Abstract
Brucella abortus is an intracellular pathogen of monocytes, macrophages, dendritic cells, and placental trophoblasts. This bacterium causes a chronic disease in bovines and in humans. In these hosts, the bacterium also invades neutrophils; however, it fails to replicate and just resists the killing action of these leukocytes without inducing significant activation or neutrophilia. Moreover, B. abortus causes the premature cell death of human neutrophils. In the murine model, the bacterium is found within macrophages and dendritic cells at early times of infection but seldom in neutrophils. Based on this observation, we explored the interaction of mouse neutrophils with B. abortus. In contrast to human, dog, and bovine neutrophils, naive mouse neutrophils fail to recognize smooth B. abortus bacteria at early stages of infection. Murine normal serum components do not opsonize smooth Brucella strains, and neutrophil phagocytosis is achieved only after the appearance of antibodies. Alternatively, mouse normal serum is capable of opsonizing rough Brucella mutants. Despite this, neutrophils still fail to kill Brucella, and the bacterium induces cell death of murine leukocytes. In addition, mouse serum does not opsonize Yersinia enterocolitica O:9, a bacterium displaying the same surface polysaccharide antigen as smooth B. abortus. Therefore, the lack of murine serum opsonization and absence of murine neutrophil recognition are specific, and the molecules responsible for the Brucella camouflage are N-formyl-perosamine surface homopolysaccharides. Although the mouse is a valuable model for understanding the immunobiology of brucellosis, direct extrapolation from one animal system to another has to be undertaken with caution.
INTRODUCTION
Polymorphonuclear neutrophil leukocytes (PMNs) are the first line of defense of the innate immune system. These cells detect microbial structures through various receptors, and the recognition of these structures influences their activation and fate, which are essential to promote inflammatory responses and host defense mechanisms.
Brucellosis is a chronic disease of domestic and wildlife mammals and a worldwide human zoonosis caused by Brucella species (1). Members of this genus are intracellular pathogens that invade monocytes (Mo), macrophages (Mϕ), and dendritic cells (DCs), as well as placental trophoblasts (1). Although in the natural host and in humans Brucella also invades PMNs (2–4), the bacterium fails to replicate in these cells and just resists their killing action without inducing significant activation (3–6). Indeed, Brucella-infected PMNs do not degranulate and induce low levels of reactive oxygen species (ROS) and cytokines, and the infection follows its course without significant neutrophilia (5, 7, 8). Likewise, the PMNs infiltration of the cervical lymph nodes after oral infection is very low, even in well-developed granulomas after 15 days of infection (9). Moreover, after 5 days of infection, the bacterium is found within Mϕ and DCs of mice but seldom inside PMNs in the target organs (10). In addition, the absence of PMNs during brucellosis promotes the activation of the Th1 adaptive immune response (11). More significantly, Brucella is capable of inducing the premature cell death of human PMNs by means of its lipopolysaccharide (Br-LPS) through a mechanism that involves CD14 and mild NADPH oxidase activation (6). This has led to the proposal that Brucella-infected PMNs function as “Trojan horses” after nonphlogistic phagocytosis by Mϕ and DCs. This would favor the spread of bacteria to different organs, fostering the chronicity of the disease (6).
The mouse has been the preferred animal model in brucellosis research to test and evaluate different hypotheses (12, 13). Here, we have explored the interaction of naive mouse PMNs with Brucella abortus and found that these cells fail to recognize this bacterium in the absence of antibodies. This is significant since the outcome of brucellosis in a given animal species may be determined during the initial stages of the infection that influence the downstream events of the immune response.
MATERIALS AND METHODS
Ethics.
Experimentation in mice was conducted with the consent of and according to guidelines established by the ‘Comité Institucional para el Cuido y Uso de los Animales de la Universidad de Costa Rica (CICUA-47-12) and in accordance with the corresponding law, Ley de Bienestar de los Animales, of Costa Rica (Law 7451 on Animal Welfare). Mice were accommodated in the animal building at the Veterinary Medicine School of the National University, Costa Rica. All animals were kept in cages with food and water ad libitum under biosafety containment conditions, previous to and during the experiment. Blood from roaming dogs kept at the shelter of the Hospital of the Veterinary Medicine School of the National University, Costa Rica, was taken for routine diagnostic purposes, according to the respective consent and approval (SIA 0434-14) and the guidelines established by the Ley de Bienestar de los Animales of Costa Rica (Law 7451 on Animal Welfare).
Human blood samples were collected from volunteer donors at the Tropical Disease Research Program (PIET), of the National University, Costa Rica, according to the corresponding institutional approval (SIA 0248-13). Accordingly, volunteers were carefully informed regarding the study and provided written consent. Samples were taken following the procedures dictated by the Costa Rican National Health system (Ley 9234, Costa Rica, La Gaceta 79, 2014) and by the World Medical Association (WMA) Declaration of Helsinki (59th WMA General Assembly, Seoul, October 2008), regarding the use of blood samples.
Experimental animals.
Wild-type Mus musculus animals were captured from the grounds of the university campus of the National University, Costa Rica. C57BL/6, BALB/c, and CD-1 mice (18 to 21 g) were provided by the following animal facilities: Instituto Clodomiro Picado, University of Costa Rica; School of Veterinary Medicine, National University, Costa Rica; and Laboratorio de Ensayos Biológicos, University of Costa Rica.
Bacterial strains and Br-LPS preparations.
Virulent B. abortus 2308, B. abortus 2308 ΔwadC, B. abortus 2308 Δper (14), B. abortus 2308 expressing green fluorescent protein (B. abortus 2308-GFP) (15), transgenic B. abortus 2308 with an integrated chromosomal gene coding for the red fluorescent protein (B. abortus 2308-RFP) from Discosoma coral (provided by Jean-Jacques Letesson, Unité de Recherche en Biologie Moléculaire, Facultés Universitaires Notre-Dame de la Paix, Namur, Belgium), Yersinia enterocolitica O:9 (16), Staphylococcus aureus (ATCC 25923), and Escherichia coli (ATCC 25922) were grown in tryptic soy broth as previously described (15). Purified Br-LPS suspensions were prepared from B. abortus 2308, as reported elsewhere (17).
Immunization and immune serum production against B. abortus.
C57BL/6 and CD-1 mice were intraperitoneally infected with 0.1 ml of phosphate-buffered saline (PBS) containing 106 CFU of virulent B. abortus 2308 bacteria. Mice were bled at different times, and antibody titration was carried out by microagglutination in 96-well round-bottom plastic plates. Briefly, Rose Bengal antigen was diluted 1/20 in PBS and used as a bacterial suspension for agglutination. Volumes of 50 μl of serum dilution were added to 50 μl of antigen suspension. Samples were incubated at 4°C for 24 h, and bacterial agglutination was recorded in the bottom of the plate. Agglutination titers beyond 1/50 were considered positive.
For immune serum production, mice were infected as described above; after 30 days of infection mice were bled, and serum was separated by centrifugation and filtered through a 0.2-μm-pore-size membrane (Millipore). Serum was then stored at −20°C in aliquots. IgGs were purified from immune mouse serum as reported elsewhere (18). Western blotting of mouse immune serum revealed that most of the antibody recognition was directed against Br-LPS (16). For ex vivo opsonization experiments, subagglutinating doses of antibodies were added to each well. In all experiments nonimmune mouse immunoglobulins were used as controls and administered at the same concentrations as the specific antibodies.
