ABSTRACT
Photobacterium damselae subsp. damselae is considered to be an emerging pathogen of marine fish of importance in aquaculture, with a notable increase in its geographical distribution during the last several years. In this study, we carried out for the first time to our knowledge a genetic and pathobiological characterization of 14 strains isolated from sea bass (Dicentrarchus labrax) reared in the Southeastern Black Sea, where high mortalities were observed at two aquaculture farms during the summer and autumn of 2011. Heterogeneity was evidenced among strains in phenotypical traits, such as sucrose fermentation, motility, and hemolysis. Although 11 of 14 isolates were hemolytic, we found that all of the isolates lacked the pPHDD1 virulence plasmid that encodes the phospholipase-D damselysin (Dly) and the pore-forming toxin PhlyP, two hemolysins previously reported to constitute major virulence factors for turbot. Subsequent PCR and sequencing analyses demonstrated that the 11 hemolytic isolates harbored a complete hlyAch gene, a chromosome I-borne gene that encodes HlyAch hemolysin, whereas the three nonhemolytic isolates contained hlyAch pseudogenes caused by insertion sequence elements. Virulence challenges with two representative strains revealed that, albeit less virulent than the pPHDD1-harboring strain RM-71, the plasmidless hlyAch-positive and hlyAch-negative Black Sea isolates were pathogenic for sea bass. A phylogenetic analysis based on the toxR gene sequence uncovered a greater diversity in the isolates, indicating that the presence of this pathogen in the Black Sea was not caused by the introduction and spread of a single virulent clone but by the proliferation of different clones.
IMPORTANCE The geographical distribution of marine bacterial pathogens is undergoing a worldwide increase. In particular, bacteria of the group vibrios are increasingly being isolated as the causative agents of disease in novel species of cultivated fish in areas where they had not been previously reported. Here we characterize for the first time to our knowledge a collection of isolates of the fish and human pathogen Photobacterium damselae subsp. damselae from diseased sea bass reared in the Black Sea. We uncovered great genetic diversity in the Black Sea isolates of this pathogen, suggesting a multiclonal origin. We also demonstrate for the first time that these isolates bear pathogenic potential for sea bass cultures by virulence challenges.
INTRODUCTION
Photobacterium damselae subsp. damselae is a marine bacterium of the family Vibrionaceae that is recognized as a pathogen for a wide variety of aquatic animals, including fish, molluscs, and crustaceans. In addition, it is a pathogen of concern for humans, as it is capable of causing fatal infections (1). Most of the reported infections in humans originated from wounds inflicted during the handling of fish and fishing tools or from exposure to marine animals or seawater. Notably, it is a primary pathogen of fish species of economical importance in aquaculture. During the last several decades, it has been isolated as the causative agent of outbreaks in turbot (Scophthalmus maximus) (2), gilthead seabream (Sparus aurata) (3), and rainbow trout (Oncorhynchus mykiss) (4), among others. Recently, this pathogen has been isolated from outbreaks in newly cultured species of marine fish, an observation that led to its consideration as an emerging pathogen in aquaculture (5). Outbreaks in sparid fish were recently reported in the Mediterranean area (6–9), affecting gilthead seabream, white seabream (Diplodus sargus), and redbanded seabream (Pagrus auriga).
In addition, recent reports mentioned the isolation of P. damselae subsp. damselae from outbreaks in sea bass (Dicentrarchus labrax) cultures in Spain (7), Egypt (9), and Tunisia (10). Recently, this pathogen was isolated from moribund sea bass cultured in the Turkish Black Sea over a 3-month period, which was coincident with episodes of fish mortality and with water temperatures close to or above 24°C (11). Thus, surveillance of P. damselae subsp. damselae in marine fish cultures has shown a notable increase in its geographical distribution during the last several years, causing disease outbreaks in countries where it had never been reported before. However, despite an increasing number of reports on the isolation of this pathogen from sea bass and from novel geographical locations, studies at the genetic level to identify the presence of virulence markers have been scant. In addition, little is known about the epidemiology of the isolates or about the clonality of the populations causing the recent outbreaks.
Recently, the virulence gene content of this subspecies has begun to be elucidated. Currently, it is recognized that highly hemolytic isolates harbor a 153-kb virulence plasmid named pPHDD1, encoding the two hemolysins damselysin (Dly) and HlyApl (12), with HlyApl recently renamed phobalysin (PhlyP) due to its differential characteristics (13). In addition, the hemolytic isolates encode in chromosome I a hemolysin dubbed HlyAch, which is responsible for the hemolytic activity of pPHDD1-negative strains (14). It is acknowledged that the synergistic effect of Dly with either PhlyP or HlyAch is responsible for maximum virulence in a turbot model (14). Interestingly, only a fraction of P. damselae subsp. damselae strains harbor pPHDD1 (15), and plasmidless strains have been routinely isolated from recent outbreaks in sparid fish (8). To date, no information is available on the virulence gene baggage of P. damselae subsp. damselae isolates from sea bass, and there is no information on the prevalence of the pPHDD1 plasmid further than in some human and turbot isolates (15). The aim of the present work was to characterize a collection of P. damselae subsp. damselae strains that was isolated from sea bass during surveys in two rearing facilities in the Black Sea in Turkey in order to carry out a study of their virulence gene baggage and to assess the pathogenicity potential for sea bass. The interest of this study is increased by the fact that the Black Sea is a particular environment, narrowly connected to other seawater masses, with warm temperatures and salinity levels below 18‰. This study constitutes the first report, to our knowledge, on the molecular characterization of this pathogen isolated from disease outbreaks in the Black Sea, and our results indicate a multiclonal origin of the P. damselae subsp. damselae isolates in this area.
MATERIALS AND METHODS
Bacterial strain isolation and characterization and culture conditions.
