K115 glucose agar plates containing 0.1 mM IPTG. pHYD3025 is the vector control for pHYD1852. The dashed arrows indicate the direction of increasing order of cell dilution.
The TetA polypeptide specifying tetracycline resistance encoded on plasmid pBR322 (also present on plasmid pACYC184) and the N-terminal 135 amino acids of the KdpA K+-translocating subunit of the Kdp complex (KdpA′) are thought to be examples of K+ carrier proteins as their expression confers upon a strain that lacks all K+ uptake systems a K+-sparing phenotype (42, 43), which is the ability of a strain to grow proficiently in media when [K+]e becomes limiting for its growth. Ptrc-directed overproduction of KdpA′ from plasmid pHYD724 with 0.1 mM IPTG in JD660 alleviated its Ks, whereas JD660 containing the vector pTrc99A displayed the Ks (Fig. 3). Furthermore, the presence of the plasmid pACYC184 but not its derivative, pHYD586, which lacks tetA, in JD660 alleviated its Ks (see Fig. S1 in the supplemental material). Transformants of JD624 (parent) bearing all of the plasmids described above did not display any overt alterations of their growth in the indicated media (Fig. 3; see Fig. S1).
A variant of the ammonium transporter AmtB bearing the H168D and H318E substitutions (AmtBK) has recently been shown to mediate selective uptake of K+ in place of its natural substrate, the ammonium ion (44). Expression of this variant (amtBK) in an otherwise wild-type strain leads to a Ks that correlates with elevated cellular K+ content in synthetic media of [K+]es of 100 mM and above (44). In addition, in a triple K+ transporter-defective strain, amtBK expression causes a Ks and K+-sparing phenotype such that this strain additionally displays an enhanced ability to grow in media containing less than 20 mM K+ (44). The aforementioned two growth phenotypes pertaining to amtBK expression were recapitulated in this study using plasmid pHYD1855 (see Table S2 in the supplemental material), which expresses amtBK from the Ptrc promoter (data not shown). We found that expression of amtBK alleviated the Ks of the ΔptsN::Kan ΔamtB double mutant JD767, whereas JD767 containing the vector or the plasmid expressing amtB displayed Ks (see Fig. S2 and Table S2 in the supplemental material).
In addition, expression of all of the K+ uptake proteins described above in the ΔptsN::Kan derivative of MC4100, JD17, alleviated its Ks, and overexpression of kup in the ΔptsN::Kan derivative of MG1655 also alleviated its Ks (data not shown). Taken together, these observations lend additional support to the notion that the Ks of the ΔptsN mutant correlates with K+ limitation.
YcgO and Ks.
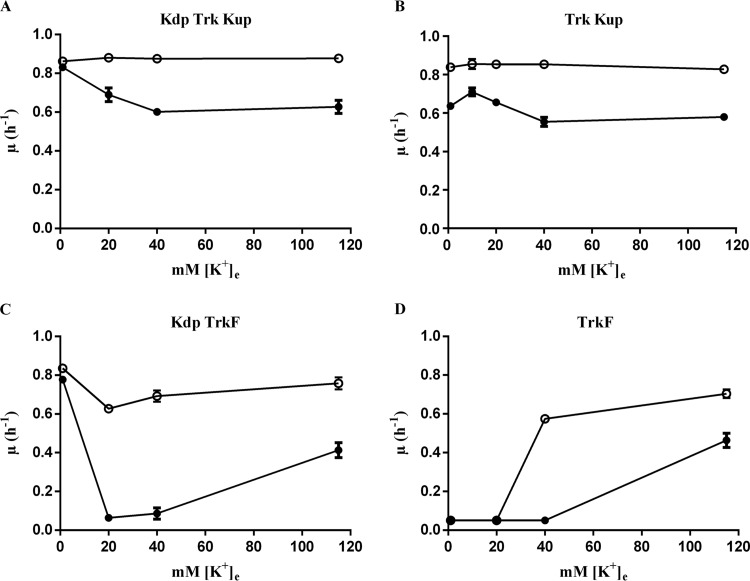
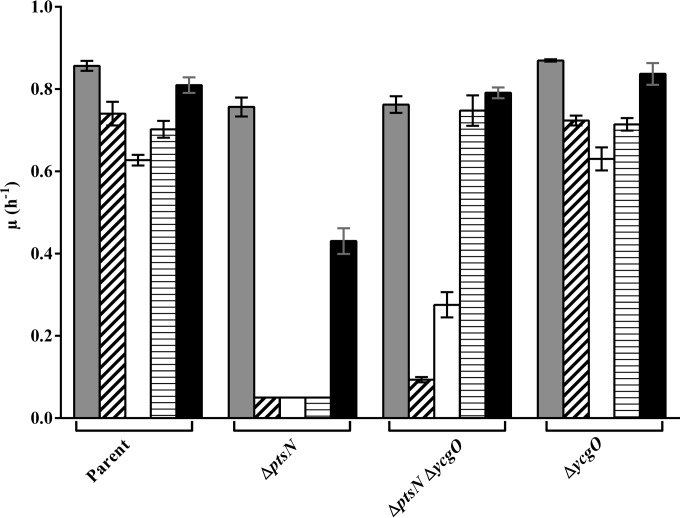
In work to be described elsewhere, we have found that MC4100 doubly defective for the nucleoid protein H-NS and either thioredoxin 1 (TrxA) or thioredoxin reductase (TrxB) also displays a Ks similar to that displayed by the ΔptsN mutant. H-NS functions as a regulator of global gene expression in E. coli, with a well-documented role as a repressor (45), and TrxB along with thioredoxin 1 (TrxA) mediates cytoplasmic protein reduction (46). The Ks in both instances is exacerbated by removal of TrkA and Kup and is suppressed by expression of kdpA′, kup, tetA, and amtBK (data not shown). We isolated a suppressor mutant of the Ks of an hns trxA double mutant after Tn10dCm transposon mutagenesis. Further studies showed that a transposon insertion located after nucleotide 1287 of ycgO that is predicted to encode an inner membrane protein implicated in cytoplasmic alkali cation homeostasis (27) caused the suppressor phenotype. Suppression resulted from a deficiency of YcgO, as the presence of a clean deletion of ycgO (ΔycgO::Kan) also suppressed the Ks of the hns trxA double mutant (data not shown). We found that the Ks of the ΔptsN mutant was also suppressed by the ΔycgO::Kan mutation (Fig. 4).
FIG 4.
Involvement of YcgO in the Ks of the ΔptsN mutant. Shown are the growth rates (μ) of the parent strain (JD624 [kdp+ trkA kup]) and its ΔptsN (JD660), ΔptsN ΔycgO (JD710), and ΔycgO (JD696) derivatives in media of various [K+]es. The indicated strains were grown in K1 (gray bars), K10 (bars with diagonal stripes), K20 (white bars), K40 (bars with horizontal stripes), and K115 (black bars) media, and their growth rates were determined.
