SUMMARY
The purpose of this report is to highlight how an unusual, outdated, unpopular and overlooked reconstructive method such as the masseter flap can be a reliable, straightforward and effective solution for oral reconstruction in selected cases. We report the transposition of the masseter crossover flap in two previously pre-treated patients presenting a second primary oral squamous cell carcinoma; excellent functional results with satisfactory cosmetic appearance were obtained in both cases. In the literature, only 60 cases of oral cavity and oropharyngeal reconstructions using the masseter flap have been reported. The possible clinical utility of this flap, even in modern head and neck reconstructive surgery, is presented and discussed. We believe that the masseter flap should enter in the armamentarium of every head and neck surgeon and be kept in mind as a possible solution since it provides an elegant and extremely simple procedure in suboptimal cases for microvascular reconstruction.
KEY WORDS: Oral cavity reconstruction, Masseter flap, Second primary tumor, Vessel depleted neck, Pedicled flap
RIASSUNTO
Lo scopo di questo lavoro è quello di evidenziare come una metodica ricostruttiva inusuale, datata ed impopolare come il lembo di massetere possa invece rappresentare, per casi selezionati, una soluzione affidabile, semplice ed efficace nelle ricostruzioni del cavo orale. Riportiamo di seguito l'utilizzo del lembo di massetere in due pazienti che presentavano un secondo tumore del cavo orale e che in precedenza erano già stati sottoposti ad intervento chirurgico nel distretto testa collo; in entrambi i casi sono stati ottenuti eccellenti risultati funzionali e soddisfacenti risultati estetici. In letteratura, fino ad oggi, sono stati riportati solo 60 casi di ricostruzione del cavo orale e dell'orofaringe con il lembo di massetere. L'utilità clinica del lembo di massetere, anche nell'ambito di un approccio moderno alle ricostruzioni del distretto testa collo, viene discussa approfonditamente in questo articolo. Riteniamo che il lembo di massetere debba far parte del bagaglio culturale di ogni chirurgo del testa-collo ed essere considerato fra le alternative proponibili, in quanto ha dimostrato di essere una metodica elegante ed estremamente semplice in casi in cui sussistono delle perplessità sulle procedure microvascolari.
Introduction
The most popular method for the management of oral cavity and oropharyngeal defects following cancer ablation is nowadays represented by the transposition of microvascular flaps 1-3, and free flaps, in fact, offer the head and neck surgeon a broad variety of available tissues (bone, muscle, skin, etc.) for optimal restoration of form and function. However, not every defect strictly requires a free flap to achieve good functional results 4 5, and not every patient is an optimal candidate for a microvascular procedure 6. Therefore, alternative pedicled flaps 7-12 may have an important role even in the free flap era when dealing with elderly patients suffering from severe comorbidities 6 or with pretreated patients presenting recurrences 13 or second primary tumours 14.
The masseter muscle has been widely used for reanimation in facial nerve palsy; on the other hand, it has been seldomly reported for oral cavity and oropharyngeal reconstruction. Conley and Gullane in 1978 first introduced the masseter muscle flap as a reconstructive method for the management of oropharyngeal defects 15. This report remained isolated and was followed 10 years later by papers from Tiwari and Snow 16 and Langdon 17. These authors highlighted the usefulness and reliability of this flap, the ease and rapidity of its harvest and the minimal technical support required for the procedure. The main reported complication, of both superiorly and inferiorly based masseter flaps, was postoperative reduction of mouth opening 18.
This paper presents two clinical cases treated at the Department of Surgery and Translational Medicine of the University of Florence in which the defect resulting from tumour resection was reconstructed by the transposition of the masseter crossover flap; the senior author (AD) performed both procedures. The advantages of this unusual reconstructive method over other more popular solutions are discussed in the light of a personalised and tailored surgical approach.
Clinical technique and cases
The masseteric branches of the maxillary artery (MbMA), facial artery (MbFA), transverse facial artery (MbTFA) and superficial temporal artery (MbSTA) supply the masseter. Based on its diameter, frequency of occurrence and distribution area, the MbTFA can be considered to be the main branch supplying the masseter muscle. This artery is never encountered during standard comprehensive or selective neck dissection, which makes the harvest of the flap perfectly reliable even after previous or concomitant neck dissection as long as the external carotid artery is not transected. Venous drainage of the masseter muscle is provided by the facial vein which flows into the internal jugular vein; in case of previous neck dissection, the pterygoid venous plexus will provide venous drainage as long as the internal jugular vein is preserved.
