Abstract
Kingella kingae is a common cause of invasive disease in young children and was recently found to produce a polysaccharide capsule containing N-acetylgalactosamine (GalNAc) and β-3-deoxy-d-manno-octulosonic acid (βKdo). Given the role of capsules as important virulence factors and effective vaccine antigens, we set out to determine the genetic determinants of K. kingae encapsulation. Using a transposon library and a screen for nonencapsulated mutants, we identified the previously identified ctrABCD (ABC transporter) operon, a lipA (kpsC)-like gene, a lipB (kpsS)-like gene, and a putative glycosyltransferase gene designated csaA (capsule synthesis type a gene A). These genes were found to be present at unlinked locations scattered throughout the genome, an atypical genetic arrangement for Gram-negative bacteria that elaborate a capsule dependent on an ABC-type transporter for surface localization. The csaA gene product contains a predicted glycosyltransferase domain with structural homology to GalNAc transferases and a predicted capsule synthesis domain with structural homology to Kdo transferases, raising the possibility that this enzyme is responsible for alternately linking GalNAc to βKdo and βKdo to GalNAc. Consistent with this conclusion, mutation of the DXD motif in the GalNAc transferase domain and of the HP motif in the Kdo transferase domain resulted in a loss of encapsulation. Examination of intracellular and surface-associated capsule in deletion mutants and complemented strains further implicated the lipA (kpsC)-like gene, the lipB (kpsS)-like gene, and the csaA gene in K. kingae capsule production. These data define the genetic requirements for encapsulation in K. kingae and demonstrate an atypical organization of capsule synthesis, assembly, and export genes.
INTRODUCTION
Kingella kingae is an emerging pediatric pathogen and is a common etiology of septic arthritis, osteomyelitis, and bacteremia in children 6 to 48 months of age. The pathogenesis of K. kingae disease begins with asymptomatic colonization of the posterior pharynx, followed by bacterial translocation across the pharyngeal epithelial barrier, invasion of the bloodstream, and dissemination to distant sites (1, 2). In previous work, our group described a series of surface factors expressed by K. kingae that influence adherence to epithelial cells in vitro, including type IV pili and a trimeric autotransporter protein called Knh. Adherence by our prototype K. kingae strain is impeded by a polysaccharide capsule containing N-acetylgalactosamine (GalNAc) and β-3-deoxy-d-manno-octulosonic acid (βKdo) with the structure [→3)-β-GalpNAc-(1→5)-β-Kdop-(2→]n, which masks the adhesive properties of Knh and is likely displaced when pili bind to the cell surface and retract (3, 4).
Polysaccharide capsules are often involved in virulence and possess functions that range from masking of bacterial surface antigens and protection against serum killing to prevention of desiccation and phagocytosis. Polysaccharide conjugate vaccines based on the capsular polysaccharides of Neisseria meningitidis, Haemophilus influenzae type b, and Streptococcus pneumoniae have dramatically reduced morbidity and mortality worldwide (5). The development of these vaccines has been among the most successful public health innovations of the last 25 years (6).
The gene clusters involved in the production of the Escherichia coli K1 and K5 polysaccharide capsules have been studied extensively and provide the basis for our current understanding of the genetic organization and regulation of so-called group 2 capsules (7). The modular, polycistronic organization of group 2 capsule gene clusters has been well established and includes region 1, region 2, and region 3 genes in E. coli (7). Region 1 and region 3 are highly conserved across different serotypes, and region 2 is serotype specific (8). Region 1 encodes components of the export apparatus called KpsF, KpsE, KpsD, and KpsU and also encodes βKdo transferases called KpsC and KpsS, which are responsible for synthesizing the poly-βKdo linker between the α-glycerophosphatidic acid capsule membrane anchor and the reducing terminus of the capsular polysaccharide polymer (7). Region 2 encodes the serotype-specific enzymes required for the synthesis of the capsule polymer. Region 3 encodes energy-dependent components of the ABC transporter called KpsM and KpsT. N. meningitidis also expresses a group 2 capsule and contains all genes involved in capsule biosynthesis at a single locus called the cps locus. In N. meningitidis, the genes in region A are responsible for the synthesis of the capsule polymer; the genes in region B are called lipA (ctrE) and lipB (ctrF) and serve the same assembly functions as E. coli kpsC and kpsS, respectively (9–12); and the genes in region C are called ctrABCD and encode the ABC-type transporter apparatus.
Given that capsules are classic virulence factors and attractive vaccine antigen candidates, we were interested in defining the machinery required for capsule biosynthesis in K. kingae. We have previously shown that K. kingae contains a ctrABCD capsule export operon that is necessary for capsule surface localization (3). However, unlike the cassette-like organization of most group 2-like capsules, there were no capsule-related genes near this K. kingae operon, suggesting that capsule genes in K. kingae may be unlinked and scattered throughout the genome. In this study, we aimed to define the genetic determinants of encapsulation in K. kingae, with a specific focus on the genes necessary for the synthesis of the GalNAc-Kdo capsular polymer in prototype strain 269-492. Using a transposon library mutagenesis screen and bioinformatic approaches, we identified a lipA (kpsC)-like gene, a lipB (kpsS)-like gene, and a putative glycosyltransferase gene designated csaA as the core genes required for encapsulation. Additional analysis demonstrated that the csaA gene product (CsaA) is a bifunctional glycosyltransferase essential for synthesizing the GalNAc-Kdo polymer.