Abstract
The role of the recently described interleukin-32 (IL-32) in Staphylococcus aureus-induced mastitis, an inflammation of the mammary gland, is unclear. We determined expression of IL-32, IL-6, and IL-8 in S. aureus- and Escherichia coli-infected bovine mammary gland epithelial cells. Using live bacteria, we found that in S. aureus-infected cells, induction of IL-6 and IL-8 expression was less pronounced than in E. coli-infected cells. Notably, IL-32 expression was decreased in S. aureus-infected cells, while it was increased in E. coli-infected cells. We identified the staphylococcal phenol-soluble modulin (PSM) peptides as key contributors to these effects, as IL-32, IL-6, and IL-8 expression by epithelial cells exposed to psm mutant strains was significantly increased compared to that in cells exposed to the isogenic S. aureus wild-type strain, indicating that PSMs inhibit the production of these interleukins. The use of genetically complemented strains confirmed this observation. Inasmuch as the decreased expression of IL-32, which is involved in dendritic cell maturation, impairs immune responses, our results support a PSM-dependent mechanism that allows for the development of chronic S. aureus-related mastitis.
INTRODUCTION
Mastitis of ruminants is an inflammation of the mammary gland commonly caused by bacterial infection; Staphylococcus aureus and Escherichia coli are among the most prevalent pathogens (1). E. coli intramammary infections often result in acute mastitis with severe clinical manifestations, while symptoms induced by S. aureus infection are usually less severe and the infection can become persistent (2). It is well documented that there are weaker proinflammatory responses to S. aureus than to E. coli in ruminants, inasmuch as differential immune responses were observed in vitro in infected bovine epithelial cells and in vivo in infected cows (3, 4).
Mammary gland epithelial cells (MEC) are the first cells that are in contact with pathogens. Pathogens can be internalized into epithelial cells and hijack host cell functions to facilitate their own propagation or circumvent host defenses (5, 6).
The cytokine network, which is fine-tuned by many regulatory steps, plays a pivotal role in the outcome of infection. The identification of members of the cytokine network may allow the prioritization of potential targets for the treatment of infection. The roles of several recently discovered cytokines during infection are not completely understood. Such cytokines include, for example, interleukin-32 (IL-32), which has been reported to play a pivotal role in the pathogenesis of infectious diseases. IL-32 is produced by T lymphocytes, natural killer (NK) cells, monocytes, and epithelial cells (7). The human IL-32 gene is organized into eight exons with six splice variants of the gene; these variants have been described as IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ε, and IL-32ζ (8). IL-32 γ induces the maturation of dendritic cells (9). Furthermore, IL-32 induces IL-1β, IL-6, tumor necrosis factor alpha (TNF-α), and IL-8 (7), which are relevant to the pathoimmunology of infections caused by S. aureus and E. coli. Thus, the comparison of IL-6, IL-8, and IL-32 gene expression by S. aureus-infected MEC with the expression of those cytokines by E. coli-infected MEC is pertinent in regard to infectious mastitis.
S. aureus-associated infections are promoted by the coordinated action of various virulence factors, among which are cell wall-associated and secreted bacterial proteins such as toxins (10, 11). Recently, it was discovered that virulence of S. aureus, in particular of community-associated methicillin-resistant S. aureus strains, depends on phenol-soluble modulins (PSMs), a family of secreted amphipathic, α-helical peptides with a variety of biological functions (12). S. aureus PSMs have potent cytolytic activity against many cell types, including neutrophils, monocytes, erythrocytes, keratinocytes, and osteoblasts (13–15). PSMs also have proinflammatory activities: they stimulate leukocytes and initiate proinflammatory responses, including neutrophil chemoattraction and activation (12, 16). Furthermore, we have shown that a PSMα-induced G2/M transition delay correlated with a decrease in the expression of several defensin genes, suggesting a role in diminution of antibacterial functions of epithelial cells (17). Moreover, PSMs are responsible for modulation of cytokine secretion. It was demonstrated that PSMs induce the release of IL-8 from neutrophils (13) and IL-18 from human keratinocytes, likely through the lytic release (15). In contrast, simultaneous treatment of dendritic cells with S. aureus cell lysate and PSMα inhibited the secretion of TNF, IL-6, and IL-12, whereas the secretion of the anti-inflammatory cytokine IL-10 was increased (18). Taking into account numerous reports and our recent findings showing that PSMs are involved in the alteration of the host defense response (17–19), we tested in this study the hypothesis that PSMs impair cytokine expression during bovine infection using an induction of specific interleukins in bovine epithelial cells as a readout.
In the present study, we found that a weaker cytokine response was associated with the exposure of bovine MEC to live S. aureus bacteria than to E. coli. We show a pivotal role for PSMs in the inhibition of the expression of major components of the cytokine network, IL-32, IL-6, and IL-8, that is induced by S. aureus, an additional feature that may contribute to PSM-mediated immune evasion and persistence of staphylococcal infections.
MATERIALS AND METHODS
Reagents.
PSMα3 peptide was synthesized by solid-phase peptide synthesis and provided by CecoLabs (Tübingen, Germany). Lactate dehydrogenase (LDH) was quantified using the Pierce LDH cytotoxicity assay (Thermopierce, Rockford, IL).
Eukaryotic cell maintenance.
A bovine mammary epithelial cell (MEC) line, BME-UV (20), was maintained in the following medium: 50% Dulbecco's modified Eagle medium (DMEM), 30%, RPMI 1640, 20% NCTC-135 supplemented with 10% fetal calf serum (FCS) (Gibco, Saint Aubin, France), 0.1% α-lactose monohydrate, 0.1% lactalbumin enzymatic hydrolysate, 1.2 mM reduced l-glutathione, 5 μg/ml of bovine insulin, 5 μg/ml of bovine holo-transferrin, 5 μg/ml of progesterone, 10−7 mol/liter of hydrocortisone, 10 μg/ml of l-ascorbic acid, and 50 IU/ml of penicillin-streptomycin (Sigma-Aldrich) at 37°C with 5% CO2. PS, a newly isolated MEC line from the secretory parenchyma (21), was maintained in advanced medium: Advanced-DMEM/F12 (Gibco) containing 20 mM HEPES, 2 mM l-glutamine (Gibco), 1 μg/ml of hydrocortisone (Sigma-Aldrich), 10 ng/ml of insulin-like growth factor (IGF), 1.5 ng/ml of fibroblast growth factor (FGF), and 5 ng/ml of epidermal growth factor (EGF). Trypsin-EDTA (Gibco) was used to release adherent BME-UV and PS cells for subculturing.
Bacterial strains and culture conditions.
Two S. aureus strains isolated from cows with mastitis were used in the in vitro studies, RF122 and Newbould 305 (NB305). These strains reproducibly induce severe or mild mastitis under experimental conditions (22).
The methicillin-resistant S. aureus USA300 (LAC wt) and its isogenic mutants LAC Δpsmα, which lacks the psmα operon encoding PSMα1 to -4, and LAC Δpsmαβhld, which lacks the psmα and psmβ operons and in which translation of the hld gene is abolished by mutation of the start codon, were obtained from the Laboratory of Bacteriology, NIH, USA (12, 23). The construction of a wild-type (WT) LAC pTXΔ16 strain harboring the control plasmid pTXΔ16, PSMα deletion mutant LAC Δpsmα pTXΔ16, and complemented strain LAC Δpsmα pTXΔα1-4, as well as PSM-deficient deletion mutant LAC Δpsmαβhld pTXΔ16 and complemented strains expressing either the four PSMα peptides (LAC Δpsmαβhld pTXΔα1-4), PSMβ (LAC Δpsmαβhld pTXΔβ1-2), or hld (LAC Δpsmαβhld pTXΔ hld), were obtained as described previously (24).