In Fig. 4, the influence of single and double deficiency of PtsN and YcgO on growth rates in media with various [K+]es is presented for a quartet of strains bearing Kdp as the sole active K+ uptake system. Among the quartet, the strain lacking YcgO, JD696, grew at rates comparable to those of the parent in all the K+-containing media employed. The ΔptsN::Kan mutant JD660 displayed a Ks that was prominent in K10, K20, and K40 media, whereas its ΔycgO derivative, JD710, grew at rates equivalent to those of the parent only in K1, K40, and K115 media but not in K10 and K20 media. A recent study has implicated a requirement for dephospho-PtsN in the optimal expression of the kdp operon in E. coli (47), which may provide a basis to explain why the ΔycgO mutation does not suppress the Ks imparted by the ΔptsN mutation in K10 and K20 media. As mentioned earlier, a kdp+ trkA kup strain experiences partial K+ limitation in media with intermediate [K+]es, due to the repression of the Kdp transporter by [K+]e, and growth of this strain in media of low (below 40 mM) [K+]es is dependent on the Kdp transporter (15, 16). If one assumes that the ΔptsN mutation in such a strain yields two distinct types of Ks, one occurring in the intermediate range of [K+]es and the other occurring (maximally) in the 40 to 115 mM range, then the absence of alleviation of the Ks of the ΔptsN mutant by the ΔycgO mutation in K10 and K20 media may be explained. The Ks caused by the ΔptsN mutation in K10 and K20 media could represent K+ limitation, arising mainly due to reduced kdp expression, and that occurring in the 40 to 115 mM range of [K+]es could represent K+ limitation related to the activity of YcgO. Furthermore, we have observed that the ΔycgO mutation does not affect the expression of a kdp-lac transcriptional fusion (data not shown), a finding that is compatible with the observation that the ΔycgO mutation does not suppress the Ks of a ΔptsN::Kan derivative of the kdp+ trkA kup strain in the 10 to 20 mM range of [K+]es.
If the aforementioned rationale is valid, then the minimal alleviation of the Ks of JD660 by alkalinization of K20 medium may also be explained (Fig. 2B), the basis being that the effect of medium alkalinization is restricted to enhancement of TrkF activity, whose magnitude is very low in the [K+]e range, below 40 mM (9, 16). The additional effect of the ΔptsN::Kan mutation in reducing kdp expression may not be subject to modulation by medium alkalinization. Thus, the poor growth of JD660 in a medium with a [K+]e of 20 mM and pH 7.8 is due to impaired kdp expression and the absence of significant TrkF activity. Overall though, the two Ks phenotypes displayed by the ΔptsN derivative of the kdp+ trkA kup strain correlate with K+ limitation because they were suppressed by overexpression of multiple K+ uptake proteins (Fig. 3; see Fig. S1 and S2B in the supplemental material).
We measured cellular K+ content in strain JD710, which is a ΔptsN::Kan ΔycgO double mutant, and strain JD696, which bears the ΔycgO mutation, after their growth in K1 medium followed by a shift for 2 h into K1, K40, and K115 media. A comparison of their K+ contents with respect to those obtained for the parent strain, JD624, and its ΔptsN::Kan derivative, JD660, permitted the following conclusions: (i) the absence of YcgO in a strain bearing the ΔptsN::Kan mutation led to restoration of cellular K+ content to a level approaching that of the parent following exposure to K40 and K115 media, and (ii) the absence of YcgO in the parent did not lead to significant alterations in cellular K+ content (Table 2).
We also documented the extent to which the ΔycgO mutation suppressed the Ks of the ΔptsN mutant in backgrounds bearing mutations in K+ uptake systems (Table 3). In the background bearing all K+ uptake systems, the ΔycgO mutation suppressed the Ks of the ΔptsN mutant with the outcome that the ΔptsN ΔycgO derivative of MC4100, JD509, grew at rates comparable to those of MC4100 in K1, K20, K40, and K115 media. In the background devoid of the Kdp transporter, the ΔycgO mutation suppressed the Ks of the ΔptsN mutant in K20, K40, and K115 media. In K1 medium, the slow growth associated with the presence of the ΔptsN mutation in this background was not altered by the ΔycgO mutation. In the triple K+ transporter-defective strain, the ΔycgO mutation suppressed the Ks of the ΔptsN mutant in K40 and K115 media. Absence of YcgO in MC4100 or in its Δkdp and Δkdp trkA kup derivatives, JD656 and JD637, respectively, did not lead to any discernible alteration in growth in media of various [K+]es (Table 3), indicating that the ΔycgO mutation does not influence K+ uptake occurring via the Kdp, TrkA, Kup, and TrkF systems.
TABLE 3.
Ks phenotype of the ΔptsN mutation and its suppression by absence of YcgO in K+ transporter-defective backgrounds
| Strain | Relevant genotype | Growth rate (h−1) on mediuma: |
|||
|---|---|---|---|---|---|
| K1 | K20 | K40 | K115 | ||
| MC4100b | kdp+ kup+trkA+ | 0.86 | 0.88 ± 0.01 | 0.88 ± 0.01 | 0.88 ± 0.01 |
| JD17b | kdp+ kup+ trkA+ ΔptsN | 0.83 ± 0.01 | 0.69 ± 0.01 | 0.60 ± 0.02 | 0.63 ± 0.03 |
| JD466 | kdp+ kup+ trkA+ ΔycgO | 0.91 ± 0.01 | 0.89 ± 0.02 | 0.91 ± 0.02 | 0.90 ± 0.03 |
| JD509 | kdp+ kup+ trkA+ ΔptsN ΔycgO | 0.83 ± 0.02 | 0.80 ± 0.02 | 0.82 | 0.84 |
| JD656b | ΔkdpA kup+ trkA+ | 0.84 ± 0.02 | 0.85 ± 0.02 | 0.85 ± 0.02 | 0.83 ± 0.02 |
| JD662b | ΔkdpA kup+ trkA+ ΔptsN | 0.64 ± 0.02 | 0.66 ± 0.01 | 0.55 ± 0.02 | 0.58 ± 0.01 |
| JD694 | ΔkdpA kup+ trkA+ ΔycgO | 0.85 ± 0.01 | 0.89 ± 0.02 | 0.89 ± 0.01 | 0.89 |
| JD725 | ΔkdpA kup+ trkA+ ΔptsN ΔycgO | 0.70 ± 0.06 | 0.81 ± 0.02 | 0.82 | 0.82 |
| JD657b,c | ΔkdpA kup trkA | <0.05 | <0.05 | 0.67 ± 0.01 | 0.83 ± 0.01 |
| JD658b,c | ΔkdpA kup trkA ΔptsN | <0.05 | <0.05 | <0.05 | 0.46 ± 0.02 |
| JD693 | ΔkdpA kup trkA ΔycgO | <0.05 | <0.05 | 0.72 ± 0.03 | 0.86 ± 0.01 |
| JD714c | ΔkdpA kup trkA ΔptsN ΔycgO | <0.05 | <0.05 | 0.76 ± 0.24 | 0.88 ± 0.01 |
Shown are growth rates (means ± standard errors) of three quartets of strains in K1, K20, K40, and K115 media. The first quartet, MC4100 to JD590, bears all K+ uptake systems, the second, JD656 to JD725, lacks the Kdp transporter, and the third, JD657 to JD714, lacks all K+ uptake systems. Growth rates for the quartet with Kdp as the sole K+ uptake system are displayed in Fig. 4. The PtsN, YcgO, and K+ transporter status with respect to Kdp, Trk, and Kup in each quartet is indicated.
