SUMMARY
There is increasing interest about all aspects of pain sensation for patients undergoing head and neck surgery, and efforts have been made to better assess, monitor and reduce the occurrence of pain. The aetiology of pain is considered to be "multifactorial", as it is defined by several features such as personal experience, quality perception, location, intensity and emotional impact. The aim of this paper is: (i) to evaluate the efficacy of analgesic treatment in patients with head and neck cancer treated by surgery, and (ii) to study the variables and predictive factors that can influence the occurrence of pain. A total of 164 patients, affected by head and neck cancer and surgically treated, between December 2009 and December 2013, were included in this study. Data collected include age, gender, assessment of anaesthetic risk, tumour localisation, pathological cancer stage, TNM stage, type of surgery performed, complexity and duration of surgery, post-operative complications, postoperative days of hospital stay and pain evaluation on days 0, 1, 3 and 5 post-surgery. We studied the appropriateness of analgesic therapy in terms of incidence and prevalence of post-operative pain; we also related pain to patient characteristics, disease and surgical treatment to determine possible predictive factors. The population studied received adequate pain control through analgesic therapy immediately post-surgery and in the following days. No associations between gender, age and post-operative pain were found, whereas pathological cancer stage, complexity of surgery and tumour site were significantly associated with the risk of post-operative pain. Adequate pain control is essential in oncological patients, and particularly in head and neck cancer patients as the prevalence of pain in this localisation is reported to be higher than in other anatomical sites. Improved comprehension of the biological and psychological factors that characterise pain perception will help to enhance its control in the future.
KEY WORDS: Pain, Head and neck cancer, Surgery, Quality of life, Pain therapy
RIASSUNTO
Negli anni è aumentata l'attenzione verso i molteplici aspetti associati alla "sfera" dolore, anche nei pazienti oncologici sottoposti a chirurgia testa-collo. Il dolore, definito infatti da diverse caratteristiche, quali l'esperienza personale, gli aspetti qualitativi della percezione, l'intensità, l'impatto emotivo, riconosce un'eziologia "multifattoriale". Scopo del presente lavoro è stato: (i) valutare l'efficacia della terapia analgesica in pazienti affetti da tumore testa-collo e sottoposti a trattamento chirurgico; (ii) studiare le possibili variabili ed i fattori predittivi che possano influenzare l'insorgenza di dolore. Sono stati studiati 164 pazienti, affetti da neoplasia maligna del distretto testa-collo, trattati chirurgicamente tra il dicembre 2009 ed il dicembre 2013. I dati raccolti comprendono l'età, il sesso, la valutazione del rischio anestesiologico, la sede del tumore, la stadiazione TNM, il tipo di intervento effettuato, la complessità e la durata dell'intervento, le eventuali complicanze post-operatorie, i giorni di degenza post-intervento, la valutazione del dolore nei giorni 0, 1, 3 e 5 post-chirurgia. L'adeguatezza della terapia analgesica è stata espressa in termini di incidenza e prevalenza del dolore post-operatorio, le variabili legate al paziente, alla malattia, al trattamento chirurgico e farmacologico, sono state poi associate all'insorgenza del dolore così da poter descrivere eventuali fattori predittivi. Dai dati ottenuti emerge che la popolazione studiata ha ricevuto un'adeguata terapia antalgica, sia nell'immediato post-operatorio che nei giorni successivi. Non sono risultate associazioni statisticamente significative tra sesso, età ed incidenza del dolore post-chirurgico, mentre lo stadio del tumore, la complessità dell'intervento chirurgico e la sede della neoplasia hanno presentano correlazione significativa con il rischio di insorgenza di dolore post-operatorio. L'elevata prevalenza del dolore in ambito oncologico testa-collo, fa sì che un'appropriata ed attenta gestione del dolore risulti fondamentale. Nel futuro pertanto si auspica una sempre migliore comprensione dei fattori biologici, sociali e psicologici che caratterizzano la percezione del dolore ai fini di migliorarne il controllo.
