ABSTRACT
The movement of influenza A viruses (IAVs) from wild bird reservoirs to domestic animals and humans is well established, but the transmission mechanisms that facilitate efficient movement across and within these host populations are not fully defined. Although predominant routes of transmission vary between host populations, the extent of environmental stability needed for efficient IAV transmission also may vary. Because of this, we hypothesized that virus stability would differ in response to varied host-related transmission mechanisms; if correct, such phenotypic variation might represent a potential marker for the emergence of novel animal or human influenza viruses. Here, the objective was to evaluate the ability of eight swine and six human IAV isolates to remain infective under various pH, temperature, and salinity conditions using a preestablished distilled water system. Swine and human viruses persisted longest at near-neutral pH, at cold temperatures, or under “freshwater” conditions. Additionally, no significant differences in persistence were observed between pandemic and nonpandemic IAVs. Our results indicate that there have been no apparent changes in the environmental stability of the viruses related to host adaptation.
IMPORTANCE This study assessed the environmental stability of eight swine and six human influenza A viruses (IAVs), including viruses associated with the 2009 H1N1 pandemic, in a distilled water system. The important findings of this work are that IAV persistence can be affected by environmental variables and that no marked changes were noted between human and swine IAVs or between pandemic and nonpandemic IAVs.
INTRODUCTION
Influenza A viruses (IAVs) have been isolated from numerous avian and mammalian hosts, and cross-species transmission commonly occurs between wild bird reservoirs, domestic animals, and humans (1). Swine are susceptible to infection by IAVs of both avian and mammalian origin (2) and are recognized as an intermediate host for the evolution and adaptation of IAVs with pandemic potential (1, 2). From 1930 to 1990, classic H1N1 swine influenza virus (SIV) underwent little genetic change, but by the late 1990s, the “triple-reassortant” SIV viruses H1N1, H3N2, and H1N2 had become predominant in swine in North America (3, 4).
The pandemic H1N1 influenza A virus (pH1N1) was first detected in April 2009 in two human cases in California (2009), and it quickly spread across the world. The emergence of this virus was subsequently traced to the reassortment of recent North American avian/human/swine triple-reassortant viruses with Eurasian swine viruses (5). This virus has since infected swine and continues to reassort with other SIVs (6, 7); pH1N1 is now a part of the endemic human influenza pool (8).
The potential for viruses such as pH1N1 to emerge as a result of reassortment between human, swine, and avian viruses involves unlikely transmission events. Transmission mechanisms for IAVs not only are poorly understood in all of these host systems but also vary between them. With birds, transmission of IAVs occurs primarily via a fecal-oral route (1, 9), in which water plays a large role. While the primary mode(s) of transmission of influenza between humans is believed to be an aerosol, droplet, or direct contact route (3, 10), indirect transmission through contaminated surfaces may also contribute to transmission. Likewise, swine influenza viruses are transmitted primarily via direct contact or through aerosols or droplets (11), but indirect contact through fomites or shared environments cannot be discounted.
In this study, the infectivities of eight swine and six human (historical, seasonal, and pandemic) influenza viruses were evaluated under variable pH, temperature, and salinity conditions using a distilled water laboratory model system (12). Although modes of transmission for swine and human viruses may not directly involve contact with contaminated water, avian-origin IAVs have been well characterized in this medium (12–18) and, as such, provide a baseline for comparison. Furthermore, the potential implications of fomites in the transmission of IAVs in mammalian systems and swine husbandry practices that utilize common drinking troughs make this laboratory investigation applicable. The mechanisms that facilitate or allow for efficient movement across different host populations are varied, but they all require some degree of environmental stability. Because of this, we hypothesized that virus stability (i.e., environmental fitness) would differ in response to varied host-related transmission mechanisms. If correct, such phenotypic variation could represent a potential marker for the emergence of novel animal or human influenza viruses.
MATERIALS AND METHODS
Viruses.