Values for growth rates from Fig. 1.
Contains the zha-203::Tn10 marker.
Overproduction of YcgO yields a Ks with similarities to that displayed by the ΔptsN mutation.
IPTG-induced overexpression of ycgO placed under the expression control of the Ptrc promoter integrated in chromosomal attB conferred a Ks that was similar to that conferred by the ΔptsN mutation. In MC4100 and its Δkdp derivative, overexpression of ycgO caused a Ks as the medium [K+]e was raised (Fig. 5A and B). In a strain that contained Kdp as the sole active K+ uptake system, overexpression of ycgO led to a Ks of increased severity in K20, K40, and K115 media (Fig. 5C). In the triple K+ transporter-defective strain, ycgO overexpression led to Ks in K40 and K115 media (Fig. 5D). Isogenic strains bearing a vector equivalent integrated in attB displayed growth commensurate with their K+ uptake capacities (Fig. 5A to D). The Ks in K40 and K115 media caused by overexpression of ycgO in the kdp+ trk kup strain JD729 was associated with reduced cellular K+ content in JD729 in comparison to its vector-bearing counterpart, JD728 (Table 4). Furthermore, co-overexpression of kup from plasmid pHYD1852 in JD732 (a strain similar to JD729) suppressed the Ks caused by Ptrc-driven expression of ycgO integrated in attB (see Fig. S3 in the supplemental material), which in turn correlated with elevation of the K+ content of JD732 in K40 and K115 media (Table 4). Overexpression of ycgO in JD732 bearing the vector pHYD3025 led to the Ks (see Fig. S3 in the supplemental material), and the K+ content of this strain in K40 and K115 media remained at a level lower than that of its counterpart that contained the plasmid pHYD1852 (Table 4). The Ks caused by overexpression of ycgO was unaffected by the PtsP/PtsO status of the strain (data not shown). These observations indicate that the Ks of ycgO overexpression is similar to that displayed by the ΔptsN mutant in more ways than one.
TABLE 4.
Reduction in the cellular K+ content by overexpression of ycgO in the parent and its restoration by overexpression of kup
| Strain | Relevant genotype | Relative K+ content after growth in mediuma: |
||
|---|---|---|---|---|
| K1 | K40 | K115 | ||
| JD728 | kdp+ kup trkA attλ::(Ptrc99A bla) | 1 (0.18) | 1 (0.12) | 1 (0.01) |
| JD729 | kdp+ kup trkA attλ::(PtrcycgO bla) | 0.73 (0.14) | 0.62 (0.04) | 0.57 (0.7) |
| JD732/pHYD3025b | kdp+ kup trkA attλ::(PtrcycgO bla::Kan) | 0.9 (0.11) | 0.8 (0.16) | 0.71 (0.08) |
| JD732/pHYD1852c | kdp+ kup trkA attλ::(PtrcycgO bla::Kan) | 1.14 (0.02) | 1.33 (0.04) | 1.07 (0.04) |
The K+ content obtained for strain JD728 bearing integration of the plasmid pTrc99A in attB [attλ::(Ptrc99A bla)] in K1, K40, and K115 media was set at 100% = 1, and the relative K+ contents for the other strains are indicated. K+ content was determined following 2 h of growth of the indicated strains in K1, K40, and K115 media containing 0.1 mM IPTG. The relative standard deviations (SD) for the measurements are indicated in parentheses. The K+ contents (mean ± SD) for JD728 following its growth in K1, K40, and K115 media were 162 ± 29.4, 147 ± 17.3, and 229 ± 3.4 nmol K+/A600, respectively.
Vector.
Plasmid bearing kup under the expression control of the Ptrc promoter.
To test if the ΔptsN mutation led to any alterations in the expression of YcgO, we constructed a strain encoding C-terminally 3× FLAG-tagged YcgO (YcgOFL) expressed from its native chromosomal location by recombineering. Appending the 3× FLAG tag to the C terminus of YcgO did not alter YcgO function as the absence of PtsN in a strain expressing YcgOFL led to Ks and a strain bearing YcgOFL did not display Ks (data not shown). YcgOFL levels in the parent strain, JD723, were comparable to those in its ΔptsN::Kan derivative, JD724, following growth in K1 medium (Fig. 5E). No differences in YcgOFL levels were seen when the aforementioned two strains were grown for 2 h in K40 medium following growth to the early exponential phase in K1 medium (data not shown). These observations indicate that the Ks caused by the ΔptsN mutation does not result from overexpression of ycgO.
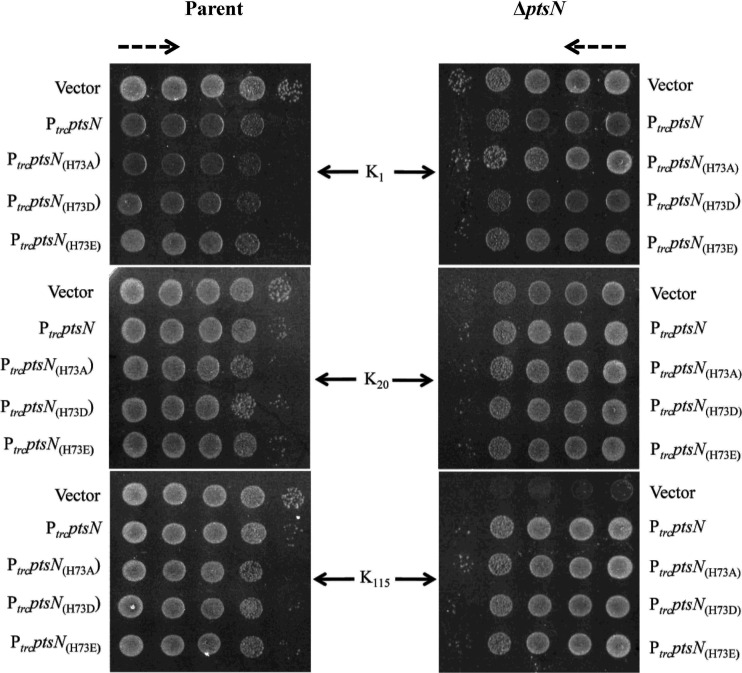
Effect of amino acid substitutions at histidine 73 of PtsN on Ks.