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are listed in Table 1. K. kingae strain 269-492 was isolated from the joint fluid of a child with septic arthritis at St. Louis Children's Hospital in St. Louis, MO, and expresses a polysaccharide capsule, as previously reported (3, 13). K. kingae strain KK01 is a stable natural variant of strain 269-492 that grows as a nonspreading, noncorroding colony type and was used as the parent strain in this study (3, 13). K. kingae strains were grown on chocolate agar (Chocolate II Agar; BD, Franklin Lakes, NJ) at 37°C with 5% CO2 supplemented with 50 μg/ml kanamycin or 2 μg/ml erythromycin, as appropriate. K. kingae strains were stored at −80°C in brain heart infusion (BHI) broth with 30% glycerol. E. coli strain DH5α was used for the construction of the gene disruptions and complementation constructs. E. coli strains were grown at 37°C on Luria-Bertani (LB) agar or in LB broth with 100 μg/ml ampicillin, 50 μg/ml kanamycin, or 500 μg/ml erythromycin, as appropriate. E. coli strains were stored at −80°C in LB broth with 15% glycerol. For expression induction experiments, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| Kingella kingae | ||
| 269-492 | K. kingae isolate from St. Louis Children's Hospital | 13 |
| KK01 | Stable nonspreading/noncorroding derivative of 269-492 | 13 |
| ctrA | KK01 with the aphA3 (Kanr) cassette inserted into ctrA, resulting in inactivation of ctrABCD | 3 |
| lipA | KK01 deletion of lipA, containing the aphA3 cassette | This study |
| lipB | KK01 deletion of lipB, containing the aphA3 cassette | This study |
| csaA | KK01 deletion of csaA, containing the aphA3 cassette | This study |
| lipA(lipA) | Inducible complement of lipA in the lipA background | This study |
| lipB(lipB) | Inducible complement of lipB in the lipB background | This study |
| csaA(csaA) | Inducible complement of csaA in the csaA background | This study |
| lipA pilA1 | lipA with the cat (Cmr) cassette disrupting pilA1, resulting in inactivation of pilA1 | This study |
| lipB pilA1 | lipB with a cat cassette disrupting pilA1, resulting in inactivation of pilA1 | This study |
| csaA pilA1 | csaA with a cat cassette disrupting pilA1, resulting in inactivation of pilA1 | This study |
| lipA(lipA) pilA1 | lipA(lipA) with a cat cassette disrupting pilA1, resulting in inactivation of pilA1 | This study |
| lipB(lipB) pilA1 | lipB(lipB) with a cat cassette disrupting pilA1, resulting in inactivation of pilA1 | This study |
| csaA(csaA) pilA1 | csaA(csaA) with a cat cassette disrupting pilA1, resulting in inactivation of pilA1 | This study |
| CsaAD99A | Amino acid change in the first D residue of the DXD motif | This study |
| CsaAD101A | Amino acid change in the second D residue of the DXD motif | This study |
| CsaAA99D* | Reversion of CsaAD99A to wild type | This study |
| CsaAA101D* | Reversion of CsaAD101A to wild type | This study |
| CsaAH518A | Amino acid change of the H residue of the HP motif | This study |
| CsaAP519A | Amino acid change of the P residue of the HP motif | This study |
| CsaAA518H* | Reversion of CsaAH518A to wild type | This study |
| CsaAA519P* | Reversion of CsaAP519A to wild type | This study |
| E. coli | ||
| DH5α | F− ϕ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1gyrA96 relA1 | 40 |
| BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm (DE3) | 41 |
| Plasmids | ||
| pUC19ctrA | ctrA disruption construct marked with the aphA3 cassette | 3 |
| pUC19lipA | lipA disruption construct marked with aphA3 | This study |
| pTrcCErmlipAcomp | Complementation vector containing full-length lipA | This study |
| pUC19lipB | lipB disruption construct marked with aphA3 | This study |
| pTrcCErmlipBcomp | Complementation vector containing full-length lipB | This study |
| pUC19csaA | csaA disruption construct marked with aphA3 | This study |
| pTrcCErmcsaAcomp | Complementation vector containing full-length csaA | This study |
| pTrcCErm | Complementation vector (pUC19) containing the trc promoter that targets recombination to complementation locus with homologous flanking regions; contains the ermC (Ermr) cassette | This study |
| pCsaA5′ | pUC19 containing the coding sequence for the CsaA N terminus and upstream 5′ sequence | This study |
| pCsaAD99A | pCsaA5′ containing a D99A point mutation | This study |
| pCsaAD101A | pCsaA5′ containing a D101A point mutation | This study |
| pCsaAA99D* | pCsaA5′ containing an A99D reversion | This study |
| pCsaAA101D* | pCsaA5′ containing an A101D reversion | This study |
| pCsaA3′ | pUC19 containing the coding sequence for the CsaA C terminus and downstream 3′ sequence | This study |
| pCsaAH518A | pCsaA5′ containing an H518A point mutation | This study |
| pCsaAP519A | pCsaA5′ containing a P519A point mutation | This study |
| pCsaAA518H* | pCsaA5′ containing an A518H reversion | This study |
| pCsaAA519P* | pCsaA5′ containing an A519P reversion | This study |
| pNtermCsaA | pET-15b containing csaA inserted into XhoI/BamHI sites | This study |
| pIND4 | Source of the ermC erythromycin resistance gene | 42 |
| pACYC184 | Source of the cat chloramphenicol resistance gene | 43 |
| pFalcon2 | Source of the aphA3 kanamycin resistance gene | 44 |
| pUC19pilA1 | pilA1 disruption construct marked with cat | This study |
Plasmid and strain construction.
Targeted gene disruptions and complementation constructs in K. kingae were generated as previously described (3, 14). Briefly, plasmid-based gene disruption constructs were created in E. coli, linearized, and introduced into K. kingae via natural transformation followed by plating onto chocolate agar containing the appropriate antibiotics. The gene disruptions and complementation constructs were confirmed by PCR. The ctrA and pamABCDE disruptions used in this study were generated previously (3, 4). To delete the lipA, lipB, and csaA loci, fragments corresponding to the surrounding 5′ and 3′ regions were PCR amplified by using the primers shown in Table 2, and a resistance cassette was ligated between the flanking regions. To complement the lipA, lipB, and csaA deletions, fragments corresponding to the open reading frames (ORFs) were PCR amplified by using primers specific to each gene (Table 2). The resulting fragments were ligated into a plasmid called ptrcCErm, which is a modified version of a previously described K. kingae complementation construct (3) and contains the Ptrc promoter and the lacI operator.
TABLE 2.
Primers used in this study
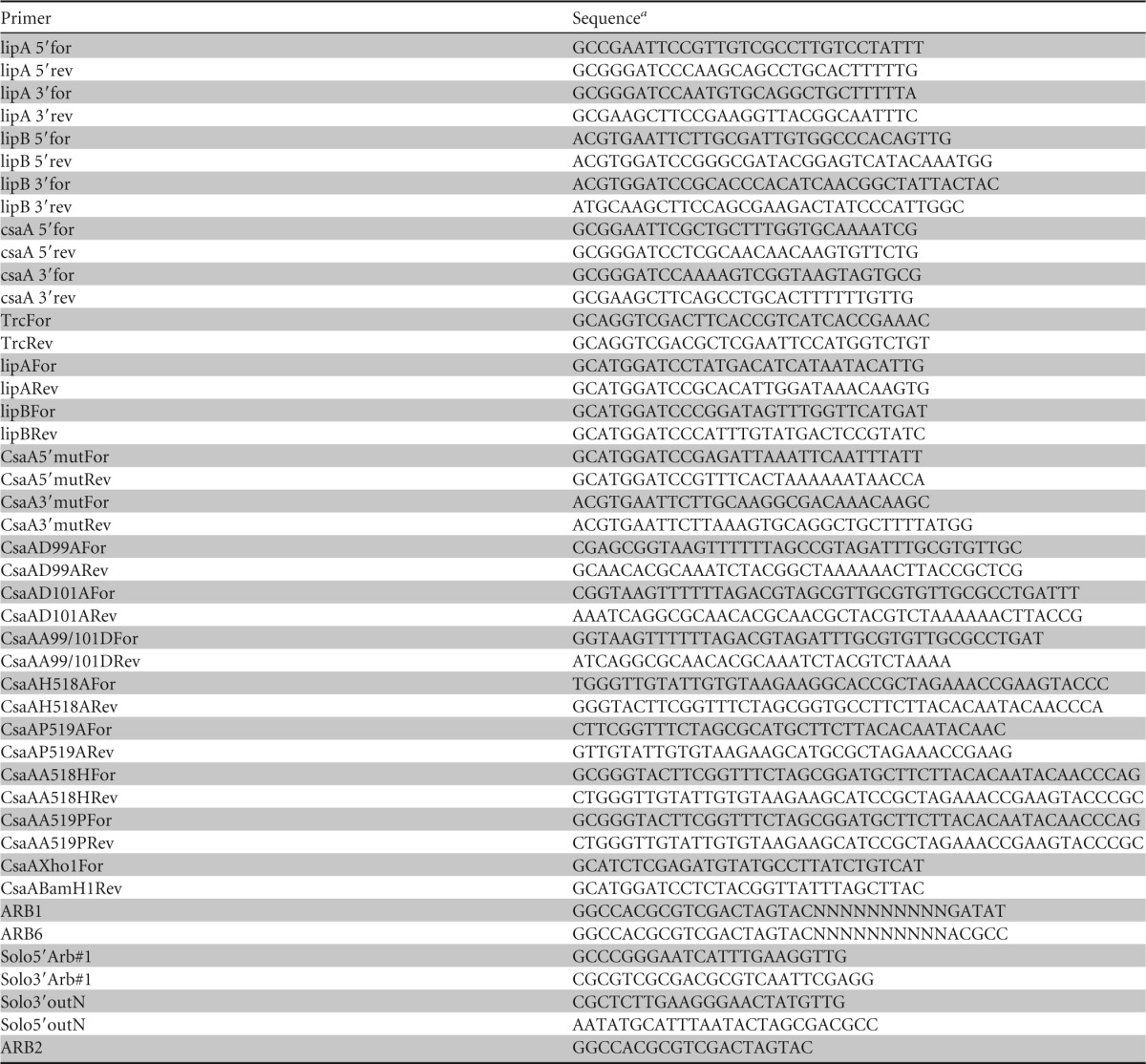
N represents any nucleotide.