One avian-origin H1N1, eight swine, and six human influenza viruses were assessed using a previously described distilled water system (12, 18); the viruses used in this study are listed in Table 1. Viruses were propagated in Madin-Darby canine kidney (MDCK) cells (ATCC CRL-2936) following the method of Szretter et al. (19) with modifications. Briefly, a 1:1,000 dilution of virus in minimal essential medium (MEM) supplemented with antibiotics and 1 mg/ml trypsin {treated with TPCK [l-(tosylamido-2-phenyl) ethyl chloromethyl ketone], Worthington Biochemical Corporation, Lakewood, NJ} was added to 75-cm2 flasks of confluent and washed MDCK cells. Cells were incubated at 35°C or 37°C, and supernatants were harvested at 75% to 90% monolayer destruction. Stock viruses were stored at −70°C; stock virus titers ranged from 106.5 to 108.1 50% tissue culture infectious dose (TCID50)/ml, as determined by titration in MDCK cells.
TABLE 1.
Description of influenza viruses used in this study
| Origin | Strain | Statusa | Passageb | Subtype | Titer (TCID50/ml) |
|---|---|---|---|---|---|
| Avian | A/Green-winged teal/Louisiana/213/1987 | Control | SPFE1/C1 | H1N1 | 6.9 |
| Swine | A/Swine/Minnesota/02719/2009 | Non-PDM | C1/E1/C1 | H3N2 | 7.6 |
| Swine | A/Swine/North Carolina/02744/2009 | Non-PDM | C1/E1/C1 | H1N2 | 7.3 |
| Swine | A/Swine/Minnesota/02746/2009 | pH1N1 | C1/E1/C1 | H1N1 | 8.3 |
| Swine | A/Swine/Minnesota/02749/2009 | pH1N1 | C1/E1/C1 | H1N1 | 8.1 |
| Swine | A/Swine/Minnesota/02751/2009 | pH1N1 | C1/E1/C1 | H1N1 | 7.7 |
| Swine | A/Swine/Illinois/02860/2009 | pH1N1 | C1/E1/C1 | H1N1 | 7.4 |
| Swine | A/Swine/Utah/02861/2009 | Non-PDM | C1/E1/C1 | H1N2 | 7.5 |
| Swine | A/Swine/Iowa/15/1930 | Non-PDM | ?/E1/C1 | H1N1 | 6.5 |
| Human | A/New Jersey/08/1976 | Non-PDM | SPFE7/E1/C1 | H1N1 | 7.7 |
| Human | A/Texas/15/2009 | pH1N1 | C2/E1/C1 | H1N1 | 7.4 |
| Human | A/Mexico/INDRE4487/2009 | pH1N1 | E2/E1/E1/C1 | H1N1 | 7.1 |
| Human | A/Brisbane/10/2007 | Non-PDM | E2/E2/E1/C1 | H3N2 | 7.9 |
| Human | A/Brisbane/59/2007 | Non-PDM | E2/E2/E1/C1 | H1N1 | 6.5 |
| Human | A/California/04/2009 | pH1N1 | C1/C1/C1 | H1N1 | 6.7 |
pH1N1, 2009 pandemic H1N1 viruses; Non-PDM, nonpandemic viruses.
Sequential passage history. C, Madin-Darby canine kidney cells; E, embryonated chicken eggs; SPFE, specific-pathogen-free embryonated chicken eggs; ?, unknown primary isolation source. Numbers indicate the number of passages within each source/passage in a new source.
Virus persistence trials.
Prior to all treatment adjustments, distilled water was buffered with 10 mM HEPES (Sigma, St. Louis, MO). Temperatures evaluated included 4°C, 10°C, 17°C, 23°C, 28°C, 32°C (human) or 35°C (swine) and 37°C. A temperature of −70°C was included as a control. Water used in temperature trials had a pH of 7.2 and a salinity of 0 ppt.