An earlier study showed that PtsN is phosphorylated at a single site, namely histidine 73 (48), whose replacement with aspartate or alanine led to absence of phosphorylated PtsN (48). We constructed a plasmid, pHYD5006, expressing under the control of the Ptrc promoter ptsN, and its derivative plasmids, pHYD5007, pHYD5008, and pHYD5009, encoding PtsN bearing the H73A, H73D, and H73E amino acid substitutions, respectively. Basal-level expression of ptsN from the aforementioned plasmids in strain JD17 (MC4100 ΔptsN::Kan) complemented its Ks, whereas JD17 bearing the vector showed the Ks (Fig. 6). Transformants of MC4100 bearing the indicated ptsN-expressing plasmids did not display the Ks (Fig. 6). We noted that in K1 medium, the presence of the indicated ptsN-expressing plasmids but not the vector in both MC4100 and JD17 caused a modest impairment of their growth (data not shown): the reasons for this at present are ill understood.
FIG 6.
Complementation of the Ks of the ΔptsN mutant by plasmids encoding PtsN and those encoding PtsN bearing amino acid substitutions of its phosphorylation site histidine. Tenfold serial dilutions of MC4100 (parent) and its ΔptsN::Kan derivative, JD17 (ΔptsN), bearing the plasmids pHYD3025 (vector), pHYD5006 (PtrcptsN), pHYD5007 (PtrcptsNH73A), pHYD5008 (PtrcptsNH73D), and pHYD5009 (PtrcptsNH73E), were spotted on the surface of K1, K20, and K115 glucose agar plates. pHYD5006 encodes PtsN, whereas pHYD5007, pHYD5008, and pHYD5009 encode PtsN bearing the H73A, H73D, and H73E amino acid substitutions, respectively. The dashed arrows indicate the direction of increasing order of cell dilution.
Ks associated with the ΔptsN mutation persists in a strain bearing acetohydroxy acid synthase II.
Besides displaying the Ks, the ΔptsN mutant has been reported to be leucine sensitive (Leus) in a minimal medium at a [K+]e of 20 mM (25, 26, 49). In E. coli K-12, the activities of the two acetohydroxy acid synthases (AHASs) encoded by the ilvBN (AHASI) and ilvHI (AHASIII) operons constitute the primary committed step in the biosynthesis of branched-chain amino acids leucine, isoleucine, and valine (reviewed in reference 50). The Leus phenotype has been proposed to arise due to a combination of elevated intracellular l-leucine and K+ levels in the ΔptsN mutant that perturb cellular AHAS activity synergistically, giving rise to a state of isoleucine pseudoauxotrophy (26, 49). E. coli K-12 lacks AHASII due to the presence of a frameshift mutation in ilvG. One group has also reported that both the Leus phenotype and the Ks of the ΔptsN mutant are absent in a strain with an intact ilvG, implying that in E. coli K-12, elevated cellular K+ content in the ΔptsN mutant perturbs AHASI and AHASII activities, and the cellular casualty in the ΔptsN mutant is the pathway of branched-chain amino acid biosynthesis (26).
We found that the Ks of the ΔptsN mutant persisted in the presence of the ilvGM+ (ilvG+) allele, obtained from E. coli strain BL21(DE3) (51). While the ilvG+ derivative of MC4100, JD271, grew at comparable rates in K1, K20, K40, and K115 media, its ΔptsN::Kan derivative, JD274, displayed a Ks similar to that displayed by its ilvG ancestor, JD17 (see Fig. S4 and Table S2 in the supplemental material). In addition, the ΔptsN::Kan derivative of MC4100 bearing ilvG+ obtained from strain NCM3722 (52) also displayed Ks (data not shown). Finally, ΔptsN::Kan derivatives of MG1655 bearing ilvG+ obtained from both BL21(DE3) and NCM3722 exhibited Ks in comparison to their isogenic parents, and 5 mM concentrations each of leucine, isoleucine, and valine did not suppress the Ks of the ΔptsN::Kan derivative of MC4100, JD17 (data not shown).
DISCUSSION
In this study, we have interrogated the physiological defect that renders a strain lacking PtsN to display Ks. Our proposal on the physiological basis for Ks contrasts with an earlier study (25) in two ways, which are that (i) the Ks correlates with K+ limitation rather than a K+ overload, and (ii) the K+ limitation mediated by YcgO causes the Ks rather than hyperactivated Trk-mediated K+ uptake. Observations of the present study are discussed below, a model for K+ limitation in the ΔptsN mutant is presented, and the probable physiological role of YcgO is discussed.
Ks of the ΔptsN mutant correlates with K+ limitation.
The present work provides credible evidence that the ΔptsN mutant experiences a K+ limitation that causes its Ks. Although for the most part we have performed comparative studies with a pair of ptsN+ and ΔptsN mutant strains bearing the repressible Kdp and the low-affinity TrkF K+ uptake systems, the notion that the Ks correlates with K+ limitation would remain unaffected despite this choice of strains. Exacerbation of the Ks by removal of the constitutive K+ uptake systems (Fig. 1) is consistent with the notion that a K+ limitation causes the Ks. If a K+ overload was causal, then one would expect its absence to alleviate the Ks, which was not the case. Persistence of the Ks in the absence of TrkA indicates that TrkA-promoted hyperactivity of the Trk K+ uptake system is not the cause of the Ks, which is contrary to the suggestion of Lee et al. (25). In addition, Lee et al. have also not tested whether trkA deficiency suppresses Ks in their mutant.
Furthermore, Ks was correlated with reduced K+ content in high-[K+]e media (Table 2) and overexpression of multiple K+ uptake proteins suppressed the Ks (Fig. 3; see Fig. S1 and S2 in the supplemental material), with suppression by overexpression of kup leading to elevation of K+ content in the ΔptsN mutant (Table 2). Alleviation of Ks by overexpression of K+ uptake proteins is analogous to the relief from K+-limited growth provided by a K+ uptake system to a triple K+ transporter-defective strain (13). Among the K+ uptake proteins, the effect of amtBK expression on Ks is perhaps noteworthy. Expression of amtBK is associated with Ks (see Fig. S2A) due to increased cytoplasmic K+ pools (44). The presence of the two Ks-causing perturbations, namely, absence of PtsN and expression of amtBK, in one strain led to their annihilation (see Fig. S2A and B), indicating that their origins must be opposing.