In a previous study aimed at determining the occurrence and frequency of bacterial pathogens in cultured sea bass, spleens and kidneys of fish belonging to the 2009, 2010, and 2011 age classes were sampled for bacteriological analyses in 2011 (11). Three cages from each of two farms were sampled (farm A located in Persembe in the Ordu Province and farm B located in Yomra in the Trabzon Province on the Turkish coast of the Black Sea) (Fig. 1), with 10 to 24 sea bass per cage. Water temperature ranged from 17.4°C to 25.8°C from June to October, although most samples were collected at water temperatures above 24.5°C. Salinity ranged from 16‰ to 17‰. A total of 14 strains (Table 1) were presumptively assigned to Photobacterium damselae subsp. damselae. P. damselae subsp. damselae and P. damselae subsp. piscicida isolates used in this study (Tables 1 and 2) were routinely grown at 25°C on tryptic soy agar supplemented with 1% NaCl (TSA-1). For hemolysis assays on agar plates, a single colony of each strain grown on a TSA-1 plate was picked with the tip of a rounded wooden pick and seeded on sheep blood agar plates (Oxoid), and pictures were taken after 24 h. Motility assays were carried out using motility agar, which consisted of LB broth supplemented with 0.25% bacteriological agar. For this assay, a single colony isolated from an 18-h culture agar plate for each strain was picked with a sterile plastic tip, and the tip was stabbed into the motility agar. Pictures were taken after 24 h. This procedure was repeated three times to ensure that the motility radius of the strains was reproducible.
FIG 1.
Geographical location of farm A (Persembe) and farm B (Yomra) in the provinces of Ordu and Trabzon, respectively, on the Turkish coast of the Black Sea. Map created with QGIS version 2.14.1 Essen.
TABLE 1.
Phenotypic and genotypic characteristics and isolation data for P. damselae subsp. damselae isolates from sea bass in the Black Sea
| P. damselae subsp. damselae straina | Geographical origin and isolation datab | Fish year class | Water temp (°C) | Organ | Nodules in spleen and kidney | dlyc | hlyAplc | hlyAch | Hemolytic halod | ureCc | Gowth in TCBSe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 154dp | Persembe, Turkey, IX-2011 | 2010 | 24.5 | Spleen | No | − | − | + | SH | + | G |
| 164dp | Persembe, Turkey, IX-2011 | 2011 | 24.5 | Spleen | Yes | − | − | + | SH | + | G |
| 144bp | Persembe, Turkey, IX-2011 | 2010 | 24.5 | Kidney | No | − | − | Ψf | NH | + | G |
| 162bp | Persembe, Turkey, IX-2011 | 2011 | 24.5 | Kidney | Yes | − | − | + | SH | + | Y |
| 158dp | Persembe, Turkey, IX-2011 | 2011 | 24.5 | Spleen | No | − | − | + | SH | + | G |
| 189bp | Persembe, Turkey, X-2011 | 2010 | 17.4 | Kidney | Yes | − | − | + | SH | + | Y |
| 82dy | Yomra, Turkey, VII-2011 | 2009 | 25.2 | Spleen | No | − | − | + | SH | + | G |
| 64bp | Persembe, Turkey, VII-2011 | 2011 | 24.5 | Kidney | Yes | − | − | Ψ | NH | + | Y |
| 156dp | Persembe, Turkey, IX-2011 | 2011 | 24.5 | Spleen | Yes | − | − | + | SH | + | G |
| 125dy | Yomra, Turkey, IX-2011 | 2011 | 24.3 | Spleen | No | − | − | + | SH | + | Y |
| 70dps | Persembe, Turkey, VII-2011 | 2010 | 22.9 | Spleen | No | − | − | + | SH | − | Y |
| 164dpbuy | Persembe, Turkey, IX-2011 | 2011 | 24.5 | Spleen | Yes | − | − | + | SH | + | G |
| 111bp | Persembe, Turkey, VIII-2011 | 2010 | 25.8 | Kidney | Yes | − | − | + | SH | + | G |
| 89dp | Persembe, Turkey, VII-2011 | 2009 | 22.9 | Spleen | No | − | − | Ψ | NH | + | Y |
Each strain was isolated from a different fish.
Roman numerals denote the month of isolation.
−, negative; +, positive.
SH, small hemolytic halo; NH, no hemolytic halo.
G, green colony on TCBS; Y, yellow colony on TCBS.
Ψ, pseudogene of hlyAch.
TABLE 2.
Other P. damselae subsp. damselae and P. damselae subsp. piscicida strains used in this study for comparative purposes and for phylogenetic analyses
| P. damselae strain | Isolation parameters (host, country, year) | Reference or sourcea |
|---|---|---|
| subsp. damselae | ||
| RM-71 | Turbot (Scophthalmus maximus), Spain, 1988 | 2 |
| RG-91 | Turbot (S. maximus), Spain, 1987 | 2 |
| RG-153 | Turbot (S. maximus), Spain, 1988 | 2 |
| RG-214 | Turbot (S. maximus), Spain, 1989 | 2 |
| RG-191 | Turbot (S. maximus), Spain, 1988 | 2 |
| RI-162 | Turbot (S. maximus), Spain, 1991 | 2 |
| LD-07 | Gilthead seabream (Sparus aurata), Spain, 1991 | 3 |
| Lb501R | Seabass (Dicentrarchus labrax), Spain, 2005 | 7 |
| subsp. piscicida | ||
| DI21 | Gilthead seabream (S. aurata), Spain, 1990 | 34 |
| PC554.2 | Sole (Solea senegalensis), Spain, 2001 | 35 |
| AQV6.1 | Sole (Solea senegalensis), Portugal, 2007 | Laboratory collection |
| MP-7801 | Seriola quinqueradiata, Japan, 1978 | T. Kitao |
| P3335 | S. quinqueradiata, Japan, 1990 | R. Kusuda |
| EPOY-8803-11 | Epinephelus akaara, Japan, 1988 | K. Muroga |
| ATCC 29690 | S. quinqueradiata, Japan, 1972 | ATCC |
T. Kitao, Department of Fisheries, Faculty of Agriculture, Miyazaki University, Miyazaki, Japan; R. Kusuda, Fish Diseases Laboratory, Faculty of Agriculture, Kochi University, Kochi, Japan; K. Muroga, Faculty of Applied Biological Science, Hiroshima University, Hiroshima, Japan.
DNA techniques.
Genomic DNA was extracted with an Easy-DNA kit (Invitrogen). Relevant PCR primers used in this study are listed in Table 3. The genetic context of the hlyAch gene was amplified by inverse PCR starting from conserved flanking genes. A nearly complete 16S gene was amplified using primers PA forward (corresponding to E. coli 16S rRNA gene positions 8 to 27) and PH reverse (E. coli 16S rRNA gene positions 1522 to 1541) as previously described (16). PCRs were routinely performed with Kapa Taq DNA polymerase (Kapa) using a T-Gradient thermocycler (Biometra) with the following thermal cycling conditions: 95°C for 5 min followed by 30 cycles of 95°C for 30 s, 57°C for 30 s, and an elongation step of 1 min at 72°C per kb. DNA sequences were determined by Sanger sequencing and a capillary DNA sequencer ABI 3730xl (Applied Biosystems).
