ABSTRACT
Five isolates from chilled food and refrigerator inner surfaces and closely related reference strains of the species Escherichia coli, Listeria monocytogenes, Staphylococcus xylosus, Bacillus cereus, Pedobacter nutrimenti, and Pedobacter panaciterrae were tested for the effect of growth temperature (30°C and 10°C) on biomass formation. Growth was monitored via optical density, and biomass formation was measured at the early stationary phase based on the following parameters in complex and defined media: viable cell count, total cell count, cell dry weight, whole-cell protein content, and cell morphology. According to the lack of growth at 1°C, all strains were assigned to the thermal class of mesophiles. Glucose and ammonium consumption related to cell yield were analyzed in defined media. Except for the protein content, temperature had a significant (t test, P < 0.05) effect on all biomass formation parameters for each strain. The results show a significant difference between the isolates and the related reference strains. Isolates achieved an increase in biomass production between 20% and 110% at the 10°C temperature, which is 15 to 25°C lower than their maximum growth rate temperatures. In contrast, reference strains showed a maximum increase of only about 25%, and some reference strains showed no increase or a decrease of approximately 25%. As expected, growth rates for all strains were higher at 30°C than at 10°C, while biomass production for isolates was higher at 10°C than at 30°C. In contrast, the reference strains showed similar growth yields at the two temperatures. This also demonstrates for mesophilic bacterial strains more efficient nutrient assimilation during growth at low temperatures. Until now, this characteristic was attributed only to psychrophilic microorganisms.
IMPORTANCE For several psychrophilic species, increased biomass formation was described at temperatures lower than optimum growth temperatures, which are defined by the highest growth rate. This work shows increased biomass formation at low growth temperatures for mesophilic isolates. A comparison with closely related reference strains from culture collections showed a significantly smaller increase or no increase in biomass formation. This indicates a loss of specific adaptive mechanisms (e.g., cold adaptation) for mesophiles during long-term cultivation. The increased biomass production for mesophiles under low-temperature conditions opens new avenues for a more efficient biotechnological transformation of nutrients to microbial biomass. These findings may also be important for risk assessment of cooled foods since risk potential is often correlated with the cell numbers present in food samples.
INTRODUCTION
Temperature has an obvious influence on the physiological functions and, consequently, on the growth and survival of bacteria (1, 2). Accordingly, bacteria have adapted to the specific temperature ranges of various environments, which can be summarized in different temperature classes.
Cold-adapted microorganisms are classified as psychrophiles. They are able to grow at temperatures close to the freezing point through various adaptation mechanisms on multiple cell levels (3). In some cases, microbial growth was reported at temperatures as low as −17°C (4). The somewhat controversial differentiation between psychrophiles and psychrotrophs was introduced due to their different optimum growth temperatures (5). The two groups grow well at 0°C, but psychrophiles have an optimum growth temperature below 20°C while psychrotrophs have an optimum growth temperature between 20 and 40°C.
Margesin (6) pointed to the ambiguous use of the term “optimum growth temperature,” which did not differentiate between the optimal temperature for growth rate and that for growth yield. Usually, this term refers to the temperature of maximum growth rate (μmax), which may be different from the temperature of maximum biomass yield. Margesin (6) demonstrated for several psychrophilic bacterial and yeast strains that biomass formation in the stationary growth phase was higher if cultures were grown 20°C below the temperature of μmax. Therefore, one cannot speak of a single optimum growth temperature in this case. The increased cell yields of psychrophilic and psychrotrophic species at temperatures below maximum growth rate temperatures have been described for strains from several taxonomic groups (7–10).
The mentioned effect differs from the prevailing conception for mesophilic bacteria. These organisms will have the largest cell yield at the temperature with the maximum growth rate. Consequently, the maximum biomass formed remains constant over the range of the maximum growth rate (11, 12). In such a case, the term “optimal growth temperature” is unambiguous. Margesin (6) confirmed increased biomass formation at low temperatures for two strains of the psychrophilic species, Pedobacter piscium (later reclassified as Pedobacter antarcticus [13]) and Pedobacter cryoconitis, but also showed a weak increase of biomass formation for a strain of the mesophilic species, Pedobacter heparinus. In contrast, various experiments done by our group with food-related bacterial isolates showed clearly increased biomass formation at low temperatures as well. All of these isolates were characterized as mesophiles by their growth rates, which are significantly higher at 30°C than at 10°C. This led us to perform further growth experiments to support and confirm the increased biomass formation of bacterial isolates from food products.
In this study, five bacterial isolates from different phyla and their closest related type strains were tested for the effects of temperature on growth parameters, including optical density (OD), viable and total cell numbers, total cell protein concentration, and cell dry mass, to identify correlations between biomass formation and growth temperature. The closest relative type strains were tested in order to identify differences in growth parameters and thereby maximum biomass formations due to their long-term storage and growth at the so-called optimal growth temperatures, temperatures with the highest growth rates. Additional growth tests in defined media enabled us to balance the carbohydrate and nitrogen consumption per milligram of cells (dry weight) and thus provided insight into the principles for elevated biomass formation at low temperatures.
MATERIALS AND METHODS
Microbial strains.
Five bacterial strains from different chilled foods and refrigerator inner surfaces were isolated in the course of previous projects, identified by fatty acid analyses and 16S rRNA gene sequencing, and used in this study. As reference strains, the most closely related type strains based on 16S rRNA gene sequence similarities were selected and included in this study.
The following isolates were tested: Bacillus cereus J1 isolated from an inner surface of a refrigerator, Escherichia coli J55 isolated from ice cream, Listeria monocytogenes Iso 11/13 isolated from chilled minced meat, Staphylococcus xylosus J70 isolated from raw milk stored in a milk tank, and Pedobacter nutrimenti J22T isolated from fresh tortellini (chilled). The respective reference strains were Bacillus cereus DSM 31T, Escherichia coli DSM 30083T, Listeria monocytogenes DSM 20600T, Staphylococcus xylosus DSM 20266T, and Pedobacter panaciterrae LMG 23400T, which were all classified as mesophilic bacteria (14–20). In this study, the term “mesophilic” was used for the tested strains because none of the strains showed growth at 1°C in a complex medium (6).
Culture media and cultivation.
Growth experiments were performed in triplicate in a complex medium as well as in defined media. The complex medium used was Caso-Bouillon (105459; Merck) and was composed of peptone from casein (17.0 g/liter), peptone from soymeal (3.0 g/liter), d-(+)-glucose (2.5 g/liter), sodium chloride (5.0 g/liter), and dipotassium hydrogen phosphate (2.5 g/liter). For the growth of L. monocytogenes strains, yeast extract (6.0 g/liter) was added.
Furthermore, each strain was cultured in an appropriate defined medium with an identical glucose concentration. Betaine was added to every defined medium to support growth at 10°C (21, 22). Defined media were used to enable subsequent determination of glucose and ammonium consumption.
E. coli strains were cultivated in M9 defined medium (modified as described by Sambrook et al. [23]), which was composed of Na2HPO4·2H2O, 90.2 mM; KH2PO4, 22.0 mM; NaCl, 8.6 mM; (NH4)2SO4, 18.7 mM; MgSO4·6H2O, 2.0 mM; CaCl2·2H2O, 0.1 mM; glucose monohydrate, 50.5 mM; and betaine, 2.0 mM.