To introduce site-directed mutations in csaA, we generated separate plasmids containing a coding sequence for the glycosyltransferase N-terminal region with a 5′-flanking sequence and a coding sequence for the C-terminal region with a 3′-flanking sequence using primers CsaA5′mutFor/CsaA5′mutRev and CsaA3′mutFor/CsaA3′mutRev, respectively. The resulting plasmids, called pCsaA5′ and pCsaA3′, were used as the templates for the mutagenesis reactions. The QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) was used to change the targeted amino acids in the DXD and HP motifs in the respective plasmids. The primers used in the mutagenesis and reversion reactions are listed in Table 2. Nucleotide sequencing was used to confirm that only the desired mutation was introduced.
Transposon screen.
DNA was purified from K. kingae strain KK01 by using the Wizard Genomic DNA kit (Promega, Madison, WI) according to the manufacturer's instructions. To create the transposon library, chromosomal DNA from K. kingae KK01 was mutagenized by using the Himar1 transposase and pFalcon2 as the source of the mariner transposon derivative containing an aphA3 kanamycin resistance cassette, as previously described (13). Following transposition, the transposed genomic DNA was dialyzed and transformed into K. kingae KK01, followed by recovery in BHI broth plus 16% horse serum for 2 h at 37°C. The reaction mixtures were then plated onto chocolate agar containing kanamycin for selection. To identify mutants lacking the polysaccharide capsule, we screened colonies by assessment of colony morphology and by alcian blue staining of colony blots. To obtain the colony blots, circular nitrocellulose membranes were applied to agar plates for 30 s and then lifted, stained with alcian blue for 1 h, and destained with 40% ethanol–5% acetic acid overnight. Following confirmation of nonencapsulated transformants, we sequenced out from the transposon to identify the insertion site using arbitrary PCR as previously described (13). The initial PCR was performed by using arbitrary primer ARB1 or ARB6 and specific primer Solo5′Arb#1 or Solo3′Arb#1 (Table 2). Solo5′Arb#1 anneals at the 5′ end of the Solo transposon, and Solo3′Arb#1 anneals at the 3′ end of the Solo transposon. In the second round of amplification, we utilized ARB2, which anneals to the 5′ end of ARB1 and ARB6, and Solo5′outN or Solo3′outN, which is external to Solo5′Arb#1 or Solo3′Arb#1, respectively. The PCR products from the second round of amplification were gel purified and sequenced (Eton Bioscience, San Diego, CA) by using either Solo5′outN or Solo3′outN, as appropriate.
Polysaccharide capsule extraction.
In preparation for capsule extraction, bacteria were grown overnight on chocolate agar. Subsequently, bacteria were resuspended in phosphate-buffered saline (PBS), washed once with PBS, and then resuspended in 50 mM Tris acetate (pH 5). After agitation for 1 h, bacteria were removed by centrifugation. The bacterial supernatants were collected, concentrated by using an Amicon Ultra centrifuge filter with a 10,000-molecular-weight cutoff, and then treated with proteinase K at 55°C for 1 h.
Thiobarbituric acid assay.
To quantify the amount of capsule extracted from the bacterial cell surface or present in whole bacterial lysates, samples were examined by using the thiobarbituric acid (TBA) assay as previously described (15, 16). Briefly, capsule material was hydrolyzed by the addition of strong acid (1.12 μl of 24% H2SO4 per 50-μl sample). A 50-μl sample of extracted polysaccharide was added to 25 μl of a 25 mM periodate solution. The tubes were inverted to mix and allowed to incubate at 37°C for 30 min. A 20-μl aliquot of 2% sodium arsenite in 0.5 N HCl was added, and the tubes were allowed to sit at room temperature until the yellow color disappeared. Subsequently, 200 μl of a 0.1 M thiobarbituric acid solution was added to the clear solution, and the tubes were placed into a boiling water bath for 7.5 min. Following boiling, the tubes were placed on ice for 5 min and then extracted with 700 μl butanol. After centrifugation at 6,000 × g, the top organic layer, consisting of a bright pink color, was measured for absorbance at 549 nm.
Staining of polysaccharide.
Aliquots of the capsule polysaccharide from K. kingae derivatives were separated on 7.5% SDS-PAGE gels and stained with 0.125% alcian blue as previously described (3, 4).
Glycosyltransferase characterization.
The full-length N-terminally His-tagged glycosyltransferase protein was induced in E. coli BL21(DE3) from plasmid pNtermCsaA. The induced protein was insoluble in inclusion bodies, which were harvested by centrifugation and separated on an SDS-PAGE gel. The major protein band corresponding to full-length CsaA was excised from the gel and sent to Cocalico Biologicals, Inc. (Reamstown, PA), for injection into a guinea pig and generation of a polyclonal antiserum (DUGP#115).
The full-length glycosyltransferase was detected in total membranes by Western blotting. Total membranes were recovered by centrifugation of cleared bacterial sonicates. The total membrane fraction was separated on a 7.5% SDS-PAGE gel, transferred to nitrocellulose, and probed with DUGP#115 at a dilution of 1:5,000, followed by an anti-guinea pig–horseradish peroxidase (HRP)-conjugated secondary antibody at a dilution of 1:10,000. The blot was developed by using a chemiluminescent substrate.
Adherence to Chang cells.
Bacterial adherence assays were performed with Chang cells (ATCC CCL-20.2) as previously described (3).
Transmission electron microscopy.
Samples of bacteria adherent to Chang cell monolayers in 24-well plates were prepared as previously described (3), except that the monolayers were grown on 7.8 mil (199 μm) Aclar embedding film inserts cut to fit the wells. Following incubation of bacteria and monolayers, samples were fixed with 5% glutaraldehyde in cacodylate buffer for 20 min at room temperature. The samples were washed once with cacodylate buffer and incubated in cacodylate buffer containing cationic ferritin (Sigma, St. Louis, MO) added to a final concentration of 1 mg/ml for 1 h at room temperature. Following incubation, the cationic ferritin solution was removed, and inserts were fixed in 5% glutaraldehyde for 2 h. The fixative was then removed, and the cells were washed 3 times with cacodylate buffer. Subsequently, the Aclar inserts were removed from the 24-well plates and placed into scintillation vials. Inserts were treated with 2% osmium tetroxide for 30 min and dehydrated in a graded series of dimethyl formamide (DMF) while progressively decreasing the temperature above the freezing point. (DMF has been reported to minimize the precipitation of polysaccharides [17].) The Aclar film was sandwiched between glass slides, infiltrated with LR white, and polymerized. After polymerization, the cells were mounted onto a resin block and sectioned. Thin sections were placed onto 200-mesh grids, stained with uranyl acetate and Reynolds lead citrate at room temperature, and examined by using a Philips CM-12 electron microscope.