All S. aureus cultures were performed as follows. Aliquots from overnight cultures in brain heart infusion (BHI) broth were diluted (1:50) in DMEM. The mutants (LAC Δpsmα and LAC Δpsmαβhld) in which the psmα operon was exchanged for a spectinomycin resistance cassette were grown in BHI containing 250 μg/ml of spectinomycin before inoculation into DMEM. The tetracycline-resistant strains harboring plasmid TDX16 were grown in BHI containing 12.5 μg/ml of tetracycline before inoculation into DMEM. The growth curves of deletion and complemented S. aureus mutants were similar to that of the wild-type strain. Strains were incubated at 37°C under anaerobic conditions until cultures reached an optical density of 0.6 at 600 nm, corresponding to approximately 108 CFU/ml. The staphylococci were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), and resuspended in the interaction medium (DMEM). Bacterial concentrations were estimated spectrophotometrically and confirmed by plate counts using a micromethod as previously described (25).
E. coli K-12 MG1655 (ATCC) was grown for 8 h in Luria broth medium, after which it was maintained under conditions as described for S. aureus strains. After dilution (1:50) in DMEM, bacterial cultures were grown to an optical density of 0.6 at 600 nm, corresponding to approximately 1.2 × 108 CFU/ml.
E. coli (1303) and S. aureus (1027) strains, which were used for the induction of experimental clinical (E. coli) and subclinical (S. aureus) mastitis as described previously (21), were isolated from cases of clinical and subclinical bovine mastitis, respectively. The numbers of bacterial strains refer to the identification numbers of the animals (26–28).
Cell culture infection.
Live E. coli and S. aureus bacteria were used for the experiments. Taking into account the difference in the doubling time between E. coli and S. aureus, we tested different multiplicities of infection (MOIs; numbers of bacteria per cell at the onset of infection) in order to determine the highest bacterial concentration that did not cause cytotoxicity toward host cells. Mock cells (cells treated the same way except without the bacteria) were used as controls.
Preliminary experiments using host cells infected with live E. coli for 2 h revealed that there was no alteration in cytokine expression at an MOI of 1:1 compared to the noninfected control cells. Usage of an MOI higher than 30:1 resulted in the development of cytotoxicity toward host cells. Consequently, 3 different MOIs (5:1, 15:1, and 30:1) were used for the experiments with E. coli-infected cells.
Preliminary assays with live S. aureus-infected cells showed that at an MOI of 20:1, there was no change in interleukin expression compared to that of the mock cells. The cytotoxic effect toward host cells was observed at an MOI higher than 160:1. Different MOIs (40:1, 80:1, and 160:1) were used in experiments.
DMEM without antibiotics was used as an infection medium. Eukaryotic cell concentrations were determined using one of the four replicate samples. The remaining samples were used for the analysis in triplicate. Unbound bacteria were removed 90 min postinfection by washing wells with PBS, followed by incubation in medium containing 20 μg/ml of lysostaphin and 100 μg/ml of gentamicin for 2 h, which eliminates the extracellular bacteria, followed by a washing in PBS and then by incubation in medium containing 25 μg/ml of gentamicin for the periods indicated below. Mock cells were used as controls.
Adhesion and internalization assays.
Adhesion assays were performed as described previously (29). Briefly, confluent monolayers of PS cells in 96-well plates (2.5 × 105 cells/well) were incubated for 90 min with an S. aureus suspension at an MOI of 80:1. After the lysis of cells using 0.01% Triton X-100, the number of CFU of adherent bacteria was determined using a micromethod (25). In order to account for possible variations in staphylococcal suspensions, the portion of the inoculum adhered to or internalized into PS cells was indicated as a percentage of the initial inoculum (i.e., of the number of input CFU).
For internalization assays, cells were exposed to S. aureus as described for the adhesion assay. After 90 min of incubation with bacteria, cells were incubated in DMEM containing 100 μg/ml of gentamicin and 20 μg/ml of lysostaphin for 2 h in order to remove extracellular bacteria and to measure internalized bacteria only. The percentage of internalized bacteria from the initial inoculum was determined as described for the adhesion assay.
There were no statistically significant differences between percentages of either adhered or internalized LAC wt strain or LAC Δpsmα and LAC Δpsmαβhld mutants (see the supplemental material).
Evaluation of the viability of epithelial cells.
The viability of eukaryotic cells was assessed at the end of the incubation with E. coli or S. aureus. The viability was estimated by cell counts using a hemocytometer combined with the trypan blue exclusion assay (30). The results were calculated as the percentage of live cells out of the total number of cells. There were no significant differences between percentages of infected and control noninfected cells at any of the concentrations used (data not shown).
Gene expression analysis by qRT-PCR.
Infection of bovine MEC was performed as indicated above. Interleukin expression was evaluated by quantitative real-time PCR (qRT-PCR), as described previously (17). Briefly, total RNA was isolated with an RNA II kit (Macherey-Nagel), and cDNA was synthesized using a qScript cDNA synthesis kit (Quanta Biosiences). Reaction mixtures devoid of reverse transcriptase and reaction mixtures containing H2O instead of cDNA were used as negative controls. Consensus sequence from an alignment of bovine mRNA IL-32 sequences available at http://www.ncbi.nlm.nih.gov (NCBI reference sequence accession numbers XM_002697870.3, XM_001790359.3, XM_002697939.2, and XM_005224638.1) were used for primer design (Table 1). The peptidyl-prolyl cis-trans isomerase A (PPIA) and RPL19 (ribosomal protein 19) housekeeping genes were used as reference genes for normalization. Amplification was performed on a CFX96 real-time system (Bio-Rad, Marne la Coquette, France).
TABLE 1.
Primer sequences for qRT-PCRa
| Primer | Sequence | Product size (bp) | NCBI accession no. |
|---|---|---|---|
| IL-32f | 5′-TCAAGAGAACAGTCCCGAAACC-3′ | 71 | Consensus sequence |
| IL-32r | 5′-AGCGTACTTCTTGCTGTGCTTC-3′ | ||
| IL-8f | 5′-TGGGCCACACTGTGAAAAT-3′ | 92 | NM_173925.2 |
| bIL-8r | 5′-TCATGGATCTTGCTTCTCAGC-3′ | ||
| IL-6f | 5′-TGCTGGTCTTCTGGAGTATC-3′ | 153 | EU276071 |
| IL-6r | 5′- GTGGCTGGAGTGGTTATTAG-3′ | ||
| RPL19f | 5′-TACTGCCAATGCTCGAATGC-3′ | 114 | NM_001040516.1 |
| RPL19r | 5′-TGATACATGTGGCGGTCAATC-3′ | ||
| PPIAf | 5′-ATGGCAAGACCAGCAAGAAG-3′ | 201 | XM_002690515.2 |
| PPIAr | 5′-CTTGGAGGGGGATAAGGAAA-3′ |
Primer sequences were designed using primer 3. The RPL19 and PPIA genes were used as housekeeping genes. All melting temperatures were 60°C. The relative quantification of the mRNA levels of the target genes was determined using CFX Manager based on the threshold cycle method.
Relative quantification after normalization refers to the PCR signal of the target transcript in a treatment group divided by the values obtained from mock cells, arbitrarily set to 1. When the expression was decreased compared to that in mock cells, data were presented as negative values.