TABLE 3.
Oligonucleotides used in this study
| Target and oligonucleotide | Sequence | No. of base pairs amplified |
|---|---|---|
| dly | ||
| Dly-5′ | CCTATGGGACATGAATGG | 549 |
| Dly-3′ | GCTCTAGGCTAAATGAATC | |
| phlyP (hlyApl) | ||
| PhlyP-5′ | GCTATAAATGAATAAGAAAA | 767 |
| PhlyP-3′ | TTGAAGCTAACTCAAAAA | |
| hlyAch (complete ORF and upstream sequences) | ||
| HlyAch-4new | ATAGAAAAGCTCATTTGGCT | Variable |
| KefA-3′ | TGTAGGTAGATATCGAGCTC | |
| hlyAch (3′-end) | ||
| HlyAcr-intR | CACATTCAGCCGTCATTACT | 131 |
| HlyA_J3G_uni1 | CTTCGTAGAGTTAATAAGGC | |
| hlyAch (5′-end) | ||
| HlyAcr_seq_5′ | AGCATCTGCTATCGGAGTATAC | 220 |
| HlyAcr_intF | CCAGATCGTATTGTATATGT | |
| pPHDD1 replication origin | ||
| Ori_pPHDD1-R | CATTACCAAAACATCTACAT | 3,052 |
| Ori_pPHDD1_F | TGGAATAACTATGAGTAACA | |
| toxR | ||
| toxR-5′ | GGGATTTTATGGTACACAAA | 985 |
| toxR-3′ | ATCATAACCAGAGAGATGCT | |
| 16S rRNA gene | ||
| PA | AGAGTTTGATCCTGGCTCAG | 1,532 |
| PH | AAGGAGGTGATCCAGCCGCA |
Experimental infection.
To test the pathogenicity of Black Sea isolates of P. damselae subsp. damselae obtained from sea bass, two strains (164dp and 64bp) were selected for experimental infection of sea bass, representing the two categories of hemolytic and nonhemolytic (NH) isolates, respectively. In addition, the highly hemolytic and pPHDD1-harboring strain RM-71 (2, 12) was used in the challenges. Fish were obtained from the Instituto Galego de Formación en Acuicultura (IGaFA) (Illa de Arousa, Galicia, Spain). Groups of 10 fish (6 ± 1.2 g) per strain tested and per dose were acclimated in 100-liter aquaria at 24°C for 1 week before the challenges were performed. The virulence tests were conducted by intraperitoneal injection of bacterial suspensions. Fish were challenged with 0.1-ml bacterial suspensions of each strain in 0.85% NaCl solution at two different doses of 5.4 × 105 and 3.4 × 107 CFU/fish. A control group was inoculated with the same volume of sterile 0.85% NaCl solution. Fish mortality was recorded daily for 10 days postchallenge. Reisolation on tryptic soy agar (TSA) and thiosulfate-citrate-bile salts-sucrose (TCBS) agar plates and identification of the bacteria from the kidneys of dead fish were performed. Colonies were confirmed by the P. damselae subsp. damselae-specific ureC gene PCR test as previously described (17). All of the protocols of animal experimentation used in this study have been reviewed and approved by the Animal Ethics Committee of the Universidade de Santiago de Compostela.
Molecular phylogenetic analysis.
The evolutionary history of the strains based on the toxR gene was inferred using the neighbor-joining method (18). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the maximum composite likelihood method (19) and are in the units of the number of base substitutions per site. The analysis involved 28 toxR nucleotide sequences. Evolutionary analyses were conducted in MEGA6 (20).
Nucleotide sequence accession numbers.
DNA sequences have been deposited in GenBank under accession numbers KU760730 to KU760757 and KU896559 to KU896561.
RESULTS
Taxonomic verification and phenotypic diversity of P. damselae subsp. damselae sea bass isolates from the Black Sea.
In a previous study (11), two sea bass farms located at a distance from each other on the Turkish coast of the Black Sea (Fig. 1) that were undergoing fish mortalities were sampled for the presence of bacteria in the kidneys and spleens of sea bass. Fourteen presumptive isolates of P. damselae subsp. damselae were isolated from erratically swimming fish, and half of the fish showed nodules in the kidney and spleen. The collection included isolates from fish born in one of three years, namely, 2009, 2010, and 2011 (Table 1). In the present study, these 14 isolates were subjected to genotypic and phenotypic characterization. Complete 16S rRNA gene sequences presumptively identified the 14 isolates as P. damselae subsp. damselae, showing 100% identity to the consensus 16S gene sequence of type strain ATCC 33539 (GenBank accession number X74700) (data not shown). Although the 16S rRNA sequences are identical between P. damselae subsp. damselae and P. damselae subsp. piscicida (16), characteristics such as growth at 37°C and the ability to grow on TCBS agar clearly distinguished these isolates as belonging to P. damselae subsp. damselae. The positive amplification of the subspecies-specific ureC gene encoding a subunit of urease enzyme (17) in all of the strains, with the exception of isolate 70dps, provided additional evidence of the identification of the isolates as P. damselae subsp. damselae (Table 1).
Differences were evident among the 14 isolates in sucrose fermentation in TCBS. Although strains of this pathogen are typically considered to produce green colonies on TCBS medium, we found that 6 sea bass isolates produced yellow colonies (Table 1). In addition, we observed diversity in the motility phenotypes of the isolates when assayed in motility agar; one strain was nonmotile (70dps) and two other strains exhibited little motility (144bp and 111bp), with the remaining strains showing different levels of spreading in motility agar after 24 h (Fig. 2A). All of these observations initially suggested that the P. damselae subsp. damselae isolates from the Black Sea might derive from different genetic lineages coexisting in this geographical area rather than constituting a clonal lineage derived from a single virulent clone.
FIG 2.
(A) Swimming motility phenotypes of the 14 P. damselae subsp. damselae strains isolated from sea bass on the Turkish coast of the Black Sea after 24 h of growth in motility agar. The motility radius was reproducible in three independent repeats of the experiment, although only one experiment is shown. (B) Hemolytic phenotypes of the 14 strains on sheep blood agar. For comparative purposes, the highly hemolytic pPHDD1-harboring strain RM-71 is included. Note that, compared to the RM-71 strain, all of the Black Sea strains are either weakly hemolytic (hemolysis due to hlyAch gene) or nonhemolytic (strains 144bp, 64bp, and 89bp contain hlyAch pseudogenes). Scale bar, 1 cm (motility assays were conducted in 15-cm petri dishes and hemolysis assays in standard 9-cm dishes).