The ΔptsN derivative of the kdp+ kup trk strain displayed an exacerbated Ks in media containing 20 to 40 mM K+ (Fig. 1C). However, the growth rate of this strain could be significantly improved by elevating the [K+]e (Fig. 1C and 2C). This growth pattern can be explained simply on the basis that as the [K+]e is increased, enhanced TrkF-mediated K+ uptake (2, 9, 16) to some extent counteracts the K+ limitation and additionally accounts for the increased growth rate of the ΔptsN derivative of the triple K+ transporter-defective strain as the [K+]e is increased from 20 to 115 mM (Fig. 1D). Enhanced TrkF activity also serves to explain the alleviation of the Ks of the ΔptsN derivative of the kdp+ kup trk strain by medium alkalinization, a positive modulator of TrkF activity (9) (Fig. 2B). Overall, these findings additionally conform to the proposal that Ks of the ΔptsN mutant correlates with K+ limitation.
YcgO is the mediator of Ks.
Suppression of the Ks of the ΔptsN mutant by the ΔycgO mutation (Fig. 4) and the Ks of ycgO overexpression (Fig. 5) are phenotypes that strongly implicate YcgO to be the mediator of the Ks of the ΔptsN mutant. Moreover, the ΔycgO mutation yielded a K+-related growth phenotype and affected cellular K+ content only in the absence of PtsN (Fig. 4; Tables 2 and 3), indicating that ordinarily YcgO activity is rendered cryptic in E. coli. The Ks of ycgO overexpression in ptsN+ strains bears striking similarities to the Ks of the ΔptsN mutant. Besides correlating with K+ limitation (Table 4), its severity could be modulated by constitutive K+ transporters (Fig. 5A to D) and suppressed by overexpression of kup (Table 4; see Fig. S3 in the supplemental material), adding credence to the notion that YcgO is the mediator of Ks. Finally, the absence of any overt alteration in the level of YcgO in the ΔptsN mutant (Fig. 5E) indicates that its Ks may result from enhanced YcgO activity. Overexpression of ycgO perhaps provokes activation of YcgO to yield the Ks.
PtsN in vivo can exist in the phosphorylated (phospho-PtsN) and the dephosphorylated (dephospho-PtsN) states, and two observations indicate that the Ks of the ΔptsN mutant may result from to the absence of dephospho-PtsN. First, the ΔptsP mutant, wherein PtsN is present in the dephosphorylated state (24, 53), did not display the Ks (data not shown), indicating that absence of phospho-PtsN does not cause Ks. Furthermore, expression of PtsN variants incapable of being phosphorylated (47, 48), and hence present in vivo in the dephosphorylated state, complemented the Ks (Fig. 6). Additional experimentation, though, will be required to clearly rule out the possibility that the Ks is not absolutely related to the absence of a particular phosphorylation state of PtsN but results from the absence of PtsN per se. We tentatively conclude that the KS results from the absence of dephospho-PtsN.
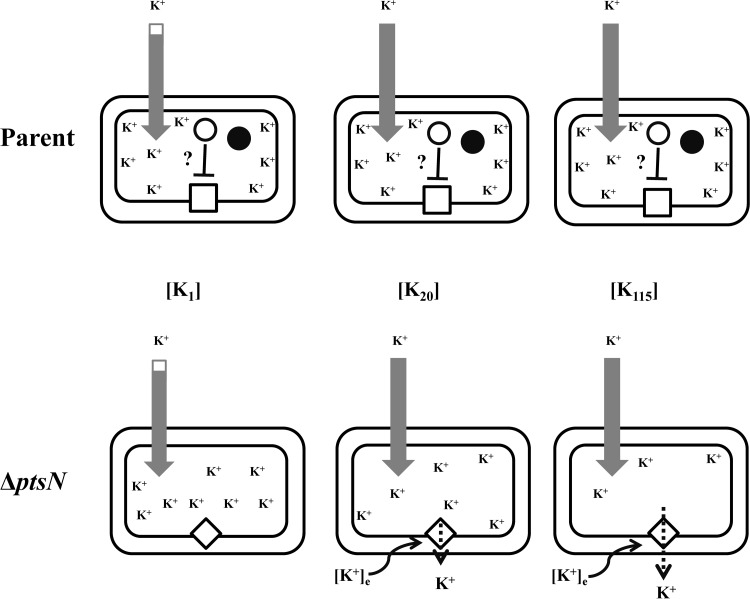
K+ limitation in the ΔptsN mutant—a model.
The Ks of the ΔptsN mutant and ycgO overexpression correlated with reduced cellular K+ content (Tables 2 and 4), which could occur if YcgO activity either inhibited overall K+ uptake or led to K+ efflux. The fact that the Ks could be modulated by the Trk and Kup status of the strain (Fig. 1 and 5A to D) and alleviated by expression of K+ uptake proteins (Fig. 3; see Fig. S1 to S3 in the supplemental material) indicates that the activity of YcgO may constitute a pathway for K+ efflux that is fettered, probably by dephospho-PtsN. If true, then a model for K+ limitation in the ΔptsN mutant can be outlined (Fig. 7). It is assumed that the magnitude of K+ efflux mediated by YcgO is lower than the flux of K+ uptake occurring separately through the Trk, Kup, and fully activated TrkF systems and is stimulated by [K+]e. A comparison of the growth rate curves for a pair of MC4100 ptsN+ and ΔptsN strains (Fig. 1A) with respect to [K+]e shows that the growth rate of the ΔptsN mutant decreases as the [K+]e is raised from 1 to 20 mM and remains at lower but a constant value with further increases in [K+]e. It is postulated that this decrease in growth rate corresponds to the onset of YcgO-mediated K+ efflux that attains a maximal value above a [K+]e of 20 mM. Growth rate decreases as described above are also apparent for the Δkdp ΔptsN double mutant JD662 (Fig. 1B) and for MC4100 and its Δkdp derivative overexpressing ycgO (Fig. 5A and B). The onset of YcgO-mediated K+ efflux is suggestive of a regulatory role for [K+]e in stimulation of YcgO activity. [K+]e-mediated regulation of the gating of the MscK (KefA) channel in E. coli (54) is a precedent for ionic regulation of transporter/channel activity. The [K+]e range above which YcgO-mediated K+ efflux reaches its maximum leads to K+ limitation and hence the Ks. Furthermore, the repression of expression of the Kdp system by [K+]e (15, 16) would serve to maintain a cytoplasmic K+ deficit. The exacerbation of the Ks of the ΔptsN mutant in the absence of TrkA and Kup (Fig. 1C) can be accounted for by this model.