HPLC-MS.
To analyze PSM production in culture filtrates of the bovine mastitis isolates, high-performance liquid chromatography–mass spectrometry (HPLC-MS) analysis of culture filtrates was performed as described previously (23).
Udder samples from E. coli- or S. aureus-infected cows.
Samples from infected animals were obtained from previous experiments conducted as described previously (21). Experiments were performed at the Centre for Clinical Veterinary Medicine, Munich, Germany, with the approval of the ethics committee of the regional government (no. 55.2-1-54-2531-108-05) Briefly, 10 healthy German Holstein Friesian heifers in midlactation were inoculated in one quarter with 500 CFU of E. coli strain 1303 (n = 5) or with 10,000 CFU of S. aureus strain 1027 (n = 5). Five heifers that received no treatment served as untreated controls. All E. coli-inoculated animals developed clinical mastitis in the affected quarter. The diagnosis of subclinical S. aureus-induced mastitis was assessed for all 5 animals based on an increase of the milk somatic cell count of >106/ml and repeated recovery of S. aureus in milk of the inoculated quarters. Animals were slaughtered 24 h postinfection (p.i.) (E. coli) or 72 h p.i. (S. aureus). RNA was isolated from 100 mg of udder tissue using TRIzol (Invitrogen) (21). Samples were reverse transcribed to cDNA using random hexamers and SuperScript RT III (Invitrogen) and were analyzed by qRT-PCR. After normalization using RPL19 and PPIA, expression of each gene was calculated relative to the values obtained from unstimulated samples.
Analysis of cytotoxic effects of PSMα3.
Bovine PS cells (15 × 103/well) were grown to 80% confluence in 96-well tissue culture plates overnight. The cells then were exposed to PSMα3 for 8 h, 24 h, and 48 h in DMEM without FCS, as described above. PSM concentrations ranged from 0.01 to 2 μg/ml. The cytotoxic activity of PSM was estimated by release of cytoplasmic lactate dehydrogenase (LDH) (LDH cytotoxicity assay kit; Pierce, USA), as described previously (17).
For controls, untreated cells were included for measurements of spontaneous LDH release and maximum LDH release was induced by the addition of lysis reagent. LDH activity in DMEM at A492 (Xenius, Safas, Monaco) alone was used as the background control. Cytotoxicity levels were calculated as follows: LDH release of treated cells (percent) = (LDH of PSM-treated cells − LDH of control untreated cells)/(maximum LDH release − LDH of control untreated cells) × 100.
An exposure to synthetic PSMα3 for 8 h and 24 h in a range of concentrations from 0.01 to 2 μg/ml and for 48 h in a range of concentrations from 0.01 to 0.5 μg/ml did not reveal a cytotoxic effect, while 10% of cytotoxic activity was observed after 48 h of incubation with 2 μg/ml of PSMα3 (data not shown).
Exposure of PS cells to synthetic PSMα3.
Bovine PS cells were exposed to synthetic PSMα3 ranging from 0.01 to 2 μg/ml for 8 h, 24 h, or 48 h. Interleukin expressions in PS cells were evaluated by qRT-PCR, as described above.
Statistical analysis.
At least three different assays were performed per experiment. The differences among the groups in in vitro experiments were assessed by analysis of variance (ANOVA). P values of <0.05 were considered to be significant. Tukey's honestly significant difference (HSD) test was applied for comparison of means between groups. The values are expressed as means ± standard deviations (SD). To analyze the tissue samples, the permutation test was performed using the StatXact software. P values of <0.05 were considered significant.
RESULTS
Upregulation of IL-32, IL-6, and IL-8 expression in E. coli-infected cells.
To analyze whether there is a difference in IL-32 expression in MEC exposed to live S. aureus versus live E. coli, we first investigated the gene expression in E. coli-infected cells, taking into account the capacity of E. coli to induce strong immune responses. Different MOIs (5:1, 15:1, and 30:1) were used for experiments with E. coli-infected cells. Results obtained with an MOI of 30:1, the highest E. coli concentration without a cytotoxic effect, are presented in Fig. 1, while Table S1 in the supplemental material shows the results obtained with other MOIs.
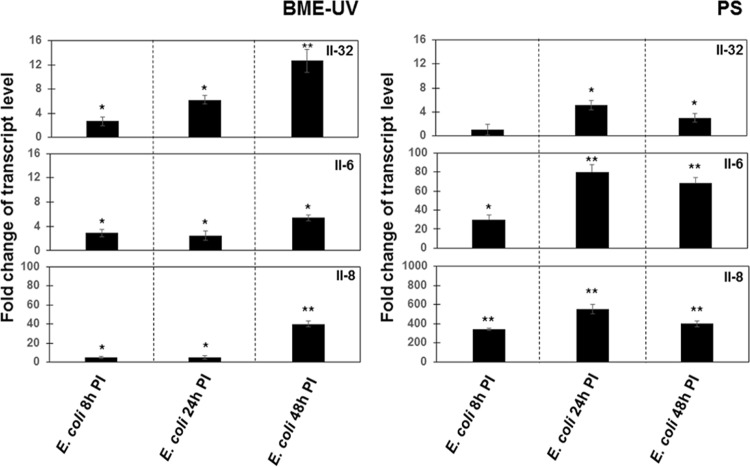
FIG 1.
Upregulation of IL-32, IL-6, and IL-8 expression in E. coli-infected cells. BME-UV or PS cells (2 × 105) were grown in six-well plates for 24 h. The cells were then exposed to the E. coli K-12 strain (MOI, 30:1) for 8 h, 24 h, and 48 h. Isolation of total RNA, synthesis of cDNA, and qRT-PCR were performed as described in Materials and Methods. After normalization using PPIA and RPL19 genes, interleukin expression at 8 h, 24 h, and 48 h p.i. was calculated relative to the values obtained from mock cells, arbitrarily set to 1. Data were calculated from three different experiments performed in triplicate. P values of <0.05 (*) and <0.01 (**) were considered significant.
There were no differences in IL-32 expression between E. coli-infected (MOI, 30:1) and uninfected BME-UV or PS cells 2 h, 4 h, and 6 h p.i. (data not shown). The expression of IL-32 by BME-UV cells was increased 3-fold after 8 h, 6-fold after 24 h, and 12-fold after 48 h of exposure compared to that in mock cells; an increase of IL-32 expression in E. coli-infected PS cells was observed after 24 h and after 48 h of exposure (Fig. 1).
To gain a more comprehensive picture of the MEC response to E. coli infection, the expression levels of IL-6 and IL-8, key regulatory mediators of immune responses, were evaluated under the same conditions. As shown in Fig. 1, the relative levels of mRNA expression of IL-6 and IL-8 were increased in both types of cells 8 h, 24 h, and 48 h p.i.; however, the level of expression was much higher in PS cells.
Similar results were obtained using MOIs of 5:1 and 15:1, as shown in Table S1.
Downregulation of IL-32 expression and upregulation of IL-6 and IL-8 expression in S. aureus-infected cells.