Introduction
Over the past years, the definition of pain has become "multidimensional", as it is considered to be not just a sensation, but has also been described by several features such as quality perception, location, intensity, emotional impact and frequency 1. Pain intensity is the most relevant clinical dimension of a painful experience; nonetheless, emotional, cognitive and behavioural aspects are part of the subjective perception of pain 1 2. The multifactorial nature of pain is particularly evident in oncological patients 3 4, as it can be accompanied by psychological, emotional and social problems 5 6. If poorly controlled, it can be associated with physical and mental deterioration, leading to increased morbidity, increased anxiety and depression, and as a result decreased quality of life.
Pain is one of the most common anxieties before and after surgery, and pain control after surgery still continues to receive little attention in the literature, particularly among head and neck patients 3 4 7-10. It has been reported that pain in head and neck cancer patients has a nociceptive origin due to the direct invasion and destruction of soft tissue and bone tissue by local invasion, but it can also be of neuropathic origin due to inflammation or compression of nervous structures. Furthermore, its intensity can be exacerbated by actions such as speech, chewing and/or swallowing 3 4 8.
The purpose of this study is: (i) to evaluate the efficacy of analgesic treatment in patients with head and neck cancer treated by surgery, and (ii) to understand the possible relation between head and neck pain and predictive factors.
Materials and methods
A total of 164 patients affected by head and neck cancer and surgically treated in the period between December 2009 and December 2013 at the ENT Department of the University Hospital of Ferrara were included in this retrospective study.
Patient history, informed consent and pre-operative anaesthesiologic evaluation data were collected for each patient. Prior to surgery, each patient received information about pain and its treatment, verbally and with paper-based information sheets given by the ENT and anaesthesiology team using a pain evaluation data sheet (incomplete or partially completed surveys were excluded).
The pre-operative exams included: blood tests, electrocardiography, chest X-ray and any further endoscopic, radiological, histopathological diagnostic investigations in order to correctly stage the tumour and to study associated comorbidities. Data collected for each patient included: gender, age, tumour site, previous treatments (radiation therapy, chemotherapy, surgery), previous analgesic therapy, assessment of anaesthetic risk (ASA), type of surgery performed, complexity of surgery according to the anaesthesiology protocols (Annex), duration of surgery, post-operative complications, post-operative days of hospital stay, drugs used in the immediate post-operative period, analgesic therapy administered during hospitalisation, pain evaluation twice a day (in the morning and in the evening) on days 0, 1, 3 and 5 post-op, definitive histological diagnosis, pTNM staging (according to UICC/AJCC Classification of Malignant Tumours 11), pathological cancer stage and any analgesic therapy prescribed at home.
All patients received antibiotic and antithrombotic prophylaxis and gastric protection during hospitalisation. Pharmacological control of pain was obtained based on a preoperative established pain program, accordingly to the type of surgery performed (minor, medium and major surgery) (Table I). This consists of multimodal therapy: a combination of opioids, non-steroidal anti-inflammatory drugs and paracetamol. The anaesthesiologist usually prescribed postoperatively analgesic therapy infused by elastomeric pump for the first 24 hours, and sometimes for 48 or 72 hours post-operatively (Table I).
Table I.
Post-operative analgesic therapy in relation to surgical complexity.
| Surgical complexity | Post-operative analgesic therapy |
|---|---|
| Minor (i.e. tracheotomy) |
|
| Medium (i.e. thyroidectomy, parotidectomy, partial glossectomy; without neck dissection) |
|
| Major (i.e. laryngectomy, neck dissections, pull-through, flap reconstructive surgery after extensive demolition) |
|
Notes:
• NSAIDs are not indicated in case of coagulopathy, hepatopathy, renal failure, or high risk of bleeding conditions.
• The use of morphine alone or in combination with other drugs is related to the expected pain.
• Morphine is not indicated in those affected by suspected (or history of) paralytic ileus, psychiatric illness, or severe respiratory disease.