L. monocytogenes strains were cultured in modified Hsiang-Ning Tsai medium (HTM) (24), which was composed of MOPS (morpholinepropanesulfonic acid) adjusted with NaOH to pH 7.4; Na2HPO4·2H2O, 4.82 mM; KH2PO4, 11.55 mM; MgSO4·6H2O, 1.70 mM; ammonium ferric citrate, 0.76 μM; glucose monohydrate, 50.5 mM; thiamine, 2.96 μM; riboflavin, 1.33 μM; biotin, 2.05 μM; lipoic acid, 24.00 pM; (NH4)2SO4, 4.55 mM; betaine, 2.0 mM; and histidine, tryptophan, leucine, isoleucine, valine, arginine, cysteine, and methionine at 100 mg liter−1 each.
For cultivation of S. xylosus strains, Hussain, Hastings and White (HHW) medium (25) was used. The HHW medium supplemented with ammonium sulfate consisted of Na2HPO4·2H2O, 44.65 mM; KH2PO4, 22 mM; glucose monohydrate, 50.5 mM; (NH4)2SO4, 18.7 mM; betaine, 2.0 mM; MgSO4·6H2O, 1.1 mM; CaCl2·2H2O, 0.05 mM; MnSO4, 33.11 μM; ammonium ferric citrate, 22.9 μM; adenine sulfate, 20 mg liter−1; guanosine, 20 mg liter−1; biotin, 0.1 mg liter−1; nicotinic acid, 2 mg liter−1; d-pantothenic acid, 2 mg liter−1; pyridoxal, 4 mg liter−1; riboflavin, 2 mg liter−1; thiamine, 2 mg liter−1; alanine, arginine, cysteine, glycine, histidine, lysine, methionine, phenylalanine, serine, tryptophan, tyrosine, 100 mg liter−1 each; and aspartic acid, glutamic acid, isoleucine, proline, threonine, valine at 150 mg liter−1 each. The final pH value was adjusted to 7.2.
B. cereus strains were grown in basic MOPS-buffered defined medium (modified as described by Milner et al. [26]), which was composed of MOPS, 50 mM; glucose monohydrate, 50.5 mM; betaine, 2 mM; KH2PO4, 7.5 mM; (NH4)2SO4, 15 mM; MgSO4, 0.8 mM; and thiamine, 10 mg liter−1. It was supplemented with micronutrients, H3BO3, 23 μM; CuSO4, 0.6 μM; MnSO4, 0.9 μM; (NH4)6Mo7O2, 44 nM; and ZnSO4, 1.5 μM, and also with the following amino acids as described by Agata et al. (27): valine, threonine, leucine, and glutamic acid at 0.3 g liter−1 each and glycine, histidine, and aspartic acid at 0.1 g liter−1.
Pedobacter strains were grown in a modified defined medium as described by Yoon et al. (19). The defined medium consisted of the following basal salts: glucose monohydrate, 50.5 mM; betaine, 2 mM; K2HPO4, 10.3 mM; KH2PO4, 7.9 mM; NH4Cl, 93.5 mM; KCl, 1.3 mM; MgSO4, 0.8 mM; and CaCl2, 0.5 mM. A vitamin solution (28), an SL-10 trace element solution (29), and a tungsten-selenite solution (30) were added to the basal salts, and the pH value was adjusted to 6.8. In addition, the same amino acids as those mentioned for the HHW defined medium were added to the medium to allow growth at 10°C.
Strains were cultured in 300-ml Erlenmeyer flasks containing 100 ml of complex or defined medium (see above) and inoculated with a preculture grown in the same medium at 30°C and 150 rpm to give an initial optical density at 625 nm (OD625) of 0.03 (complex medium) or 0.01 (defined medium). Each strain was cultured at 30°C and 10°C at 150 rpm. The growth was documented by optical density at 625 nm.
Analytical methods.
Optical density (OD) was measured with a UV-visible (UV-Vis) spectrophotometer (Genesys 10 UV scanning; Thermo Fisher Scientific, Waltham, MA, USA) at 625 nm. Numbers of viable cells were determined by the plate count method on tryptic soy agar (105458; Merck). To support growth of L. monocytogenes strains, yeast extract (6.0 g/liter) was added. CFU were counted after 24 h at 30°C for strains of the species E. coli, S. xylosus, and B. cereus, after 1 to 2 days at 30°C for L. monocytogenes strains, and after 2 days at 30°C for strains of the species P. panaciterrae and P. nutrimenti. Plates containing 10 to 300 colonies were used for statistically valid enumeration. To determine cell dry mass, cultures were centrifuged at 12,800 × g and 4°C for 30 min. The pellet was lyophilized overnight at −56°C and 30 Pa before weighing.
Total cell counts were determined by epifluorescent direct counting as reviewed by Kepner and Pratt (31). Cells were stained with fluorochrome 4′,6-diamidino-2-phenylindole (DAPI). For an even distribution of bacteria on filter surfaces and better DAPI staining, cells were immediately fixed with ethanol at 4°C for 1 to 2 h. For this purpose, samples were mixed with filter-sterilized 70% ethanol (pore size, 0.2 μm) to a total volume of 30 ml. For quantification, cells were collected on cellulose acetate filters (47-mm diameter and 0.22-μm pore size; Sartorius, Germany) using a vacuum pump (type N035AN.18; KNF Neuberger, Germany). After air drying, 50 to 100 μl DAPI (1 μg/ml) was applied to the filter and stained for 10 min at 4°C in the dark. Stained filters were washed with filter-sterilized double-distilled water (ddH2O) and air dried again. The cut filters were placed on microscope slides. The enumeration and determination of cell sizes were performed at 1,000× magnification using an Axio Scope.A1 microscope with an HXP 120-V microscope illuminator (Zeiss, Germany) and an AxioCam MRm microscope camera (1 in., 1.0×; Zeiss, Germany) and the corresponding software ZEN 2012 blue edition (Zeiss, Germany). The total cell count per milliliter was estimated from 10 to 20 randomly chosen microscope fields, containing 10 to 50 cells. A total of at least 200 cells were counted. For determination of cell sizes, the parameters fiber length, width, diameter (for cocci), and cell area were calculated based on the contrast difference between cells and matrix using the software ZEN 2012 blue edition (Zeiss, Germany). Approximately 100 to 200 cells were measured per batch, and results were summarized and are presented in box plots.
The protein content of the cells was determined using the biuret method of Robinson and Hodgen (32) as modified by Stickland (33). Prior to the analyses, samples were washed twice with cold ddH2O.
The glucose concentration of the medium was analyzed from the supernatant of the cultures after centrifugation at 12,800 × g and 4°C for 30 min and after acetylation of the saccharide according to Li et al. (34). An aliquot of 100 μl of supernatant was diluted 1:10 in dimethyl sulfoxide (DMSO), and 500 μl of this dilution was mixed with 375 μl of acetic anhydride and 75 μl of 1-methylimidazole in a glass tube and stirred for 10 min. The excess of acetic anhydride was removed by the addition of 1.5 ml ddH2O. Acetylated saccharide was extracted after addition of 500 μl dichloromethane. Then, the tubes were centrifuged for 1 min to separate the organic phase. One microliter of the lower methylene chloride phase was injected for gas chromatography-mass spectrometry (GC-MS) analysis. Glucose standards with concentrations of 50, 25, 12.5, 6.25, 2.5, 1.0, and 0.5 ng/μl diluted in DMSO were acetylated and analyzed in parallel with the supernatant samples. A 7890A GC system connected to a 5975C inert MSD triple-axis detector, a 7683 series autosampler, and a 7683B series injector (Agilent, Santa Clara, CA, USA) was used for the quantification of acetylated glucose derivatives on a 5% phenylmethyl silicone capillary column (0.25 mm by 30 m, 0.25-μm film thickness; Agilent, USA). Mass detection was carried out in full scan mode and in selected ion monitoring (SIM) mode simultaneously. The oven temperatures were adjusted as follows: initiation at 100°C and then gradually ramped up to 190°C (12°C/min) and held for 6 min, ramped up to 250°C (30°C/min) and held for 6 min, and ramped up to 280°C (40°C/min) and held for 10 min. The temperature at the injector port was programmed to 250°C, and the temperature at the detector port was programmed to 260°C. Helium was used as the carrier gas at a constant flow rate of 1.0 ml/min. ChemStation software (Agilent, Santa Clara, CA, USA) was used for data acquisition and analysis. Glucose was identified according to the retention time of the standard and the main fragment ions (α-d-glucopyranose acetyl ester; m/z, 115, 157, and 98).