FIG 7.
A model for K+ limitation mediated by YcgO in the ΔptsN mutant. Scheme depicting cellular K+ content in the parent strain and its reduction in the ΔptsN mutant. K+ content in the two strains is shown in media with [K+]es of 1 ([K1]), 20 ([K20]), and 115 ([K115]) mM. A double-colored arrow represents the contribution to cellular K+ content due to K+ uptake mediated by the Kdp (white) and the Trk plus Kup (gray) transporters, whereas a single-colored gray arrow represents K+ uptake occurring via the Trk and Kup transporters and reflects the repression of the Kdp system by [K+]e. The contribution of the TrkF activity to the cellular K+ content in strains bearing all K+ uptake systems is considered to be negligible. K+ efflux mediated by YcgO is represented as a dashed arrow, and its stimulation by [K+]e is represented by a wavy arrow. The heights of the arrows representing K+ uptake and efflux are proportional to their K+ transport fluxes. Dephospho-PtsN, presumed to fetter YcgO, and the phosphorylated form of PtsN are represented as open and solid circles, respectively. The open square and diamond, respectively, represent the fettered and unfettered states of YcgO.
In the Δkdp background, the ΔptsN mutation additionally retarded the growth rate in the [K+]e range below 10 mM (Fig. 1B), which was not suppressed by the ΔycgO mutation (Table 3). The reason for this growth retardation is not known, but it is indicative of a K+ limitation because it was absent in a Kdp+ strain (Fig. 1A) and may represent another aspect of K+ ion metabolism that is regulated by PtsN, one that occurs independent of YcgO and is apparent in the absence of Kdp.
A probable physiological role for YcgO.
The present work implies that in E. coli, YcgO is rendered cryptic, probably by dephospho-PtsN, whose absence leads to K+ limitation via YcgO-mediated K+ efflux. That the phenotype of the ΔycgO mutant was seen only in the absence of PtsN (Table 3) is consistent with this implication and is indicative of a specialized physiological role for YcgO. What is the likely physiological role of YcgO? An earlier study implicated a role for YcgO in growth adaptation to low medium osmolarity, because the ΔycgO mutant displayed highly impaired growth in certain low-osmolarity media (27). However, we did not observe any significant differences in the growth rates between MC4100 and its ΔycgO derivative in the low-osmolarity media employed in the aforementioned study (data not shown). YcgO belongs to the CPA1 family of proteins that mediate monovalent cation/proton antiport (55), and its orthologs in Vibrio parahaemolyticus (56) and Vibrio cholerae (57) have been reported to mediate K+ efflux by functioning as K+/H+ antiporters. It is thus plausible that YcgO-mediated K+ efflux may occur via K+/H+ antiport.
A role for K+ efflux as an adaptive, stress-relieving mechanism has previously been documented for the KefG/B and KefF/C K+/H+ (Kef) antiporters. K+ efflux via Kef proteins has been referred to as protective K+ efflux because it is thought to provide adaptation to cytoplasmic electrophile stress (8). Allowing for the notion that dephospho-PtsN fetters YcgO, by analogy to the Kef system it is possible that certain stresses alter the balance of the phosphorylation status of PtsN toward phospho-PtsN and lead to a decline in growth via YcgO-mediated K+ limitation. Impaired kdp expression resulting from the absence or lowered levels of dephospho-PtsN reported earlier (47) may then be rationalized as a factor in maintenance of K+ limitation. E. coli thus can reduce its growth rate via K+ limitation, serving as an adaptive response for stress tolerance. Our results therefore suggest that perhaps one of the functions of the PtsP-PtsO-PtsN phosphorelay is in the control of a stress-responsive K+ efflux pathway.
The PtsP-PtsO-PtsN phosphorelay appears to possess a sensing attribute, which is the presence of a GAF domain thought to function as a sensor of small molecules (58), located in the N terminus of PtsP (22). Recently glutamine and α-ketoglutarate were shown to reciprocally affect the phosphorylation status of PtsN, with the former elevating and the latter decreasing the level of dephospho-PtsN (24). Glutamine and α-ketoglutarate were shown to exert their effects by inhibiting and stimulating, respectively, a GAF domain-dependent autophosphorylation of PtsP (24). The ratio of glutamine and α-ketoglutarate is thought to be a measure of the state of the cell with regard to its nitrogen sufficiency or deficiency, with the ratios being high and low in nitrogen-rich and nitrogen-limiting environments, respectively (59). Given this, it has been proposed that the PtsP-PtsO-PtsN phosphorelay serves as a sensor of nitrogen availability (24). However, the reason or reasons for a linkage between nitrogen metabolism, the PtsP-PtsO-PtsN phosphorelay, and K+ metabolism remain somewhat obscure. Perhaps such a linkage may be realized in the context of a microbial community setting such as a biofilm, wherein a metabolic stress signal(s) conveyed via YcgO-mediated K+ efflux could be a means of propagating the signal within the community. Under this scenario, the postulated stimulation of YcgO K+ efflux by [K+]e could serve to amplify the signal. Recently, K+ efflux via the YugO K+ channel and its dispersal from the interior to the periphery of the Bacillus subtilis biofilm have been proposed to coordinate glutamate utilization by cells in the two spatially separated zones of the biofilm (60).
PtsN and AHAS activity.
In this study, performed with E. coli K-12 strains, K+ limitation mediated by YcgO is implicated to be the cause of the Ks of the ΔptsN mutant. However, an earlier study (26) implicates impaired AHASI and AHASIII activities in the E. coli K-12 ΔptsN mutant to be the cellular casualty of its Ks, due to the observation that the Ks could be alleviated by the presence of a functional AHASII that otherwise is absent in E. coli K-12 (50). That study made two presumptions, namely (i) that cellular K+ content is elevated in the ΔptsN mutant that perturbs AHASI and AHASIII activities and (ii) that AHASII is insensitive to elevated cellular K+ content (26). We found that the Ks persisted in the ΔptsN mutant bearing a functional AHASII (see Fig. S4 in the supplemental material) and was not alleviated by leucine, isoleucine, and valine supplementation, indicating that impaired AHAS activity may not be the sole correlate of the physiological defect in the ΔptsN mutant. The reason or reasons for this discrepancy are at present unclear but may be the result of a genetic variation or variations between the AHASII-bearing strains used in this study and the aforementioned study, which remain to be characterized.