For pH trials, water pH was adjusted from 5.4 to 9.0 at 0.4-unit increments with the addition of 1 N HCl or NaOH. A pH of 7.2 was also included in the analysis. All pH trials were completed at 17°C and a salinity of 0 ppt. Each pH treatment was measured at the start of the study and confirmed at the completion of each trial. In all cases, it did not vary more than 0.1 unit from the starting pH.
Salinity trials were completed in water at 17°C and pH 7.2. Salinity was adjusted with commercially available sea salt (Morton, Chicago, IL) to 0, 5, 10, 15, 20, 25, and 30 ppt.
For each trial, virus stock was diluted 1:25 to 1:100 in the respective water treatments to achieve a starting titer of approximately 105.0 to 106.0 TCID50/ml. Virus-inoculated water was aliquoted as single-use 1-ml volumes into 5-ml polystyrene tubes which were allowed to incubate in environmental chambers or water baths at defined temperatures; tubes were removed from the respective temperatures at the predetermined sampling time points and disposed of following titration. For each trial, virus-inoculated water was titrated at the time of inoculation (0 days postinoculation [dpi]) and at variable time points (from 1 to 14 days); these varied by treatment and were based on data from previous infectivity assays (12, 13, 17, 18). Sampling frequencies ranged from daily (under conditions that have been shown to quickly inactivate viruses) to monthly (under conditions in which viruses have been shown to be long-lived). The numbers of time points recorded are indicated in Tables S1 to S3 in the supplemental material. Virus titrations with MDCK cells were performed as previously described (12). Endpoints were measured via hemagglutination assay using 0.5% chicken red blood cells as described previously (20).
Statistical analysis.
Titers were calculated by using the method of Reed and Muench (21) and reported as TCID50/ml. Linear regression was used to determine a 90% reduction time (Rt) for each virus-treatment combination; Rt values correspond to the time required for a decrease in viral titer by 1 log10 TCID50/ml. Regression equations are shown in Tables S1 to S3 in the supplemental material. The minimum detectable limit for this procedure is 101.8 TCID50/ml.
Because Rt values were not normally distributed and their variances differed across environmental conditions, separate nonparametric comparisons of viruses originating from swine and humans and of pandemic and nonpandemic viruses were performed for each condition using the Wilcoxon rank sum test. All tests assumed a two-sided alternative hypothesis, and a P value of <0.05 was considered statistically significant. Analyses were performed using commercially available software (JMP, version Pro 12, 1989-2007; SAS Institute, Cary, NC).
RESULTS
Temperature.
The MDCK-adapted swine and human viruses persisted longest at cold temperatures and were inactivated at temperatures greater than 17°C (Fig. 1); this pattern of responses is similar to that seen with the single avian control isolate (data shown in Table S1 in the supplemental material). Treatments of 32°C and 35°C were excluded from the statistical comparisons, because none of the swine isolates were evaluated at 32°C, and only one of the human isolates was evaluated at 35°C. No reduction in persistence over the course of the trial (up to 300 days) was observed for viruses held at −70°C (data not shown). The variations in response within swine viruses and within human viruses were greatest at low temperatures. At 4°C, Rt values ranged from 55 to 250 days for swine viruses and from 30 to 160 days for human viruses; at 37°C, Rt values ranged from 0.5 to 3.4 days for swine viruses and 0.9 to 4 days for human viruses. Swine viruses persisted longer than human viruses at 10°C and 23°C (P = 0.045 and 0.010, respectively). There were no significant differences in environmental persistence between pandemic and nonpandemic viruses (Fig. 2).
FIG 1.