Ammonium content was analyzed photometrically based on the Nessler reaction from supernatants according to Streuli and Averell (35). A calibration curve with ammonium chloride concentrations of 500 μM, 250 μM, 125 μM, 62.5 μM, 31.25 μM, 15.625 μM, and 0 μM was used. The absorbance was measured at 430 nm with the Genesys 10 UV scanning spectrophotometer (Thermo Fisher Scientific, USA).
Statistical evaluation.
Differences in growth parameters for the two temperatures were tested for their significance. For this purpose, normal distribution of the data was confirmed by the Shapiro-Wilk test. Potential outlier values were determined using Grubbs outlier tests (36). The evaluation of the data was performed using a two-sample t test (P < 0.05). Differences between the two temperatures were considered significant if the P value was <0.05.
RESULTS
Growth in complex medium.
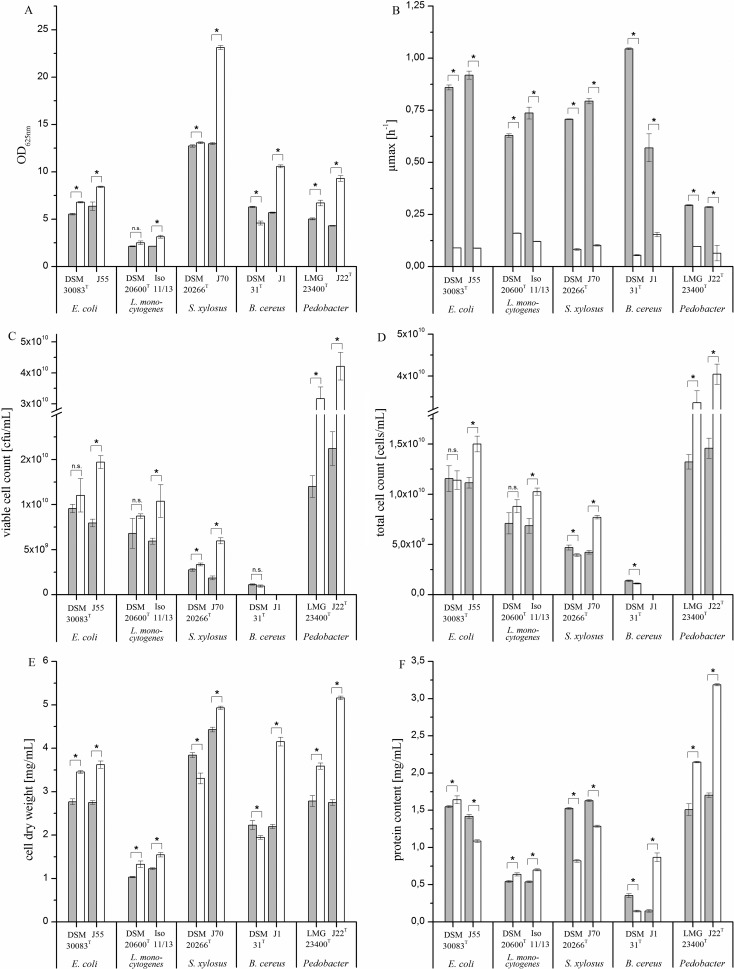
Growth of isolates and reference strains was monitored from inoculation until the stationary growth phase (data not shown). Points of maximum optical density in the early stationary growth phase were used for analysis of viable and total cell numbers, total cell protein, cell dry mass, and cell size. The results of the Shapiro-Wilk test showed a normal distribution for all sampling groups. This allowed subsequent significance tests based on two-sample t tests. Growth temperature had a significant (P < 0.05) effect on all growth parameters (OD625, growth rate, viable and total cell count, cell dry weight, and whole-cell protein concentration) for each strain. As expected, maximum growth rates were higher at 30°C than at 10°C for all strains. The measured growth rates confirmed the classification of the species tested as mesophilic microorganisms. Growth rates at 30°C were 3-fold to 17-fold higher than the growth rates at 10°C. In contrast, maximum growth parameter indicators of biomass formation were higher at 10°C for the isolates E. coli J55, L. monocytogenes Iso 11/13, S. xylosus J70, B. cereus J1, and P. nutrimenti J22T. Increases in biomass formation, based on optical density, of between 20% and 100% were recorded for these isolates. The reference strains showed no change (S. xylosus DSM 20266T), a slight increase of about 25% (L. monocytogenes DSM 20600T, E. coli DSM 30083T, P. panaciterrae LMG 23400T), or a slight decrease of about 25% (B. cereus DSM 31T) in optical density (Fig. 1A). No cell counts were determined for B. cereus J1 because of filamentous growth on solid and liquid media.
FIG 1.
Effect of temperature (30°C, gray bars; 10°C, white bars) on maximum values of optical density (A), growth rate (B), viable cell counts (C), total cell counts (D), cell dry weight (E), and whole-cell protein content (F) of strains tested in a complex medium at the stationary growth phase. Data represent mean values (n = 3) of the isolates E. coli J55, L. monocytogenes Iso 11/13, S. xylosus J70, B. cereus J1, and P. nutrimenti J22T and the reference strains E. coli DSM 30083T, L. monocytogenes DSM 20600T, S. xylosus DSM 20266T, B. cereus DSM 31T, and P. panaciterrae LMG 23400T. No cell counts were determined for B. cereus J1 because of filamentous growth on solid and liquid media. *, statistically significant differences (t test, P < 0.05) between the temperatures for each strain. Values marked with “n.s.” are not significantly different.
The increases in cell yields for the isolates at 10°C, indicated by OD625 values, corresponded well to viable cell count, total cell count, and cell dry weight values and partly to the protein concentration of whole cells. For the latter parameter, E. coli J55 and S. xylosus J70 showed inverse behaviors compared to those of the other growth parameters (Fig. 1A to F).
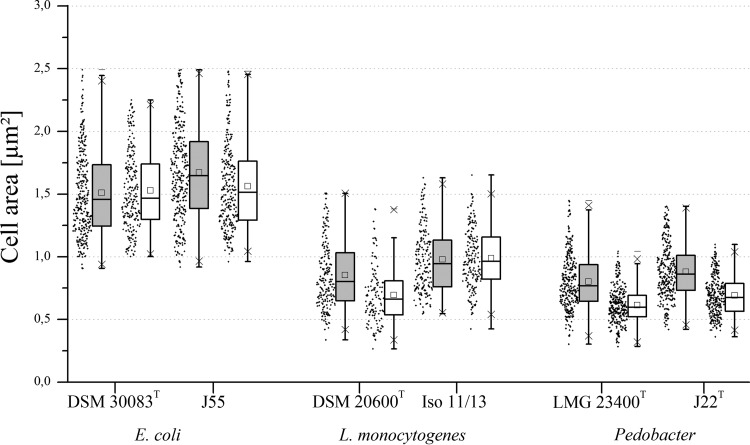
Because OD values can be affected significantly by cell morphology, temperature-dependent changes in cell morphology were checked by microscopy. Temperature-dependent deformations or other unusual cell morphologies were not observed. Because temperature-dependent changes in cell length, width, and area were consistent for each strain, only data for cell area are presented in Fig. 2. While S. xylosus and B. cereus strains showed no changes in cell area at different temperatures (data not shown), E. coli J55, L. monocytogenes DSM 20600T, and the two Pedobacter strains showed only a marginal decrease in cell dimensions at 10°C versus 30°C (Fig. 2).