In conclusion, this study implicates a role for PtsN in preventing K+ limitation in E. coli by a mechanism that may involve fettering of YcgO-mediated K+ efflux, probably by dephospho-PtsN. It is speculated that K+ limitation may be conditional such that under favorable growth conditions, futile efflux of K+ is prevented. YcgO-mediated K+ limitation may occur under certain physiological stresses that may exert its effects on the PtsP-PtsO-PtsN phosphorelay by shifting the balance between the two phosphorylation states of PtsN toward phospho-PtsN. The ensuing K+ limitation and reduced growth may serve as means to tolerate the stress. Future studies in this regard will need to establish unambiguously the phosphorylation state of PtsN that negatively regulates YcgO activity, in particular, whether YcgO displays selective interaction with a phosphorylated state of PtsN, and obtain direct evidence that YcgO functions as a K+ efflux protein.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lionello Bossi, Marc Dreyfus, Wolfgang Epstein, R. Harinarayanan, J. Gowrishankar, J. Krishna Leela, the late Sydney Kustu, H. Mori, and Barry Wanner for providing strains and plasmids used in this study and J. Gowrishankar and members of the Laboratory of Bacterial Genetics for advice. The hospitality of Kan Tanaka for providing laboratory space is gratefully acknowledged. We also thank Aatif Mehraj Kababi for construction of the chromosomal λInCh strains and the technical department of the Centre for Advanced Materials Analysis, Tokyo Institute of Technology, Yokohama, Japan.
R.S. and S.U. are recipients, respectively, of fellowships from the Department of Biotechnology and the University Grants Commission, Government of India, and are registered under the academic program of Manipal University.
Funding Statement
This work was supported by a Centre of Excellence in Microbial Biology research grant from the Department of Biotechnology, Government of India (to A.A.S.), and grants from the Department of Science and Technology, Government of India (to A.A.S.), and the Japan Society for the Promotion of Science (to T.S.) under the India-Japan Cooperative Science Programme.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01029-15.
REFERENCES
- 1.Csonka LN, Epstein W. 1996. Osmoregulation, p 1210–1223. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology 2nd ed, vol 1 American Society for Microbiology, Washington, DC. [Google Scholar]
- 2.Epstein W. 2003. The roles and regulation of potassium in bacteria. Prog Nucleic Acid Res Mol Biol 75:293–320. doi: 10.1016/S0079-6603(03)75008-9. [DOI] [PubMed] [Google Scholar]
- 3.Epstein W, Schultz SG. 1965. Cation transport in Escherichia coli. V. Regulation of cation content. J Gen Physiol 49:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richey B, Cayley DS, Mossing MC, Kolka C, Anderson CF, Farrar TC, Record MT Jr. 1987. Variability in the intracellular ionic environment of Escherichia coli: differences between in vitro and in vivo effects of ion concentrations on protein-DNA interactions and gene expression. J Biol Chem 262:7157–7164. [PubMed] [Google Scholar]
- 5.Dinnbier U, Limpinsel E, Schmid R, Bakker EP. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol 150:348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- 6.Cayley S, Lewis BA, Guttman HJ, Record MT Jr. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity: implications for protein-DNA interactions in vivo. J Mol Biol 222:281–300. doi: 10.1016/0022-2836(91)90212-O. [DOI] [PubMed] [Google Scholar]
- 7.Ninfa AJ. 2007. Regulation of carbon and nitrogen metabolism: adding regulation of ion channels and another second messenger to the mix. Proc Natl Acad Sci U S A 104:4243–4244. doi: 10.1073/pnas.0700325104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth IR. 29 March 2005. Glycerol and methylglyoxal metabolism. EcoSal Plus 2005 doi: 10.1128/ecosalplus.3.4.3. [DOI] [PubMed] [Google Scholar]
- 9.Buurman ET, McLaggan D, Naprstek J, Epstein W. 2004. Multiple paths for nonphysiological transport of K+ in Escherichia coli. J Bacteriol 186:4238–4245. doi: 10.1128/JB.186.13.4238-4245.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker EP, Booth IR, Dinnbier U, Epstein W, Gajewska A. 1987. Evidence for multiple K+ export systems in Escherichia coli. J Bacteriol 169:3743–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein W, Whitelaw V, Hesse J. 1978. A K+ transport ATPase in Escherichia coli. J Biol Chem 253:6666–6668. [PubMed] [Google Scholar]
- 12.Hesse JE, Wieczorek L, Altendorf K, Reicin AS, Dorus E, Epstein W. 1984. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A 81:4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laimins LA, Rhoads DB, Epstein W. 1981. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A 78:464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asha H, Gowrishankar J. 1993. Regulation of kdp operon expression in Escherichia coli: evidence against turgor as signal for transcriptional control. J Bacteriol 175:4528–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roe AJ, McLaggan D, O'Byrne CP, Booth IR. 2000. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol Microbiol 35:1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x. [DOI] [PubMed] [Google Scholar]
- 16.Laermann V, Cudic E, Kipschull K, Zimmann P, Altendorf K. 2013. The sensor kinase KdpD of Escherichia coli senses external K+. Mol Microbiol 88:1194–1204. doi: 10.1111/mmi.12251. [DOI] [PubMed] [Google Scholar]
- 17.Postma PW, Lengeler JW, Jacobson GR. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saier MH., Jr 2001. The bacterial phosphotransferase system: structure, function, regulation and evolution. J Mol Microbiol Biotechnol 3:325–327. [PubMed] [Google Scholar]
- 19.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutscher J, Ake FM, Derkaoui M, Zebre AC, Cao TN, Bouraoui H, Kentache T, Mokhtari A, Milohanic E, Joyet P. 2014. The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiol Mol Biol Rev 78:231–256. doi: 10.1128/MMBR.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell BS, Court DL, Inada T, Nakamura Y, Michotey V, Cui X, Reizer A, Saier MH Jr, Reizer J. 1995. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. Enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J Biol Chem 270:4822–4839. [DOI] [PubMed] [Google Scholar]
- 22.Reizer J, Reizer A, Merrick MJ, Plunkett G III, Rose DJ, Saier MH Jr. 1996. Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an enzyme I homologue that possesses a putative sensory transduction domain. Gene 181:103–108. doi: 10.1016/S0378-1119(96)00481-7. [DOI] [PubMed] [Google Scholar]
- 23.Rabus R, Reizer J, Paulsen I, Saier MH Jr. 1999. Enzyme INtr from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J Biol Chem 274:26185–26191. [DOI] [PubMed] [Google Scholar]