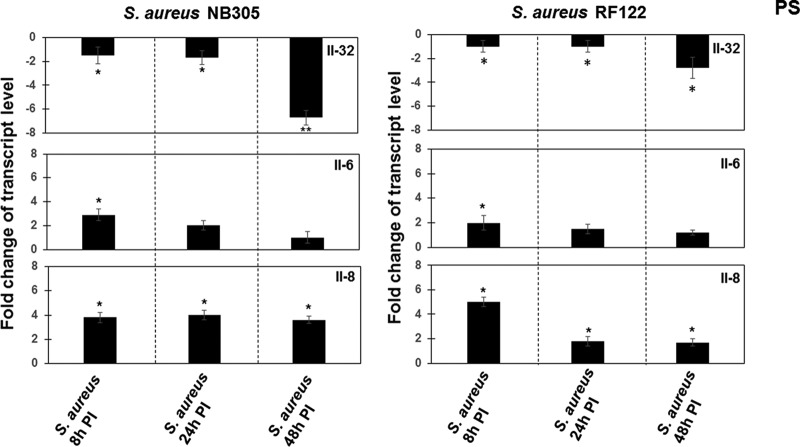
Since the responsiveness of PS cells was higher than that of BME-UV cells (Fig. 1), PS cells were employed for the analysis of cytokine expression after challenge with the two S. aureus strains RF122 and NB305. MOIs of 40:1, 80:1, and 160:1 were used in the experiments. The results obtained with an MOI of 80:1 (the highest S. aureus concentration without a cytotoxic effect toward host cells) are presented in Fig. 2, while Table S2 in the supplemental material shows results obtained with the 3 different MOIs.
FIG 2.
Analysis of IL-32, IL-6, and IL-8 expression in S. aureus-infected cells. PS cells (2 × 105) were grown for 24 h. The cells were then exposed to S. aureus NB305 or RF122 strains (MOI, 80:1) for 8 h, 24 h, and 48 h. Isolation of total RNA, synthesis of cDNA, and qRT-PCR were performed as described in Materials and Methods. After normalization using PPIA and RPL19 genes, interleukin expression at 8 h, 24 h, and 48 h p.i. was calculated relative to the values obtained from mock cells, arbitrarily set to 1. When the expression was decreased compared to that in unstimulated mock cells, data were presented as negative values. Data were calculated from three different experiments performed in triplicate. P values of <0.05 (*) and <0.01 (**) were considered significant.
There was no alteration in IL-32 expression at 2 h, 4 h, or 6 h postinfection; hence, the expression of IL-32 was investigated from 8 h to 48 h postinfection. As shown in Fig. 2, there were slight decreases in IL-32 expression after 8 h or 24 h of exposure of PS cells to either S. aureus strain (Fig. 2). The decreases in IL-32 expression were stronger after 48 h of exposure, namely, 7-fold and 3-fold for strains RF122 and NB305, respectively. To gain a more complete picture of the cytokine response during S. aureus infection, the levels of expression of IL-6 and IL-8 were analyzed at 8 h, 24 h, and 48 h postinfection. A slight increase in IL-6 expression was observed in cells after 8 h of exposure to either the NB305 or RF122 strain. There were no differences between infected and noninfected cells at 24 h or 48 h postinfection (Fig. 2). The level of IL-8 expression was approximately 4-fold higher in cells infected for 8 h than in noninfected control cells. After 24 h and 48 h, the IL-8 expression was only twice as high as the expression in control cells (Fig. 2). Similar results were obtained using MOIs ranging from 40:1 to 160:1 (see Table S2 in the supplemental material).
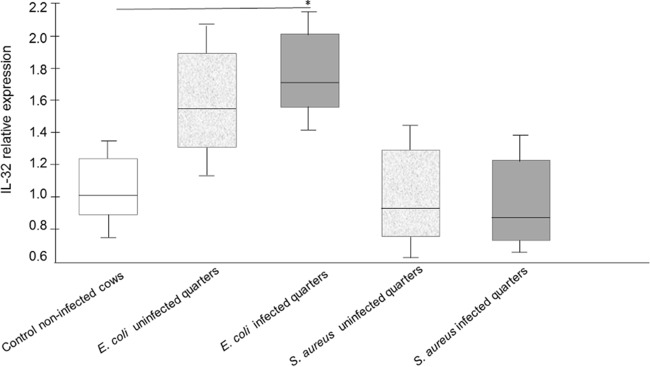
IL-32 expression in E. coli- and S. aureus-infected udder tissue resembles in vitro responses.
To verify whether IL-32 expression was increased in the udders of experimentally infected cows, E. coli- or S. aureus-infected udder samples, prepared as described previously (21), were analyzed by qRT-PCR. A significant increase of IL-32 expression was observed in E. coli-infected quarters. There was a slight increase in IL-32 expression in uninfected quarters from E. coli-infected cows; however, the difference did not reach statistical significance (Fig. 3). In contrast, there were no differences in IL-32 expression between udder samples of control cows and samples of either infected or uninfected quarters of udders of S. aureus-infected cows (Fig. 3).
FIG 3.
Increased IL-32 expression during experimentally E. coli-induced mastitis. Cows were infected either with E. coli or S. aureus as described in Materials and Methods. IL-32-specific qRT-PCRs were performed with RNA extracted from tissue samples of udder quarters from uninfected or bacteria-infected cows. Data presented are values from five animals; a statistical significance (P < 0.05 [*]) of E. coli-infected versus uninfected cows was observed using exact permutation tests after global comparison using a Kruskal-Wallis test.
The bovine mastitis isolates have low-level expression of PSMα peptides but high expression of δ-toxin.
To analyze PSM production in the bovine mastitis isolates used in this study, we performed HPLC-MS analysis of culture filtrates (23) in comparison to a culture filtrate of the USA300 isolate LAC, which was previously described as a high PSM producer (12). Taking into account that all tested bovine mastitis isolates and LAC USA300 have similar growth curves, all strains were grown as described in Materials and Methods until cultures reached an optical density of 0.6 at 600 nm.
As shown in Fig. 4, the bovine mastitis isolates showed production of δ-toxin in a range similar to that of strain LAC, but PSMα and PSMβ levels were considerably reduced compared to those in that strain.
FIG 4.
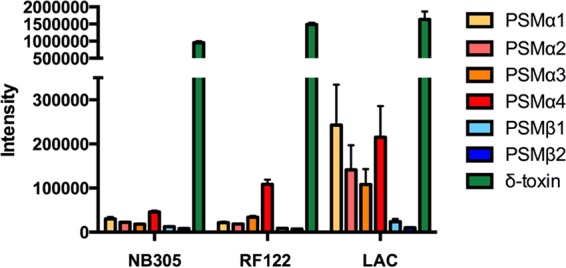
PSM production in bovine mastitis isolates. PSMs were analyzed in culture filtrates of cultures inoculated from precultures and grown for 8 h in tryptic soy broth. HPLC-MS detection was performed essentially as described previously (23). Intensity values are based on the integration of the extracted ion chromatogram using the two most abundant m/z peaks of every single PSM. Intensities can thus be directly compared between strains for a specific PSM, but only in a limited way for different PSMs. The assay was performed in triplicate. Error bars show standard deviations.