FIG 2.
Cell areas of E. coli, L. monocytogenes, and Pedobacter strains in complex medium. Comparison of the cell sizes based on cell areas in square micrometers at 30°C (gray boxes) and 10°C (white boxes). Graphical representation of key values, such as mean, median, standard deviation, and maxima, from summary statistics via box plots (each data set, n = 200 to 300). Dots to the left of the box plots represent the distribution of the measured data values.
The absence of significant temperature-dependent changes in cell size indicated that OD is adequate to monitor bacterial growth. All data, except protein concentration, consistently confirmed the substantial increase in biomass formation and cell concentration for the isolates with low growth temperatures. In contrast, for the closely related reference strains, this effect was much smaller or even absent. Thus, B. cereus J1 and P. nutrimenti J22 showed a doubling of biomass formation at 10°C compared to that at 30°C, whereas the reference strains achieved only a maximum increase of about 25% of growth parameters (Fig. 1C). In contrast to the cell yield, the growth rates were higher for all isolates and reference strains at 30°C. This demonstrates that the maxima of cell yield and growth rates were linked to different growth temperatures for mesophilic isolates. However, this phenomenon was not observed for mesophilic reference strains.
Growth in defined medium.
Preliminary experiments with the E. coli strains demonstrated the enhancing effect of betaine supplement on growth yield at 10°C (Table 1). Without betaine supplementation, strain E. coli DSM 30083T attained an approximately 70% lower optical density at 10°C than at 30°C, and the isolate E. coli J55 achieved at 10°C the same OD values as those at 30°C. Since it is known that compatible solutes can act as a part of cold adaptation (37), M9 medium was supplemented with 2 mM betaine. Supplementation with 2 mM betaine increased the biomass formation of E. coli strains more than 2-fold in the defined M9 medium and therefore was used as the standard supplement for all defined media (Table 1).
TABLE 1.
Effect of betaine supplementation on the growth parameters μmax and OD625 at 30°C and 10°C for E. coli strains in a defined mediuma
| Strain | μmax (h−1) |
OD625 |
Δ Optical density (%)b | ||
|---|---|---|---|---|---|
| 30°C | 10°C | 30°C | 10°C | ||
| E. coli DSM 30083T (without betaine) | 0.45 | 0.024 | 1.43 | 0.46 | −68 |
| E. coli J55 (without betaine) | 0.47 | 0.053 | 1.47 | 1.46 | −1 |
| E. coli DSM 30083T | 0.44 | 0.061 | 2.02 | 2.46 | +22 |
| E. coli J55 | 0.51 | 0.050 | 2.07 | 3.27 | +58 |
Data represent mean values (n = 3).
Δ Optical density, change in the optical density at 10°C compared to that at 30°C.
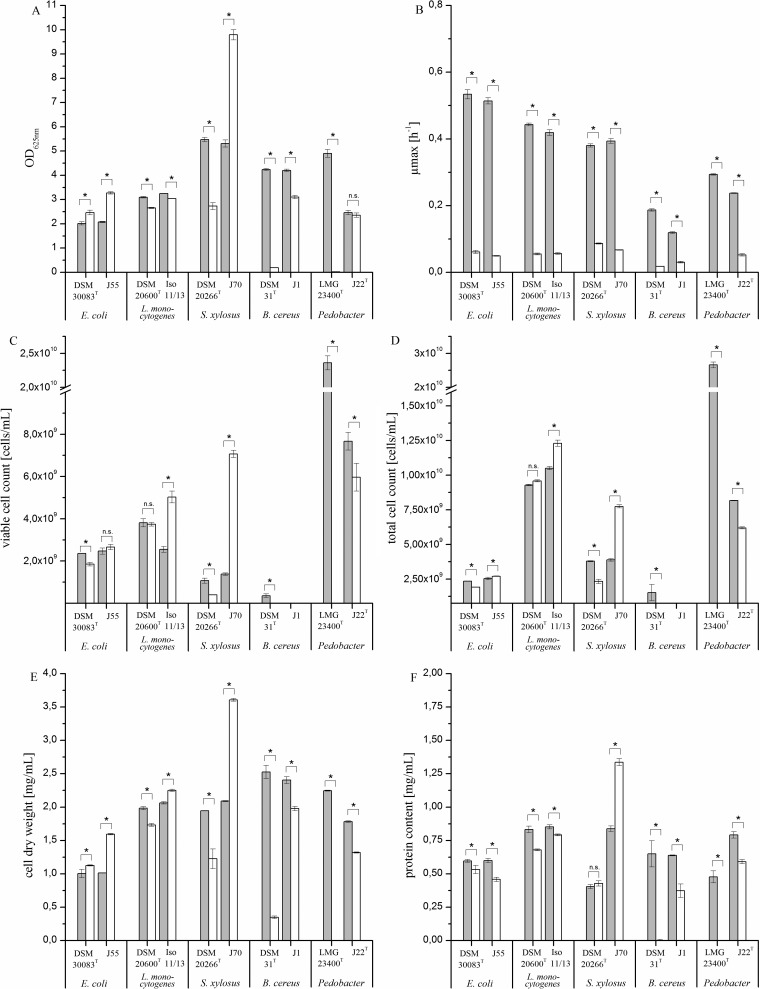
Also, for growth in a defined medium, temperature had a significant (P < 0.05) impact on the majority of the growth parameters (OD625, growth rate, viable and total cell counts, cell dry weight, and whole-cell protein content) for each strain. The only exceptions were the OD of P. nutrimenti J22, the cell counts of E. coli J55 and L. monocytogenes DSM 20600T, and the protein content of S. xylosus DSM 20266T (Fig. 3). Pedobacter panaciterrae LMG 23400T showed no growth at all at 10°C. As expected, μmax values were lower than those in the complex medium, but the growth rate ratios between the two temperatures were comparable to those observed in the complex medium. In contrast to the growth in the complex medium, only the two E. coli strains and the isolate S. xylosus J70 showed increased maximum OD values at 10°C in defined media. For the B. cereus and Pedobacter strains, none of the growth parameters indicated an increase in biomass formation at low temperatures. No increase in growth parameters was observed for L. monocytogenes DSM 20600T. Interestingly, the isolate L. monocytogenes Iso 11/13 showed an increase in viable cell count, total cell numbers, and cell dry weight at 10°C, even though optical density decreased slightly at low temperatures. Generally, the OD625 values corresponded well to viable cell counts, total cell counts, and cell dry weights, with some exceptions probably related to the changes in cell size. The protein content decreased for all isolates and reference strains, except the two S. xylosus strains (Fig. 3F). The experiments performed in defined medium showed that the increased biomass production under low-temperature growth is much more pronounced in the complex medium.
FIG 3.
Effect of temperature (30°C, gray bars; 10°C, white bars) on maximum values of optical density (A), growth rate (B), viable cell counts (C), total cell counts (D), cell dry weight (E), and whole-cell protein content (F) of strains tested in defined media at the stationary growth phase. Data represent the mean values (n = 3) of the isolates E. coli J55, L. monocytogenes Iso 11/13 A, S. xylosus J70, B. cereus J1, and P. nutrimenti J22T and the reference strains E. coli DSM 30083T, L. monocytogenes DSM 20600T, and S. xylosus DSM. No values were determined for Pedobacter panaciterrae LMG 23400T because of nonexistent growth at 10°C. No cell counts were determined for B. cereus J1 because of filamentous growth on solid and liquid media. Cell counts of B. cereus DSM 31T at 10°C are not visible due to their low numbers (viable cell count, 7 × 106 CFU/ml); total cell count, 3 × 107. *, statistically significant differences (t test, P < 0.05) between the temperatures for each strain. Values marked with “n.s.” are not significantly different.