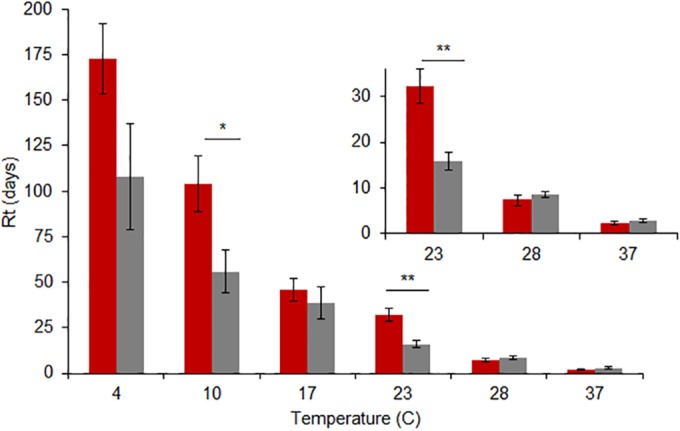
Mean Rt values (± standard error [SE]) for eight swine (red) and six human (gray) viruses in distilled water at temperatures ranging from 4°C to 37°C. The pH was held constant at 7.2, and salinity was 0 ppm. Significant differences in the responses for swine and human viruses exist at temperatures of 10°C (*, P < 0.05) and 23°C (**, P < 0.01). No other statistically significant differences were observed at an α value of 0.05.
FIG 2.
Mean (±SE) Rt values for seven pandemic (blue) and seven nonpandemic (white) viruses in distilled water at temperatures ranging from 4°C to 37°C. The pH was held constant at 7.2, and salinity was 0 ppm. No statistically significant differences were observed in the responses for pandemic and nonpandemic viruses at any temperature at an α value of 0.05.
pH.
All swine viruses included in this study were most stable at near-neutral pH and were quickly inactivated at the extremes of the ranges tested; viruses of human origin showed a similar response (Fig. 3). There was rapid inactivation at pHs less than 6.2 and greater than 8.2 for all swine and human viruses, and the majority of viruses in all the groups were most stable at pH 7.2. There were no significant differences in the responses of swine and human viruses at any pH. Seasonal and pandemic human viruses also did not differ significantly in their responses at any pH (Fig. 4). Rt values for all of the individual viruses and pH levels are provided in Table S2 in the supplemental material.
FIG 3.
Mean (±SE) Rt values for eight swine (red) and six human (gray) viruses in distilled water at pHs ranging from 5.4 to 9. The temperature was held constant at 17°C, and salinity was 0 ppm. No statistically significant differences were observed in the responses for swine and human viruses at any pH at an α value of 0.05.
FIG 4.
Mean (±SE) Rt values for seven pandemic (blue) and seven nonpandemic (white) viruses in distilled water at pHs ranging from 5.4 to 9. The temperature was held constant at 17°C, and salinity was 0 ppm. No statistically significant differences were observed in the responses for pandemic and nonpandemic viruses at any pH at an α value of 0.05.
Salinity.
Increased salinity had a detrimental effect on virus stability. The Rt values of all viruses assessed, regardless of host origin, were greatest in freshwater at a salinity of 0 ppt. Persistence of viruses from either host group (swine or human) showed similar declines with increasing salinity, with a marked decrease in stability as salinity was raised from 0 to 5 ppt (Fig. 5); the response was similar for the single avian control isolate (see Table S3 in the supplemental material). Swine viruses persisted longer than human viruses at 20 ppt (P = 0.010), but the two groups did not differ significantly at any other saline concentration. As was the case at colder temperatures, viruses within each host group were most variable in their persistence at a salinity of 0 ppt. Excluding A/Swine/Utah/02861/2009 (H1N2), with an Rt value of 6.3 days at a salinity of 0 ppt, the range of Rt values for all other swine viruses, regardless of subtype, was 33 to 72 days. While A/Mexico/INDRE/2009 (H1N1) was the least stable at 0 ppt, with an Rt value of 14 days, all other human viruses, seasonal and pandemic combined, had Rt values ranging from 21 to 46 days in freshwater. As was the case with temperature and pH, pandemic and nonpandemic viruses responded similarly to salinity, with no significant differences between groups at any saline concentration (Fig. 6). Regression equations for all viruses in the salinity trials are provided in Table S3 in the supplemental material.