Animal studies.
K. kingae strains were grown on chocolate agar for 17 to 18 h, and the bacteria were swabbed from plates and resuspended in PBS to a final density of 7.5 × 107 CFU/100 μl. Groups of eight nursing 5-day-old Sprague Dawley rat pups (Charles River Laboratories, Wilmington, MA) were injected via the intraperitoneal route with 100 μl of the appropriate bacterial suspension or 100 μl of PBS alone (control) using a 27 1/2-gauge needle and then returned to their cage with a lactating dam. The experimental and control groups were housed separately with a lactating dam and monitored for mortality and signs of illness twice daily for a total of 5 days. Animals that were found to be moribund were euthanized by using CO2 inhalation followed by secondary decapitation.
Ethics statement.
The animal studies were approved by The Children's Hospital of Philadelphia Institutional Animal Care and Use Committee (protocol IAC 13-001050) according to all federal and institutional guidelines.
RESULTS
Capsule biosynthesis genes are not restricted to a single locus.
To identify the genetic determinants of encapsulation in K. kingae strain 269-492, we generated a random transposon library in strain KK01, a stable nonspreading/noncorroding derivative of 269-492 with a prominent mucoid colony morphology. Nonencapsulated mutants of KK01 were identified based on a nonmucoid colony morphology and lack of staining with alcian blue in colony blots (18, 19). The resulting mutants were subjected to sequencing of the transposon insertion sites, revealing insertions in the ctrABCD capsule export operon that we described previously (3), a gene with homology to the N. meningitidis lipA gene and the E. coli kpsC gene involved in capsule assembly, a gene with homology to the N. meningitidis lipB gene and the E. coli kpsS gene involved in capsule assembly, and a gene encoding a hypothetical protein. Analysis of the homology of the hypothetical protein to other proteins revealed similarity to known and predicted glycosyltransferases, suggesting that this protein is involved in capsule polysaccharide synthesis. Further analysis revealed two distinct domains, including one in the N-terminal region with homology to GalNAc transferases and another in the middle region with homology to Kdo transferases. With this information in mind, we designated the gene encoding the hypothetical protein csaA for capsule synthesis region a gene A.
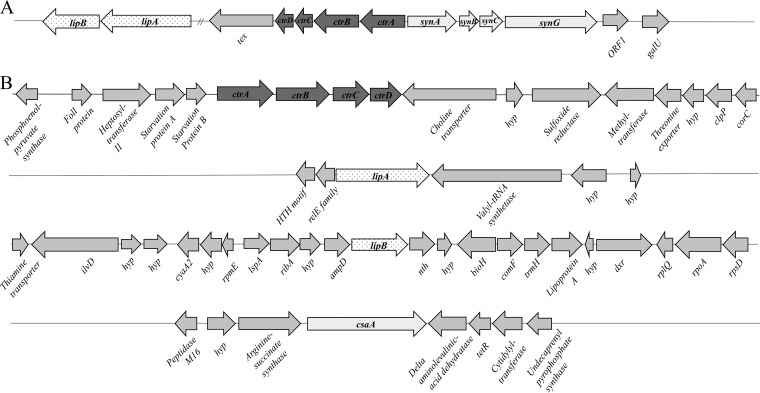
As shown in Fig. 1, the genes identified in our screen that are involved in capsule export, assembly, and probable synthesis are present in unlinked loci on the K. kingae chromosome, thus differing from the organization of capsule genes in N. meningitidis (20), E. coli, and other group 2 encapsulated bacteria.
FIG 1.
Location of the K. kingae capsule export/assembly/synthesis genes reveals an atypical organization. (A) The N. meningitidis serogroup W-135 capsule locus is composed of contiguous region A to C genes, including export (ctrABCD) (dark gray), assembly (lipA and lipB) (white with stippling), and synthesis (light gray) genes. (B) The homologous genes in K. kingae are shown in their disparate genetic locations in the corresponding shades. HTH, helix-turn-helix.
LipA and LipB affect capsule assembly, and CsaA affects polysaccharide synthesis.
To confirm that the genes identified in our screen are involved in encapsulation, we generated targeted deletions in lipA, lipB, and csaA. These deletions resulted in a loss of the mucoid colony phenotype, consistent with a loss of encapsulation. To extend these findings, surface polysaccharides were acid extracted from these mutants, separated on an SDS-PAGE gel, and stained with alcian blue to visualize extractable polysaccharide capsule. As shown in Fig. 2, targeted deletion of lipA, lipB, or csaA resulted in a loss of alcian blue staining of surface polysaccharide material compared to parent strain KK01, indicating a loss of encapsulation, analogous to a disruption of the ctrABCD operon.
FIG 2.
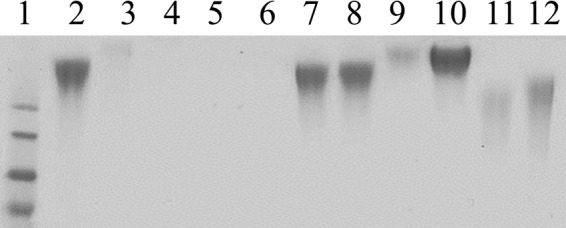
Alcian blue-stained gel of purified capsule material. Lane 1, ladder; lane 2, prototype strain KK01; lane 3, ctrA mutant; lane 4, lipA mutant; lane 5, lipB mutant; lane 6, csaA mutant; lane 7, lipA mutant complemented with intact lipA [lipA(lipA)]; lane 8, lipA mutant complemented with intact lipA induced with IPTG [lipA(lipA) IPTG]; lane 9, lipB mutant complemented with intact lipB [lipB(lipB)]; lane 10, lipB mutant complemented with intact lipB induced with IPTG [lipB(lipB) IPTG]; lane 11, csaA mutant complemented with intact csaA [csaA(csaA)]; lane 12, csaA mutant complemented with intact csaA induced with IPTG [csaA(csaA) IPTG].
Complementation of the lipA, lipB, and csaA deletions at a separate locus on the chromosome restored encapsulation, as visualized by colony morphology and alcian blue staining of surface polysaccharide material (Fig. 2). Slight differences in the mobility and intensity of the bands were observed for the surface extracts from the lipB(lipB) and csaA(csaA) complemented strains. Capsule material appeared to migrate through the gel slower than KK01 capsule in the lipB(lipB) complemented strain and faster than KK01 capsule in the csaA(csaA) complemented strain. Mass spectrometry analysis of the capsule material obtained from the complemented strains confirmed that the polysaccharides contained both GalNAc and Kdo, as expected (data not shown). Other investigators have suggested that the altered stoichiometry of LipA/KpsC to other capsule biosynthetic components may result in aberrant capsule chain length (21), raising the possibility that altered stoichiometry of capsule synthesis components through complementation at a nonnative locus may result in a similar change in capsule chain length.