Post-operative pain trend, in terms of number of episodes and intensity, during the entire hospital stay (on the day of surgery and then on the following days, until discharge) was recorded. Pain intensity during mornings (M) and evenings (E), as well as the maximum value of numeric rating scale (NRS) reached, was recorded each day.
Pain evaluation data sheet
The pain evaluation datasheet was completed on admission for all patients, updated daily and after patient discharge, and was archived together with the patient's medical records. Pain evaluation was performed through several methods. Visual analogue scale (VAS) measured post-operative intensity pain immediately after surgery, while Numeric Rating Scale (NRS) was used during the remaining post-operatory hospital stay. The Behavioural Pain Scale (BPS) was used if patients were held in the intensive care unit in the immediate post-operative period 12-15. Different sections composed the pain evaluation datasheet adopted for this study.
Section I. Includes patient personal data and medical diagnosis. Other information collected in this part include: pain at admission (assessed by NRS), type of pain (acute vs chronic); pain management/therapy at home; type of surgical procedure and anaesthesia. Expected pain and pain management plan proposed by the anaesthesiologist and type of surgical plan (with the aim of tailoring pain management for each patient and its specific disease) is also reported in this section.
Section II. This part is dedicated to pain measurement and recording procedures. These measures were performed at different post-operative days. It was generally measured and recorded twice a day after surgery, and there is an extra section to report episodes of acute pain. Only if NRS > 3 can extra analgesic drugs can be administrated to patients on the basis of the anaesthesiologist prescriptions.
Statistical analysis
Statistical analysis was performed using contingency tables analysed by chi-square (χ2) or by Fisher's test to compare small groups of data. To investigate the possible contribution of different variables in multivariate methods, binary logistic processing procedures were used. The calculations were performed using the statistical system Systat v.5 (Systat Inc. Evanstone, IL USA).
If not otherwise specified, p values < 0.05 were considered statistically significant, while p values between 0.06 and 0.1 were considered of borderline significance.
Results
A total of 164 patients were enrolled in this study. There were 101 males (61.5%) and 63 females (38.5%). Age range was 16 and 86 years, with a mean of 62.84 years. Patients were divided into two age groups: 0-60 years (56 patients, 34.1%) and > 60 years (108 patients, 65.9%; Table II).
Table II.
Demographic and clinical characteristics of patients.
| Patients N (%) |
Mean | ||
|---|---|---|---|
| Demographic data |
Gender | ||
| • Male • Female |
101 (61.5) 63 (38.4) |
||
| Age | 62.84 | ||
| • 0-60 years • ≥ 60 years |
56 (34.1) 108 (65.9) |
||
| Clinical features |
Tumour site | ||
| • Pharynx-larynx • Neck-thyroid-salivary glands • Oral cavity |
64 (39.1) 58 (35.4) 32 (19.5) 10 (6.1) |
||
| Stage | |||
| • Low (0-I-II) • High (III-IV) |
52 (36.8) 89 (63.2) |
||
| Previous therapies | |||
| • No • Yes |
114 (69.5) 50 (30.5) |
||
| ASA | |||
| • 1 • 2 • 3 • 4 |
5 (3.1) 59 (36.0) 86 (52.5) 14 (8.6) |
||
| Surgical complexity | |||
| • Minor • Moderate • Major |
1 (0.6%) 56 (34.2) 107 (65.2) |
||
| Post-operative complications | |||
| • No • Yes |
138 (84.1) 26 (15.9) |
||
| Hospital stay | 11.46 | ||
| • ≤ 5 days • 6-20 days • ≥ 21 days |
63 (38.5) 77 (46.9) 24 (14.6) |
||
| Post-op analgesic therapy | |||
| • Opioids • Opioids+NSAIDs/paracetamol • NSAIDs |
27 (16.5) 121 (73.7) |
||
| 16 (9.8) | |||
| Analgesic therapy at discharge | |||
| • No • Yes • Data not available |
69 (42.0) 90 (54.9) 5 (3.1) |
The primary cancer sites were very different and patients were grouped into four cohorts identified by a number: (1) pharyngeal and laryngeal tumours (64 patients, 39.1%); (2) thyroid, salivary gland and neck tumours (58 patients, 35.4%); (3) oral cavity tumours (32 patients, 19.5%); (4) tumours at other sites (i.e. head and neck skin or occult carcinomas to neck nodes; 10 patients, 6.1%; Table II).