Bone marrow-derived PMNs.
Murine bone marrow cells were isolated essentially as described by Boxio et al. (19). Briefly, bone marrow was collected from the femurs and tibiae of BALB/c mice and suspended in 1 ml of Hanks' balanced salt solution (HBSS; no calcium and no magnesium) containing 2 mM EDTA and 2% inactivated fetal calf serum. After samples were washed once with 2 ml of HBSS, cell concentration was determined with a Neubauer chamber, and the PMN percentage was calculated by Giemsa staining after cytospin centrifugation (Shandon Cytospin 2) or by flow cytometry using Guava easyCyte (Millipore). Data were analyzed with FlowJo software, version 10.0.7 (Tree Star, Inc.), as described previously (6). For some experiments, direct observation of spleen cells from B. abortus 2308-GFP-infected mice was performed as described elsewhere (20).
PMN phagocytosis assay.
Aliquots of 350 μl of human, canine, or murine fresh heparinized blood were incubated with bacteria or ∼2-μm latex beads (Sigma-Aldrich) at 37°C for 1 h under mild agitation, at the multiplicity of infection (MOI) indicated in the figure legends. Alternatively, bone marrow-derived mouse PMNs suspended in 350 μl HBSS or mouse serum were incubated with bacteria at 37°C for 1 h under mild agitation, at the MOI indicated in the figure legends. Blood smears in three glass slides were fixed with methanol, centrifuged in a cytospin, mounted with ProLong Gold Antifade reagent with 4′,6′-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific), and observed under a fluorescence microscope. Before cell staining on glass slides, bone marrow cell suspensions were fixed with BD fluorescence-activated cell sorter (FACS) lysing solution or 3.5% paraformaldehyde. At least 50 PMNs were counted per slide, and the number of intracellular fluorescent bacteria/PMN was determined.
Ex vivo bactericidal activity of serum and PMNs.
Bacteria (105 to 106 CFU) were incubated with 350 μl of normal, heat-inactivated (56°C for 30 min), and yeast-consumed and -inactivated (37°C for 1 h) serum or with immune mouse serum at 37°C at different times. After incubation, aliquots were dispersed on Trypticase soy agar plates and incubated at 37°C for 48 to 72 h, and CFU counts were determined. Aliquots of 350 μl of fresh mouse heparinized blood or bone marrow PMNs (suspended in HBSS or mouse serum) were mixed at an MOI of 2 or 5 bacteria/PMN under mild agitation at 37°C for up to 90 min. In some cases the blood was supplemented with 0.25% anti-Brucella murine immune serum. After incubation, cells were lysed with 0.2% Triton X-100 and 1,000 U/ml DNase (Sigma); aliquots were dispensed on Trypticase soy agar plates and incubated at 37°C for 48 to 72 h, and CFU counts were determined.
PMN cell death assays.
Aliquots of 350 μl of fresh human or mouse heparinized blood were mixed with different concentrations of B. abortus or Br-LPS under mild agitation at 37°C for 2 h. After incubation, red blood cells were lysed by mixing 100 μl of heparinized blood with 1,500 μl of red blood cell lysis buffer (8.02 g of NH4Cl, 0.84 g of NaHCO3, and 0.37 g/liter EDTA, pH 7.2) for 5 min. Then the remaining leukocytes were washed with ice-cold PBS to remove cell debris and resuspended in 100 μl of annexin V binding buffer (Life Technologies). Volumes of 5 μl of annexin V and 2 μl of AquaDead (Invitrogen) diluted 1/20 in PBS were added and incubated for 30 min on ice in the dark. Cells were washed once with ice-cold PBS and resuspended in 500 μl of BD FACS lysing solution. Samples were then subjected within 1 h to flow cytometry analysis using Guava easyCyte (Millipore), and data were analyzed using FlowJo software, version 10.0.7 (Tree Star, Inc.), as described previously (6).
Adsorption of serum components by B. abortus and protein identification.
For serum adsorption, 5 × 1010 CFU of the corresponding B. abortus strain was incubated with 3 ml of murine or human serum at 37°C for 45 min under mild agitation. Control bacteria were incubated with only PBS, pH 7.2. Bacteria were washed four times with PBS, pH 7.2. All bacterial preparations were treated with 0.1 M glycine-HCl (pH 2.7) to remove adsorbed proteins or with PBS for control purposes. The eluted supernatants were neutralized with 1 M Tris-HCl (pH 9) and precipitated with methanol-chloroform. Samples were subjected to 10% SDS-PAGE under reducing conditions. The Coomassie blue-stained protein bands were excised and subjected to reduction, alkylation, and in-gel tryptic digestion, followed by matrix-assisted laser desorption ionization–two-stage time of flight (MALDI-TOF-TOF) mass spectrometry analysis on a Proteomics Analyzer 4800 Plus mass spectrometer (Applied Biosystems), as described elsewhere (21). Resulting fragmentation spectra were searched against the corresponding mouse or human UniProt databases (www.uniprot.org) using ProteinPilot, version 4.0, and the Paragon algorithm (ABsciex) for protein identification at ≥95% confidence.
Complement and fibronectin detection.
For Western blotting, samples were transferred to a polyvinylidene difluoride (PVDF) membrane after SDS-PAGE. The membranes were blocked and incubated for the detection of murine complement C3 with 1:500 diluted goat anti-mouse C3 antibody (Thermo Scientific) and further with 1:1,000 protein G-peroxidase (Life Technologies). For the detection of murine fibronectin, membranes were incubated with 1:500-diluted goat IgG anti-fibronectin antibodies (Sigma) and further with 1:1,000 protein G-peroxidase (Life Technologies). Proteins were detected with enhanced chemiluminescence (Roche).
Statistics.
Analysis of variance (ANOVA) or Student's t test was used to determine statistical significance in the different assays (JASP Software, 2015 [https://jasp-stats.org/]). Data were processed in Microsoft Office Excel 2013.
RESULTS
B. abortus is not recognized by naive murine PMNs.
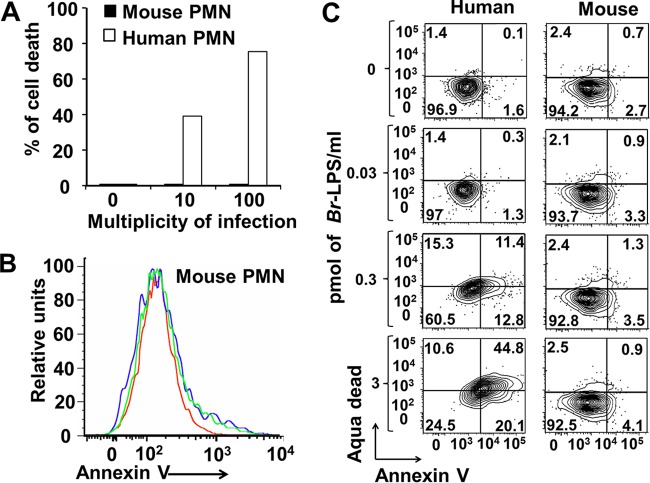
We have previously shown that B. abortus is phagocytized by human PMNs and induces the premature death of these leukocytes through the action of its LPS (6). This has led to the hypothesis that infected PMNs function as Trojan horses that spread the Brucella infection in different organs (6). Based on this, we asked whether B. abortus and its LPS could induce the same effect in murine PMNs. As shown in the experiment presented in Fig. 1, while human PMNs died after contact with B. abortus or its LPS, mouse PMNs did not show any signs of cell death after exposure to these components.