The flap can be harvested as a crossover flap by maintaining the superior zygomatic attachments, or as an island flap by transecting both insertions. The only careful step is elevation of the parotid gland and terminal branches of the facial nerve from the superficial aspect of the muscle. This step, however, is easily performed with adequate exposure; the fascia of the masseter just above the angle of the mandible is incised and dissected free along with the cheek flap to preserve the branches of the facial nerve, and the muscle is freed along its posterior margin from the parotid gland. The detachment of the mandibular or zygomatic insertions is very quickly obtained with electrocautery and the muscle is ready to be transposed.
Patient 1
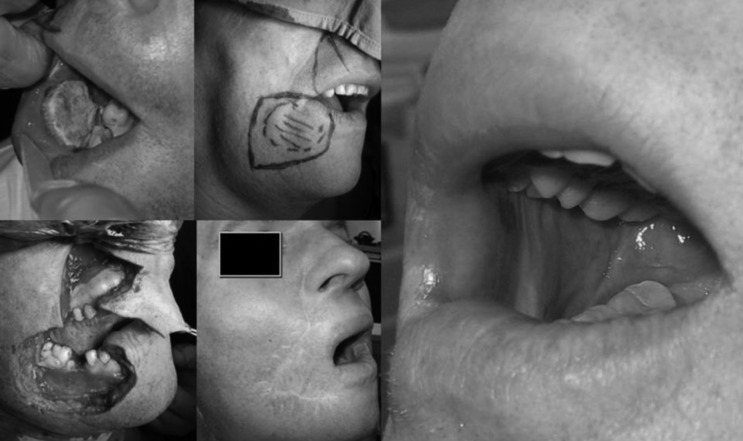
At routine follow-up consultation a second primary tumour in the retromolar trigone/posterior alveolar ridge on the right side was detected in a 64-year-old man. Biopsy revealed adenosquamous cell carcinoma, contrast enhanced CT scan was acquired and preoperative staging was cT1N0M0. Five years previously he had undergone full thickness resection of the cheek and labial commissure at the right hand side with bilateral neck dissection (levels I-V ipsilateral, and I-III contralateral) and postoperative radiotherapy for a cT4aN2bM0/pT4aN1 oral squamous cell carcinoma. Reconstruction at that time was achieved with an Abbé-Estlander flap from the upper lip and a facial artery musculomucosal flap (Fig. 1).
Fig. 1.
Full thickness resection of the right cheek and labial commissure, bilateral neck dissection and reconstruction with Abbé-Estlander + FAMM flap.
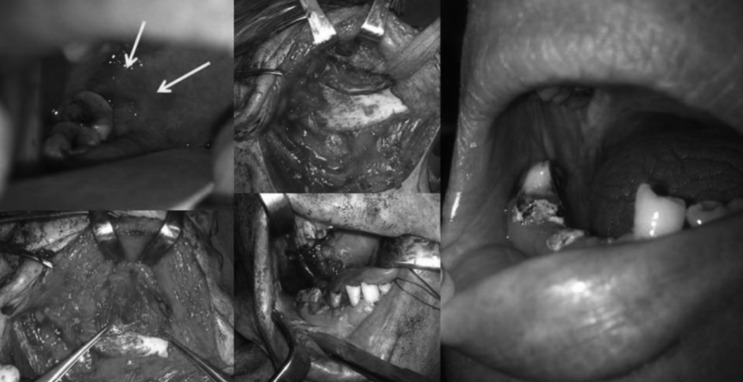
The area of the second primary tumour was approached via lateral visor flap, and tumour resection included marginal mandibulectomy; reconstruction was performed by transposition of the masseter crossover flap. Healing was uncomplicated and the flap epithelialised within 3 weeks. The pathological report confirmed adenosquamous cell carcinoma pT1, which was radically removed. The patient remains free of disease at 23 months follow-up (Fig. 2).
Fig. 2.
Resection of the second primary tumour (arrows) and reconstruction with the masseter crossover flap, complete reepithelisation of the muscle and full coverage of the remaining mandible was obtained.
Patient 2
At routine follow-up consultation a second primary squamous cell carcinoma of the right superior retromolar trigone was detected in a 56-year-old woman. Biopsy revealed a squamous cell carcinoma, contrast enhanced CT scan was acquired and preoperative staging was cT4aN0M0.
Nine years previously she had undergone resection of a cT2N0M0 squamous cell carcinoma of the right inferior alveolar ridge and floor of mouth via inferior labiotomy and lower cheek flap approach, together with selective neck dissection (levels I-III); the defect was closed primarily. The pathological report confirmed a pT2N0 squamous cell carcinoma with negative surgical margins, R0.