TNM stage was available in only 141 patients (as lymphomas, skin tumours and neck metastases from occult cancers were excluded). Therefore, 2 patients were classified as Tis (1.4%), 58 as T1 (41.1%), 28 as T2 (19.8%), 31 as T3 (22%) and 22 as T4 (15.6%). On the base of tumour staging, 2 patients (1.4%) had oncological stage 0, 38 (27.2%) had stage I, 12 (8.5%) had stage II, 39 (27.7%) stage III and 50 (35.5%) stage IV (Fig. 1). For statistical analysis, patients were divided into those with low tumour stage (stages 0, I and II: 52 patients) and advanced tumour stage (stages III and IV: 89 patients; Table II).
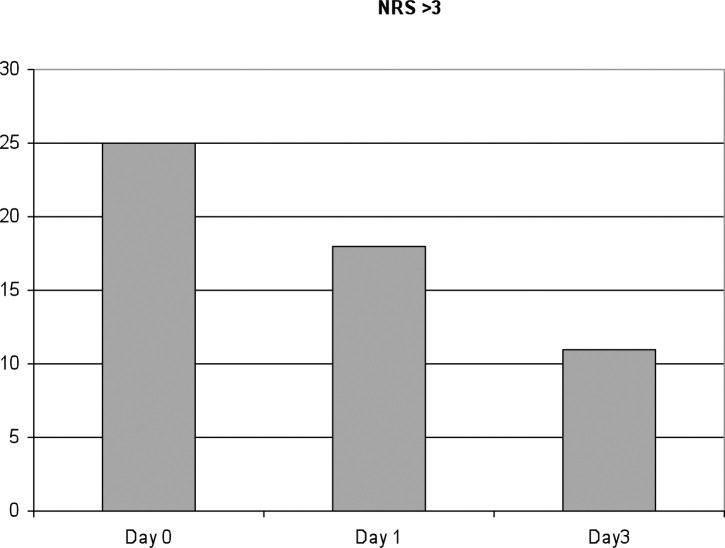
Fig 1.
Incidence of uncontrolled pain. There is a trend of a gradual pain decrease during hospital stay, with a peak on the day of surgery.
A total of 114 patients were previously untreated (69.5%), while 50 (30.5%) had received previous treatment with radio- chemotherapy (n = 3), surgery (n = 42), or both (n = 5). Surgical procedures were grouped as minor (1 patient, 0.6%), moderate surgery (56 patients, 34.2%) and major surgery (107 patients, 65.2%). Possible complications in the postoperative period were classified as local (13 cases) and systemic (13 cases; Table II).
The average duration of hospitalisation was 11.46 days; patients were classified into 3 groups based on duration of hospitalisation: first group, ≤ 5 days (63 patients, 38.5%); second group, 6-20 days (77 patients, 46.9%); third group, ≥ 21 days (24 patients, 14.6%). In relation to anaesthesiological risk, 5 patients were ASA 1 (3.1%), 59 patients ASA 2 (36%), 86 ASA 3 (52.5%) and 14 patients were ASA 4 (8.6%) (Table II).
Considering pain therapy administered, 27 (16.5%) patients received opioids in the immediate postoperative period, 121 (73.7%) patients received a combination of opioids, non-steroidal anti-inflammatory drugs and/or paracetamol, 16 (9.8%) patients received only non-steroidal anti-inflammatory drugs (Table II). Analgesic pain therapy on discharge was prescribed to 90 of 164 patients.