Hemolytic activity and hemolysin gene baggage.
The three main recognized virulence factors of P. damselae subsp. damselae so far are hemolysin genes. The two hemolysins, damselysin (Dly) and the pore-forming toxin PhlyP, are encoded in the pPHDD1 plasmid, whereas a third hemolysin HlyAch is encoded in chromosome I. In a previous study, we found that P. damselae subsp. damselae strains can be divided into categories depending on the width of their haloes of beta-hemolysis (large halo [LH], medium halo [MH], small halo [SH], and nonhemolytic [NH]) (12). In the present study, we found that the sea bass strains exhibited either a small beta-hemolytic halo (SH) (11 isolates) or no halo (NH) (3 isolates) in sheep blood agar plates, in contrast to the pPHDD1-harboring highly hemolytic strain RM-71 isolated from turbot that we used as a control for the LH phenotype (Fig. 2B). Thus, the hemolytic tests seem to indicate that none of the sea bass isolates from the Black Sea contain plasmid pPHDD1 and suggest that the 11 SH isolates harbor the hlyAch gene in their genomes. In order to test these hypotheses, we carried out a PCR screening of the dly, phlyP, and hlyAch genes, and the results were compared with their hemolytic phenotypes. We found that all of the 14 Black Sea strains tested negative for the dly and phlyP genes (Table 1), and they all yielded negative results for a PCR test targeted to the pPHDD1 replication origin (data not shown). These results thus indicate that the pPHDD1 plasmid is not present in the Black Sea isolates from sea bass.
We therefore tested for the presence of the hlyAch gene using different primer combinations that allow for detection of partial and complete genes. Interestingly, we found that the 11 SH strains tested positive for both partial and complete hlyAch genes, whereas the 3 NH strains, 64bp, 89dp, and 144bp, tested negative for the complete gene but still exhibited partial amplification products of this gene. These results thus suggested that the three nonhemolytic strains may contain hlyAch pseudogenes.
Nonhemolytic P. damselae subsp. damselae strains have undergone genetic modifications.
In a previous study (15), we reported that the genomic context of hlyAch contained an unexpected genetic diversity and suggested that hlyAch is located in an unstable chromosomal region, which in some cases contains typical features of mobile DNA. Invariably, the chromosomal region upstream of the hlyAch promoter includes two serine tRNA genes and the conserved gene kefA encoding a putative ion channel (VDA_002421 in ATCC 33539 genome annotation), present in all P. damselae subsp. damselae strains analyzed to date. Therefore, to investigate the genetic basis of the absence of a beta-hemolytic phenotype in strains 144bp, 64bp, and 89dp, we accomplished the complete sequencing of the genome region located between the hlyAch transcriptional terminator and the 3′-end of the conserved gene kefA in all of the isolates. We found that the 11 hemolytic SH strains, plus the two NH strains 64bp and 89dp, invariably contained the structure of hlyAch-tRNA-tRNA-kefA genes almost identical to the sequence previously described in the strain RM-71 (Fig. 3). Strain 144bp, on the contrary, showed a different genetic context with two additional open reading frames (ORFs) between hlyAch and kefA and was 99% identical to the homologous region previously reported in P. damselae subsp. damselae strain LD-07 (15), a Spanish isolate from gilthead seabream from 1991 (3).
FIG 3.
Variable chromosomal region containing the hlyAch gene in the 14 P. damselae subsp. damselae isolates from sea bass in the Black Sea analyzed in this study. Conserved genes encoding two serine tRNAs and the KefA protein are present in all the strains. The hemolytic phenotypes are depicted in the right panels. (A) The 11 isolates with a small hemolytic halo (SH) contain a complete hlyAch gene. (B) In the two nonhemolytic strains 64bp and 89dp, hlyAch is disrupted by an insertion sequence (IS10) element. (C) The nonhemolytic strain 144bp, in addition to an IS10 element disrupting hlyAch, contains two open reading frames (CDS_11 and CDS_12) between hlyAch and the tRNAs. ΨhlyAch denotes hlyAch pseudogene.
Regarding the three NH strains 64bp, 89dp, and 144bp, we found that their lack of hemolysis was attributable to the presence of an insertion sequence element of the IS10 family disrupting the coding sequence of the hlyAch gene (Fig. 3). This IS10 was shown to be identical to an IS10 element previously described in a multicopy fashion in the conjugative plasmid pAQU1 from a P. damselae subsp. damselae isolate from Japan (21) and different from the previously reported insertion element (IS) that disrupts the hlyAch gene in P. damselae subsp. damselae isolate 501H (15).
Interestingly, we uncovered that the insertion of IS10 took place at exactly the same position in the two isolates 64bp and 89dp, corresponding to position 1586 in the open reading frame of the hlyAch gene, in a codon that encodes Asn529 in the HlyAch protein, whereas the insertion of IS10 in strain 144bp took place in the codon that encodes Tyr410 in the HlyAch protein (Fig. 3). Altogether, the data on the hlyAch gene context point to the existence of different genetic lineages among the P. damselae subsp. damselae isolates from sea bass reared in the Black Sea.
toxR gene-based phylogenetic analysis: evidence for a multiclonal origin of the Black Sea population of P. damselae subsp. damselae associated with sea bass farms.
We wanted to obtain further evidence of the multiclonal origin of these isolates since this study represents the first demonstrated report to our knowledge on the occurrence of this pathogen in the Black Sea. The toxR gene, encoding a transmembrane transcriptional regulator involved in many aspects of virulence regulation, is considered to be a valuable molecular clock for fine-tuned discrimination of taxa within the Vibrionaceae due to its high variability, and a 9% divergence has been previously reported between P. damselae subsp. damselae and P. damselae subsp. piscicida toxR gene sequences (22). Therefore, we amplified and sequenced the complete toxR gene in all of the isolates from the Black Sea, and for comparative purposes, we also sequenced this gene in other P. damselae subsp. damselae and P. damselae subsp. piscicida isolates from our laboratory collection. The comparative phylogenetic analysis revealed a high level of identity among the P. damselae subsp. piscicida isolates, clustering together in a well-defined branch (Fig. 4). This observation is of special interest since these P. damselae subsp. piscicida strains include isolates from Europe as well as from Japan, and some of them were isolated from distant locations with many years between isolations (Table 2). Interestingly, we observed that this gene constitutes a pseudogene in all seven of the P. damselae subsp. piscicida isolates due to the existence of a stop codon that truncates the open reading frame (data not shown).