- 24.Lee CR, Park YH, Kim M, Kim YR, Park S, Peterkofsky A, Seok YJ. 2013. Reciprocal regulation of the autophosphorylation of enzyme INtr by glutamine and alpha-ketoglutarate in Escherichia coli. Mol Microbiol 88:473–485. doi: 10.1111/mmi.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CR, Cho SH, Yoon MJ, Peterkofsky A, Seok YJ. 2007. Escherichia coli enzyme IIANtr regulates the K+ transporter TrkA. Proc Natl Acad Sci U S A 104:4124–4129. doi: 10.1073/pnas.0609897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reaves ML, Rabinowitz JD. 2011. Characteristic phenotypes associated with ptsN-null mutants in Escherichia coli K-12 are absent in strains with functional ilvG. J Bacteriol 193:4576–4581. doi: 10.1128/JB.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verkhovskaya ML, Barquera B, Wikstrom M. 2001. Deletion of one of two Escherichia coli genes encoding putative Na+/H+ exchangers (ycgO) perturbs cytoplasmic alkali cation balance at low osmolarity. Microbiology 147:3005–3013. doi: 10.1099/00221287-147-11-3005. [DOI] [PubMed] [Google Scholar]
- 28.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 30.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein W, Davies M. 1970. Potassium-dependent mutants of Escherichia coli K-12. J Bacteriol 101:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein W, Kim BS. 1971. Potassium transport loci in Escherichia coli K-12. J Bacteriol 108:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer M, Baker TA, Schnitzler G, Deischel SM, Goel M, Dove W, Jaacks KJ, Grossman AD, Erickson JW, Gross CA. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev 53:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amann E, Ochs B, Abel KJ. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 35.Chang AC, Cohen SN. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 37.Kleckner N, Bender J, Gottesman S. 1991. Uses of transposons with emphasis on Tn10. Methods Enzymol 204:139–180. doi: 10.1016/0076-6879(91)04009-D. [DOI] [PubMed] [Google Scholar]
- 38.Groisman EA, Casadaban MJ. 1986. Mini-mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol 168:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papp-Wallace KM, Maguire ME. 2008. Regulation of CorA Mg2+ channel function affects the virulence of Salmonella enterica serovar Typhimurium. J Bacteriol 190:6509–6516. doi: 10.1128/JB.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frymier JS, Reed TD, Fletcher SA, Csonka LN. 1997. Characterization of transcriptional regulation of the kdp operon of Salmonella typhimurium. J Bacteriol 179:3061–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pathania A, Sardesai AA. 2015. Distinct paths for basic amino acid export in Escherichia coli: YbjE (LysO) mediates export of l-lysine. J Bacteriol 197:2036–2047. doi: 10.1128/JB.02505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dosch DC, Salvacion FF, Epstein W. 1984. Tetracycline resistance element of pBR322 mediates potassium transport. J Bacteriol 160:1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sardesai AA, Gowrishankar J. 2001. Improvement in K+-limited growth rate associated with expression of the N-terminal fragment of one subunit (KdpA) of the multisubunit Kdp transporter in Escherichia coli. J Bacteriol 183:3515–3520. doi: 10.1128/JB.183.11.3515-3520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall JA, Yan D. 2013. The molecular basis of K+ exclusion by the Escherichia coli ammonium channel AmtB. J Biol Chem 288:14080–14086. doi: 10.1074/jbc.M113.457952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon SC, Dorman CJ. 2010. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 46.Ortenberg R, Beckwith J. 2003. Functions of thiol-disulfide oxidoreductases in E. coli: redox myths, realities, and practicalities. Antioxid Redox Signal 5:403–411. doi: 10.1089/152308603768295140. [DOI] [PubMed] [Google Scholar]
- 47.Luttmann D, Heermann R, Zimmer B, Hillmann A, Rampp IS, Jung K, Gorke B. 2009. Stimulation of the potassium sensor KdpD kinase activity by interaction with the phosphotransferase protein IIANtr in Escherichia coli. Mol Microbiol 72:978–994. doi: 10.1111/j.1365-2958.2009.06704.x. [DOI] [PubMed] [Google Scholar]
- 48.Zimmer B, Hillmann A, Görke B. 2008. Requirements for the phosphorylation of the Escherichia coli EIIANtr protein in vivo. FEMS Microbiol Lett 286:96–102. doi: 10.1111/j.1574-6968.2008.01262.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee CR, Koo BM, Cho SH, Kim YJ, Yoon MJ, Peterkofsky A, Seok YJ. 2005. Requirement of the dephospho-form of enzyme IIANtr for derepression of Escherichia coli K-12 ilvBN expression. Mol Microbiol 58:334–344. doi: 10.1111/j.1365-2958.2005.04834.x. [DOI] [PubMed] [Google Scholar]
- 50.Salmon KA, Yang CR, Hatfield GW. 28 February 2006. Biosynthesis and regulation of the branched-chain amino acids. EcoSal Plus 2006 doi: 10.1128/ecosalplus.3.6.1.5. [DOI] [PubMed] [Google Scholar]
- 51.Studier FW, Moffatt BA. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 52.Soupene E, van Heeswijk WC, Plumbridge J, Stewart V, Bertenthal D, Lee H, Prasad D, Paliy O, Charernnoppakul P, Kustu S. 2003. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J Bacteriol 185:5611–5626. doi: 10.1128/JB.185.18.5611-5626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bahr T, Lüttmann D, März W, Rak B, Görke B. 2011. Insight into bacterial phosphotransferase system-mediated signaling by interspecies transplantation of a transcriptional regulator. J Bacteriol 193:2013–2026. doi: 10.1128/JB.01459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. 2002. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J 21:5323–5330. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saier MH Jr, Reddy VS, Tamang DG, Västermark A. 2014. The transporter classification database. Nucleic Acids Res 42(Database issue):D251–D258. doi: 10.1093/nar/gkt1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radchenko MV, Waditee R, Oshimi S, Fukuhara M, Takabe T, Nakamura T. 2006. Cloning, functional expression and primary characterization of Vibrio parahaemolyticus K+/H+ antiporter genes in Escherichia coli. Mol Microbiol 59:651–663. doi: 10.1111/j.1365-2958.2005.04966.x. [DOI] [PubMed] [Google Scholar]
- 57.Resch CT, Winogrodzki JL, Patterson CT, Lind EJ, Quinn MJ, Dibrov P, Häse CC. 2010. Putative Na+/H+ antiporter of Vibrio cholerae, Vc-NhaP2, mediates the specific K+/H+ exchange in vivo. Biochemistry 49:2520–2528. doi: 10.1021/bi902173y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez SE, Beavo JA, Hol WG. 2002. GAF domains: two-billion-year old molecular switches that bind cyclic nucleotides. Mol Interv 2:317–323. doi: 10.1124/mi.2.5.317. [DOI] [PubMed] [Google Scholar]
- 59.Senior PJ. 1975. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous-culture technique. J Bacteriol 123:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Suel GM. 2015. Ion channels enable electrical communication in bacterial communities. Nature 527:59–63. doi: 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.