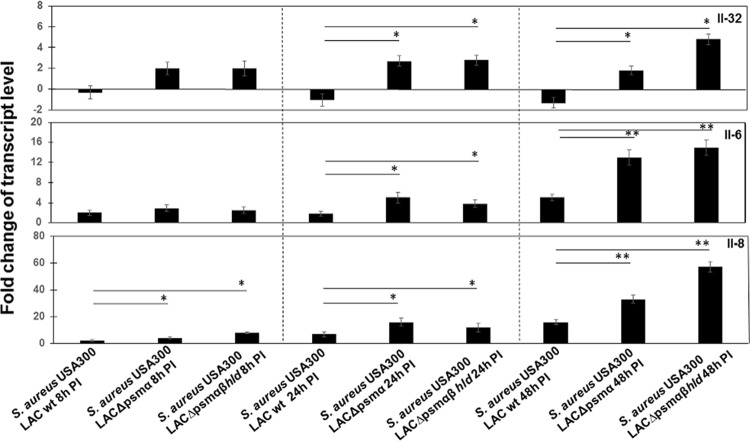
S. aureus PSM deletion mutants induce higher interleukin expression than the wild-type strain.
Interleukin expression levels exposed to various strains were compared to those of mock cells, and then the differences between those of cells exposed to the LAC wt strain and deletion mutants were evaluated. Expression levels of IL-32, IL-6, and IL-8 in cells exposed either to LAC wt or to corresponding deletion mutants with MOI 80:1 from 8 h to 48 h differ from those of mock cells. As shown in Fig. 5, the LAC wt strain decreased IL-32 expression 24 h and 48 h postinfection, while its isogenic mutants LAC Δpsmα and LAC Δpsmαβhld increased expression levels compared to those in mock cells (Fig. 5). To assess the impact of PSMs on interleukin expression, we compared interleukin expressions in LAC wt-treated cells to those in cells stimulated by the LAC Δpsmα or LAC Δpsmαβhld. ANOVA analysis followed by Tukey's HSD test showed that there was a significant difference between IL-32 expression levels in cells when stimulated either by the LAC Δpsmα mutant or LAC Δpsmαβhld compared to LAC wt at 24 h and 48 h postinfection (Fig. 5).
FIG 5.
Analysis of the expression of IL-32, IL-6, and IL-8 in cells exposed to S. aureus LAC wt and its mutants. PS cells (2 × 105) were grown for 24 h. The cells were then exposed to LAC wt and to the deletion mutants LAC Δpsmα and LAC Δpsmαβhld at an MOI of 80:1 for 8 h, 24 h, and 48 h. An mRNA expression was measured in total RNA preparation by qRT-PCR. After normalization using PPIA and RPL19 genes, interleukin expression was calculated relative to the values obtained from mock cells, arbitrarily set to 1. According to ANOVA with post hoc Tukey's HSD test, there was a statistically significant difference between interleukin expression levels in cells exposed either to LAC wt or deletion mutants and mock cells, except for IL-32 expression in LAC wt-treated cells for 8 h. There were also statistically significant differences between interleukin expression levels in cells exposed to LAC wt and corresponding deletion mutants as indicated with asterisks. Data were calculated from three different experiments performed in triplicate. P values of <0.05 (*) and <0.01 (**) were considered significant.
Additionally, there were statistically significant differences between IL-6 and IL-8 expression levels in cells exposed to either LAC wt or deletion mutants: IL-6 expression was twice as great in cells exposed to LAC Δpsmα or LAC Δpsmαβhld mutants than in cells exposed to LAC wt 24 h postinfection and 3-fold higher at 48 h postinfection. IL-8 was increased 2.8-fold in LAC Δpsmα-treated cells and 4-fold in LAC Δpsmαβhld-treated cells compared to LAC wt-treated cells. Similar results were obtained with other MOIs (see Table S3 in the supplemental material).
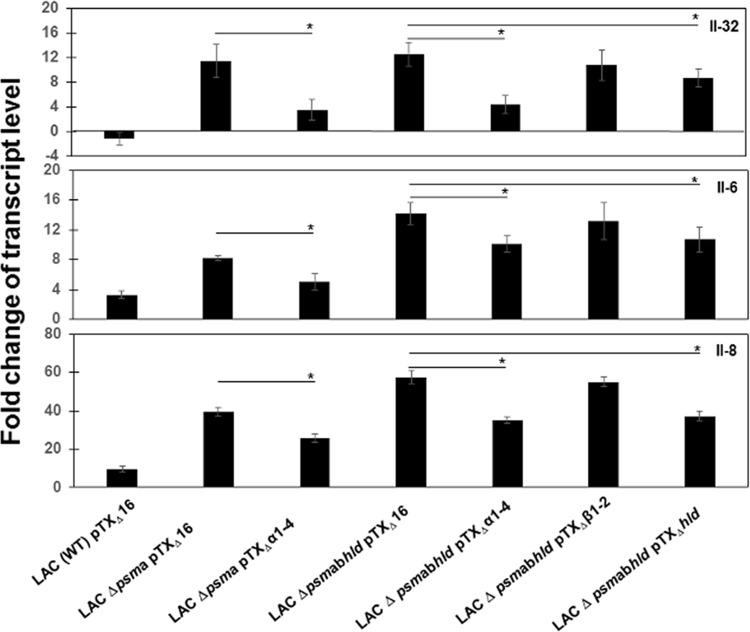
To confirm the findings, we compared interleukin expression levels induced by a wild-type (WT) LAC pTXΔ16 strain harboring the control plasmid pTXΔ16 to those of corresponding deletion mutants as well as genetically complemented strains, which are described in Materials and Methods. It has to be noted that interleukin expression levels in cells exposed to all strains from 8 h to 48 h were different from those of mock cells: the IL-32 expression level was downregulated, while IL-6 and IL-8 expression levels were upregulated (Fig. 6). Interleukin expression levels in cells exposed to a LAC (WT) pTXΔ16 strain was, similar to those induced by LAC wt (USA300). Exposure to psmα deletion mutant LAC Δpsmα pTXΔ16 resulted in increased expression of all tested interleukins, similar to the increase of expression induced by the deletion mutant LAC Δpsmα, while exposure to the complemented strain LAC Δpsmα pTXΔα1-4 resulted in a decrease of expression levels. Comparison of expression levels in cells exposed to LAC (WT) pTXΔ16 to those in cells exposed to corresponding deletion and complemented mutants showed that interleukin expression levels were significantly higher in cells exposed to complemented strain LAC Δpsmα pTXΔα1-4 than in cells infected with LAC (WT) pTXΔ16 but lower than those from cells infected with deletion mutant LAC Δpsmα pTXΔ16.
FIG 6.
Analysis of the expression of IL-32, IL-6, and IL-8 in cells exposed to S. aureus LAC WT pTXD16, corresponding deletion mutants, and complemented strains. PS cells (2 × 105) were grown for 24 h. The cells were then exposed to LAC (WT) pTXΔ16, to the deletion PSMα-deficient mutant LAC Δpsmα pTXΔ16, and to the complemented LAC Δpsmα pTXΔα1-4 strain, as well as to the PSM-deficient mutant LAC Δpsmαβhld pTXΔ16 and complemented strains LAC Δpsmαβhld pTXΔα1-4, LAC Δpsmαβhld pTXΔβ1-2, and LAC Δpsmαβhld pTXΔηhld at an MOI of 80:1 for 24 h. mRNA expression was measured in total RNA preparations by qRT-PCR. After normalization using PPIA and RPL19 genes, interleukin expression was calculated relative to the values obtained from mock cells, arbitrarily set to 1. According to ANOVA with post hoc Tukey's HSD test, there was a statistically significant difference between interleukin expression levels in cells exposed to either LAC (WT) pTXΔ16, the deletion mutants, or complemented strains and mock cells. Statistical analysis demonstrated that interleukin expression levels were significantly higher in cells infected with deletion mutants than in those from cells infected either with LAC (WT) pTXΔ16 or complemented strains. Statistically significant differences between interleukin expression levels in cells exposed to deletion mutants and corresponding complemented strains are indicated with asterisks. Data were calculated from three different experiments performed in triplicate. P values of <0.05 (*) and <0.01 (**) were considered significant.