FIG 1.
B. abortus and its LPS do not induce the cell death of mouse PMNs. (A) Heparinized blood of humans or C57BL/6 mice was incubated with B. abortus 2308-GFP at the indicated MOI for 2 h, and the PMN population was gated and analyzed by cytometry using annexin V as a cell death marker. (B) Heparinized blood of C57BL/6 mice was incubated with B. abortus 2308-GFP at an MOI of 10 (green line) or an MOI 100 (blue line) or with PBS (red line) for 2 h, and the PMN population was gated and analyzed by cytometry using annexin V as a cell death marker. (C) Heparinized blood of humans or C57BL/6 mice was incubated with Br-LPS at the indicated concentrations for 2 h, and the PMN population was gated and analyzed by cytometry for cell death markers using AquaDead and annexin V. The proportions of PMNs positive for the respective marker are presented with each section of the corresponding cytogram. While human PMNs acquire death cell markers, mouse PMNs remain unaffected. The figure represents one experiment of at least four repetitions.
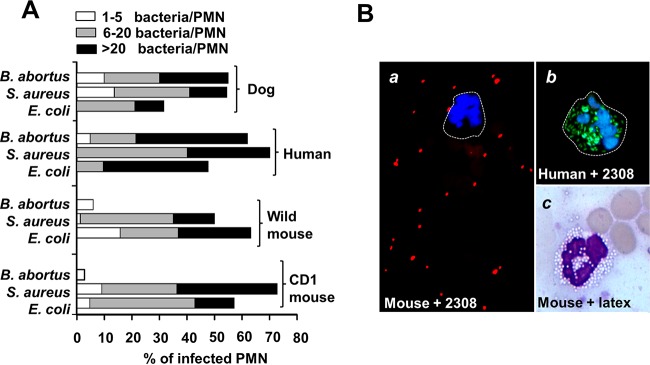
In order to understand this phenomenon, we then explored the early interaction of murine PMNs with B. abortus and compared it with that of PMNs of other animals. It has been shown that naive mouse PMNs readily ingest S. aureus and E. coli in the presence or absence of complement opsonization (22, 23). In agreement with this, we also observed phagocytosis of S. aureus and E. coli by PMNs of an outbred CD-1 strain and wild Mus musculus (Fig. 2A). Likewise, human and dog PMNs readily ingested these two bacterial species. In contrast to PMNs of humans and dogs, murine PMNs failed to ingest B. abortus (Fig. 2A). Phagocytosis of Brucella by murine PMNs was seldom detected even at a high MOI (∼100). Moreover, at this concentration, some bacteria may remain on the cell surface and not be internalized (Fig. 2B). The absence of Brucella recognition was specific since murine PMNs were capable of ingesting not only other bacteria but also large numbers of latex beads (Fig. 2B). Similar observations were obtained with PMNs from inbred C57BL/6 (Fig. 2B) and BALB/c mice. Consistent with previous results (5), B. abortus was isolated from the blood of infected mice as early as 1 h after infection, and bacteremia persisted for at least 2 days. In spite of this, we did not detect Brucella-infected PMNs in blood or in target organs during the first 4 days of infection, corroborating previous results (10).
FIG 2.
Naive murine PMNs do not phagocytize B. abortus. (A) Blood from the indicated species was incubated with B. abortus-GFP, S. aureus, or E. coli at an MOI of 20 for 1 h. Blood smears were then fixed and mounted with ProLong Gold Antifade reagent with DAPI. At least 50 PMNs were counted per sample, and the number of intracellular bacteria in each PMN and the proportion of phagocytosis were estimated under fluorescence microscopy. (B) Comparison between C57BL/6 mouse PMNs incubated with B. abortus 2308-RFP at an MOI of 100, human PMNs incubated with B. abortus-GFP at an MOI of 50, and Giemsa staining of C57BL/6 mouse PMNs incubated with latex beads at an MOI of 100, as indicated. Magnifications, ×200 (frame a) and ×400 (frames b and c). Images were cut from the microscope field, contrasted, and saturated using the hue tool to obtain suitable color separation. Similar results were obtained with BALB/c mice.
Altogether these results demonstrate that the interaction of murine PMNs with B. abortus significantly departs from that of PMNs from humans, guinea pigs, cows, goats, and dogs, which are capable of phagocytizing Brucella in the absence or presence of complement (3, 4, 24–26).
Mouse PMNs ingest B. abortus after the development of adaptive immunity.
Antibodies are well-known opsonizing elements. We then explored the ability of murine PMNs to phagocytize B. abortus during development of the adaptive immune response. At the onset of infection (first 2 days) Brucella was not internalized by murine PMNs (Fig. 3A); however, at later times the bacterium was readily phagocytized by these leukocytes (Fig. 3A). This internalization correlated with a quick rise in murine antibodies against Br-LPS (the main Brucella antigen) (27). Both immune mouse serum and specific IgG anti-Brucella promoted the phagocytosis of B. abortus by murine PMNs (Fig. 3B and C). As indicated previously (5), under the microscope, these infected leukocytes did not show obvious signs of alterations at early times of infection (<1 h). These results indicate that phagocytosis of B. abortus by murine PMNs is efficiently mediated by opsonizing antibodies through Fc receptors at the surface of these leukocytes.
FIG 3.
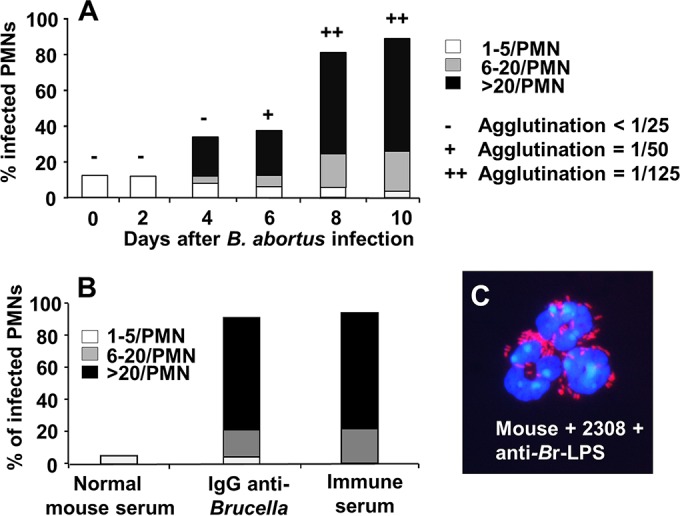
Mouse PMNs phagocytize B. abortus after antibodies are generated. CD-1 mice were infected intraperitoneally with 106 CFU; then, mouse blood was collected at different days of infection and incubated with B. abortus 2308-RFP (MOI of 50) for 1 h. Blood smears were then fixed and mounted with ProLong Gold Antifade reagent with DAPI. At least 50 PMNs were counted per sample, and the number of intracellular bacterial in each PMN was determined. The relative agglutination titer for the day evaluated after intraperitoneal injection with B. abortus is indicated at the top of the bars according to the legend on the figure. (B) Mouse PMNs of CD-1 mice were incubated with B. abortus 2308-RFP at an MOI of 50 for 2 h and under different conditions of opsonization. Then, the number of phagocytized bacteria was recorded by fluorescence microscopy. (C) CD-1 mouse PMNs incubated with B. abortus 2308-RFP and anti-Brucella mouse serum at an MOI of 100. The image was cut from the microscope field, contrasted, and saturated using hue tool to obtain suitable color separation (magnification, ×200). Similar results were obtained with C57BL/6 and BALB/c mice.