Prevalence and incidence of pain episodes during post-operative recovery
When evaluating the effectiveness of post-operative pain therapy, we firstly considered the prevalence of post-operative pain that was uncontrolled by therapy (NRS > 3) in the total group of patients. Therefore, 46 of 164 (28.1%) patients had at least one episode of post-operative pain that was not controlled by therapy (NRS > 3) during the hospital stay. Of these 46 patients, 31 had a single episode of pain (NRS > 3), 11 had 2 episodes, 2 had 3 episodes and 2 had 4 episodes.
Regarding the incidence of uncontrolled pain on the day of surgery and the first three post-operative days, we recorded a total of 25 episodes during the day of surgery (M+E), 18 episodes (M+E) in the day after surgery and 11 episodes (M+E) in the third day of hospital staying. These data show that there is a trend of a gradual pain decrease during hospitalisation, with a peak on the day of surgery (Fig. 1).
According to the PMI (Pain Management Index), patients were divided into 4 categories in relation to pain episodes and into 4 groups in relation to the analgesic drugs administered. The results showed adequate analgesic prescription, with a prevalence of cases with a negative PMI (4.3%) (Table III).
Table III.
The PMI (Pain Management Index) was used to evaluate the effectiveness of pain medications administered immediately after surgery (at days 0 and 1): in 95.7%, the analgesic prescription was adequate.
| No drugs |
No opioids |
Weak opioids |
Strong opioids |
|
|---|---|---|---|---|
| No pain | 0 | 2 | 1 | 16 |
| Mild pain | 0 | 8 | 28 | 71 |
| Moderate pain | 0 | 6 | 6 | 21 |
| Severe pain | 0 | 0 | 1 | 4 |
Variables and predictive factors that can influence the occurrence of pain
Multivariate binary logistic regressions were employed on different days and different times within a single day (morning/evening) from surgery (days 0, 1, 2, 3) in order to determine any possible associations between post-operative pain and parameters such as gender, age, site of disease, tumour stage and complexity of intervention. A dependent binary variable was therefore defined, first as to the simple presence of pain, then as to a pain level higher than 3, (pain scale ranging from 0: no pain to 10: maximum level pain).
The simple pain presence (NRS > 3) showed monovariate association, at day 0, with malignant localisation. In particular, at M0, neck, thyroid and salivary glands tumours were at greater risk of inducing pain (p < 0.04) than those located at the pharynx and larynx (OR 4.2; 95% CI: 0.07- 16.3). Moreover, at M0, at monovariate analysis there was a significant reduction (p < 0.05) of pain in relation to the increase of hospital stay (OR: 0.86; 95% CI 0.74-0.99). At M0, there was a borderline association (p < 0.07) between surgery complexity and post-operative pain, with a higher risk for moderate surgery (OR: 0.4; 95% CI 0.12-1.1). At M3, there was a correlation between pain occurrence and gender, as women had higher risk of developing pain (p < 0.03) (OR: 6.2; 95% CI: 1.2-30.8).
Finally, when considering days 1 and 5 post-surgery, there was no significant association between pain and the different variables examined.
Discussion
There are few studies evaluating pain sensation and quality of life in head and neck cancer patients. Moreover, most of the reports suggest that the management of acute postoperative pain, in particular, is still sub-optimal 3 4 7-10 16-18. According to a 2007 review, the prevalence of pain in patients with head and neck tumour is reported to be 70% 17. Keefe FJ reported that the incidence of pain in patients suffering from head and neck cancer ranges between 40% and 70% 7. Literature data concerning incidence of postoperative pain in patients undergoing surgery for malignant head and neck disease show that 48% of patients present a pain intensity greater than 4 at VAS 18.
Moreover, considering the effectiveness of analgesic therapy for head and neck surgery patients, the data available are limited. A meta-analysis by Deandrea et al. found that 43% of cancer patients from 26 different studies had insufficient control of pain 19. Orgill et al. hypothesised that the cause of the inadequateness of analgesic therapy in a laryngectomy series (35% of patients) was the incorrect prescription and/or use of a suboptimal drug dosage (in relation to the extent and type of surgery) 20. Inhestern et al. described that many patients with head and neck cancer have moderate or severe pain within 24 hours after surgery, and that pain can be related to factors such as duration of surgery and presence of preoperative pain 21.