To examine the role of other PSMs, interleukin expression levels in cells exposed to LAC (WT) pTXΔ16 were compared to expression levels in cells exposed to the PSM-deficient deletion mutant LAC Δpsmαβhld pTXΔ16 and to the complemented mutants LAC Δpsmαβhld pTXΔα1-4, LAC Δpsmαβhld pTXΔβ1-2, and LAC Δpsmαβhld pTXΔhld. As shown in Fig. 6, exposure to deletion mutant LAC Δpsmαβhld pTXΔ16 increased interleukin expression compared to that of the LAC (WT) pTXΔ16 strain expressing PSMs. Exposure to the complemented strains LAC Δpsmαβhld pTXΔα1-4 and LAC Δpsmαβhld pTXΔhld resulted in lower interleukin expression than did exposure to the PSM-deficient LAC Δpsmαβhld pTXΔ16 strain, while no significant difference was observed when the psmβ locus was complemented (with plasmid pTXΔpsmβ1-2) (Fig. 6).
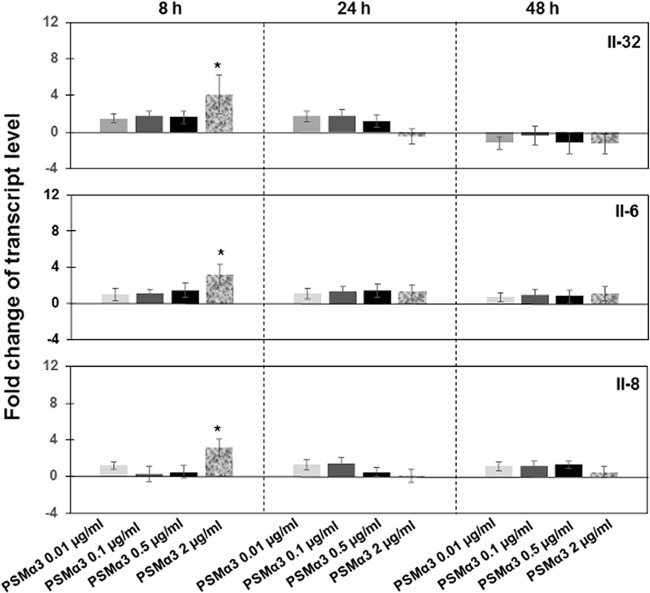
Additionally to the analysis of interleukin expression by the cells exposed to the mutantand genetically complemented bacterial strains, interleukin expression was evaluated in the cells treated with PSMα3, one of the most cytolytically active and well-characterized PSMs (12). As shown in Fig. 7, expression levels of IL-32, IL-6, and IL-8 were significantly increased after 8 h of exposure to 2 μg/ml of PSMα3.
FIG 7.
Analysis of the expression of IL-32, IL-6, and IL-8 in cells treated with synthetic PSM peptides. PS cells (2 × 105) were grown for 24 h and then exposed to synthetic PSMα3 ranging from 0.01 to 2 μg/ml for 8 h, 24 h, or 48 h. mRNA expression was measured in total RNA preparation by qRT-PCR. After normalization using PPIA and RPL19 genes, interleukin expression was calculated relative to the values obtained from mock cells, arbitrarily set to 1. According to ANOVA with post hoc Tukey's HSD test, there were statistically significant differences between interleukin expression levels in cells treated with 2 μg/ml of PSMα3 for 8 h compared to those in mock cells. Data were calculated from three different experiments performed in triplicate. P values of <0.05 (*) were considered significant.
DISCUSSION
Mammary epithelial cells (MEC) represent the first line of contact with microorganisms and are crucial for the early defense against intramammary pathogens. They produce various antimicrobial substances and inflammatory mediators that enhance effector functions of innate immunity and stimulate adaptive immunity, playing a pivotal role in the resolution of infection (27). A great number of studies dedicated to the analysis of the defensive response of MEC used killed bacteria or bacterial supernatants; however, those models have a significant limitation, since there is a great difference in the immune responses initiated by live bacteria, killed bacteria, bacterial supernatants, and purified bacterial components (26, 31, 32). Using live E. coli and S. aureus bacteria, we set up in vitro infection models that assessed the effects of these Gram-negative and Gram-positive bacteria on expression of IL-32, IL-6, and IL-8.
In in vitro MEC challenges, the increased level of E. coli-induced IL-6 and IL-8 expression compared to the reduction induced by S. aureus observed in our experiments was in agreement with data obtained by others (26).
The role of the newly discovered proinflammatory IL-32 during inflammation and in several types of infections is well documented (33, 34). However, to our knowledge there is no information about the involvement of IL-32 during exposure to live S. aureus, and there are no data regarding infections associated with S. aureus or E. coli in the context of mastitis. We observed decreased IL-32 expression in S. aureus-infected cells compared to that in E. coli-infected cells. This is important new information contributing to our understanding of the molecular mechanisms that underlie the divergent host responses during S. aureus versus E. coli infection.
The increase of IL-32 expression in E. coli-infected cells or in the udder tissue of E. coli-infected cows was consistent with other studies of IL-32 in response to Gram-negative pathogens and endotoxin (35–37). In contrast, expression of IL-32 by S. aureus-infected cells was decreased. Taking into account that IL-32α plays an intracellular mediatory role in IL-6 production in promonocytic cells (34) and considering the capacity of human IL-32γ to induce the maturation of dendritic cells (38), we speculate that decreased IL-32 expression during S. aureus infection could be associated with an attenuated immune response and may promote the progression of chronic infection.
The extraordinary virulence of S. aureus depends on its ability to effectively compromise host defense mechanisms by a variety of strategies: S. aureus may inhibit complement activation, block and destroy phagocytic cells, and modify host B-cell and T-cell responses (39). Several factors contribute to the pathogenicity of S. aureus, including the well-established virulence determinants accessory gene regulator and alpha-hemolysin (Hla) (40, 41). The immunomodulatory actions of staphylococcal enterotoxins are also reported (42, 43). Additionally, PSMs have recently emerged as a novel toxin family that contributes to increased virulence and the spread of S. aureus infection (12, 44). PSMs are divided into two groups, depending on their size: the short (20- to 25-amino-acid) α-type peptides (PSMα1 to PSMα4 and δ-toxin) and the long (44-amino-acid) β-type peptides (PSMβ1 and PSMβ2) (13). Prompted by those results, we examined in this study the role of PSMs in S. aureus-induced interleukin expression. Comparison of clinically related LAC wt strain to PSM deletion mutants allows the correlation of the findings with those obtained by using of RF122 and NB305 strains isolated from cows with mastitis. Infection of cells with PSM deletion mutants resulted in an increase of IL-6, IL-8, and IL-32 expression, in contrast to the findings with LAC wt (Fig. 5). Utilization of wild-type, deletion, and complemented strains harboring plasmids allowed us to comprehend the impact of different PSMs. Exposure of cells to a strain expressing the four PSMα peptides significantly decreased interleukin expression compared to that obtained with a PSMα deletion mutant (Fig. 6), suggesting that PSMα expression by internalized S. aureus inhibits interleukin expression. Since the LAC Δpsmαβhld mutant induced a higher level of interleukin expression than the LAC Δpsmα mutant, we examined whether PSMβ peptides or, more likely, the highly expressed δ-toxin may be involved in the inhibition of interleukin expression in addition to the PSMα peptides. Results from strains specifically expressing only the δ-toxin or the PSMβ peptides showed that also the δ-toxin has a significant effect, while no decrease of interleukin expression was observed with the PSMβ-expressing strain, a result that may be explained either by the different structure of the considerably longer PSMβ peptides or by their overall lower expression levels. The observed inhibitory effect likely depends on the presence of PSMs and not different numbers of bacteria, which were adhered to or internalized into cells, since we observed similar adherence and internalization rates between LAC wt and its mutants.