Surface N-formyl-perosamine homopolysaccharides are responsible for Brucella camouflage.
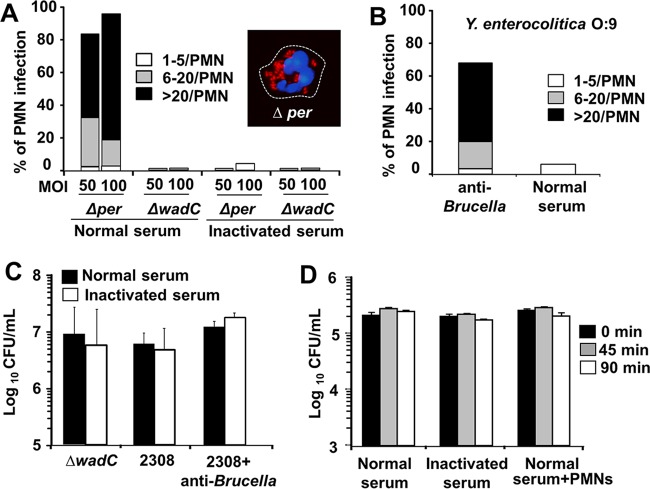
Since innate mouse opsonins failed to promote the phagocytosis of smooth B. abortus by murine PMNs, we then explored the presence of blocking components on the bacterial surface. First, we tested the ability of murine PMNs to phagocytize the rough B. abortus 2308 Δper mutant lacking surface N-formyl-perosamine sugars (Br-LPS O chain and native hapten [NH] polysaccharide) and the smooth B. abortus 2308 ΔwadC mutant displaying a defect in the core oligosaccharide (Fig. 4). While the core ΔwadC mutant was not recognized by murine PMNs and behaved as the parental strain did, the rough Δper mutant was readily phagocytized in the presence of normal but not inactivated mouse serum (Fig. 5A). High numbers of intracellular rough B. abortus Δper bacteria did not cause obvious alterations in mouse PMNs at early times (<1 h) of infection (Fig. 5A, inset), paralleling the results obtained with antibody-opsonized smooth brucellae (Fig. 3C).
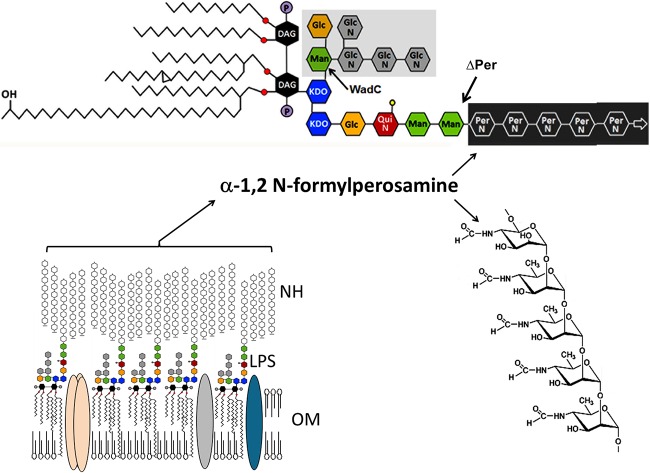
FIG 4.
Schematic structure of B. abortus outer membrane showing the LPS and NH. The O polysaccharide and native hapten (NH) are unbranched linear homopolymers of α-1,2-linked 4,6-dideoxy-4-formamido-d-mannopyranosyl units (N-formyl-perosamine) with an average chain length of 96 to 100 glycosyl subunits (28). While the NH is not directly bound to the lipid membrane, the O polysaccharide is linked to a core bifurcating oligosaccharide composed of βGlcN-6-βGlcN-4-βGlcN(-6-βGlcN)-3-αMan(-6-αGlc)-5-KDO1(−1-KDO1)-lipid A immersed in the outer membrane. Branching from KDO2 is αPerNFo-(-2PerNFo)n-2PerNFo-2-αMan-3-αMan-3-βQuiNAc-4-βGlc-4-KDO2-4-KDO2 (59). The KDO1 is linked to the lipid A composed of a backbone of diaminoglucose (DAG) disaccharide, substituted with phosphates (P) and amide and ester-linked long-chain saturated (C16:0 to C18:0) and hydroxylated (3-OH-C12:0 to 29-OH-C30:0) fatty acids (17, 60). The lipid A is bound to the outer membrane. KDO, ketodeoxyoctulosonic acid; Man, mannose; QuiN, acetyl-quinovosamine; Glc, glucose (Glc); PerNFo, N-formyl-perosamine. The ΔwadC mutation precludes the incorporation of the βGlcN-6-βGlcN-4-βGlcN(-6-βGlcN)-3-αMan(-6-αGlc)-oligosaccharide to the KDO1 (marked by a gray area) while the Δper mutation precludes the incorporation of αPerNFo-(-2PerNFo)n-2PerNFo-homopolymer (61).
FIG 5.
Surface N-formyl-perosamine polysaccharides are responsible for Brucella camouflage. (A) Smooth B. abortus ΔwadC and rough Δper mutants were incubated with BALB/c bone marrow PMNs at the indicated MOIs in the presence of normal or inactivated mouse serum, and phagocytosis was recorded as described in the legend of Fig. 2. The inset corresponds to a PMN with internalized B. abortus Δper (MOI of 50); the infected cell was fixed and mounted with ProLong Gold Antifade reagent with DAPI and observed under the fluorescence microscope at a magnification of ×200. The image was cut from the microscope field, contrasted, and saturated using the hue tool to obtain suitable color separation. (B) Y. enterocolitica O:9 (MOI of 100) was incubated with BALB/c bone marrow PMNs in the presence of normal serum or serum with anti-Brucella antibody (2%), and the phagocytosis was recorded. (C) B. abortus 2308 and the ΔwadC mutant (106 CFU) were incubated with normal or inactivated mouse serum or normal mouse serum containing antibodies against Brucella, and bacterial viability was recorded after 45 min of incubation. (D) B. abortus Δper was incubated in the presence of normal or inactivated mouse serum or with BALB/c bone marrow PMNs in normal serum, and the bacterial viability was estimated at the indicated times.
To confirm the role of N-formyl-perosamine sugars in the Brucella camouflage, we then tested the phagocytosis of Yersinia enterocolitica O:9. The O chain and NH surface molecules of this bacterium are identical to those of B. abortus (16, 28). Y. enterocolitica O:9 was not phagocytized by mouse PMNs in the presence of normal mouse serum (Fig. 5B). However, this bacterium was readily internalized in the presence of anti-Brucella antibodies. These results demonstrate that the surface N-formyl-perosamine polysaccharides were the moieties responsible for the B. abortus camouflage for opsonization.