FIG 4.
Neighbor-joining tree showing the phylogenetic relationships of the 14 Black Sea sea bass isolates of P. damselae subsp. damselae (names in red) to other P. damselae subsp. damselae (names in black) and P. damselae subsp. piscicida (names in blue) strains. Numbers at the nodes show bootstrap values (%). Only bootstrap values of >70% are shown.
In addition, we observed that six P. damselae subsp. damselae isolates from closely related epizootic outbreaks reported between 1987 and 1991 in turbot farms in Galicia (northwestern Spain) were closely associated. Four of them (RG-91, RG-153, RG-214, and RG-191) clustered together with toxR sequences that were 100% identical, although they were isolated in different years between 1987 and 1989. On the contrary, the P. damselae subsp. damselae isolates from the Black Sea showed a different picture, with a dispersed distribution in several branches of the phylogenetic tree constituting six different groups. One group (cluster A) included six isolates with sequence similarities between their toxR sequences of 100%. Notably, among the 8 isolates that were not included in cluster A, the pair 70dps/111bp (cluster B) and the pair 64bp/89dp (cluster C) formed two well-defined branches, and each pair showed a conspicuous phenotypical trait. Cluster B is represented by the two strains with less-motile phenotypes, whereas cluster C includes the two nonhemolytic isolates that share the same IS10 insertion mutation in the hlyAch gene. Interestingly, strain 125dy was shown to be closely associated with the turbot isolates from the northwestern Spain outbreaks that occurred between 1987 and 1991, whereas 162bp and 189bp (cluster D) were phylogenetically associated with strain Lb07070501R that was isolated from sea bass in the Mediterranean area and to the P. damselae subsp. piscicida isolates. Strain 144bp, as mentioned above, has a genetic context upstream of the hlyAch gene that is almost identical to that previously described in strain LD-07, and the toxR gene sequences of these two strains were also 100% identical (Fig. 4).
Another result that deserves attention is the distribution of sequence types throughout the year classes of sea bass in the farms. Fish from the 2009 year class were at the fish farm for 3 years and bacteria were isolated in 2011, whereas fish from the 2011 year class were juveniles and they likely become infected after they were transferred. Cluster A includes isolates from year classes 2010 and 2011 (Table 1), suggesting that fish from different years might have been reinfected by bacteria that were already present in the fish farm environment. As can be deduced from the phylogenetic tree, the fish from the 2011 year class in the Persembe farm were infected by as many as 3 sequence types (isolates in clusters A, C, and D). Regarding geographical location, it is also pertinent to say that the two isolates from the Yomra farm (82dy and 125dy) do not represent a single clone but branch far apart from each other.
Virulence for sea bass of P. damselae subsp. damselae Black Sea isolates.
In order to assess the virulence of P. damselae subsp. damselae for sea bass, one strain with a small hemolytic halo (164dp) and one strain without a hemolytic halo (64bp) were used in challenge studies. We also included in the challenges the highly hemolytic strain RM-71 (2), from which the pPHDD1 virulence plasmid was first described (12). All of the challenges were conducted in fish kept at a water temperature of 24°C and used two different doses of 5.4 × 105 and 3.4 × 107 CFU/fish. Strain RM-71 containing the pPHDD1 virulence plasmid was highly virulent for sea bass, with mortality rates of 100% at the two doses (Fig. 5). Remarkably, this strain caused the death of 100% of the animals in less than 24 h, indicating not only a strong pathogenic potential but also a lack of stringent host specificity since RM-71 was isolated from turbot epizootics in 1988 (2). The virulence data obtained from strain 164dp (small hemolytic halo and hlyAch positive) demonstrated the pathogenic potential for sea bass of the plasmidless strains isolated from the Black Sea (Fig. 5). The mortality rates for 164dp were 20% and 80% at doses of 5.4 × 105 and 3.4 × 107 CFU/fish, respectively, and the temporal progression of the mortality differed with respect to that of the RM-71 strain. For the nonhemolytic strain 64bp (hlyAch gene disrupted by an insertion element), the mortality rates at the same two assayed doses were 0% and 20%, respectively, indicating that this strain exhibits only a low degree of virulence under the conditions tested. Almost all dead fish exhibited hemorrhagic areas around the mouth, fins, and anus. P. damselae subsp. damselae could be reisolated from the kidneys of all dead fish postchallenge, and colonies were proven to belong to this subspecies, as they tested positive in a colony PCR for the subspecies-specific ureC gene (data not shown). These results demonstrate the pathogenic potential of the plasmidless P. damselae subsp. damselae from the Black Sea for sea bass.
FIG 5.
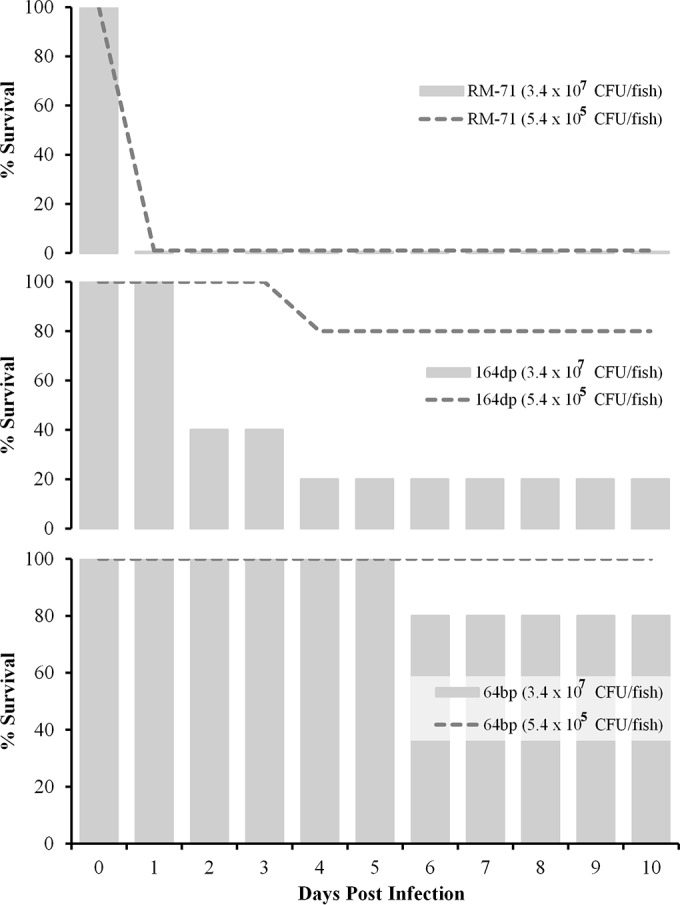
Survival (%) of sea bass intraperitoneally challenged with two different doses of three P. damselae subsp. damselae isolates (n = 10 fish per dose and strain). RM-71 is a highly virulent, pPHDD1-harboring turbot isolate from Galicia (northwestern Spain). Strains164dp (hemolytic, hlyAch-positive) and 64bp (nonhemolytic, hlyAch-negative) are two sea bass isolates from the Turkish Black Sea that are characterized in the present study. A control group of 10 fish inoculated with saline solution exhibited a 100% survival rate (data not shown).