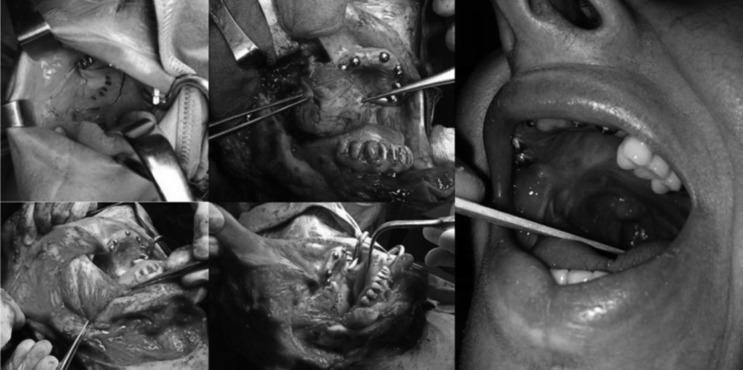
The area of the second primary tumour was approached through the previous inferior labiotomy and harvesting a lower cheek flap. The resection of the coronoid process of the mandible provided lateral access to the pterygoid plates to ensure an adequate posterior margin, and inferior posterior maxillectomy with wide macroscopic margins was performed. The resection resulted in a class Ib postmaxillectomy defect (Okay classification) with extension to a full thickness resection of the lateral quarter of the soft palate; reconstruction was very easily achieved by transposition of a masseter crossover flap (Fig. 3).
Fig. 3.
Inferior-posterior maxillectomy with masseter crossover flap reconstruction, complete re-epithelisation and tight separation between the oral cavity and maxillary sinus was obtained.
The pathological report confirmed bony involvement of the squamous cell carcinoma, pT4a, with negative surgical margins (mucosal and bony, R0), with an indication for adjuvant radiotherapy. The postoperative course was complicated by the onset of a sialocele, which was managed by weekly transcutaneous needle evacuations for 3 weeks. Despite this minor complication, healing was uneventful, the defect underwent spontaneous epithelisation and the patient completed postoperative radiotherapy.
Discussion
The masseter flap has been seldomly reported for oral cavity and oropharyngeal reconstructions, and in the literature only the results of 60 cases are available (Table I) 16- 18. In the series reported by Tiwari and Snow 16, the flap survived in 23 of 24 cases. In two cases, there was a temporary cutaneous fistula in the neck. Three patients had temporary trismus. One patient had persistent trismus. Two patients had unexplained postoperative pain over the temporomandibular area in the first week, but it improved with time until complete recovery.
Table I.
Overview of previously-reported cases.
| Author | No. of flaps |
Site* | Stage of tumour |
Previous neck RT |
Masseter Muscle Flap† |
Adjuvant treatment‡ |
Complications after surgery |
Patients requiring further surgery** |
|---|---|---|---|---|---|---|---|---|
| Tiwari 1988 | 24 | RTr: 10 LFM: 6 PF: 3 LBMT: 3 AFM: 2 SP: 1 |
T2: 16 T3: 8 |
NR | NR | None: 16 RT: 8 |
None: 14 Fistula: 2 Trismus: 2 PO-pain: 2 BoA: 1 WI: 1 |
VP: 6 |
| Langdon 1989 | 14 | EA: 1 PFM+RTr:1 SP+HP: 1 NR: 11 |
NR | NR | NR | NR | Fistula: 1 Trismus: 1 None: 13 |
CF: 1 None: 2 NR: 11 |
| Antoniades 2005 | 22 | NR | T2: 2 T3: 13 T4: 7 |
None: 22 | SBMF: 12 IMF: 10 |
CT+RT: 15 RT: 7 |
SI: 2 SH: 1 Trismus: NR |
NR |
NR: Not Reported
RTr: Retromolar trigone LFM: lateral floor of mouth PF: palatoglossal fold LBMT: lateral border of the middle third of the tongue AFM: anterior floor of the mouth SP: soft palate EA: edentulous alveolus PFM: posterior floor mouth HP: hard palate
SBMF: superiorly based masseter muscle flap IMF: island masseter muscle flap
RT: radiotherapy CT+RT: chemo radiation therapy
PO-pain: postoperative pain BoA: Breakdown of anastomoses WI: Wound infection SI: Superficial infection SH: small haematoma
VP: Vestibuloplasty CF: closure fistula
In the series by Langdon 17, there were no complications related to the flap and in all cases the bare muscle epithelialised spontaneously with no breakdown of the suture margins. No complications were reported either with previous or adjuvant radiotherapy.