FIG 5.
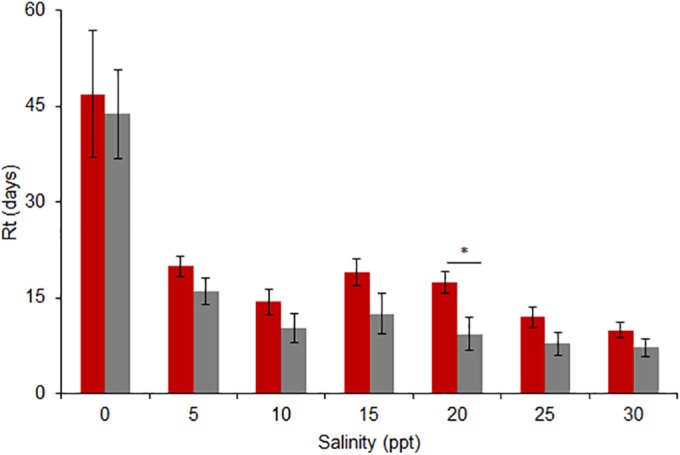
Mean (±SE) Rt values for eight swine (red) and six human (gray) viruses in distilled water at salinities ranging from 0 to 30 ppt. The temperature was held constant at 17°C, and the pH was 7.2. Significant differences in the responses for swine and human viruses existed at 20 ppt (*, P < 0.05). No statistically significant differences were observed in the responses for swine and human viruses at any other saline concentration at an α value of 0.05.
FIG 6.
Mean (±SE) Rt values for seven pandemic (blue) and seven nonpandemic (white) viruses in distilled water at salinities ranging from 0 to 30 ppt. The temperature was held constant at 17°C, and the pH was 7.2. No statistically significant differences were observed in the responses for pandemic and nonpandemic viruses at any salinity at an α value of 0.05.
DISCUSSION
Understanding the environmental stability of IAVs has relevance in defining transmission risks both within the avian reservoir and across domestic poultry and mammalian species. Persistence in water may represent a critical factor in virus maintenance and transmission in wild avian populations but also may play a minor role in the transmission of human and swine IAVs, which are transmitted primarily by contact and respiratory droplets. Although transmission mechanisms differ between these host groups, our results indicate no consistent or significant adaptations related to changes in environmental stability as determined by temperature, pH, and salinity. The general trends described for swine and human IAVs are similar to the well-documented responses of avian IAVs.
The interspecies and intraspecies transmission and maintenance of IAVs are dependent on factors at the host, viral, and environmental levels. To be transmitted and maintained, viruses must remain infectious. It has been well established that IAVs persist longest at cold temperatures (12, 13, 17, 22, 23); human and swine viruses analyzed in this study lasted longest at 4°C, and results were consistent with those previously reported for pandemic virus A/Paris/2590/2009 (H1N1), which had a reported Rt value of 178 days at 4°C (22). The swine and human viruses included in this study, however, demonstrated greater persistence at low temperatures than did a suite of viruses of avian origin that were previously analyzed (12). This result may be an artifact of how the human and swine viruses tested in our study were propagated; it has been shown that both human and avian viruses grown on MDCK cells are more stable at higher temperatures than are the same viruses when grown in chicken eggs (24). At higher temperatures, all the viruses assessed, regardless of their subtype and origin, were quickly inactivated, especially at temperatures higher than 28°C; this is similar to the reduction in persistence seen for a 2009 pandemic H1N1 and a 1999 seasonal H1N1 from >150 days at 4°C to just 2 days at 35°C (22). While swine viruses persisted significantly longer than human viruses at 10°C (P = 0.045) and 23°C (P = 0.010) in this study, such differences were not consistent across the broader range of temperatures evaluated, and given the small sample sizes, the statistical power is low. This study evaluates the thermal stability of swine influenza viruses at a range of temperatures likely encountered in both laboratory and natural settings. Thermal neutrality is important in swine production systems and is dependent largely on age strata and weight (25). Generally, preferred thermal conditions for swine range from 10°C to 32°C (26); under the laboratory conditions of this study, some currently circulating swine influenza viruses can remain infectious for several weeks (35°C) to more than 1 year (10°C) within this temperature range. The temperature stability of these viruses at temperatures consistent with host environments, such as swine production systems, may suggest that environmental adaptation is not necessary for movement of the virus across species barriers, despite the disparate body temperatures seen in avian (42°C), swine (39°C), and human (37°C) hosts.