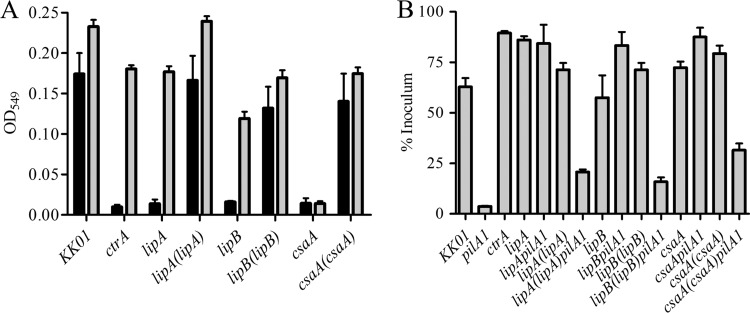
As another approach to quantify the amount of polysaccharide in the mutant and complemented strains, we measured thiobarbituric acid (TBA) reactivity. The TBA assay relies on measurement of sialic acids and related products, including Kdo, which represents half of the sugar content in the strain KK01 polysaccharide capsule. As shown in Fig. 3A, disruption of ctrA, lipA, or lipB resulted in a 90% reduction in TBA reactivity of surface-extractable material and a 20 to 60% reduction in TBA reactivity of whole-cell extracts in these mutants compared to the parent KK01 strain. Complementation of the lipA and lipB mutations restored TBA reactivity of surface extracts and whole-cell extracts to wild-type levels. We were unable to generate a complementation construct for the ctrA mutant, as previously reported (3). Unlike the ctrA, lipA, and lipB mutants, the csaA mutant demonstrated a marked reduction in TBA reactivity of both surface extracts and whole-cell extracts, suggesting the absence of intracellular polysaccharide pools. TBA reactivity was fully restored by complementation of the csaA deletion [csaA(csaA)].
FIG 3.
TBA reactivity and adherence as functional readouts of capsule loss and complementation. (A) TBA reactivity of mutant and complemented strains. Values for surface-extractable material are shown in black, and whole-cell reactivity values are shown in gray. OD549, optical density at 549 nm. (B) Adherence to Chang cells by mutant and complemented strains as a percentage of the inoculum. Bars represent the mean and standard error measurements from assays performed in biological and technical triplicate.
In previous work, we established that K. kingae encapsulation masks the adhesive properties of the Knh autotransporter protein when type IV pili are absent (3). To create a functional readout of encapsulation in the mutant and complemented strains, we eliminated pili by insertionally inactivating the pilA1 structural pilin gene and then measured Knh-mediated adherence to Chang epithelial cells. We predicted that mutant strains would be fully adherent and that the complemented strains would produce a capsule that masks Knh-mediated adherence like the capsule in the parent KK01 strain. As shown in Fig. 3B, the complemented lipA(lipA), lipB(lipB), and csaA(csaA) strains were fully adherent, similarly to parent strain KK01. In contrast, the lipA(lipA) pilA1, lipB(lipB) pilA1, and csaA(csaA) pilA1 complemented strains were minimally adherent, similarly to nonpiliated parent strain KK01, indicating the presence of a capsule capable of masking Knh in the absence of pili.
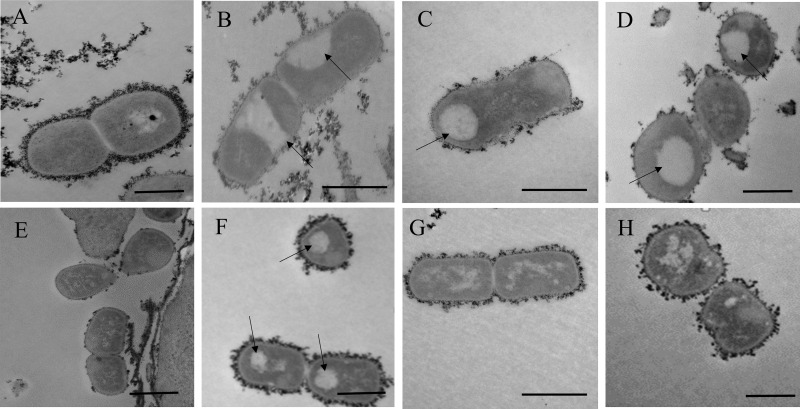
In order to visualize surface-associated capsule, we stained bacteria with cationic ferritin and performed thin-section transmission electron microscopy (Fig. 4). As shown in Fig. 4A, we observed a layer of cationic ferritin-stained material around parent strain KK01, as evidenced by the enhanced electron density. For the ctrA mutant, we observed a lack of cationic ferritin-stained surface polysaccharide (Fig. 4B). Instead, this mutant had intracellular pools of polysaccharide (lacunae) that appeared to be membrane associated, likely indicating that the intracellular polysaccharide is assembled and lipidated but is unable to be exported. For the lipA (Fig. 4C) and lipB (Fig. 4D) mutants, we also observed a lack of surface capsule and the presence of lacunae. Interestingly, in these mutants, the lacunae were in the interior of the cell and were not associated with the inner membrane, suggesting that the intracellular polysaccharide is unable to link to the inner membrane when LipA or LipB is absent, as observed for E. coli when KpsS or KpsC is absent (19). For the csaA mutant (Fig. 4E), we observed a lack of surface capsule and a lack of lacunae, supporting the conclusion that csaA plays a role in capsule synthesis.
FIG 4.
Thin-section transmission electron microscopy images of cationic ferritin-stained K. kingae. (A) KK01 parent; (B) ctrA mutant; (C) lipA mutant; (D) lipB mutant; (E) csaA mutant; (F) lipA mutant complemented with intact lipA [lipA(lipA)]; (G) lipB mutant complemented with intact lipB [lipB(lipB)]; (H) csaA mutant complemented with intact csaA [csaA(csaA)]. Arrows highlight lacunae. Bars, 500 nm.
All of the complemented strains had a restoration of surface capsule, as assessed by cationic ferritin staining of surface polysaccharide, with some strain-to-strain variability in the quantity of capsule. Complementation of lipA (Fig. 4F) resulted in an even layer of cationic ferritin staining on the cell surface despite some persistence of the intracellular pools. Complementation of lipB (Fig. 4G) resulted in a layer of cationic ferritin that resembled that of parent strain KK01 and small lacunae. Complementation of csaA (Fig. 4H) resulted in an interrupted layer of cationic ferritin.
Mutation of the CsaA DXD or HP motif results in loss of polysaccharide capsule.
Given the novelty of the CsaA glycosyltransferase, we used BLAST and PHYRE2 to compare its amino acid sequence and predicted protein structure to those of other known glycosyltransferases. At the N terminus of CsaA, we found a GT-A-type glycosyltransferase superfamily domain that has homology to the N-terminal domain of α-N-acetylgalactosaminyltransferase-T1, which catalyzes the transfer of α-N-acetylgalactosamine from UDP-GalNAc to Ser or Thr residues of core proteins to form the Tn antigen (GalNAc-α-1-O-Ser/Thr) in mice, suggesting that CsaA is a GalNAc transferase (22). In support of this hypothesis, CsaA has Asp residues at positions 99 and 101, similarly to the DXD motif in GT-A structural superfamily glycosyltransferases (23). To assess whether these Asp residues represent a critical DXD domain, we mutated these residues and examined the effects on encapsulation. Mutation of either Asp residue to Ala resulted in the loss of surface-extractable alcian blue staining and TBA-reactive material. Reversion of each Ala residue back to Asp restored capsule production (Fig. 5B and C). In the middle region of CsaA, we found a capsule synthesis domain with homology to the Acinetobacter baumannii WaaA Kdo transferase, which is responsible for the addition of Kdo from the activated CMP-Kdo to the 4′-phosphorylated lipid A precursor (24–26). This domain contains a His residue at position 518 and a Pro residue at position 519, suggesting an HP motif similar to the HP motif present in some sialyltransferases and the KpsC/LipA and KpsS/LipB βKdo transferases (21, 27). As shown in Fig. 5B and C, mutation of either the His residue at position 518 or the Pro residue at position 519 to Ala resulted in a loss of encapsulation. Reversion of these mutations restored encapsulation (Fig. 5B and C). None of the introduced mutations affected the CsaA protein level, as assessed by Western blotting (Fig. 5A). Together, these results indicate that the N-terminal and middle regions of CsaA likely function together in the synthesis of the GalNAc-Kdo polysaccharide capsule.