In the series reported by Antoniades et al. 18, the viability of the flap was excellent in all patients and epithelisation was completed within 3 weeks. The authors stated how the island masseter muscle flap was more flexible and pliable than the crossover version. The island masseter flap is free to pivot around its pedicle with increased mobility and is useful for oropharyngeal defects; in the crossover masseter flap, the superior zygomatic insertions are maintained with obvious limitations in the mobility, and it is therefore used for more adjacent defects, mainly the retromolar trigone. In these series, the masseter flap was a safe one-stage procedure, which does not require elaborate techniques or postoperative care, and results in acceptable aesthetic loss.
In our opinion, the disadvantages of the masseter muscle flap, which restricted its wider use, are represented by the close vicinity to the primary tumour, which often results in its inclusion with the resected specimen, and by the dimensional limitations and limited mobility that make it inadequate for large or complex defects.
In the cases presented, the masseter cross-over flap was chosen instead of a fasciocutaneous free flap or a temporal myofascial flap for several reasons. Both cases were considered suboptimal for a microvascular procedure; in fact, both had an ipsilateral vessel depleted neck which raised some concerns about recipient vessels. Furthermore, since both second primaries were ipsilateral to the previously dissected neck and distant from the midline, there were no indications for an additional neck dissection. Reconstruction by means of an alternative pedicled flap was sought, and the masseter cross-over flap was favoured over the temporal flap in both cases. By approaching the tumour through a lateral visor flap and a lower cheek flap, the masseter muscle could be harvested immediately and very easily in both cases, without the need for an additional incision. Both surgical procedures were conducted without temporary tracheotomy, and hospitalisation lasted for 9 and 8 days, respectively; no further reduction in mouth opening was recorded for patient #1 and no postoperative trismus was seen in patient #2.
Conclusions
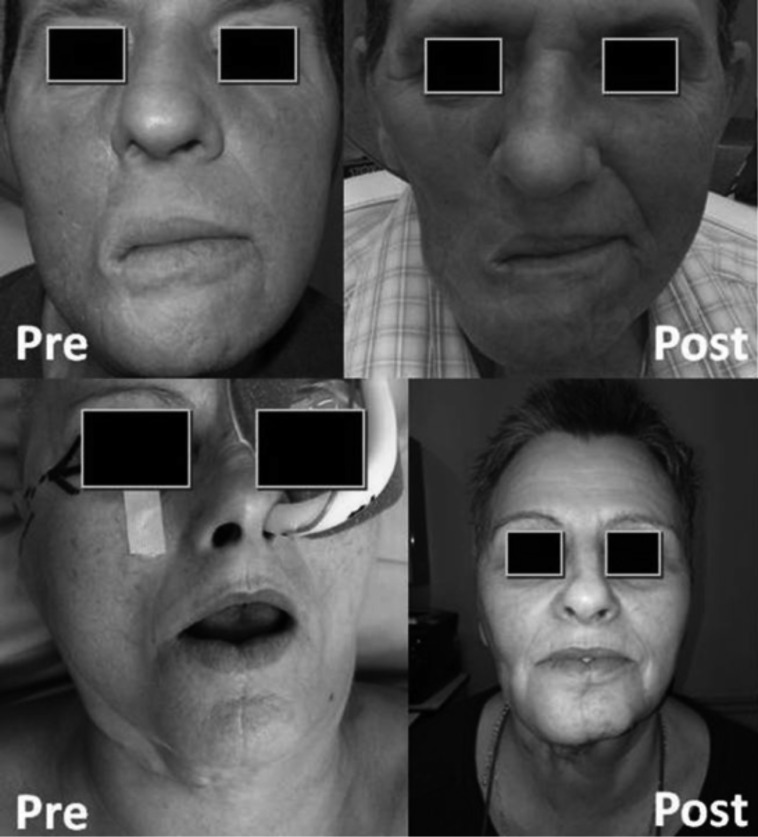
The masseter flap offers a reliable method for oral cavity and oropharyngeal reconstruction in selected cases; it is a safe, single stage procedure, which does not require elaborate technique or postoperative care. Especially advantageous are the low postoperative morbidity, low rate of postoperative complications and good functional results with acceptable cosmetic donor site morbidity (Fig. 4).
Fig. 4.
Pre- and postoperative appearance of both patients; the aesthetic deformity following a masseter flap reconstruction is minimal.