The role of pH in IAV hemagglutinin (HA) membrane fusion has been well characterized (27–30), and pH might play a role in adaptation of IAV to a new host. From an environmental perspective, the effect of low pH on the integrity of external proteins on the surface of the virus may serve as a preemptive trigger, rendering HAs inactive (31). In the present study, human and swine viruses were most stable within a neutral pH range, as has been found with avian viruses (12). We observed some subtle and nonsignificant differences in persistence across hosts and subtypes, but these differences did not provide clear evidence of pH-related environmental adaptation. Very small differences in the pH of fusion (between 5.0 and 5.7) have been associated with IAV host adaptation and cross-species transmission (32, 33). Changes in environmental stability at pH 7.4 also have been reported with recombinant H5N1 viruses where a 0.5-unit change in the pH of fusion was shown to decrease environmental stability more than 45 days, while a decrease by the same amount increased persistence nearly 20 days compared to that of the wild type (34). While we did not detect any clear evidence of differences in pH tolerance between swine and human IAVs, additional evaluation may be necessary to detect fine-scale differences that may occur early in host adaption, especially those related to virus adaptation from avian to mammalian hosts.
Of the three variables investigated here, salinity is the least understood in its effect or mechanism of action relating to influenza stability in water. As was expected from results of previous studies, virus persistence decreased as the salinity of water increased for all viruses assessed. This loss in infectivity was most marked from 0 to 5 ppt for all viruses except A/Swine/Utah/02861/2009 (H1N2). Increasing osmotic pressure might serve to disrupt the integrity of the virus membrane and/or lead to premature inactivation. The response of individual avian virus stocks to increasing salinity has been shown to take on a number of forms, from negative log-linear to Gaussian (12). While many avian viruses tend to persist longest in moderately saline water, most viruses in our study showed greatest persistence in water with a salinity of 0 ppt. This difference might be due to the host from which the lipid bilayer of the virus was derived, the glycosylation moieties of the surface proteins (also a function of the host cell), or potentially a marker of adaptation. Assessing these markers of adaptation, as well as determining the potential relevance of increased persistence of swine over human viruses at 20 ppt (P = 0.045) in this study, would require further investigation with a larger diversity of viruses. Given that IAV transmission in human and swine systems often involves respiratory secretions, salt concentrates in droplets likely play a role in virus viability. Yang et al. (35) proposed three conditions related to relative humidity (RH)—physiological, concentrated, and dry—that play a role in the infectivity of influenza in droplets. Under this schema, virus stability was most jeopardized under conditions of RH between 50% and 99%, at which the evaporation of a given droplet of medium with salts led to an increase in the concentration of solutes; the interaction of mucus and RH yielded a similar effect on virus persistence.
This study provides general response models for the individual effects of temperature, pH, and salinity on the ability of IAVs of different subtypes and from different hosts to remain infective in a distilled water milieu. Altogether, these results shed some light on the dynamics of these avian, swine, human, and pandemic H1N1 viruses under laboratory-simulated “environmental” conditions. Our data indicate that influenza persistence can be modulated by environmental variables and support previous findings for avian influenza viruses (12, 13, 17, 18). Furthermore, and perhaps of greater relevance to the swine and 2009 H1N1 outbreak strains, these data might inform decisions regarding preventive and management practices in both the laboratory and the field.