B. abortus is resistant to the bactericidal action of mouse immune serum and PMNs.
Since it has been shown that B. abortus ΔwadC and Δper mutants are more sensitive to the bactericidal action of bovine serum than the parental strain (14), we then asked whether murine serum components and PMNs were capable of killing intracellular Brucella. The ΔwadC mutant was resistant to the action of normal mouse serum, as was the virulent B. abortus 2308 strain (Fig. 5C). Moreover, the presence of anti-Brucella antibodies did not have a significant effect on the viability of the bacteria (Fig. 5C). Although serum components opsonized the rough Δper mutant, no bactericidal activity was recorded by mouse serum in the absence or presence of PMNs (Fig. 5D).
N-Formyl-perosamine homopolysaccharides hamper the binding of murine heat-labile serum components.
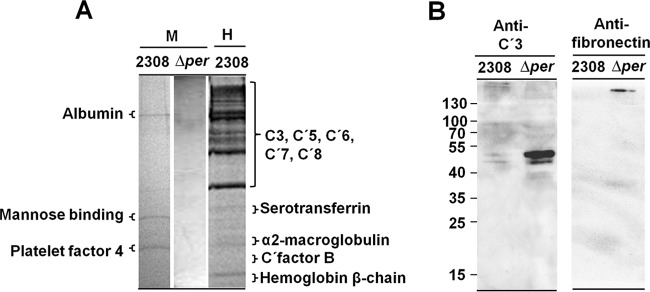
Since bacterial opsonization commonly occurs via activation and binding of various heat-labile serum factors such as complement and fibronectin (29), we then asked whether B. abortus was capable of interacting with these components. As presented in Fig. 6A, B. abortus was capable of adsorbing a significant number of human serum proteins, including complement components. Under the same experimental conditions mouse serum constituents were barely adsorbed by smooth or rough B. abortus strains (Fig. 6A). The small amounts of mouse serum proteins adsorbed by B. abortus strains, revealed as faint bands, corresponded to serum albumin, platelet factor 4, and mannose binding protein. With the exception of the last protein, no other opsonins or complement-related proteins were detected by this proteomic approach.
FIG 6.
Normal mouse serum proteins do not opsonize smooth B. abortus. (A) Coomassie blue-stained 10% SDS-PAGE gel of mouse (M) and human (H) proteins (10 μg/well) eluted from the surface of B. abortus 2308 or the Δper mutant after incubation of the respective serum with viable bacterial cells. Individual lines, corresponding to the different eluted fractions, were cut out and separated from the main gel as indicated in the figure; then each gel line was horizontally sliced (about 2-mm slices, from top to bottom), and the identities of proteins in each slice were determined by proteomic analysis. (B) Western blotting of mouse proteins (10 μg/well) eluted from the surface of B. abortus 2308 or the Δper mutant after incubation of the respective serum with viable bacterial cells. Western blots were developed with monoclonal antibodies against mouse C′3 or monospecific rabbit IgG anti-fibronectin.
In spite of the overall lack of affinity of the Brucella cell envelope for mouse serum factors, it was revealed by Western blotting that the Δper mutant adsorbed heat-labile complement and fibronectin in larger amounts than the smooth counterpart strain 2308 (Fig. 6B). This was consistent with the phagocytosis of B. abortus Δper by murine PMNs in the presence of normal but not inactivated serum (Fig. 5A). These results demonstrate that phagocytosis of the Δper strain by murine PMNs occurs through opsonization of small amounts of heat-labile serum components such as complement and fibronectin and that the O chain and NH polysaccharides hamper the access of these components to surface bacterial molecules.
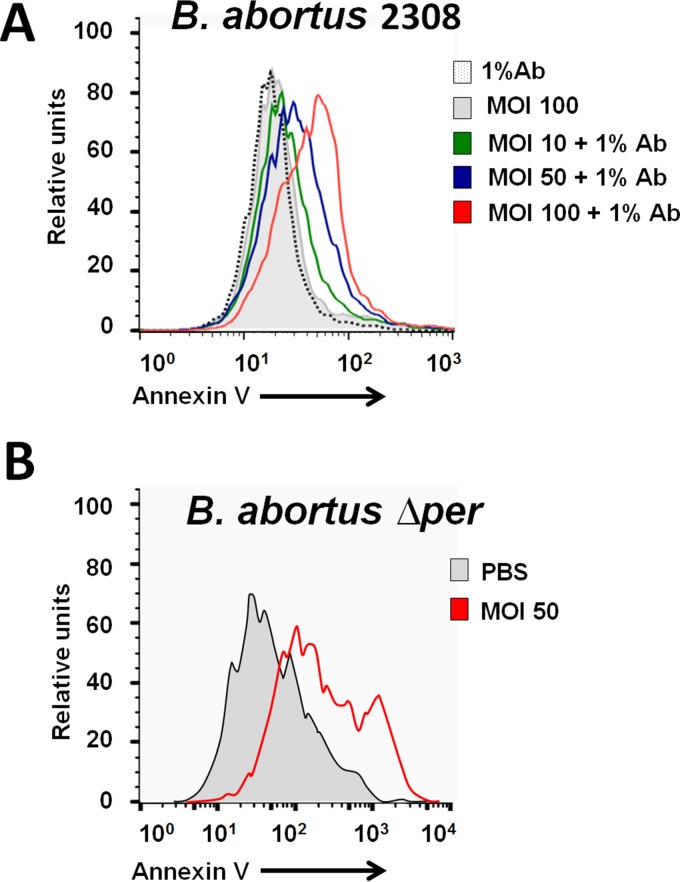
Intracellular B. abortus induces the cell death of mouse PMNs.
We have demonstrated that B. abortus induces premature cell death of human PMNs (6). Therefore, we explored the effect of both the antibody-opsonized smooth B. abortus 2308 and the rough mutant B. abortus Δper on mouse PMNs. As shown in Fig. 7, both internalized bacteria readily induce the cell death of murine PMNs, paralleling the phenomenon observed with human PMNs.
FIG 7.
Induction of mouse PMN cell death after phagocytosis of antibody-opsonized smooth B. abortus 2308 or the rough Δper mutant. (A) Heparinized blood of C57BL/6 mice was incubated with B. abortus 2308-GFP or with B. abortus 2308-GFP opsonized with antibody (Ab) against Br-LPS at the indicated MOIs for 2 h, and then the PMN population was gated and analyzed by cytometry using annexin V as a cell death marker. Negative controls were treated with either PBS or antibody alone. Similar results were observed with bone marrow-derived PMNs. (B) Bone marrow PMNs of BALB/c mice were incubated with the rough mutant B. abortus Δper at an MOI of 50 or with PBS for 4 h, and the PMN population was gated and analyzed by cytometry using annexin V as a cell death marker. The figure represents one experiment of at least four repetitions.
DISCUSSION
It has been shown that naive human, guinea pig, bovine, rat, caprine, and canine PMNs readily phagocytize both smooth and rough Brucella species (3–5, 24–26, 30). Moreover, PMNs from some of these animals (humans, guinea pigs, rats, and cows) are capable of ingesting Brucella in the presence of inactivated normal serum or even in the absence of serum (3, 4, 6). In general, the overall behavior of human PMNs is rather similar to that observed with bovine PMNs (4), a phenomenon that is commensurate with the coevolution of B. abortus and its host.