The observed inhibition of IL-6, IL-8, and IL-32 expression in bovine epithelial cells by staphylococcal PSMs differs from the observations showing increased release of IL-8 by human neutrophils upon addition of PSMs (12, 45). This difference could be explained by different conditions of experiments, including the exposure time and PSM concentrations, or by the different transduction pathways induced by PSMs in different types of cells. Furthermore, cell lysis may also explain the release of IL-8, as the PSM concentrations used in those experiments were in the cytolytic range (12). Similarly, the stimulation of PSM-induced IL-1β and IL-18 release from human keratinocytes, highly specialized epithelial cells, was also likely associated with cell lysis (15). Treatment of bovine PS cells with PSMα3 at 2 μg/ml for 8 h resulted in increased interleukin expression in the absence of a cytotoxic effect (Fig. 7). These findings suggest a difference between transduction pathways induced by a synthetic PSM and a PSM produced by internalized S. aureus bacteria.
An analysis of epithelial intestinal HT-29 cells showed an increase in IL-8 secretion after exposure to TNF-α in contrast to the absence of IL-8 secretion in PSM-stimulated cells (46). Interleukin inhibition by internalized bacteria, observed in our experiments, is in accordance with data showing the inhibition of IL-6, IL-12, and TNF-α expression in dendritic cells by PSMα and data demonstrating the inhibition of TNF-α secretion by the USA300 wild-type strain harboring PSMs in contrast to the mutant strain deficient in all PSMs (18), and it shows that this effect extends to the “front line” defenses of the epithelium. The epithelium is in permanent contact with various microorganisms, resulting in the host's defense mechanisms, including expressing and secreting inflammatory cytokines that recruit inflammatory cells. Thus, it was shown that TNF-α and IL-1β were secreted by the primary cultured rat epithelia infected with S. aureus (47). Human corneal epithelial cells react to S. aureus infection by enhancing the secretion of IL-6 (48).
In conclusion, we found that in mammary epithelial cells S. aureus PSMs decrease the expression of the cytokine IL-32, which is responsible for the maturation of dendritic cells and plays an essential role in linking innate and adaptive immunity. This mechanism may contribute to the attenuated immune response generally observed during staphylococcal infection. These findings may be important for the development of new anti-infective and anti-inflammatory strategies. Finally, we cannot exclude the existence of various isoforms of bovine IL-32 (with differential similarity to human IL-32) with different expression levels of the expression; a more detailed assessment of those differences forms an avenue for future investigation. As a final point, it has to be noted that the findings obtained with bovine cells and tissue likely may be extended to human medicine: it would be appropriate to determine the level of IL-32 in patients with mastitis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the French National Institute for Agricultural Research (INRA), Metaprogram Gestion Integrée de la Santé des Animaux (GISA), Ruminflame grant no. P10552 (to N.B., M.D., Y.L.L, P.R., and P.G.), ERA-NET ANIHWA KOLIMASTIR, Convention ANR-13-ANWA-0003-06 (to N.B., P.G., P.R., L.A., and K.S.) and OD1718 (to D.G.E.S.), and the National Institutes of Health, National Institute of Allergy and Infectious Diseases Intramural Research Program (to M.O.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01330-15.
REFERENCES
- 1.Thompson-Crispi K, Atalla H, Miglior F, Mallard BA. 2014. Bovine mastitis: frontiers in immunogenetics. Front Immunol 5:493. doi: 10.3389/fimmu.2014.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schukken YH, Gunther J, Fitzpatrick J, Fontaine MC, Goetze L, Holst O, Leigh J, Petzl W, Schuberth HJ, Sipka A, Smith DG, Quesnell R, Watts J, Yancey R, Zerbe H, Gurjar A, Zadoks RN, Seyfert HM. 2011. Host-response patterns of intramammary infections in dairy cows. Vet Immunol Immunopathol 144:270–289. doi: 10.1016/j.vetimm.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Fu Y, Zhou E, Liu Z, Li F, Liang D, Liu B, Song X, Zhao F, Fen X, Li D, Cao Y, Zhang X, Zhang N, Yang Z. 2013. Staphylococcus aureus and Escherichia coli elicit different innate immune responses from bovine mammary epithelial cells. Vet Immunol Immunopathol 155:245–252. doi: 10.1016/j.vetimm.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Jensen K, Gunther J, Talbot R, Petzl W, Zerbe H, Schuberth HJ, Seyfert HM, Glass EJ. 2013. Escherichia coli- and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genomics 14:36. doi: 10.1186/1471-2164-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alekseeva L, Huet D, Femenia F, Mouyna I, Abdelouahab M, Cagna A, Guerrier D, Tichanne-Seltzer V, Baeza-Squiban A, Chermette R, Latge JP, Berkova N. 2009. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol 9:33. doi: 10.1186/1471-2180-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida RA, Dogan B, Klaessing S, Schukken YH, Oliver SP. 2011. Intracellular fate of strains of Escherichia coli isolated from dairy cows with acute or chronic mastitis. Vet Res Commun 35:89–101. doi: 10.1007/s11259-010-9455-5. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. 2005. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity 22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. 2004. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A 101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung MY, Son MH, Kim SH, Cho D, Kim TS. 2011. IL-32gamma induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J Immunol 186:6848–6859. doi: 10.4049/jimmunol.1003996. [DOI] [PubMed] [Google Scholar]
- 10.Painter KL, Krishna A, Wigneshweraraj S, Edwards AM. 2014. What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia? Trends Microbiol 22:676–685. doi: 10.1016/j.tim.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 13.Peschel A, Otto M. 2013. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11:667–673. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasigade JP, Trouillet-Assant S, Ferry T, Diep BA, Sapin A, Lhoste Y, Ranfaing J, Badiou C, Benito Y, Bes M, Couzon F, Tigaud S, Lina G, Etienne J, Vandenesch F, Laurent F. 2013. PSMs of hypervirulent Staphylococcus aureus act as intracellular toxins that kill infected osteoblasts. PLoS One 8:e63176. doi: 10.1371/journal.pone.0063176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed AK, Reed TJ, Clark KL, Boles BR, Kahlenberg JM. 2015. Staphylococcus aureus phenol-soluble modulins stimulate the release of proinflammatory cytokines from keratinocytes and are required for induction of skin inflammation. Infect Immun 83:3428–3437. doi: 10.1128/IAI.00401-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. 2010. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deplanche M, Filho RA, Alekseeva L, Ladier E, Jardin J, Henry G, Azevedo V, Miyoshi A, Beraud L, Laurent F, Lina G, Vandenesch F, Steghens JP, Le LY, Otto M, Gotz F, Berkova N. 2015. Phenol-soluble modulin alpha induces G2/M phase transition delay in eukaryotic HeLa cells. FASEB J 29:1950–1959. doi: 10.1096/fj.14-260513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiner J, Kretschmer D, Klenk J, Otto M, Buhring HJ, Stevanovic S, Wang JM, Beer-Hammer S, Peschel A, Autenrieth SE. 2013. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J Immunol 190:3417–3426. doi: 10.4049/jimmunol.1202563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsman H, Christenson K, Bylund J, Dahlgren C. 2012. Receptor-dependent and -independent immunomodulatory effects of phenol-soluble modulin peptides from Staphylococcus aureus on human neutrophils are abrogated through peptide inactivation by reactive oxygen species. Infect Immun 80:1987–1995. doi: 10.1128/IAI.05906-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavizion B, van DM, Schaeffer W, Politis I. 1996. Establishment and characterization of a bovine mammary epithelial cell line with unique properties. In Vitro Cell Dev Biol Anim 32:138–148. doi: 10.1007/BF02723679. [DOI] [PubMed] [Google Scholar]