Supplementary Material
ACKNOWLEDGMENTS
The influenza A virus A/New Jersey/8/1976 (H1N1) NR-21667 reagent was obtained through BEI Resources, NIAID, NIH. All other human-origin viruses were received from the Centers for Disease Control and Prevention (Atlanta, GA).
We are grateful to Marie Culhane at the University of Minnesota (St. Paul, MN) for providing all swine viruses. We thank the faculty and staff of the Southeastern Cooperative Wildlife Disease Study at the University of Georgia, especially Alex Byas, Deborah Carter, and Clara Kienzle, for their technical assistance.
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Department of Health and Human Services, under contracts HHSN266200700007C and HHSN272201400006C.
The funding agencies did not have any involvement in the implementation or publication of this study, and the research presented here represents our opinions but not necessarily the opinions of the funding agencies.
We declare no competing interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00133-16.
REFERENCES
- 1.Webster RG, Hinshaw VS, Bean WJ Jr, Turner B, Shortridge KF. 1977. Influenza viruses from avian and porcine sources and their possible role in the origin of human pandemic strains. Dev Biol Stand 39:461–468. [PubMed] [Google Scholar]
- 2.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol Rev 56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen CW. 2002. The emergence of novel swine influenza viruses in North America. Virus Res 85:199–210. doi: 10.1016/S0168-1702(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 4.Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. 2008. Swine influenza viruses: a North American perspective. Adv Virus Res 72:127–154. doi: 10.1016/S0065-3527(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 5.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 6.Ducatez MF, Hause B, Stigger-Rosser E, Darnell D, Corzo C, Juleen K, Simonson R, Brockwell-Staats C, Rubrum A, Wang D, Webb A, Crumpton JC, Lowe J, Gramer M, Webby RJ. 2011. Multiple reassortment between pandemic (H1N1) 2009 and endemic influenza viruses in pigs, United States. Emerg Infect Dis 17:1624–1629. doi: 10.3201/1709.110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijaykrishna D, Poon LLM, Zhu HC, Ma SK, Li OTW, Cheung CL, Smith GJD, Peiris JSM, Guan Y. 2010. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science 328:1529. doi: 10.1126/science.1189132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. 2014. Recommended composition of influenza virus vaccines for use in the 2014-2015 Northern Hemisphere influenza season. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 9.Ito T, Kawaoka Y. 2000. Host-range barrier of influenza A viruses. Vet Microbiol 74:71–75. doi: 10.1016/S0378-1135(00)00167-X. [DOI] [PubMed] [Google Scholar]
- 10.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. 2007. Transmission of influenza A in human beings. Lancet Infect Dis 7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 11.Olsen CW, Brown IH, Easterday BC, Van Reeth K. 2006. Swine influenza, p 469–482. In Diseases of swine, 9th ed Blackwell Publishing, Ames, IA. [Google Scholar]
- 12.Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. 2009. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet Microbiol 136:20–26. doi: 10.1016/j.vetmic.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Brown JD, Swayne DE, Cooper RJ, Burns RE, Stallknecht DE. 2007. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis 51:285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- 14.Stallknecht DE, Brown JD. 2009. Tenacity of avian influenza viruses. Rev Sci Tech 28:59–67. doi: 10.20506/rst.28.1.1880. [DOI] [PubMed] [Google Scholar]
- 15.Lebarbenchon C, Sreevatsan S, Lefevre T, Yang M, Ramakrishnan MA, Brown JD, Stallknecht DE. 2012. Reassortant influenza A viruses in wild duck populations: effects on viral shedding and persistence in water. Proc Biol Sci 279:3967–3975. doi: 10.1098/rspb.2012.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebarbenchon C, Yang M, Keeler SP, Ramakrishnan MA, Brown JD, Stallknecht DE, Sreevatsan S. 2011. Viral replication, persistence in water and genetic characterization of two influenza A viruses isolated from surface lake water. PLoS One 6:e26566. doi: 10.1371/journal.pone.0026566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stallknecht DE, Kearney MT, Shane SM, Zwank PJ. 1990. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis 34:412–418. doi: 10.2307/1591429. [DOI] [PubMed] [Google Scholar]