In this work we have shown that mouse normal serum components do not opsonize smooth B. abortus and that mouse PMNs, in the absence of specific antibodies, do not phagocytize this bacterium. This phenomenon is specific, and the molecules responsible for the Brucella camouflage are N-formyl-perosamine surface homopolysaccharides (Fig. 4). This is relevant since in addition to the hindrance function, these perosamine polysaccharides display other biological properties related to virulence, such as dimerization of major histocompatibility complex (MHC) class II, blocking of MHC class II antigen presentation, and protection against a collection of bactericidal substances (16, 31–33).
The absence of NH and O-chain polysaccharides uncovers potential targets in the Brucella cell envelope, such as outer membrane proteins and phospholipids, as well as ketodeoxyoctulosonic acid (KDO) and lipid A phosphate groups, present in the innermost sections of the core moiety (14, 34). Still, mouse PMNs fail to ingest the rough B. abortus Δper mutant in the absence of classical heat-sensitive serum opsonins, such as complement and fibronectin. This is relevant since mouse PMNs are capable of ingesting latex beads and other bacteria, such as S. aureus and E. coli, in the presence or absence of complement, albeit the phagocytosis is more efficient in the former case (22, 23). In the absence of opsonization by specific antibodies or complement, PMNs may also ingest microorganisms through the pathways of other PMN pattern recognition receptor (PRRs), such as β2 integrins, C-type lectins, or scavenger or pentraxin receptors (22, 35, 36). This explains the failure of mouse PMN PRRs to recognize putative B. abortus pathogen-associated molecular patterns (PAMPs), such as outer membrane lipoproteins, adhesion-like proteins, ornithine-containing lipids, flagellar structures, and phospholipids, among the most conspicuous elements on the surface of brucellae, which in other bacteria are constitutive PAMPs and targets for recognition (7, 8, 14, 27, 37).
Although Brucella organisms are more resistant than other Gram-negative bacteria to the killing action of human serum and PMNs, these components are still able to kill about 20 to 30% of virulent smooth B. abortus bacteria after 90 min (3–5, 38). This bactericidal activity is even more conspicuous and efficient in the case of rough B. abortus bacteria (3). In contrast, both smooth and rough B. abortus strains were totally resistant to the killing actions of mouse PMNs and complement even in the presence of antibodies.
Therefore, it seems that the lack of B. abortus recognition and the failure to kill this bacterium work on at least three different levels of the innate immune system: (i) the absence of binding of natural opsonins to the surface of smooth Brucella bacteria, (ii) the lack of recognition of putative PAMPs by murine PMN PRRs, and (iii) the virtually absolute resistance of Brucella to the killing action of mouse complement—by either the alternative or classical pathway—and PMNs.
Regarding the interaction of Brucella with human complement, it has been demonstrated that the bacteria are considerably more resistant to the killing action of human serum than other Gram-negative bacteria (5, 39). In spite of this, the binding of human complement components to the surface of B. abortus was conspicuous and commensurate with the opsonization of this bacterium by normal serum. This is in clear contrast to the absence of opsonization and brucellicidal activity observed by mouse serum. This is striking since it has been proposed that bacterial opsonization by mouse complement is similar to that of human complement and that the mouse has significant quantities of complement activity and high serum levels of C3 as well as other complement proteins (40). However, genetic and structural differences have been demonstrated between human and mouse complement C3 and C4 components (41). For instance, while human C3 is readily inhibited by compstatin, mouse C3 is resistant to this compound due to structural differences in amino acid residues 329 to 534 (42). Likewise, mouse C4 does not have classical C5 convertase activity due to differences with the human beta-chain segments of C4 and other regions of the molecule contributing to C5 binding (43).
Although the differences between murine and human surface PMN PRRs have not been explored in detail, there are some discrete features that may be relevant. While in humans the complement receptor proteins CR1 and CR2 are two different molecules, in mouse PMNs, they constitute a single chimeric CR1/CR2 molecule with different affinities for various complement components (44). In addition, the affinity of the G protein-coupled receptor for the chemoattractant and activator formyl-Met-Leu-Phe (fMLP) is lower in mouse than in human PMNs (45). Likewise, while the human PMN receptor CXCR1 uses as the substrate CXCL8, the mouse counterpart uses CXCL6 (46). Finally, some receptors present in mouse PMNs (e.g., the sialic acid receptor CD33, FcγRIIIA, FcγRIII, and FcγRIV) are absent in humans, and vice versa (e.g., Toll-like receptor 10 [TLR-10], l-selectin binding to E-selectin, and FcγRIIA) (47–52).
Finally, the almost absolute resistance to the killing action of mouse complement and PMNs is linked to the absence of activation of these elements by the putative Brucella PAMPs (5, 7, 8, 14, 27, 37), as well as to the bacterial resistance to the microbicidal substances of PMNs (33, 34). Regarding this, decreased lytic activity of mouse complement in comparison to that of the human counterpart has been described (53). With respect to PMNs, there are several differences in microbicidal components between human and mouse. For instance, mouse PMNs lack defensins (54), and the functions of several proteases and ROS activation differ between PMNs of mice and humans (55–57). Whether some of these differences are related to the absence of Brucella recognition and killing by murine serum components and PMNs remains to be investigated.
We have previously shown that Brucella induces premature death of human PMNs (6). Commensurate with this, B. abortus also induces the death of these leukocytes once the bacteria have been internalized via Fc receptors or, in the case of the Δper mutant strain, via other serum opsonins. As proposed (6), this phenomenon may favor the nonphlogistic removal of infected dying PMNs by Mϕ and DCs, favoring the dispersion of Brucella in the organism following a Trojan horse effect. In this sense, the premature death of mouse PMNs parallels that of human PMNs, and therefore it is a useful model to explore this hypothesis.
The mouse model has provided a substantial body of information concerning the pathobiology and immunology of brucellosis (12, 13). Still, our approach has revealed significant differences between mice and other hosts in front-line elements of innate immunity that may have a profound influence on downstream mechanisms of the immune response. This is not trivial since the outcome of brucellosis in a given animal species may be determined during the initial stages of immune recognition. Therefore, the differences between the immune system of mice and that of other mammals (58) should prevent us from making direct extrapolations and encourage us to dissect the mechanisms behind them.