DISCUSSION
Turkey is one of the major sea bass producers in Europe, and this fish is the second most favored cultured fish species, after rainbow trout, in the Turkish coastal brackish waters of the Black Sea. Several infectious diseases affect the cultivation of sea bass in this environment, including viruses such as viral erythrocytic infection virus (VEIV) (23), parasites such as Diplectanum aequans (24), and bacteria such as P. damselae subsp. damselae and Aeromonas veronii bv. sobria (11).
P. damselae subsp. damselae is being increasingly isolated from disease outbreaks in sea bass as well as in gilthead seabream and other sparid species in the Mediterranean area. These reports led to the consideration of P. damselae subsp. damselae as an emerging pathogen in marine aquaculture (5). The first report of the presence of this pathogen in the Black Sea (23) was not accompanied by a genetic characterization of the isolates. More recent reports described the presence of this bacterium in sea bass in the Black Sea (11). Until now, no studies had been conducted to our knowledge to characterize sea bass isolates of this emerging pathogen, and indeed there was a complete lack of data on virulence challenges of P. damselae subsp. damselae for sea bass since most data on virulence were obtained using turbot (2), redbanded seabream (8), and rainbow trout (4) as models of infection.
We determined that the collection of Black Sea isolates of this pathogen exhibited variable phenotypes and genotypes. Although P. damselae subsp. damselae is recognized as a urease-positive subspecies in most reports (2–4, 25, 26), previous studies already reported the existence of urease-negative isolates (27). In our study, we found that isolate 70dps was the unique isolate that tested negative for the ureC gene. Moreover, although strains of this pathogen are typically reported to exhibit green colonies on TCBS medium (2, 9, 25, 28), we found that 6 Black Sea sea bass isolates produced yellow colonies on TCBS medium, an observation to be added to a previous study that also reported the existence of yellow colonies of this pathogen (29). Interestingly, we observed diversity in the motility of the Black Sea strains as demonstrated by motility agar assays, to our knowledge the first reported evidence of variability in this phenotypical trait in this pathogen to date. Since a large number of genes are supposed to be involved in flagellar motility in Vibrionaceae, including structural and regulatory flagellar genes as well as genes of chemotaxis systems (30), this observation also points to the existence of genetic heterogeneity among the Black Sea populations of P. damselae subsp. damselae.
Virulence experiments using the highly hemolytic strain RM-71 indicate that the pPHDD1 plasmid is a major virulence factor for sea bass, as this strain caused 100% fish death in the first 24 h after inoculation. The two pPHDD1-encoded hemolysins Dly and HlyApl (recently renamed PhlyP) are considered to be major virulence factors for turbot, and HlyAch is believed to contribute to virulence for turbot to a lesser extent (14). Here we have demonstrated that the pPHDD1 plasmid is absent in all of the Black Sea isolates from sea bass, confirming a trend anticipated in a recent study where the pPHDD1 plasmid was restricted to 20% of 44 isolates tested (15). It is becoming evident that pPHDD1-negative isolates of P. damselae subsp. damselae are pathogenic for numerous fish species. Recent studies reported that P. damselae subsp. damselae strains lacking pPHDD1 were virulent in a redbanded seabream model (8). Another study carried out with P. damselae subsp. damselae strains isolated from rainbow trout in Denmark revealed a more than 1,000-fold difference in 50% lethal dose (LD50) between highly hemolytic strains (presumptively harboring pPHDD1) and low-hemolytic strains (presumptively encoding only HlyAch), but these low-hemolytic strains were still virulent for trout (4). In this regard, we have demonstrated that the pPHDD1-negative isolates from the Black Sea environment bear pathogenic potential for sea bass, and virulence was greatly diminished in the strain bearing a mutated hlyAch gene version. Taken together, the evidence clearly supports the hypothesis that pPHDD1-negative P. damselae subsp. damselae isolates pose a health risk for many fish species of interest in aquaculture and point at the role of HlyAch hemolysin in virulence. Our discovery that the 3 nonhemolytic isolates from the Black Sea contain pseudogenes of the hlyAch gene reinforces the hypothesis that the chromosomal region encoding HlyAch is a hot spot for recombination and IS element insertion in the P. damselae subsp. damselae genome and opens the question of why a gene encoding a virulence factor of this subspecies is so prone to undergo genetic inactivation events. To date, only two P. damselae subsp. damselae isolates were proven negative for this gene and were isolated from places separated by a considerable distance (15).
We used the gene encoding the transmembrane transcriptional regulator ToxR as a phylogenetic marker in order to provide clues about the phylogeography of the P. damselae subsp. damselae Back Sea isolates. The toxR sequence is increasingly being used as a valuable molecular clock for fine-tuned discrimination of taxa within the Vibrionaceae due to its high variability and the existence of intrasubspecies variation in P. damselae subsp. damselae (22, 31). The phylogeny based on the toxR gene unveiled the existence of genetic heterogeneity among the Black Sea isolates, and we found interesting correlations between identity at the toxR gene level and occurrence of the same phenotypic or genotypic features. Of special interest is the observation that strain 144bp shared the same genetic context upstream of the hlyAch locus with strain LD-07 as well as 100% identity in the toxR gene. These two strains were isolated thousands of kilometers apart from different host species 20 years apart. Similarly, the two nonhemolytic strains 64bp and 89dp contain exactly the same IS10 insertion that disrupts the hlyAch gene, and their toxR gene sequences are 99.9% identical. The observation that cluster A isolates share 100% identical toxR gene sequences suggests that this specific group of strains is clonal in origin. If this is the case, the differential features observed between strain 144bp and the rest of the cluster A strains in the hlyAch locus may correspond to recent genetic events. On the one hand, the acquisition of an IS10 element disrupting hlyAch is a distinctive and unique feature among the 14 Black Sea isolates and may constitute a recent transposition event. On the other hand, the presence of two open reading frames upstream of hlyAch in 144bp but not in the rest of the cluster A isolates may be explained by a gene loss event since that DNA sequence is flanked by two large sequence repeats that are believed to constitute hotspots for gene recombination (15).