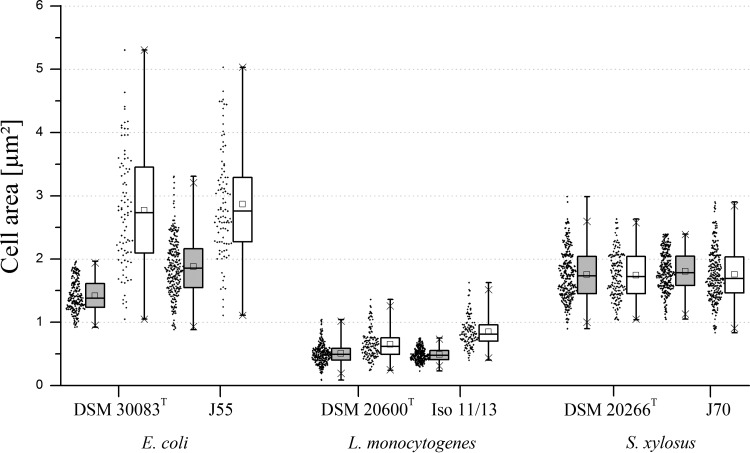
Also, for growth in defined media, deformations or other unusual cell morphologies were not observed at the two temperatures. Pedobacter strains showed no change in cell size at the two temperatures. B. cereus J1 cell dimensions were not determined due to the formation of long cell filaments without clearly separated single cells (data not shown). As in the complex medium, the temperature had no effect on the cell size of the two S. xylosus strains. In contrast, E. coli and L. monocytogenes strains showed significant increases in cell area at 10°C compared to that at 30°C, in which the mean values had nearly doubled (Fig. 4). Due to the small L. monocytogenes cells at 30°C, the increase in cell size at 10°C had also resulted in a cell shape change from coccoidal to ellipsoidal.
FIG 4.
Cell areas of E. coli, L. monocytogenes, and S. xylosus strains in defined medium. Comparison of the cell sizes based on measured areas in square micrometers at 30°C (gray boxes) and 10°C (white boxes). Graphical representation of key values, such as mean, median, standard deviation, and maxima, from summary statistics via box plots (each data set, n = 100 to 300). Dots to the left of the box plots represent the distribution of the measured data values.
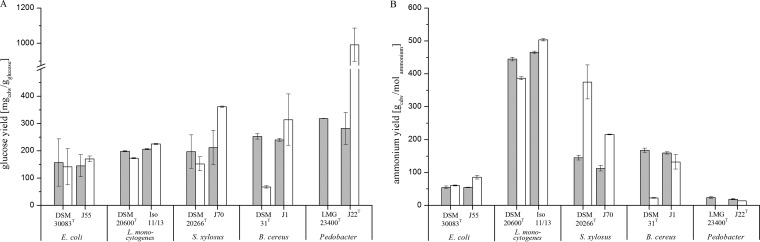
Defined media were used to analyze the C and N source consumption in more detail. Final concentrations of glucose and ammonium were determined at stationary phase, and subsequently, the cell yield per unit of consumed carbon or nitrogen source was calculated. Figure 5A shows the cell yields in grams of cells (dry weight) per gram of consumed glucose at 30°C and 10°C. At 30°C, the cell yields of isolates and reference strains were at the same level. In contrast, at 10°C, cell yields increased for the isolates and decreased for the reference strains. The elevated cell yields correlated well with the growth parameters for E. coli J55, L. monocytogenes Iso 11/13, and S. xylosus J70 as shown in Table 2 and Fig. 3A to F.
FIG 5.
Cell yields in milligrams of cells (dry weight) (cdw) per gram glucose consumed (A) and cell yields in milligrams of cells (dry weight) per mole ammonium consumed (B). Gray bars, 30°C; white bars, 10°C. No values were determined for Pedobacter panaciterrae LMG 23400T because of nonexistent growth at 10°C.
TABLE 2.
Changes in growth parameters and cell yields of the reference strains after a temperature decrease from 30°C to 10°C in a defined medium
| Strain | % change at 10°C compared to 30°C |
||||||
|---|---|---|---|---|---|---|---|
| OD625 | Viable cell count | Total cell count | Cell area | Cell dry wt | Cell dry wt per g glucose consumed | Cell dry wt per mol ammonium consumed | |
| E. coli DSM 30083T | +22 | −22 | −19 | +100 | +13 | −10 | +11 |
| E. coli J55 | +58 | +8 | +7 | +50 | +60 | +17 | +56 |
| L. monocytogenes DSM 20600T | −17 | −2 | +3 | +30 | −13 | −13 | −13 |
| L. monocytogenes Iso 11/13 | −7 | +97 | +17 | +70 | +9 | +9 | +8 |
| S. xylosus DSM 20266T | −53 | −62 | −64 | *a | −37 | −23 | +158 |
| S. xylosus J70 | +85 | +400 | +90 | * | +73 | +70 | +91 |
*, not significantly different.
Figure 5B shows cell yields per mole of ammonium consumed. For this mass balance, only ammonia nitrogen was considered. Betaine, amino acids, and other N sources that were supplemented were not considered. These supplemented amines represent between 10% and 50% of total nitrogen content. Therefore, yields are only comparable between strains cultivated in the same medium.
Analogous to glucose utilization, the ammonium cell yields at 30°C were almost identical for each isolate and its corresponding type strain. At 10°C, the isolates showed a more heterogeneous ratio of biomass production per unit of ammonium consumption than per unit of glucose consumption. Only three isolates, E. coli J55, L. monocytogenes Iso 11/13, and B. cereus J1, showed higher yields than their reference strains. In contrast, isolate S. xylosus J70 showed a lower yield than its respective reference strain. The ammonium-related cell yields correlated very well with temperature-dependent changes in growth parameters for the isolates as well as for the closely related type strains. For example, the increase in cell yield per unit of consumed ammonium at 10°C for E. coli DSM 30083T (11%) and for E. coli J55 (56%) is similar to the increases in OD and cell dry weight (Table 2; Fig. 5B).
DISCUSSION
All of the growth parameters measured in the complex medium indicate that the analyzed mesophilic isolates and corresponding reference strains are able to grow at the two temperatures tested. The significantly decreased growth rates at 10°C support the classification of the tested species as mesophiles. However, a significant difference between the isolates and their related reference strains was noticeable. Biomass formation was significantly higher for isolates than for the reference strains at 10°C.
Furthermore, this study demonstrated that temperatures of high growth rates are different from those temperatures that produce high concentrations of cells or biomass. This was demonstrated for the isolates by measuring optical density, viable cell counts, total cell counts, and cell dry weight. An anomaly was observed for L. monocytogenes Iso 11/13 in a defined medium. Despite reduced OD values at 10°C, cell number and cell dry weight values indicated an increase in biomass production (Fig. 3). This may be explained by the change in cell size and shape at 10°C. Koch (38) showed that the change from a spherical to an ellipsoidal shape can result in a reduction of optical density of about 15% (Table 2). Due to the individual change in cell size at low temperatures for each strain and the concomitant impact on the growth parameters OD625, viable cell count, and total cell count, we abstain from a correlation analysis of these parameters (Table 2). For those strains that showed a temperature-independent constant cell size, viable and total cell counts also correlated well with cell dry weight. The whole-cell protein content, however, showed only a low correlation with temperature and thus was not suitable as a growth parameter for the characterization of increased biomass formation at low temperatures.