ACKNOWLEDGMENTS
We thank the research teams of PIET of the Universidad Nacional, CIET of the Universidad de Costa Rica, Ignacio Moriyón and Raquel Conde (University of Navarra, Pamplona, Spain) for providing LPS samples, wadC, and per constructs, Alexandra Rucavado (ICP, University of Costa Rica) for the antifibronectin antibody and fibronectin control, and Caterina Guzmán-Verri for her helpful discussions.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Moreno E, Moriyón I. 2006. The genus Brucella, p 315–456. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrant E (ed), The prokaryotes, vol 5 Springer Verlag, New York, NY. [Google Scholar]
- 2.Ackermann MR, Cheville NF, Deyoe BL. 1988. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet Pathol 25:28–35. doi: 10.1177/030098588802500104. [DOI] [PubMed] [Google Scholar]
- 3.Kreutzer DL, Dreyfus LA, Robertson DC. 1979. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun 23:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley LK, Robertson DC. 1984. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun 46:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzmán-Verri C, Chacón-Díaz C, Rucavado A, Moriyón I, Moreno E. 2007. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One 2:e631. doi: 10.1371/journal.pone.0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barquero-Calvo E, Mora-Cartín R, Arce-Gorvel V, de Diego JL, Chacón-Díaz C, Chaves-Olarte E, Guzmán-Verri C, Buret AG, Gorvel JP, Moreno E. 2015. Brucella abortus induces the premature death of human neutrophils through the action of its lipopolysaccharide. PLoS Pathog 11:e1004853. doi: 10.1371/journal.ppat.1004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barquero-Calvo E, Conde-Álvarez R, Chacón-Díaz C, Quesada-Lobo L, Martirosyan A, Guzmán-Verri C, Iriarte M, Mancek-Keber M, Jerala R, Gorvel JP, Moriyón I, Moreno E, Chaves-Olarte E. 2009. The differential interaction of Brucella and Ochrobactrum with innate immunity reveals traits related to the evolution of stealthy pathogens. PLoS One 4:e5893. doi: 10.1371/journal.pone.0005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palacios-Chaves L, Conde-Álvarez R, Gil-Ramírez Y, Zúñiga-Ripa A, Barquero-Calvo E, Chacón-Díaz C, Chaves-Olarte E, Arce-Gorvel V, Gorvel JP, Moreno E, de Miguel MJ, Grilló MJ, Moriyón I, Iriarte M. 2011. Brucella abortus ornithine lipids are dispensable outer membrane components devoid of a marked pathogen-associated molecular pattern. PLoS One 6:e16030. doi: 10.1371/journal.pone.0016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Bargen K, Gagnaire A, Arce-Gorvel V, de Bovis B, Baudimont F, Chasson L, Bosilkovski M, Papadopoulos A, Martirosyan A, Henri S, Mège JL, Malissen B, Gorvel JP. 2014. Cervical lymph nodes as a selective niche for Brucella during oral infections. PLoS One 10:e0121790. doi: 10.1371/journal.pone.0121790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copin R, Vitry MA, Hanot-Mambres D, Machelart A, De Trez C, Verwinden JM, Magez S, Akira S, Ryffel B, Carlier Y, Letesson JJ, Muraille E. 2012. In situ microscopy analysis reveals local innate immune response developed around Brucella infected cells in resistant and susceptible mice. PLoS Pathog 8:e1002575. doi: 10.1371/journal.ppat.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barquero-Calvo E, Martirosyan A, Ordoñez-Rueda D, Arce-Gorvel V, Alfaro-Alarcón A, Lepidi H, Malissen B, Malissen M, Gorvel JP, Moreno E. 2013. Neutrophils exert a suppressive effect on Th1 responses to intracellular pathogen Brucella abortus. PLoS Pathog 9:e1003167. doi: 10.1371/journal.ppat.1003167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grilló MJ, Blasco JM, Gorvel JP, Moriyón I, Moreno E. 2012. What have we learned from brucellosis in the mouse model? Vet Res 43:29. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva TM, Costa EA, Paixão TA, Tsolis RM, Santos RL. 2011. Laboratory animal models for brucellosis research. J Biomed Biotechnol 2011:518323. doi: 10.1155/2011/518323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conde-Álvarez R, Arce-Gorvel V, Iriarte M, Manček-Keber M, Barquero-Calvo E, Palacios-Chaves L, Chacón-Díaz C, Chaves-Olarte E, Martirosyan A, von Bargen K, Grilló MJ, Jerala R, Brandenburg K, Llobet E, Bengoechea JA, Moreno E, Moriyón I, Gorvel JP. 2012. The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog 8:e1002675. doi: 10.1371/journal.ppat.1002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chacón-Díaz C, Muñoz-Rodríguez M, Barquero-Calvo E, Guzmán-Verri C, Chaves-Olarte E, Grilló MJ, Moreno E. 2011. The use of green fluorescent protein as a marker for Brucella vaccines. Vaccine 29:577–582. doi: 10.1016/j.vaccine.2010.09.109. [DOI] [PubMed] [Google Scholar]
- 16.Aragón V, Díaz R, Moreno E, Moriyón I. 1996. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J Bacteriol 178:1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno E, Stackebrandt E, Dorsch M, Wolters J, Busch M, Mayer H. 1990. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol 172:3569–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinney MM, Parkinson A. 1987. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods 96:271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- 19.Boxio R, Bossenmeyer-Pourié C, Steinckwich N, Dournon C, Nüsse O. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol 75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 20.Chacón-Díaz C, Altamirano-Silva P, González-Espinoza G, Medina M-C, Alfaro-Alarcón A, Bouza-Mora L, Wong M, Barquero-Calvo E, Rojas N, Guzmán-Verri C, Moreno E, Chaves-Olarte E. 2015. Brucella canis is an intracellular pathogen inducing a lower proinflammatory response than smooth zoonotic counterparts. Infect Immun 83:4861–4870. doi: 10.1128/IAI.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomonte B, Tsai WC, Ureña-Díaz JM, Sanz L, Mora-Obando D, Sánchez EE, Fry BG, Gutiérrez JM, Gibbs HL, Calvete JJ. 2014. Venomics of New World pit vipers: genus-wide comparisons of venom proteomes across Agkistrodon. J Proteomics 96:103–116. doi: 10.1016/j.jprot.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson KE, Boyle KB, Davidson K, Chessa TA, Kulkarni S, Jarvis GE, Sindrilaru A, Scharffetter-Kochanek K, Rausch O, Stephens LR, Hawkins PT. 2008. CD18-dependent activation of the neutrophil NADPH oxidase during phagocytosis of Escherichia coli or Staphylococcus aureus is regulated by class III but not class I or II PI3Ks. Blood 112:5202–5211. doi: 10.1182/blood-2008-04-149450. [DOI] [PubMed] [Google Scholar]
- 23.Hart PH, Spencer LK, Nikoloutsopoulos A, Lopez AF, Vadas MA, McDonald PJ, Finlay-Jones JJ. 1986. Role of cell surface receptors in the regulation of intracellular killing of bacteria by murine peritoneal exudate neutrophils. Infect Immun 52:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canning PC, Deyoe BL, Roth JA. 1988. Opsonin-dependent stimulation of bovine neutrophil oxidative metabolism by Brucella abortus. Am J Vet Res 49:160–163. [PubMed] [Google Scholar]
- 25.Meador VP, Deyoe BL, Cheville NF. 1989. Pathogenesis of Brucella abortus infection of the mammary gland and supramammary lymph node of the goat. Vet Pathol 26:357–368. [DOI] [PubMed] [Google Scholar]
- 26.Victor J, Pollack AD, Raymond R, Valliant JR. 1952. Studies on phagocytosis determination of blood opsonin for Brucella. J Bacteriol 64:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno E, Kurtz RS, Berman DT. 1984. Induction of immune and adjuvant immunoglobulin G responses in mice by Brucella lipopolysaccharide. Infect Immun 46:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bundle DR, Cherwonogrodzky JW, Gidney MA, Meikle PJ, Perry MB, Peters T. 1989. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun 57:2829–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vuento M, Salonen E, Salminen K, Pasanen M, Stenman UK. 1980. Immunochemical characterization of human plasma fibronectin. Biochem J 191:719–727. doi: 10.1042/bj1910719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallego MC, Lapeña MA. 1990. The interaction of Brucella melitensis 16-M and caprine polymorphonuclear leukocytes. Comp Immunol Microbiol Infect Dis 13:59–65. doi: 10.1016/0147-9571(90)90517-W. [DOI] [PubMed] [Google Scholar]