References
- 1.Tarsitano A, Ciocca L, Cipriani R, et al. Mandibular reconstruction using fibula free flap harvested using a customised cutting guide: how we do it. Acta Otorhinolaryngol Ital. 2015;35:198–201. [PMC free article] [PubMed] [Google Scholar]
- 2.Berrone M, Crosetti E, Succo G. Repositioning template for mandibular reconstruction with fibular free flaps: an alternative technique to pre-plating and virtual surgical planning. Acta Otorhinolaryngol Ital. 2014;34:278–282. [PMC free article] [PubMed] [Google Scholar]
- 3.Pellini R, Pichi B, Marchesi P, et al. External monitor for buried free flaps in head and neck reconstructions. Acta Otorhinolaryngol Ital. 2006;26:1–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Deganello A, Manciocco V, Dolivet G, et al. Infrahyoid fascio-myocutaneous flap as an alternative to free radial forearm flap in head and neck reconstruction. Head Neck. 2007;29:285–291. doi: 10.1002/hed.20512. [DOI] [PubMed] [Google Scholar]
- 5.Deganello A, Gitti G, Parrinello G, et al. Cost analysis in oral cavity and oropharyngeal reconstructions with microvascular and pedicled flaps. Acta Otorhinolaryngol Ital. 2013;33:380–387. [PMC free article] [PubMed] [Google Scholar]
- 6.Deganello A, Gitti G, Parrinello G, et al. Infrahyoid flap reconstruction of oral cavity and oropharyngeal defects in elderly patients with severe general comorbidities. Head Neck. 2012;34:1299–1305. doi: 10.1002/hed.21913. [DOI] [PubMed] [Google Scholar]
- 7.Deganello A, Leemans CR. The infrahyoid flap: a comprehensive review of an often overlooked reconstructive method. Oral Oncol. 2014;50:704–710. doi: 10.1016/j.oraloncology.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Deganello A, Gitti G, Struijs B, et al. Palliative combined treatment for unresectable cutaneous basosquamous cell carcinoma of the head and neck. Acta Otorhinolaryngol Ital. 2013;33:353–356. [PMC free article] [PubMed] [Google Scholar]
- 9.Deganello A, Gallo O, Cesare JM, et al. Surgical management of surgery and radiation induced peristomal neck ulcerations. B-ENT. 2008;4:169–174. [PubMed] [Google Scholar]
- 10.Giordano L, Bondi S, Toma S, et al. Versatility of the supraclavicular pedicle flap in head and neck reconstruction. Acta Otorhinolaryngol Ital. 2014;34:394–398. [PMC free article] [PubMed] [Google Scholar]
- 11.Leite AK, Matos LL, Belli M, et al. Pectoralis major myocutaneous flap for head and neck reconstruction: risk factors for fistula formation. Acta Otorhinolaryngol Ital. 2014;34:389–393. [PMC free article] [PubMed] [Google Scholar]
- 12.Bussu F, Gallus R, Navach V, et al. Contemporary role of pectoralis major regional flaps in head and neck surgery. Acta Otorhinolaryngol Ital. 2014;34:327–341. [PMC free article] [PubMed] [Google Scholar]
- 13.Deganello A, Franchi A, Sardi I, et al. Genetic alterations between primary head and neck squamous cell carcinoma and recurrence after radiotherapy: recurrence, genetically related cancer, or second primary? Cancer. 2010;116:1291–1297. doi: 10.1002/cncr.24854. [DOI] [PubMed] [Google Scholar]
- 14.Deganello A, Gitti G, Mannelli G, et al. Risk factors for multiple malignancies in the head and neck. Otolaryngol Head Neck Surg. 2013;149:105–111. doi: 10.1177/0194599813484273. [DOI] [PubMed] [Google Scholar]
- 15.Conley J, Gullane PJ. The masseter muscle flap. Laryngoscope. 1978;88:605–612. doi: 10.1002/lary.1978.88.4.605. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari RM, Snow GB. Role of masseter crossover flap in oropharyngeal reconstruction. J Laryngol Otol. 1989;103:298–301. doi: 10.1017/s0022215100108758. [DOI] [PubMed] [Google Scholar]
- 17.Langdon JD. The masseter muscle cross-over flap: a versatile flap for reconstruction in the oral cavity. Br J Oral Maxillofac Surg. 1989;27:124–131. doi: 10.1016/0266-4356(89)90059-4. [DOI] [PubMed] [Google Scholar]
- 18.Antoniades K, Lasaridis N, Vahtsevanos K, et al. Superiorly based and island masseter muscle flaps for repairing oropharyngeal defects. J Craniomaxillofac Surg. 2005;33:334–339. doi: 10.1016/j.jcms.2005.04.008. [DOI] [PubMed] [Google Scholar]