The results obtained in this study add a new fact to the already known effect of increased cell yield at low temperatures, which has been reported several times, e.g., by Herbert and Bell (7), Feller et al. (8), Isaksen and Jørgensen (11), and Bakermans and Nealson (10), for a wide range of taxonomic groups, e.g., the genera Arthrobacter, Alteromonas, Bacillus, Moraxella, Pseudomonas, Psychrobacter, and Rhodococcus (8, 10, 39, 40). All of these groups are psychrotrophic or psychrophilic isolates from cold habitats (Polar Sea, deep sea, and cold soils).
Margesin (6) compared the increases in growth parameters at low temperatures between psychrophilic and mesophilic microorganisms. For psychrophilic strains, she demonstrated a significant increase in maximum biomass formation under low-temperature growth, while this effect was considerably lower for mesophilic strains. The tested mesophilic strains showed maximum biomass formation at temperatures close to their maximum growth rate temperatures. This finding corresponds only partially to the results of this study for the reference strains. While we can confirm the results of Margesin (6) for the reference strains, the tested isolates revealed a significant increase in biomass yield. This increase ranged between 80% and 110% at 10°C versus 30°C. All of these isolates were mesophilic strains according to the definition of Margesin (6) and failed to growth at 1°C. The observed biomass increases at a low temperature for mesophilic isolates are similar to the growth characteristics of psychrophilic strains described before (6, 7, 9–11).
Previously, it was assumed that mesophilic bacteria achieved maximum cell yields and maximum growth rates at almost the same temperature, and in this case, the term optimum growth temperature is used unambiguously (11, 12). However, this was not true for the mesophilic isolates analyzed in this study since the highest biomass yields and growth rates were not equally correlated with growth temperature (Fig. 1A to F and 3A to F). Consequently, the term “optimal growth temperature” is ambiguous for the investigated mesophilic bacterial isolates as was already demonstrated for psychrophilic and psychrotrophic microorganisms (6).
Our results demonstrate clearly the different growth characteristics of mesophilic isolates and closely related mesophilic reference strains. The results indicate that microorganisms from strain collections may lose specific traits, like increased biomass formation at low temperatures, probably due to their long-term cultivation under artificial conditions. These cultivation conditions, particularly growth temperatures, are usually optimized for maximal growth rates but not for the most effective transformation of nutritional resources into biomass. For several habitats with limited resources, the latter strategy may be at least similar in importance to the strategy of high growth rates, which may be more successful in nutrient-rich habitats like artificial growth media. This demonstrates the limitations of studies using reference strains with a long-term culture collection history. Remarkably, the conclusions of Margesin (6) were largely based on the mesophilic strain Pedobacter heparinus DSM 2366T and the two psychrophilic strains Pedobacter antarcticus (formerly piscium) DSM 11725 and Pedobacter cryoconitis DSM 14825T. These strains not only differ by their growth temperatures but also by their duration in culture collections. While the mesophilic strain P. heparinus DSM 2366T was already described in 1956 (41), the psychrophilic strains P. antarcticus DSM 11725 and P. cryoconitis DSM 14825T were isolated in 1966 and 2003, respectively (42, 43). In addition to thermal class, different duration times in artificial media may also have an effect on the different growth behavior of these strains. For instance, Liu et al. (44) showed that the type strain of the species Listeria monocytogenes had lost its initial virulence with successive laboratory use. Most of the reference strains used in this study had a long history on artificial media and may have lost some of their mechanisms of adaptation to their original environments. The reference strains used here were isolated in 1941 (E. coli DSM 30083T [45]), 1924 (L. monocytogenes DSM 20600T [46]), 1975 (S. xylosus DSM 20266T [47]), 1916 (B. cereus DSM 31T [48]), and 2007 (P. panaciterrae LMG 23400T [19]).
There are very few reports about the mechanistic basis for biomass increase under suboptimum growth rate temperature conditions. Bakermans and Nealson (10) detected a doubling of the cell yield for a “Psychrobacter cryopegella” isolate (later described as Psychrobacter cryohalolentis [49]) between 0°C and 4°C compared with that at higher temperatures. They assumed a more efficient RNA and protein synthesis that maximized cell yield at low temperatures.
Thereby, the optimization of the growth processes may be induced by cold adaptation mechanisms, such as upregulation of cold shock and cold acclimation proteins, changes in protein synthesis, and adaptations of membrane enzymes and protein structures (3). In particular, cold shock proteins are able to greatly modify protein expression patterns and thus change basal cell functions/pathways. Moreover, the term optimum growth temperature is often equated to the temperature at which the highest growth rates occur (50), which does not necessarily mean that all biological processes run under optimum conditions. In Pseudomonas fluorescens, an increased protein degradation rate was shown for temperatures above 17°C (39), pointing to detrimental heat effects at the optimum growth temperature. This suggests that growth at the maximum growth rate may represent one possible adaptation in specific habitats but is at the same time unfavorable for other biological processes, as has been described for protein synthesis, membrane permeability, enzyme production, and extent of cellular stress (40, 50–53). This hypothesis is further supported by a mathematical growth model published by Corkrey et al. (54). This model links a thermodynamic enzyme-catalyzed growth rate with temperature-dependent protein stability fitting 95 growth rate data sets for various Bacteria, Archaea, and Eukarya (54). It was found that the minimum amount of protein denaturation was predicted at lower temperatures than the optimum growth rate temperature. These Tmes (temperature of maximum enzyme stability) values were 10 to 15°C lower than the temperatures at which maximum growth rates occur (55). More energy is conserved because of the most efficient conditions for correct protein folding and protein turnover, which may result in improved biomass formation.
For a more in-depth analysis of yield-related carbon and nitrogen assimilation, we used defined media. It was shown that the increased biomass formation correlated with a more efficient glucose and ammonia assimilation at low temperatures (Table 2). In particular, the cell yield per mole of ammonium correlated well with percentage increases in growth parameters. These results may confirm the hypothesis of streamlined growth processes, which means improving the efficiency of substrate utilization by lowering the energy demand for cell maintenance (for instance, a lower macromolecule synthesis rate per cell division), as proposed by Bakermans and Nealson (10). This assumption is also supported by Herbert and Bell (7). Their data show that a minimal respiratory quotient for Vibrio sp. strain AF-1 was coincident with the temperature of maximum cell yield and not maximum growth rate.
In defined media, increased biomass formation at low temperatures could only be reproduced for three isolates (Fig. 3A to F; Table 2). Preliminary growth tests showed that E. coli DSM 30083T was significantly limited in the M9 medium and that E. coli J55 was not able to reach higher OD values at 10°C versus 30°C (Table 1). Supplementation of M9 medium with betaine resulted in significant increases in cell yield (Table 1). Thereupon, all minimum media were supplemented with betaine for all strains. The poor growth in the defined medium at low temperatures may be due to the fact that defined media are usually composed and used for cultivation at the temperature with the highest growth rate and are not optimized for low growth temperatures (19, 24–27). It has been shown that compatible solutes function as part of the cold adaptation mechanism and thus improve cell yields, growth rates, and cell viability at low temperatures, including E. coli and L. monocytogenes (21, 22, 37, 56). We performed no comparative test without betaine supplementation for the other strains used in this study. However, for all species, corresponding genes encoding the betaine transporter have been detected before (57–60). For the species P. panaciterrae and P. nutrimenti, information about the synthesis, import, and use of compatible solutes is not available due to their recent description (19, 20).