- 21.Roussel P, Cunha P, Porcherie A, Petzl W, Gilbert FB, Riollet C, Zerbe H, Rainard P, Germon P. 2015. Investigating the contribution of IL-17A and IL-17F to the host response during Escherichia coli mastitis. Vet Res 46:56. doi: 10.1186/s13567-015-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vautor E, Cockfield J, Le MC, Le LY, Chevalier M, Robinson DA, Thiery R, Lindsay J. 2009. Difference in virulence between Staphylococcus aureus isolates causing gangrenous mastitis versus subclinical mastitis in a dairy sheep flock. Vet Res 40:56. doi: 10.1051/vetres/2009039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joo HS, Otto M. 2014. The isolation and analysis of phenol-soluble modulins of Staphylococcus epidermidis. Methods Mol Biol 1106:93–100. doi: 10.1007/978-1-62703-736-5_7. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GY, Li M, Otto M. 2013. Essential Staphylococcus aureus toxin export system. Nat Med 19:364–367. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron F, Cochet MF, Ablain W, Grosset N, Madec MN, Gonnet F, Jan S, Gautier M. 2006. Rapid and cost-effective method for micro-organism enumeration based on miniaturization of the conventional plate-counting technique. Lait 86:251–257. doi: 10.1051/lait:2006005. [DOI] [Google Scholar]
- 26.Günther J, Esch K, Poschadel N, Petzl W, Zerbe H, Mitterhuemer S, Blum H, Seyfert HM. 2011. Comparative Kinetics of Escherichia coli- and Staphylococcus aureus-specific activation of key immune pathways in mammary epithelial cells demonstrates that S. aureus elicits a delayed response dominated by interleukin-6 (IL-6) but not by IL-1A or tumor necrosis factor alpha. Infect Immun 79:695–707. doi: 10.1128/IAI.01071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitterhuemer S, Petzl W, Krebs S, Mehne D, Klanner A, Wolf E, Zerbe H, Blum H. 2010. Escherichia coli infection induces distinct local and systemic transcriptome responses in the mammary gland. BMC Genomics 11:138. doi: 10.1186/1471-2164-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petzl W, Zerbe H, Gunther J, Yang W, Seyfert HM, Nurnberg G, Schuberth HJ. 2008. Escherichia coli, but not Staphylococcus aureus triggers an early increased expression of factors contributing to the innate immune defense in the udder of the cow. Vet Res 39:18. doi: 10.1051/vetres:2007057. [DOI] [PubMed] [Google Scholar]
- 29.Almeida RA, Patel D, Friton GM, Oliver SP. 2007. Intracellular killing of mastitis pathogens by penethamate hydriodide following internalization into mammary epithelial cells. J Vet Pharmacol Ther 30:151–156. doi: 10.1111/j.1365-2885.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 30.Berkova N, Gilbert C, Goupil S, Yan J, Korobko V, Naccache PH. 1999. TNF-induced haptoglobin release from human neutrophils: pivotal role of the TNF p55 receptor. J Immunol 162:6226–6232. [PubMed] [Google Scholar]
- 31.Gilbert FB, Cunha P, Jensen K, Glass EJ, Foucras G, Robert-Granie C, Rupp R, Rainard P. 2013. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet Res 44:40. doi: 10.1186/1297-9716-44-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreilhon C, Gras D, Hologne C, Bajolet O, Cottrez F, Magnone V, Merten M, Groux H, Puchelle E, Barbry P. 2005. Live Staphylococcus aureus and bacterial soluble factors induce different transcriptional responses in human airway cells. Physiol Genomics 20:244–255. doi: 10.1152/physiolgenomics.00135.2004. [DOI] [PubMed] [Google Scholar]
- 33.Hou J, OGvan Groothuismink ZM, Claassen MA, Kreefft K, Zaaraoui-Boutahar F, van IJcken WF, Osterhaus AD, Janssen HL, Andeweg AC, de Knegt RJ, Boonstra A. 2014. Gene expression profiling to predict and assess the consequences of therapy-induced virus eradication in chronic hepatitis C virus infection. J Virol 88:12254–12264. doi: 10.1128/JVI.00775-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JW, Park YS, Lee DH, Kim JH, Kim MS, Bak Y, Hong J, Yoon DY. 2012. Intracellular interaction of interleukin (IL)-32alpha with protein kinase Cepsilon (PKCepsilon) and STAT3 protein augments IL-6 production in THP-1 promonocytic cells. J Biol Chem 287:35556–35564. doi: 10.1074/jbc.M112.400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama M, Niki Y, Kawasaki T, Takeda Y, Ikegami H, Toyama Y, Miyamoto T. 2013. IL-32-PAR2 axis is an innate immunity sensor providing alternative signaling for LPS-TRIF axis. Sci Rep 3:2960. doi: 10.1038/srep02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouhara K, Kawai T, Silva MJ, Fujita T, Hayashida K, Karimbux NY, Kajiya M, Shiba H, Kawaguchi H, Kurihara H. 2012. Expression levels of novel cytokine IL-32 in periodontitis and its role in the suppression of IL-8 production by human gingival fibroblasts stimulated with Porphyromonas gingivalis. J Oral Microbiol 4:10.3402/jom.v4i0.14832. doi: 10.3402/jom.v4i0.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakitani K, Hirata Y, Hayakawa Y, Serizawa T, Nakata W, Takahashi R, Kinoshita H, Sakamoto K, Nakagawa H, Akanuma M, Yoshida H, Maeda S, Koike K. 2012. Role of interleukin-32 in Helicobacter pylori-induced gastric inflammation. Infect Immun 80:3795–3803. doi: 10.1128/IAI.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenk M, Krutzik SR, Sieling PA, Lee DJ, Teles RM, Ochoa MT, Komisopoulou E, Sarno EN, Rea TH, Graeber TG, Kim S, Cheng G, Modlin RL. 2012. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med 18:555–563. doi: 10.1038/nm.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. 2015. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berube BJ, Bubeck WJ. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bubeck WJ, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 42.Krakauer T, Stiles BG. 2013. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence 4:759–773. doi: 10.4161/viru.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinchuk IV, Beswick EJ, Reyes VE. 2010. Staphylococcal enterotoxins. Toxins (Basel) 2:2177–2197. doi: 10.3390/toxins2082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung GY, Joo HS, Chatterjee SS, Otto M. 2014. Phenol-soluble modulins–critical determinants of staphylococcal virulence. FEMS Microbiol Rev 38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, Otto M, Inohara N, Nunez G. 2013. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol 170:1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 47.Zhao YT, Guo JH, Wu ZL, Xiong Y, Zhou WL. 2008. Innate immune responses of epididymal epithelial cells to Staphylococcus aureus infection. Immunol Lett 119:84–90. doi: 10.1016/j.imlet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Arranz-Valsero I, Schulze U, Contreras-Ruiz L, Garcia-Posadas L, Lopez-Garcia A, Paulsen F, Diebold Y. 2013. Involvement of corneal epithelial cells in the Th17 response in an in vitro bacterial inflammation model. Mol Vis 19:85–99. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.