- 18.Stallknecht DE, Shane SM, Kearney MT, Zwank PJ. 1990. Persistence of avian influenza viruses in water. Avian Dis 34:406–411. doi: 10.2307/1591428. [DOI] [PubMed] [Google Scholar]
- 19.Szretter KJ, Balish AL, Katz JM. 2006. Influenza: propagation, quantification, and storage. Curr Protoc Microbiol Chapter 15:Unit 15G.1. doi: 10.1002/0471729256.mc15g01s3. [DOI] [PubMed] [Google Scholar]
- 20.Killian ML. 2008. Hemagglutination assay for the avian influenza virus. Methods Mol Biol 436:47–52. doi: 10.1007/978-1-59745-279-3_7. [DOI] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench H. 1938. A simple method for estimating fifty percent endpoints. Am J Hyg 27:493–497. [Google Scholar]
- 22.Dublineau A, Batejat C, Pinon A, Burguiere AM, Leclercq I, Manuguerra JC. 2011. Persistence of the 2009 pandemic influenza A (H1N1) virus in water and on non-porous surface. PLoS One 6:e28043. doi: 10.1371/journal.pone.0028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negovetich NJ, Webster RG. 2010. Thermostability of subpopulations of H2N3 influenza virus isolates from mallard ducks. J Virol 84:9369–9376. doi: 10.1128/JVI.01170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shigematsu S, Dublineau A, Sawoo O, Batejat C, Matsuyama T, Leclercq I, Manuguerra JC. 2014. Influenza A virus survival in water is influenced by the origin species of the host cell. Influenza Other Respir Viruses 8:123–130. doi: 10.1111/irv.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ames D. 1980. Thermal environment affects production efficiency of livestock. Bioscience 30:457–460. doi: 10.2307/1307947. [DOI] [Google Scholar]
- 26.Baker JE. 2004. Effective environmental temperature. J Swine Health Prod 12:140–143. [Google Scholar]
- 27.Maeda T, Ohnishi S. 1980. Activation of influenza virus by acidic media causes hemolysis and fusion of erythrocytes. FEBS Lett 122:283–287. doi: 10.1016/0014-5793(80)80457-1. [DOI] [PubMed] [Google Scholar]
- 28.Matlin KS, Reggio H, Helenius A, Simons K. 1981. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol 91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skehel JJ, Bayley PM, Brown EB, Martin SR, Waterfield MD, White JM, Wilson IA, Wiley DC. 1982. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A 79:968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doms RW, Helenius A, White J. 1985. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem 260:2973–2981. [PubMed] [Google Scholar]
- 31.Junankar PR, Cherry RJ. 1986. Temperature and pH dependence of the haemolytic activity of influenza virus and of the rotational mobility of the spike glycoproteins. Biochim Biophys Acta 854:198–206. doi: 10.1016/0005-2736(86)90111-2. [DOI] [PubMed] [Google Scholar]
- 32.Galloway SE, Reed ML, Russell CJ, Steinhauer DA. 2013. Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: implications for host range and adaptation. PLoS Pathog 9:e1003151. doi: 10.1371/journal.ppat.1003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotter CR, Jin H, Chen Z. 2014. A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability and infectivity. PLoS Pathog 10:e1003831. doi: 10.1371/journal.ppat.1003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed ML, Bridges OA, Seiler P, Kim JK, Yen HL, Salomon R, Govorkova EA, Webster RG, Russell CJ. 2010. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity and transmissibility in ducks. J Virol 84:1527–1535. doi: 10.1128/JVI.02069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W, Elankumaran S, Marr LC. 2012. Relationship between humidity and influenza A viability in droplets and implications for influenza's seasonality. PLoS One 7:e46789. doi: 10.1371/journal.pone.0046789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