FIG 5.
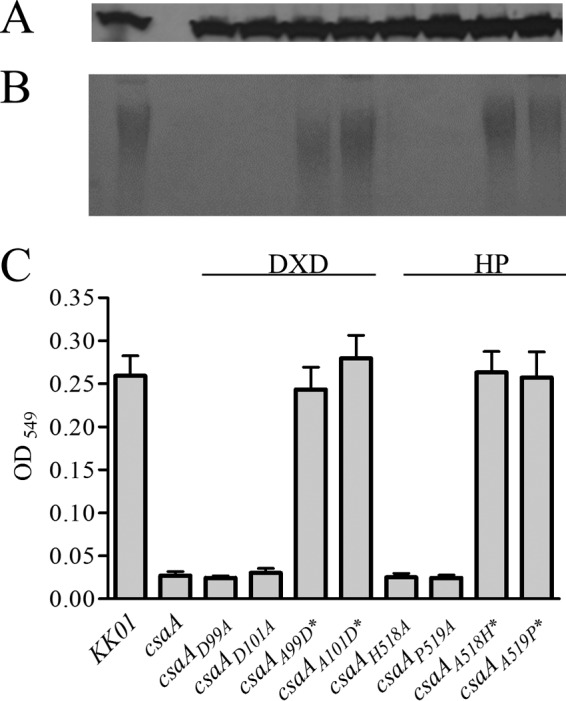
Characterization of the CsaA glycosyltransferase domain mutations. (A) CsaA Western blot analysis of total membrane preparations from K. kingae strains. (B) Alcian blue staining of acid-extracted capsule preparations resolved on an SDS-PAGE gel. (C) TBA reactivity of acid-extracted capsule preparations. Reversions are indicated with asterisks. DXD refers to the Asp-X-Asp motif, and HP refers to the His-Pro motif.
Capsule is required for K. kingae virulence in a juvenile rat infection model.
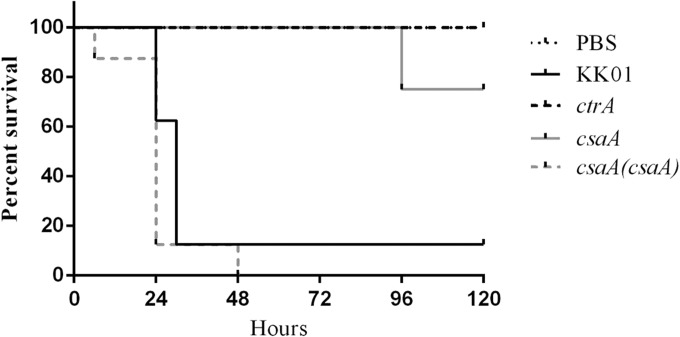
To begin to address the role of capsule in K. kingae virulence, we took advantage of the previously described juvenile rat infection model (28). Five-day-old Sprague-Dawley rats in groups of eight were injected via the intraperitoneal route with 7.5 × 107 CFU of the K. kingae KK01, ctrA, csaA, or csaA(csaA) strain or with PBS only as a control. Among pups infected with an encapsulated strain [KK01 or csaA(csaA)], all except one succumbed to infection during the 5-day observation period [7/8 pups infected with KK01 and 8/8 pups infected with the csaA(csaA) strain] (Fig. 6). Conversely, among pups infected with a nonencapsulated strain (ctrA or csaA), only 2 of 16 died (0/8 pups infected with the ctrA strain and 2/8 pups infected with the csaA strain). The 2 pups that died due to csaA strain infection succumbed at 96 h postinfection, while all 15 pups that died due to an encapsulated strain succumbed within 48 h of infection (Fig. 6). These results indicate that an intact csaA gene and encapsulation are essential for full K. kingae virulence in the juvenile rat infection model.
FIG 6.
Effect of encapsulation on virulence in the juvenile rat intraperitoneal infection model. Shown are Kaplan-Meyer survival curves for groups of eight 5-day-old Sprague-Dawley rats injected via the intraperitoneal route with 100 μl of PBS or 7.5 × 107 CFU of the KK01, ctrA, csaA, or csaA(csaA) strain in 100 μl PBS. The PBS control and the ctrA mutant have overlapping survival curves with 100% survival.
DISCUSSION
The pathogenesis of K. kingae disease is believed to begin with asymptomatic colonization of the upper respiratory tract, mediated via type IV pili and a trimeric autotransporter adhesin called Knh. Using prototype K. kingae strain 269-492, we have demonstrated that Knh is masked by a polysaccharide capsule with the structure [→3)-β-GalpNAc-(1→5)-β-Kdop-(2→]n. Given this unusual structure and the likely role of the capsule in K. kingae virulence, we set out to characterize the determinants of capsule biosynthesis. In this study, we used a transposon screen and identified genes involved in the synthesis, assembly, and export of the K. kingae polysaccharide capsule. Targeted gene deletion and complementation studies established that the csaA polysaccharide synthesis gene, the lipA and lipB capsule assembly genes, and the ctrABCD capsule export genes are required for encapsulation. Point mutations in csaA established that the CsaA glycosyltransferase is a novel bifunctional enzyme that creates a polymer of GalNAc and Kdo and is essential for creating both the β-GalpNAc-(1→5)-β-Kdop linkage and the β-Kdop-(2→3)-β-GalpNAc linkage.
Our results established that the csaA, lipA, lipB, and ctrABCD loci are located in four disparate regions of the chromosome and are surrounded by genes with no clear predicted function in capsule expression. While capsule genes are typically clustered together at a single locus on the chromosome, there are reports of atypical genetic arrangements of capsule genes in other bacteria. For example, a transposon screen in Vibrio vulnificus identified a region with 4 ORFs essential for capsule expression outside the central capsule polysaccharide locus (29). Similarly, a glycosyltransferase responsible for linking the heparosan backbone in the Pasteurella multocida capsule polysaccharide is encoded by a gene outside the capsule biosynthesis locus and is flanked by two metabolic genes with no known role in capsule polymer synthesis (alanine racemase and glucose-6-phosphate isomerase) (30). A glycosyltransferase required for encapsulation in Xanthomonas citri subsp. citri is encoded by the gpsX gene, which is also outside the locus with the other capsule biosynthesis genes (31). These examples highlight that capsule synthesis genes may reside in distinct regions of the chromosome and underscore the importance of understanding how the K. kingae capsule genes are coordinately regulated.