Analysing the data obtained from our group, it is interesting to note that pain therapy was adequate. Assuming that values of NRS > 3 indicate inadequate pain control, only 26.5% of patients in the days examined had at least one episode of pain that was not effectively controlled by therapy. In our group, pain intensity presented a peak on the day of surgery and then decreased in the following days. This trend is consistent with the results in the literature. In fact, Mom et al. also described a peak of pain intensity in the immediately post-surgery period (median value VAS = 7), and a value < 3 at 30 hours after surgery 9. When considering the type of analgesics administered, in our patient group, opioids alone or in combination with other analgesics (paracetamol and/or non-steroidal antiinflammatory drugs), are the most commonly used agents on days 0 and 1. In particular, these drugs were used in 94.8% of patients: in 93.1% of cases there was a continuous administration via elastomeric pump that was effective in 95.7% of cases (Table III). No side effects due to the administration via infusion pump were reported. Therefore, opioids can be considered as the most effective drugs within the series presented, as for other series in the literature 22.
Other reports have investigated whether there are predictive factors that might influence post-operative pain outcome in head and neck oncology 4 8 23-26. In general, it has been observed that pain is more intense in older patients and in those with comorbidities. In particular, alcohol abuse, smoking and depression were found to be associated with greater intensities of pain in head and neck cancer patients 4 23 24. Neck surgery was found to be associated to an increased risk of developing pain 25; according to Talmi, this can be attributed to the interruption of the nerve fibres, and differentiation may also result in other symptoms such as of dysaesthesia, paraesthesia and hypersensitivity 3. In our study, there were no significant differences between gender, age and the risk of developing post-operative pain. Pathological cancer stage, complexity of surgery and tumour site, however, were significantly associated with a increased risk of post-operative pain. In particular, patients with advanced stages of cancer (III and IV) had a lower risk of developing post-operative pain compared to patients with low-stage tumours (0, I, II). This could be explained since the latter received less effective analgesic therapy, and patients with advanced stages of cancer are planned to receive a more potent analgesic therapy due to the more complex and invasive surgical procedures programmed.
In conclusion, analysis of the data herein show that:
the population studied received adequate pain control immediately post-surgery and in the following days;
there was a significant correlation between occurrence of post-operative pain and sex, tumour site, tumour stage and complexity of the intervention.
We believe that the good pain control overall registered in our series can be attributable to the good counselling performed for each patient of the present series; we tried to tailor, for each patient, analgesic therapy to the expected post-operative pain, thus considering patient characteristics, comorbidities and type of surgery.
The limitations of this study are:
the number of parameters studied; with a greater number of patients it would be possible to include other features such as chronic pain prior to surgery, diabetes mellitus, alcohol and/or smoking.
mental status prior to surgery was not evaluated (i.e. those affected by chronic pain have been reported to be more prone to pain perception in the literature 21).
Conclusions
In conclusion, there is growing attention about quality of life and particularly of pain perception for head and neck surgery patients, and several efforts have been made in order to better assess, monitor and minimise the incidence of pain. Hopefully, better comprehension of the biological and psychological factors that characterise the pain sensation will help to enhance its control in the future, therefore increasing the quality of life of these patients.
Acknowledgements
The authors would like to thank Dr. Giuseppe Gilli for his assistance with statistical analysis.