Another effect of low-temperature growth in a defined medium was the significant increase in the cell size of E. coli and L. monocytogenes. Russel and Fukunaga (61) evaluated the increase in cell size and alteration of cell morphology under low-temperature conditions as adaptation mechanisms that improve substrate uptake at low temperatures.
This work shows the effect of the increased biomass formation at low growth temperatures for mesophilic isolates. Compared with the mesophilic isolates, the described effect was significantly lower or was not detectable in the closely related reference strains from culture collections. This indicates the presence of adaptive mechanisms for growth under low temperatures for mesophiles, which can be lost during long-term cultivation of these reference strains under artificial laboratory conditions. The increased biomass production for mesophiles under low-temperature conditions opens new aspects for a more efficient biotechnological transformation of nutrients to microbial biomass or specific microbial cell compounds under energy-saving conditions. For the risk assessment of cooled foods, it will be fundamental to include the potential production of higher cell concentrations under low-temperature conditions into the relevant risk assessment models for spoiling food and pathogenic microorganisms since risk potential is often correlated with cell numbers present in food samples.
ACKNOWLEDGMENT
We thank Tizian Bajon for experimental support during a practical student course.
REFERENCES
- 1.Ingraham JL, Bailey GF. 1959. Comparative study of effect of temperature on metabolism of psychrophilic and mesophilic bacteria. J Bacteriol 77:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratkowsky DA, Olley J, McMeekin TA, Ball A. 1982. Relationship between temperature and growth rate of bacterial cultures. J Bacteriol 149:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer S, Neuhaus K. 2006. Life at low temperatures, p 210–262. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes. A handbook on the biology of bacteria: ecophysiology and biochemistry, 3rd ed, vol 2 Springer, New York, NY. [Google Scholar]
- 4.Panikov NS, Sizova MV. 2007. Growth kinetics of microorganisms isolated from Alaskan soil and permafrost in soil media frozen down to −35°C. FEMS Microbiol Ecol 59:500–512. doi: 10.1111/j.1574-6941.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 5.Russell NJ, Harrisson P, Johnston IA, Jaenicke R, Zuber M, Franks F, Wynn-Williams D. 1990. Cold adaptation of microorganisms. Philos Trans R Soc Lond B Biol Sci 326:595–611. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- 6.Margesin R. 2009. Effect of temperature on growth parameters of psychrophilic bacteria and yeasts. Extremophiles 13:257–262. doi: 10.1007/s00792-008-0213-3. [DOI] [PubMed] [Google Scholar]
- 7.Herbert RA, Bell CR. 1977. Growth characteristics of an obligately psychrophilic Vibrio sp. Arch Microbiol 113:215–220. doi: 10.1007/BF00492028. [DOI] [PubMed] [Google Scholar]
- 8.Feller G, Narinx E, Arpigny JL, Zekhnini Z, Swings J, Gerday C. 1994. Temperature dependence of growth, enzyme secretion and activity of psychrophilic Antarctic bacteria. Appl Microbiol Biotechnol 41:477–479. doi: 10.1007/s002530050176, doi:. [DOI] [Google Scholar]
- 9.Knoblauch C, Jørgensen BB. 1999. Effect of temperature on sulphate reduction, growth rate and growth yield in five psychrophilic sulphate-reducing bacteria from Arctic sediments. Environ Microbiol 1:457–467. doi: 10.1046/j.1462-2920.1999.00061.x. [DOI] [PubMed] [Google Scholar]
- 10.Bakermans C, Nealson KH. 2004. Relationship of critical temperature to macromolecular synthesis and growth yield in Psychrobacter cryopegella. J Bacteriol 186:2340–2345. doi: 10.1128/JB.186.8.2340-2345.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaksen MF, Jørgensen BB. 1996. Adaptation of psychrophilic and psychrotrophic sulfate-reducing bacteria to permanently cold marine environments. Appl Environ Microbiol 62:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamberg K, Kask S, Laht TM, Paalme T. 2003. The effect of temperature and pH on the growth of lactic acid bacteria: a pH-auxostat study. Int J Food Microbiol 85:171–183. doi: 10.1016/S0168-1605(02)00537-8. [DOI] [PubMed] [Google Scholar]
- 13.Farfán M, Montes MJ, Marqués AM. 2014. Reclassification of Sphingobacterium antarcticum Shivaji et al. 1992 as Pedobacter antarcticus comb. nov. and Pedobacter piscium (Takeuchi and Yokota 1993) Steyn et al. 1998 as a later heterotypic synonym of Pedobacter antarcticus. Int J Syst Evol Microbiol 64:863–868. doi: 10.1099/ijs.0.054965-0. [DOI] [PubMed] [Google Scholar]
- 14.Logan NA, De Vos P. 2009. Genus I. Bacillus Cohn 1872, 174AL, p 21–128. In De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FR, Schleifer K-H, Whitman WB (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 3 Springer, New York, NY. [Google Scholar]
- 15.Scheutz F, Strockbine NA. 2005. Genus I. Escherichia Castellani and Chalmers 1919, 941TAL, p 607–624. In Brenner DJ, Krieg NR, Staley JT, Garrity GM (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 Springer, New York, NY. [Google Scholar]
- 16.McLauchlin J, Rees CED. 2009. Genus I. Listeria Pirie 1940a, 383AL, p 244–256. In De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FR, Schleifer K-H, Whitman WB (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 3 Springer, New York, NY. [Google Scholar]
- 17.Low JC, Donachie W. 1997. A review of Listeria monocytogenes and listeriosis. Vet J 153:9–29. doi: 10.1016/S1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 18.Schleifer K-H, Bell JA. 2009. Genus I. Staphylococcus Rosenbach 1884, 18AL (Nom. Cons. Opin 17 Jud. Comm. 1958, 153.) p 392–420. In DeVos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FR, Schleifer K-H, Whitman WB (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 3 Springer, New York, NY. [Google Scholar]
- 19.Yoon MH, Ten LN, Im WT, Lee ST. 2007. Pedobacter panaciterrae sp. nov., isolated from soil in South Korea. Int J Syst Evol Microbiol 57:381–386. doi: 10.1099/ijs.0.64693-0. [DOI] [PubMed] [Google Scholar]
- 20.Derichs J, Kämpfer P, Lipski A. 2014. Pedobacter nutrimenti sp. nov., isolated from chilled food. Int J Syst Evol Microbiol 64:1310–1316. doi: 10.1099/ijs.0.058677-0. [DOI] [PubMed] [Google Scholar]
- 21.Angelidis AS, Smith GM. 2003. Role of the glycine betaine and carnitine transporters in adaptation of Listeria monocytogenes to chill stress in defined medium. Appl Environ Microbiol 69:7492–7498. doi: 10.1128/AEM.69.12.7492-7498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wemekamp-Kamphuis HH, Sleator RD, Wouters JA, Hill C, Abee T. 2004. Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol 70:2912–2918. doi: 10.1128/AEM.70.5.2912-2918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 24.Tsai HN, Hodgson DA. 2003. Development of a synthetic minimal medium for Listeria monocytogenes. Appl Environ Microbiol 69:6943–6945. doi: 10.1128/AEM.69.11.6943-6945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain M, Hastings JGM, White PJ. 1991. A chemically defined medium for slime production by coagulase-negative staphylococci. J Med Microbiol 34:143–147. doi: 10.1099/00222615-34-3-143. [DOI] [PubMed] [Google Scholar]
- 26.Milner JL, Raffel SJ, Lethbridge BJ, Handelsman J. 1995. Culture conditions that influence accumulation of zwittermicin A by Bacillus cereus UW85. Appl Microbiol Biotechnol 43:685–691. doi: 10.1007/BF00164774. [DOI] [PubMed] [Google Scholar]