The multiclonality hypothesis is reinforced by the observation that the toxR gene sequences among the different clusters of P. damselae subsp. damselae isolates from the Black Sea show a much higher degree of variability among them than the cluster of P. damselae subsp. piscicida strains or the cluster of turbot isolates from Spain of P. damselae subsp. damselae.
The phylogenetic data, together with the phenotypic diversity and the existence of different gene patterns in the vicinity of the hlyAch loci, clearly indicate that the P. damselae subsp. damselae circulating in the environment of sea bass-rearing facilities in the Turkish Black Sea originated not from the clonal spread of a single strain but from the simultaneous occurrence of several clones. Previous studies using different molecular typing methods pointed to the idea that P. damselae subsp. damselae is a genetically diverse subspecies. A study analyzing 50 isolates from seafood in Taiwan encountered as many as 42 typeable profiles by pulsed-field gel electrophoresis (PFGE) (32). Similarly, a high number of PFGE profiles were reported among 16 isolates from rainbow trout in Denmark, a finding that led the authors to conclude that the disease outbreaks in Danish rainbow trout were caused by a multiclonal population of this pathogen (4).
The genetic diversity that we found among bacteria from the two sea bass farms in the Black Sea suggests that the infectious agent was already present in the environment, likely waiting for the appearance of stressful conditions, such as an increase in the water temperature, in order to cause infection. Global warming is causing bacterial species previously unreported in certain ecological areas to be increasingly reported and not only as mere environmental bacteria but as pathogens of either humans or animals or both (33). Actually, the pathogen was recovered from sea bass kidneys and spleens only in the period between July and October, i.e., in the warm season, with water temperatures ranging from 22.9 to 25.8°C at most sampling points. The presence of stressful conditions and of changes in environmental variables, namely, temperature (2, 25), is increasingly suggested to be associated with the pathogenicity of P. damselae subsp. damselae in fish. It is thus possible that P. damselae subsp. damselae behaves as an opportunistic pathogen, not as a clonal pathogen, for sea bass in the Black Sea environment.
ACKNOWLEDGMENTS
We thank Bernardo Fernández Souto and the Instituto Galego de Formación en Acuicultura (IGaFA) (Illa de Arousa, Galicia, Spain) for their valuable support in providing sea bass for the virulence challenges.
Mateus S. Terceti thanks the Brazilian Ministry of Education and CAPES (Coordenaçao de Aperfeiçoamento de Pessoal de Nível Superior) for a predoctoral studentship.
Funding Statement
This work was funded by grant AGL2013-48353-R from the Ministerio de Economía y Competitividad (MINECO) of Spain, cofunded by the FEDER Programme of the European Union, to C.R.O.
REFERENCES
- 1.Rivas AJ, Lemos ML, Osorio CR. 2013. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front Microbiol 4:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouz B, Larsen JL, Nielsen B, Barja JL, Toranzo AE. 1992. Characterization of Vibrio damsela strains isolated from turbot Scophthalmus maximus in Spain. Dis Aquat Org 12:155–166. doi: 10.3354/dao012155. [DOI] [Google Scholar]
- 3.Vera P, Navas JI, Fouz B. 1991. First isolation of Vibrio damsela from seabream (Sparus aurata). Bull Eur Assoc Fish Pathol 11:112–113. [Google Scholar]
- 4.Pedersen K, Skall HF, Lassen-Nielsen AM, Bjerrum L, Olesen NJ. 2009. Photobacterium damselae subsp. damselae, an emerging pathogen in Danish rainbow trout, Oncorhynchus mykiss (Walbaum), mariculture. J Fish Dis 32:465–472. doi: 10.1111/j.1365-2761.2009.01041.x. [DOI] [PubMed] [Google Scholar]
- 5.Labella A, Berbel C, Manchado M, Castro D, Borrego JJ. 2011. Photobacterium damselae subsp. damselae, an emerging pathogen affecting new cultured marine fish species in southern Spain, p 135–152. In Aral F. (ed), Recent advances in fish farms. InTech, Rijeka, Croatia. [Google Scholar]
- 6.Labella A, Vida M, Alonso MC, Infante C, Cardenas S, López-Romalde S, Manchado M, Borrego JJ. 2006. First isolation of Photobacterium damselae ssp. damselae from cultured redbanded seabream, Pagrus auriga Valenciennes, in Spain. J Fish Dis 29:175–179. doi: 10.1111/j.1365-2761.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 7.Labella A, Manchado M, Alonso MC, Castro D, Romalde JL, Borrego JJ. 2010. Molecular intraspecific characterization of Photobacterium damselae ssp. damselae strains affecting cultured marine fish. J Appl Microbiol 108:2122–2132. [DOI] [PubMed] [Google Scholar]
- 8.Labella A, Sanchez-Montes N, Berbel C, Aparicio M, Castro D, Manchado M, Borrego JJ. 2010. Toxicity of Photobacterium damselae subsp. damselae strains isolated from new cultured marine fish. Dis Aquat Org 92:31–40. doi: 10.3354/dao02275. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Aziz M, Eissa AE, Hanna M, Okada MA. 2013. Identifying some pathogenic Vibrio/Photobacterium species during mass mortalities of cultured gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) from some Egyptian coastal provinces. Int J Vet Sci Med 1:87–95. doi: 10.1016/j.ijvsm.2013.10.004. [DOI] [Google Scholar]
- 10.Khouadja S, Lamari F, Bakhrouf A, Gaddour K. 2014. Virulence properties, biofilm formation and random amplified polymorphic DNA analysis of Photobacterium damselae subsp. damselae isolates from cultured sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax). Microb Pathog 69–70:13–19. [DOI] [PubMed] [Google Scholar]
- 11.Uzun E, Ogut H. 2015. The isolation frequency of bacterial pathogens from sea bass (Dicentrarchus labrax) in the Southeastern Black Sea. Aquaculture 437:30–37. doi: 10.1016/j.aquaculture.2014.11.017. [DOI] [Google Scholar]