References
- 1.Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Second edition. Washington, DC: IASP Press; 1994. pp. 209–214. [Google Scholar]
- 2.Frank-Stromborg M, Olsen O. Instruments for Clinical Health-Care Research. Burlington, MA: Jones & Bartlett; 1999. pp. 528–564. [Google Scholar]
- 3.Talmi YP, Horowitz Z, Pfeffer MR, et al. Pain in the neck after neck dissection. Otolaryngol Head Neck Surg. 2000;123:302–306. doi: 10.1067/mhn.2000.104946. [DOI] [PubMed] [Google Scholar]
- 4.Chen SC, Liao CT, Chang JT. Orofacial pain and predictors in oral squamous cell carcinoma patients receiving treatment. Oral Oncology. 2011;47:131–135. doi: 10.1016/j.oraloncology.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Ghei A, Khot S. Pain management in patients with head and neck carcinoma. Otorhinolaryngology Clinics: An International Journal. 2010;2:69–75. [Google Scholar]
- 6.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1659–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 7.Keefe FJ, Manuel G, Brantley A, et al. Pain in the head and neck cancer patient: changes over treatment. Head Neck Surg. 1986;8:169–176. doi: 10.1002/hed.2890080308. [DOI] [PubMed] [Google Scholar]
- 8.Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck. 1999;21:531–537. doi: 10.1002/(sici)1097-0347(199909)21:6<531::aid-hed6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Mom T, Commun F, Derbal C, et al. Postoperative pain evaluation in the surgery of head and neck cancers. Rev Laryngol Otol Rhinol (Bord) 1996;117:93–96. [PubMed] [Google Scholar]
- 10.Ziv G, Darryl B, Nissim M, et al. Treatment of pain after head and neck surgeries: control of acute pain after head and neck oncological surgeries. Otolaryngol Head Neck Surg. 2006;135:182–188. doi: 10.1016/j.otohns.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 12.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 13.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is a moderate pain in millimeters. Pain. 1997;72:95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 14.Chanques G, Payen JF, Mercier G, et al. Assessing pain in non-intubated critically ill patients unable to self report: an adaptation of the Behavioral Pain Scale. Intens Care Med. 2009;35:2060–2067. doi: 10.1007/s00134-009-1590-5. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez CS, McMillan S, Yarandi H. Pain measurement in older adults with head and neck cancer and communication impairments. Cancer Nurs. 2004;27:425–433. doi: 10.1097/00002820-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39:259–267. doi: 10.1016/j.jpainsymman.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Beuken- Van Everdingen MH, Rijke JM, Kessels AG, et al. Prevalence of pain in patient with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 18.Sommer M, Geurts JW, Stessel B, et al. Prevalence and predictors of postoperative pain after ear, nose and throat surgery. Arch Otolaryngol Head Neck Surg. 2009;135:124–130. doi: 10.1001/archoto.2009.3. [DOI] [PubMed] [Google Scholar]
- 19.Deandrea S, Montanari M, Moja L, et al. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19:1985–1991. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orgill R, Krempl GA, Medina JE. Acute pain management following laryngectomy. Arch Otolaryngol Head Neck Surg. 2002;128:829–832. doi: 10.1001/archotol.128.7.829. [DOI] [PubMed] [Google Scholar]
- 21.Inhestern J, Schuerer J, Illge C, et al. Pain on the first postoperative day after head and neck cancer surgery. Eur Arch Otorhinolaryngol. 2015;272:3401–3409. doi: 10.1007/s00405-014-3307-9. [DOI] [PubMed] [Google Scholar]
- 22.Cavalcanti IL, Carvalho AC, Musauer MG, et al. Safety and tolerability of controlled-release oxycodone on postoperative pain in patients submitted to the oncologic head and neck surgery. Rev Col Bras Cir. 2014;41:393–399. doi: 10.1590/0100-69912014006003. [DOI] [PubMed] [Google Scholar]
- 23.Shuman AG, Terrell JE, Light E, et al. Predictors of pain among patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2012;138:1147–1154. doi: 10.1001/jamaoto.2013.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy SA, Ronis DL, Valenstein M, et al. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–148. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 25.Gellrich NC, Schimming R, Schramm A, et al. Pain, function, and psychologic outcome before, during, and after intraoral tumor resection. J Oral Maxillofac Surg. 2002;60:772–777. doi: 10.1053/joms.2002.33244. [DOI] [PubMed] [Google Scholar]
- 26.Bianchini C, Maldotti F, Crema L, et al. Pain in head and neck cancer: prevalence and possible predictive factors. J Buon. 2014;19:592–597. [PubMed] [Google Scholar]