In recent work, Willis and Whitfield demonstrated that KpsS (LipB) and KpsC (LipA) in E. coli and N. meningitidis are βKdo transferases that synthesize a poly-βKdo linker onto a phosphatidylglycerol (PG) lipid moiety, serving as the capsule membrane anchor (21). KpsS/LipB adds a single βKdo residue to PG, and KpsC/LipA adds a short chain of βKdo residues onto the first βKdo residue. Current evidence suggests that the capsule polymer is then assembled onto the terminal βKdo residue of the poly-βKdo linker (21). Based on alcian blue staining of capsule extracts, TBA analysis, and transmission electron microscopy data, we conclude that LipA and LipB likely serve the same roles in K. kingae. The phenotypes of the lipA and lipB deletion mutants as assessed by TBA assays and transmission electron microscopy are consistent with previously reported observations of other organisms (21, 32, 33), including the lack of surface capsule and the presence of cytosolic lacunae. It is speculated that these lacunae result from aberrant initiation events, allowing a nonlipidated polymer to form and accumulate in the cytoplasm (21, 32).
Comparison of the K. kingae lipA complemented strain with the parent strain revealed no appreciable change in polysaccharide size on SDS-PAGE gels. Interestingly, functional complementation of an E. coli kpsC mutation with either E. coli kpsC or N. meningitidis lipA resulted in a longer polysaccharide. Comparison of the K. kingae lipB complemented strain with the parent strain revealed a difference in the mobility of the polysaccharide, suggesting that the polysaccharide length might be altered in this strain. However, the E. coli kpsS mutation complemented with either E. coli kpsS or N. meningitidis lipB did not result in a shift in mobility (21). One possible explanation for the difference in polysaccharide mobility observed in the lipB complemented strain is that the stoichiometry between the polysaccharide synthesis and transporter components may be altered from the wild-type state, a factor known to be important for capsule expression (34). Given that complemented genes were expressed under an IPTG-inducible promoter, competition between synthesis and transport could have affected our complementation strategy. Changes in transcriptional levels or stability could also alter other unknown targets, affecting capsule in subtle ways that we could not appreciate in our assays.
Based on homology analysis, the CsaA glycosyltransferase contains a putative GalNAc transferase domain and a putative βKdo transferase domain. The presence of these two domains suggests that CsaA is a bifunctional enzyme and is essential for synthesizing the GalNAc-βKdo-containing capsule polysaccharide, creating both the β-GalpNAc-(1→5)-β-Kdop linkage and the β-Kdop-(2→3)-β-GalpNAc linkage. Several other examples of bacterial glycosyltransferases with bifunctional activities have been identified over the years. In particular, the Streptococcus pyogenes Cap3B synthase (35) and HasA hyaluronan synthase have bifunctional activity (36). In addition, the E. coli KfiC protein is involved in the biosynthesis of the K5 capsule containing both α- and β-glycosyltransferase activities responsible for the sequential addition of glucuronic acid and GlcNAc to the growing polysaccharide chain (37). In S. pneumoniae, the Tts β-glucosyltransferase has been suggested to catalyze the 1,2- and 1,3-β-glucosidic capsule linkages (38).
The mariner transposon mutagenesis strategy used in this study generated ∼10,000 transposon insertion mutants. A thorough screen of this library identified the ctrABCD locus, lipA, lipB, and csaA as the only nonessential genes required for surface capsule expression in K. kingae strain 269-492. Interestingly, most group 2 capsule synthesis loci in E. coli contain a gene encoding a putative glycosyltransferase of unknown function (21). Willis and Whitfield speculated that this gene product is a capsule type-specific component of the synthesis machinery necessary to add the first sugar residue onto the terminal βKdo residue of the poly-βKdo linker to serve as an appropriate acceptor for the synthesis of the capsular polymer repeating unit (21). We speculate that the N-terminal domain of CsaA is able to directly catalyze the addition of GalNAc onto the terminal βKdo residue of the poly-βKdo linker, in particular given that the normal acceptor sugar for the GalNAc transferase activity is βKdo, which is added by the middle region domain of CsaA. The possibility that CsaA by itself is sufficient for the synthesis of the capsule polymer onto the poly-βKdo linker as well as the polymerization of the repeating unit would explain why we did not identify any other glycosyltransferases in our screen for nonencapsulated mutants. Other genes involved in modulating capsule length or levels of expression may not have been identified in our screen for various reasons, including lethality, redundancy, compensatory mutations, or partial phenotypes that we could not appreciate using our screening approach.
Most encapsulated bacteria exhibit intraspecies capsule-type diversity, with different strains producing capsules composed of a variety of sugars and glycosidic linkages. This phenomenon has been well characterized for many human pathogens, including E. coli, N. meningitidis, H. influenzae, and S. pneumoniae, among others. Bendaoud et al. reported a different capsule polysaccharide in K. kingae strain PYKK181, with the structure [→6)-α-d-GlcNAcp-(1→5)-β-Kdo-(2→]n (39), indicating that K. kingae expresses at least two distinct capsule types. In future studies, it will be important to examine larger collections of clinical isolates to define the diversity of capsule types in K. kingae.
ACKNOWLEDGMENTS
We are grateful to Sara Miller and Neil Medvitz in the Duke Electron Microscopy Service for assistance with transmission electron microscopy and to Parastoo Azadi at the Georgia Complex Carbohydrate Research Center for assistance with carbohydrate composition analysis.
REFERENCES
- 1.Yagupsky P, Porsch E, St Geme JW III. 2011. Kingella kingae: an emerging pathogen in young children. Pediatrics 127:557–565. doi: 10.1542/peds.2010-1867. [DOI] [PubMed] [Google Scholar]
- 2.Yagupsky P, Porat N, Pinco E. 2009. Pharyngeal colonization by Kingella kingae in children with invasive disease. Pediatr Infect Dis J 28:155–157. doi: 10.1097/INF.0b013e318184dbb8. [DOI] [PubMed] [Google Scholar]
- 3.Porsch E, Kehl-Fie T, St Geme JW III. 2012. Modulation of Kingella kingae adherence to human epithelial cells by type IV pili, capsule, and a novel trimeric autotransporter. mBio 3:e00372–12. doi: 10.1128/mBio.00372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starr KF, Porsch EA, Heiss C, Black I, Azadi P, St Geme JW III. 2013. Characterization of the Kingella kingae polysaccharide capsule and exopolysaccharide. PLoS One 8:e75409. doi: 10.1371/journal.pone.0075409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldblatt D. 2000. Conjugate vaccines. Clin Exp Immunol 119:1–3. doi: 10.1046/j.1365-2249.2000.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiden MCJ. 2013. The impact of protein-conjugate polysaccharide vaccines: an endgame for meningitis? Philos Trans R Soc Lond B Biol Sci 368:20120147. doi: 10.1098/rstb.2012.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts IS. 1996. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol 50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 8.Clarke BR, Pearce R, Roberts IS. 1999. Genetic organization of the Escherichia coli K10 capsule gene cluster: identification and characterization of two conserved regions in group III capsule gene clusters encoding polysaccharide transport functions. J Bacteriol 181:2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng Y, Datta A, Strole C, Lobritz M, Carlson R, Stephens D. 2005. Translocation and surface expression of lipidated serogroup B capsular polysaccharide in Neisseria meningitidis. Infect Immun 73:1491–1505. doi: 10.1128/IAI.73.3.1491-1505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitfield C, Roberts IS. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol Microbiol 31:1307–1319. doi: 10.1046/j.1365-2958.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 11.Whitfield C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 12.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, Frasch CE, Stephens DS, Feavers I, Frosch M, Parkhill J, Vogel U, Quail MA, Bentley SD, Maiden MCJ. 2013. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis 19:566–573. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehl-Fie TE, St Geme JW III. 2007. Identification and characterization of an RTX toxin in the emerging pathogen Kingella kingae. J Bacteriol 189:430–436. doi: 10.1128/JB.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehl-Fie TE, Porsch EA, Miller SE, St Geme JW III. 2009. Expression of Kingella kingae type IV pili is regulated by sigma 54, PilS, and PilR. J Bacteriol 191:4976–4986. doi: 10.1128/JB.00123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren L. 1959. The thiobarbituric acid assay of sialic acids. J Biol Chem 234:1971–1975. [PubMed] [Google Scholar]