- 31.Escola JM, Moreno E, Chavrier P, Gorvel JP. 1994. The O-chain of Brucella abortus lipopolysaccharide induces SDS-resistant MHC class II molecules in mouse B cells. Biochem Biophys Res Commun 203:1230–1236. doi: 10.1006/bbrc.1994.2314. [DOI] [PubMed] [Google Scholar]
- 32.Forestier C, Deleuil F, Lapaque N, Moreno E, Gorvel JP. 2000. Brucella abortus lipopolysaccharide in murine peritoneal macrophages acts as a down-regulator of T cell activation. J Immunol 165:5202–5210. doi: 10.4049/jimmunol.165.9.5202. [DOI] [PubMed] [Google Scholar]
- 33.Freer E, Moreno E, Moriyón I, Pizarro-Cerdá J, Weintraub A, Gorvel JP. 1996. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J Bacteriol 178:5867–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez de Tejada G, Pizarro-Cerdá J, Moreno E, Moriyón I. 1995. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect Immun 63:3054–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottazzi B, Doni A, Garla C, Mantovani A. 2010. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol 28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 36.Futosi K, Fodor S, Mócsai A. 2013. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martirosyan A, Moreno E, Gorvel JP. 2011. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol Rev 240:211–234. doi: 10.1111/j.1600-065X.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Prada CM, Zelazowska EB, Nikolich M, Hadfield TL, Roop RM II, Robertson GL, Hoover DL. 2003. Interactions between Brucella melitensis and human phagocytes: bacterial surface O-polysaccharide inhibits phagocytosis, bacterial killing, and subsequent host cell apoptosis. Infect Immun 71:2110–2119. doi: 10.1128/IAI.71.4.2110-2119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Prada CM, Nikolich M, Vemulapalli R, Sriranganathan N, Boyle SM, Schurig GG, Hadfield TL, Hoover DL. 2001. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect Immun 69:4407–4416. doi: 10.1128/IAI.69.7.4407-4416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osmers I, Szalai AJ, Tenner AJ, Barnum SR. 2006. Complement in BuB/BnJ mice revisited: serum C3 levels and complement opsonic activity are not elevated. Mol Immunol 43:1722–1725. doi: 10.1016/j.molimm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Odink KG, Fey G, Wiebauer K, Diggelmann H. 1981. Mouse complement components C3 and C4. Characterization of their messenger RNA and molecular cloning of complementary DNA for C3. J Biol Chem 256:1453–1458. [PubMed] [Google Scholar]
- 42.Tamamis P, Pierou P, Mytidou C, Floudas CA, Morikis D, Archontis G. 2011. Design of a modified mouse protein with ligand binding properties of its human analog by molecular dynamics simulations: the case of C3 inhibition by compstatin. Proteins 79:3166–3179. doi: 10.1002/prot.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebanks RO, Isenman DE. 1996, Mouse complement component C4 is devoid of classical pathway C5 convertase subunit activity. Mol Immunol 33:297–309. doi: 10.1016/0161-5890(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson AC, Wei JH. 2008. Comparative functional evolution of human and mouse CR1 and CR2. J Immunol 181:2953–2959. doi: 10.4049/jimmunol.181.5.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao JL, Murphy PM. 1993. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem 268:25395–25401. [PubMed] [Google Scholar]
- 46.Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA, Hipkin RW. 2007. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem 282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- 47.Brinkman-Van der Linden EC, Angata T, Reynolds SA, Powell LD, Hedrick SM, Varki A. 2003. CD33/Siglec-3 binding specificity, expression pattern, and consequences of gene deletion in mice. Mol Cell Biol 23:4199–4206. doi: 10.1128/MCB.23.12.4199-4206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruhns P. 2012. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 49.Hasan U, Chaffois C, Gaillard C, Saulnie V, Merck E, Tancredi S, Guiet C, Brière F, Vlach J, Lebecque S, Trinchieri G, Bates EE. 2005. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol 174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 50.Jönsson F, Mancardi DA, Zhao W, Kita Y, Iannascoli B, Khun H, van Rooijen N, Shimizu T, Schwartz LB, Daëron M, Bruhns P. 2012. Human FcγRIIA induces anaphylactic and allergic reactions. Blood 119:2533–2544. doi: 10.1182/blood-2011-07-367334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marois L, Paré G, Vaillancourt M, Rollet-Labelle E, Naccache PH. 2011. FcγRIIIb triggers raft-dependent calcium influx in IgG-mediated responses in human neutrophils. J Biol Chem 286:3509–3519. doi: 10.1074/jbc.M110.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zöllner O, Lenter MC, Blanks JE, Borges E, Steegmaier M, Zerwes HG, Vestweber D. 1997. l-Selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol 136:707–716. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ish C, Ong GL, Desai N, Mattes MJ. 1993. The specificity of alternative complement pathway-mediated lysis of erythrocytes: a survey of complement and target cells from 25 species. Scand J Immunol 38:113–122. doi: 10.1111/j.1365-3083.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 54.Eisenhauer PB, Lehrer RI. 1992. Mouse neutrophils lack defensins. Infect Immun 60:3446–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bagaitkar J, Matute JD, Austin A, Arias AA, Dinauer MC. 2012. Activation of neutrophil respiratory burst by fungal particles requires phosphatidylinositol 3-phosphate binding to p40phox in humans but not in mice. Blood 120:3385–3387. doi: 10.1182/blood-2012-07-445619. [DOI] [PubMed] [Google Scholar]
- 56.Kalupov T, Brillard-Bourdet M, Dadé S, Serrano H, Wartelle J, Guyot N, Juliano L, Moreau T, Belaaouaj A, Gauthier F. 2009. Structural characterization of mouse neutrophil serine proteases and identification of their substrate specificities: relevance to mouse models of human inflammatory diseases. J Biol Chem 284:34084–34091. doi: 10.1074/jbc.M109.042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiesner O, Litwiller RD, Hummel AM, Viss MA, McDonald CJ, Jenne DE, Fass DN, Specks U. 2005. Differences between human proteinase 3 and neutrophil elastase and their murine homologues are relevant for murine model experiments. FEBS Lett 579:5305–5312. doi: 10.1016/j.febslet.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 58.Mestas J, Hughes CC. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 59.Kubler-Kielb J, Vinogradov E. 2013. The study of the core part and non-repeating elements of the O-antigen of Brucella lipopolysaccharide. Carbohydr Res 366:33–37. doi: 10.1016/j.carres.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iriarte M, González D, Delrue RM, Monreal D, Conde R, López-Goñi I, Letesson JJ. 2004. Brucella lipopolysaccharide: structure, biosynthesis and genetics, p152–183. López-Gõni I, Moriý on I (ed), Brucella: molecular and cellular biology. Horizon Bioscience, Wymondham, United Kingdom. [Google Scholar]
- 61.Gil-Ramírez Y, Conde-Álvarez R, Palacios-Chaves L, Zúñiga-Ripa A, Grilló MJ, Arce-Gorvel V, Hanniffy S, Moriyón I, Iriarte M. 2014. The identification of wadB, a new glycosyltransferase gene, confirms the branched structure and the role in virulence of the lipopolysaccharide core of Brucella abortus. Microb Pathog 73:53–59. doi: 10.1016/j.micpath.2014.06.002. [DOI] [PubMed] [Google Scholar]