- 27.Agata N, Ohta M, Mori M, Shibayama K. 1999. Growth conditions of and emetic toxin production by Bacillus cereus in a defined medium with amino acids. Microbiol Immunol 43:15–18. doi: 10.1111/j.1348-0421.1999.tb02367.x. [DOI] [PubMed] [Google Scholar]
- 28.Widdel F, Bak F. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p 3352–3378. In Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (ed), The prokaryotes, 2nd ed Springer-Verlag, New York, NY. [Google Scholar]
- 29.Widdel F, Kohring GW, Mayer F. 1983. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. Arch Microbiol 134:286–294. doi: 10.1007/BF00407804. [DOI] [PubMed] [Google Scholar]
- 30.Tschech A, Pfennig N. 1984. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol 137:163–167. doi: 10.1007/BF00414460. [DOI] [Google Scholar]
- 31.Kepner RL, Pratt JR. 1994. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol Rev 58:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson HW, Hodgen CG. 1940. The biuret reaction in the determination of serum proteins. 1. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J Biol Chem 135:707–725. [Google Scholar]
- 33.Stickland LH. 1951. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol 5:698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- 34.Li K, Liu S, Tan Y, Chao N, Tian X, Qi L, Powell WA, Jiang X, Gai Y. 2013. Optimized GC-MS method to simultaneously quantify acetylated aldose, ketose, and alditol for plant tissues based on derivatization in a methyl sulfoxide/1-methylimidazole system. J Agric Food Chem 61:4011–4018. doi: 10.1021/jf3053862. [DOI] [PubMed] [Google Scholar]
- 35.Streuli CA, Averell PR. 1970. The analytical chemistry of nitrogen and its compounds, p 52–62. In Elving PJ, Kolthoff IM (ed), Chemical analysis, vol 28 Wiley-Interscience, New York, NY. [Google Scholar]
- 36.Grubbs FE. 1950. Sample criteria for testing outlying observations. Ann Math Stat 21:27–58. doi: 10.1214/aoms/1177729885. [DOI] [Google Scholar]
- 37.Kandror O, DeLeon A, Goldberg AL. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc Natl Acad Sci U S A 99:9727–9732. doi: 10.1073/pnas.142314099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch AL. 1961. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta 51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- 39.Guillou C, Guespin-Michel JF. 1996. Evidence for two domains of growth temperature for the psychrotrophic bacterium Pseudomonas fluorescens MF0. Appl Environ Microbiol 62:3319–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margesin R, Fonteyne PA, Redl B. 2005. Low-temperature biodegradation of high amounts of phenol by Rhodococcus spp. and basidiomycetous yeasts. Res Microbiol 156:68–75. doi: 10.1016/j.resmic.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Payza AN, Korn ED. 1956. Bacterial degradation of heparin. Nature 177:88–89. doi: 10.1038/177088a0. [DOI] [PubMed] [Google Scholar]
- 42.Komagata K, Ogawa H. 1966. Microbiological studies on frozen foods III. Determination of Gram-negative bacteria isolated from frozen foods. Food Hyg Safety Sci 7:239–247. (In Japanese.) [Google Scholar]
- 43.Margesin R, Sprör C, Schumann P, Schinner F. 2003. Pedobacter cryoconitis sp. nov., a facultative psychrophile from alpine glacier cryoconite. Int J Syst Evol Microbiol 53:1291–1296. doi: 10.1099/ijs.0.02436-0. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Ainsworth AJ, Austin FW, Lawrence ML. 2003. Characterization of virulent and avirulent Listeria monocytogenes strains by PCR amplification of putative transcriptional regulator and internalin genes. J Med Microbiol 52:1065–1070. doi: 10.1099/jmm.0.05358-0. [DOI] [PubMed] [Google Scholar]
- 45.Kauffmann F. 1944. Zur Serologie der Coli-Gruppe. Acta Pathol Microbiol Scand 21:20–45. [PubMed] [Google Scholar]
- 46.Murray EGD, Webb RA, Swann MBR. 1926. A disease of rabbits characterised by a large mononuclear leucocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes (n. sp.). J Pathol Bacteriol 29:407–439. doi: 10.1002/path.1700290409. [DOI] [Google Scholar]
- 47.Schleifer KH, Kloos WE. 1975. Isolation and characterization of staphylococci from human skin I. Amended descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus and descriptions of three new species: Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus. Int J Syst Bacteriol 25:50–61. [Google Scholar]
- 48.Lawrence JS, Ford WW. 1916. Spore-bearing bacteria in milk. J Bacteriol 1:277–320. [PMC free article] [PubMed] [Google Scholar]
- 49.Bakermans C, Ayala-del-Río HL, Ponder MA, Vishnivetskaya T, Gilichinsky D, Thomashow MF, Tiedje JM. 2006. Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov, isolated from Siberian permafrost. Int J Syst Evol Microbiol 56:1285–1291. doi: 10.1099/ijs.0.64043-0. [DOI] [PubMed] [Google Scholar]
- 50.Feller G, Gerday C. 2003. Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1:200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- 51.Jaouen T, De E, Chevalier S, Orange N. 2004. Pore size dependence on growth temperature is a common characteristic of the major outer membrane protein OprF in psychrotrophic and mesophilic Pseudomonas species. Appl Environ Microbiol 70:6665–6669. doi: 10.1128/AEM.70.11.6665-6669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Amico S, Collins T, Marx JC, Feller G, Gerday C. 2006. Psychrophilic microorganisms: challenges for life. EMBO Rep 7:385–389. doi: 10.1038/sj.embor.7400662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feller G. 2007. Life at low temperatures: is disorder the driving force? Extremophiles 11:211–216. doi: 10.1007/s00792-006-0050-1. [DOI] [PubMed] [Google Scholar]
- 54.Corkrey R, Olley J, Ratkowsky D, McMeekin T, Ross T. 2012. Universality of thermodynamic constants governing biological growth rates. PLoS One 7(2):e32003. doi: 10.1371/journal.pone.0032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corkrey R, McMeekin TA, Bowman JP, Ratkowsky DA, Olley J, Ross T. 2014. Protein thermodynamics can be predicted directly from biological growth rates. PLoS One 9(5):e96100. doi: 10.1371/journal.pone.0096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajkumari K, Gowrishankar J. 2001. In vivo expression from the RpoS-dependent P1 promoter of the osmotically regulated proU operon in Escherichia coli and Salmonella enterica serovar Typhimurium: activation by rho and hns mutations and by cold stress. J Bacteriol 183:6543–6550. doi: 10.1128/JB.183.22.6543-6550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucht JM, Bremer E. 1994. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system proU. FEMS Microbiol Rev 14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 58.Ko R, Smith LT. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl Environ Microbiol 65:4040–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dordet-Frisoni E, Dorchies G, De Araujo C, Talon R, Leroy S. 2007. Genomic diversity in Staphylococcus xylosus. Appl Environ Microbiol 73:7199–7209. doi: 10.1128/AEM.01629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.den Besten HM, Mols M, Moezelaar R, Zwietering MH, Abee T. 2009. Phenotypic and transcriptomic analyses of mildly and severely salt-stressed Bacillus cereus ATCC 14579 cells. Appl Environ Microbiol 75:4111–4119. doi: 10.1128/AEM.02891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russell NJ, Fukunaga N. 1990. A comparison of thermal adaptation of membrane lipids in psychrophilic and thermophilic bacteria. FEMS Microbiol Lett 75:171–182. doi: 10.1111/j.1574-6968.1990.tb04093.x. [DOI] [Google Scholar]