- 12.Rivas AJ, Balado M, Lemos ML, Osorio CR. 2011. The Photobacterium damselae subsp. damselae hemolysins damselysin and HlyA are encoded within a new virulence plasmid. Infect Immun 79:4617–4627. doi: 10.1128/IAI.05436-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivas AJ, Von Hoven G, Neukirch C, Meyenburg M, Qin Q, Füser S, Boller K, Lemos ML, Osorio CR, Husmann M. 2015. Phobalysin, a small β-pore-forming toxin of Photobacterium damselae subsp. damselae. Infect Immun 83:4335–4348. doi: 10.1128/IAI.00277-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivas AJ, Balado M, Lemos ML, Osorio CR. 2013. Synergistic and additive effects of chromosomal and plasmid-encoded hemolysins contribute to hemolysis and virulence in Photobacterium damselae subsp. damselae. Infect Immun 81:3287–3299. doi: 10.1128/IAI.00155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivas AJ, Labella A, Borrego JJ, Lemos ML, Osorio CR. 2014. Evidence for horizontal gene transfer, gene duplication and genetic variation as driving forces of the diversity of haemolytic phenotypes in Photobacterium damselae subsp. damselae. FEMS Microbiol Lett 355:152–162. doi: 10.1111/1574-6968.12464. [DOI] [PubMed] [Google Scholar]
- 16.Osorio CR, Collins MD, Toranzo AE, Barja JL, Romalde JL. 1999. 16S rRNA gene sequence analysis of Photobacterium damselae and nested PCR method for rapid detection of the causative agent of fish pasteurellosis. Appl Environ Microbiol 65:2942–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osorio CR, Toranzo AE, Romalde JL, Barja JL. 2000. Multiplex PCR assay for ureC and 16S rRNA genes clearly discriminates between both subspecies of Photobacterium damselae. Dis Aquat Org 40:177–183. doi: 10.3354/dao040177. [DOI] [PubMed] [Google Scholar]
- 18.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 19.Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A 101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nonaka L, Maruyama F, Miyamoto M, Miyakoshi M, Kurokawa K, Masuda M. 2012. Novel conjugative transferable multiple drug resistance plasmid pAQU1 from Photobacterium damselae subsp. damselae isolated from marine aquaculture environment. Microbes Environ 27:263–272. doi: 10.1264/jsme2.ME11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osorio CR, Klose KE. 2000. A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among Vibrio species. J Bacteriol 182:526–528. doi: 10.1128/JB.182.2.526-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timur G, Timur M, Akayli T, Korun J, Erkan M. 2008. The first observation of viral erythrocytic infection associated with pathogenic bacteria and some ectoparasites in cultured Mediterranean sea bass (Dicentrarchus labrax L.) in Turkey. Bull Eur Assoc Fish Pathol 28:58–65. [Google Scholar]
- 24.Ogut H, Uzun E. 2014. Incidence and prevalence of Diplectanum aequans and its influence on the fitness of juvenile sea bass (Dicentrarchus labrax) in the Black Sea. Aquacult Res 45:742–748. doi: 10.1111/are.12015. [DOI] [Google Scholar]
- 25.Pedersen K, Dalsgaard I, Larsen JL. 1997. Vibrio damsela associated with diseased fish in Denmark. Appl Environ Microbiol 63:3711–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi H, Miya S, Kimura B, Yamane K, Arakawa Y, Fujii T. 2008. Difference of genotypic and phenotypic characteristics and pathogenicity potential of Photobacterium damselae subsp. damselae between clinical and environmental isolates from Japan. Microb Pathog 45:150–158. doi: 10.1016/j.micpath.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Botella S, Pujalte MJ, Macian MC, Ferrus MA, Hernandez J, Garay E. 2002. Amplified fragment length polymorphism (AFLP) and biochemical typing of Photobacterium damselae subsp. damselae. J Appl Microbiol 93:681–688. doi: 10.1046/j.1365-2672.2002.01748.x. [DOI] [PubMed] [Google Scholar]
- 28.Vaseeharan B, Sundararaj S, Murugan T, Chen JC. 2007. Photobacterium damselae ssp. damselae associated with diseased black tiger shrimp Penaeus monodon Fabricius in India. Lett Appl Microbiol 45:82–86. doi: 10.1111/j.1472-765X.2007.02139.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao DH, Sun JJ, Liu L, Zhao HH, Wang HF, Liang LQ, Liu LB. 2009. Characterization of two phenotypes of Photobacterium damselae subsp. damselae isolated from diseased juvenile Trachinotus ovatus reared in cage mariculture. J World Aquacult Soc 40:281–289. doi: 10.1111/j.1749-7345.2009.00251.x. [DOI] [Google Scholar]
- 30.Zhu S, Kojima S, Homma M. 2013. Structure, gene regulation and environmental response of flagella in Vibrio. Front Microbiol 4:410 10.3389/fmicb.2013.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins P, Navarro RVV, Coelho FJRC, Gomes NCM. 2015. Development of a molecular methodology for fast detection of Photobacterium damselae subspecies in water samples. Aquaculture 435:137–142. doi: 10.1016/j.aquaculture.2014.09.028. [DOI] [Google Scholar]
- 32.Chiu TH, Kao LY, Chen ML. 2013. Antibiotic resistance and molecular typing of Photobacterium damselae subsp. damselae, isolated from seafood. J Appl Microbiol 114:1184–1192. doi: 10.1111/jam.12104. [DOI] [PubMed] [Google Scholar]
- 33.Le Roux F, Wegner KM, Baker-Austin C, Vezzulli L, Osorio CR, Amaro C, Ritchie JM, Defoirdt T, Destoumieux-Garzón D, Blokesch M, Mazel D, Jacq A, Cava F, Gram L, Wendling CC, Strauch E, Kirschner A, Huehn S. 2015. The emergence of Vibrio pathogens in Europe: ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front Microbiol 6:830. doi: 10.3389/fmicb.2015.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magariños B, Romalde JL, Bandín I, Fouz B, Toranzo AE. 1992. Phenotypic, antigenic, and molecular characterization of Pasteurella piscicida strains isolated from fish. Appl Environ Microbiol 58:3316–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magariños B, Romalde JL, López-Romalde S, Moriñigo MA, Toranzo AE. 2003. Pathobiological characterisation of Photobacterium damselae subsp. piscicida isolated from cultured sole (Solea senegalensis). Bull Eur Assoc Fish Pathol 23:183–190. [Google Scholar]