- 16.Straus DC, Lonon MK, Woods DE, Garner CW. 1990. 3-Deoxy-D-manno-2-octulosonic acid in the lipopolysaccharide of various strains of Pseudomonas cepacia. J Med Microbiol 33:265–269. doi: 10.1099/00222615-33-4-265. [DOI] [PubMed] [Google Scholar]
- 17.Bayer ME, Carlemalm E, Kellenberger E. 1985. Capsule of Escherichia coli K29: ultrastructural preservation and immunoelectron microscopy. J Bacteriol 162:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austrian R. 1953. Morphologic variation in pneumococcus: an analysis of the basis for morphologic variation in pneumococcus and description of a hitherto undefined morphologic variant. J Exp Med 98:21–34. doi: 10.1084/jem.98.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radke KL, Siegel EC. 1971. Mutation preventing capsular polysaccharide synthesis in Escherichia coli K-12 and its effect on bacteriophage resistance. J Bacteriol 106:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan-Livengood JM, Miller YK, Martin LE, Urwin R, Stephens DS. 2003. Genetic basis for nongroupable Neisseria meningitidis. J Infect Dis 187:1616–1628. doi: 10.1086/374740. [DOI] [PubMed] [Google Scholar]
- 21.Willis LM, Whitfield C. 2013. KpsC and KpsS are retaining 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules. Proc Natl Acad Sci U S A 110:20753–20758. doi: 10.1073/pnas.1312637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz TA, Hurley JH, Trinh L-B, Shiloach J, Tabak LA. 2004. The beginnings of mucin biosynthesis: the crystal structure of UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltransferase-T1. Proc Natl Acad Sci U S A 101:15307–15312. doi: 10.1073/pnas.0405657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breton C, Šnajdrová L, Jeanneau C, Koča J, Imberty A. 2006. Structures and mechanisms of glycosyltransferases. Glycobiology 16:29R–37R. [DOI] [PubMed] [Google Scholar]
- 24.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. 2015. The Phyre2 Web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth KM, Marchant A. 2013. Conservation of the 2-keto-3-deoxymanno-octulosonic acid (Kdo) biosynthesis pathway between plants and bacteria. Carbohydr Res 380:70–75. doi: 10.1016/j.carres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt H, Hansen G, Singh S, Hanuszkiewicz A, Lindner B, Fukase K, Woodard RW, Holst O, Hilgenfeld R, Mamat U, Mesters JR. 2012. Structural and mechanistic analysis of the membrane-embedded glycosyltransferase WaaA required for lipopolysaccharide synthesis. Proc Natl Acad Sci U S A 109:6253–6258. doi: 10.1073/pnas.1119894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freiberger F, Claus H, Günzel A, Oltmann-Norden I, Vionnet J, Mühlenhoff M, Vogel U, Vann WF, Gerardy-Schahn R, Stummeyer K. 2007. Biochemical characterization of a Neisseria meningitidis polysialyltransferase reveals novel functional motifs in bacterial sialyltransferases. Mol Microbiol 65:1258–1275. doi: 10.1111/j.1365-2958.2007.05862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basmaci R, Yagupsky P, Ilharreborde B, Guyot K, Porat N, Chomton M, Thiberge J-M, Mazda K, Bingen E, Bonacorsi S, Bidet P. 2012. Multilocus sequence typing and rtxA toxin gene sequencing analysis of Kingella kingae isolates demonstrates genetic diversity and international clones. PLoS One 7:e38078. doi: 10.1371/journal.pone.0038078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AB, Siebeling RJ. 2003. Identification of genetic loci required for capsular expression in Vibrio vulnificus. Infect Immun 71:1091–1097. doi: 10.1128/IAI.71.3.1091-1097.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeAngelis PL, White CL. 2004. Identification of a distinct, cryptic heparosan synthase from Pasteurella multocida types A, D, and F. J Bacteriol 186:8529–8532. doi: 10.1128/JB.186.24.8529-8532.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Wang N. 2012. The gpsX gene encoding a glycosyltransferase is important for polysaccharide production and required for full virulence in Xanthomonas citri subsp. citri. BMC Microbiol 12:31. doi: 10.1186/1471-2180-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steenbergen SM, Vimr ER. 2008. Biosynthesis of the Escherichia coli K1 group 2 polysialic acid capsule occurs within a protected cytoplasmic compartment. Mol Microbiol 68:1252–1267. doi: 10.1111/j.1365-2958.2008.06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cieslewicz M, Vimr E. 1996. Thermoregulation of kpsF, the first region 1 gene in the kps locus for polysialic acid biosynthesis in Escherichia coli K1. J Bacteriol 178:3212–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield C, Amor PA, Koplin R. 1997. Modulation of the surface architecture of Gram-negative bacteria by the action of surface polymer:lipid A-core ligase and by determinants of polymer chain length. Mol Microbiol 23:629–638. doi: 10.1046/j.1365-2958.1997.2571614.x. [DOI] [PubMed] [Google Scholar]
- 35.Arrecubieta C, López R, García E. 1996. Type 3-specific synthase of Streptococcus pneumoniae (Cap3B) directs type 3 polysaccharide biosynthesis in Escherichia coli and in pneumococcal strains of different serotypes. J Exp Med 184:449–455. doi: 10.1084/jem.184.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeAngelis PL, Weigel PH. 1994. Immunochemical confirmation of the primary structure of streptococcal hyaluronan synthase and synthesis of high molecular weight product by the recombinant enzyme. Biochemistry 33:9033–9039. doi: 10.1021/bi00197a001. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths G, Cook NJ, Gottfridson E, Lind T, Lidholt K, Roberts IS. 1998. Characterization of the glycosyltransferase enzyme from the Escherichia coli K5 capsule gene cluster and identification and characterization of the glucuronyl active site. J Biol Chem 273:11752–11757. doi: 10.1074/jbc.273.19.11752. [DOI] [PubMed] [Google Scholar]
- 38.Llull D, Garcia E, Lopez R. 2001. Tts, a processive beta-glucosyltransferase of Streptococcus pneumoniae, directs the synthesis of the branched type 37 capsular polysaccharide in pneumococcus and other gram-positive species. J Biol Chem 276:21053–21061. doi: 10.1074/jbc.M010287200. [DOI] [PubMed] [Google Scholar]
- 39.Bendaoud M, Vinogradov E, Balashova N, Kadouri D, Kachlany S, Kaplan J. 2011. Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J Bacteriol 193:3879–3886. doi: 10.1128/JB.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 41.Studier F, Moffatt B. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton HL, Schwartz KJ, Dillard JP. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J Bacteriol 183:4718–4726. doi: 10.1128/JB.183.16.4718-4726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang AC, Cohen SN. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendrixson D, Akerley B, DiRita V. 2001. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol Microbiol 40:214–224. doi: 10.1046/j.1365-2958.2001.02376.x. [DOI] [PubMed] [Google Scholar]