ABSTRACT
Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) strains may be responsible for food-borne infections in humans. Twenty-eight STEC and 75 EPEC strains previously isolated from French shellfish-harvesting areas and their watersheds and belonging to 68 distinguishable serotypes were characterized in this study. High-throughput real-time PCR was used to search for the presence of 75 E. coli virulence-associated gene targets, and genes encoding Shiga toxin (stx) and intimin (eae) were subtyped using PCR tests and DNA sequencing, respectively. The results showed a high level of diversity between strains, with 17 unique virulence gene profiles for STEC and 56 for EPEC. Seven STEC and 15 EPEC strains were found to display a large number or a particular combination of genetic markers of virulence and the presence of stx and/or eae variants, suggesting their potential pathogenicity for humans. Among these, an O26:H11 stx1a eae-β1 strain was associated with a large number of virulence-associated genes (n = 47), including genes carried on the locus of enterocyte effacement (LEE) or other pathogenicity islands, such as OI-122, OI-71, OI-43/48, OI-50, OI-57, and the high-pathogenicity island (HPI). One O91:H21 STEC strain containing 4 stx variants (stx1a, stx2a, stx2c, and stx2d) was found to possess genes associated with pathogenicity islands OI-122, OI-43/48, and OI-15. Among EPEC strains harboring a large number of virulence genes (n, 34 to 50), eight belonged to serotype O26:H11, O103:H2, O103:H25, O145:H28, O157:H7, or O153:H2.
IMPORTANCE The species E. coli includes a wide variety of strains, some of which may be responsible for severe infections. This study, a molecular risk assessment study of E. coli strains isolated from the coastal environment, was conducted to evaluate the potential risk for shellfish consumers. This report describes the characterization of virulence gene profiles and stx/eae polymorphisms of E. coli isolates and clearly highlights the finding that the majority of strains isolated from coastal environment are potentially weakly pathogenic, while some are likely to be more pathogenic.
INTRODUCTION
Escherichia coli is a commensal aerobic bacterium of the warm-blooded animal intestinal microbiota and is used as a fecal indicator in the environment to classify shellfish-harvesting and bathing areas (1). However, E. coli can become pathogenic through the acquisition of mobile genetic elements such as bacteriophages, pathogenicity islands, and plasmids. Among pathogenic E. coli strains are the Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) strains.
STEC can cause infections ranging from uncomplicated diarrheas to hemorrhagic colitis (HC) and hemolytic-uremic syndrome (HUS). Several STEC serotypes have been involved in numerous food-borne outbreaks worldwide (2, 3), and these strains have been identified as enterohemorrhagic E. coli (EHEC). Although E. coli O157:H7 has been the main serotype implicated in HC and HUS since the early 1980s, recent studies have shown that non-O157 serotypes are also responsible for numerous human STEC infections. The serogroups most commonly implicated in human STEC infections in the United States are O26, O45, O103, O111, O121, O145, and O157 (4), whereas in Europe, five major EHEC serogroups (the top five; O157, O26, O103, O111, and O145) dominate (5).
The primary virulence factor of STEC is the Shiga toxin, encoded by a lambdoid bacteriophage, which inhibits host cell protein synthesis (6). Within the two major types of Shiga toxin, namely, Stx1 and Stx2, three subtypes of the stx1 gene (stx1a, stx1c, and stx1d) and seven subtypes of stx2 gene (stx2a, stx2b, stx2c, stx2d, stx2e, stx2f, and stx2g) have been identified (7). Specific stx subtypes are involved in human infections (e.g., stx2a, stx2c, and stx2d are often isolated from patients with HUS) (8, 9), whereas others are related to nonhuman animal infections (e.g., stx2e, causing edema disease in pigs [10]). Certain stx subtypes present within a strain may indicate its source: stx2c is prevalent in cattle (11), whereas stx2f is associated mainly with pigeons (12).
The STEC strains implicated in the major cases of human infection (also referred to as “typical EHEC” strains) possess the LEE (locus of enterocyte effacement) pathogenicity island, which is involved in attaching and effacing (A/E) lesions on intestinal epithelial cells (13). Eighteen types and nine subtypes of the eae gene, namely, α, α2, β1 to β3, γ1, γ2, δ, ε, ε2 to ε4, ζ, η, η2, θ, ι, ι2, κ, λ, μ, ν, ξ, ο, π, ρ, and σ, have been deposited in the GenBank database (14). The eae subtypes are responsible for some host tissue cell tropisms (15) and are related to human infections; eae subtypes β, γ, ε, and θ are those most frequently associated with human infections (16, 17).
However, the emergence of human infections linked to LEE-negative STEC strains indicates that this pathogenicity island is not the only factor responsible for the adherence of bacteria and suggests the presence of other virulence factors carried by other pathogenicity islands or plasmids (18). Additional proteins associated with attachment have been proposed as adhesive factors. For example, Paa is involved in intimate attachment of the bacteria to enterocytes and induced typical A/E lesions in pigs (19). EhaA, an enterohemorrhagic E. coli autotransporter, is involved in attachment to biotic and abiotic surfaces (20). Saa is an autoagglutinating adhesin unique to LEE-negative STEC (21). Long polar fimbriae (Lpf) facilitate the attachment of the bacteria to murine Peyer's patches (22).
In addition to genes located on the LEE, a large number of non-LEE effector genes located on other pathogenicity islands (nleA, nleB, nleC, nleD, nleE, nleG, etc.) have been identified in strains responsible for human infections. These genes are involved in various functions, such as the inhibition of phagocytosis, disruption of host innate immune responses, and blockage of cell division (23).
Besides Shiga toxins, other hemolysins or toxins have been identified in the pathogenesis of STEC strains. These include, for example, the enterohemolysin, encoded by the ehxA gene, which is linked to cytotoxic effects on endothelial cells (24), the enteroaggregative E. coli (EAEC) heat-stable enterotoxin, encoded by the astA gene (25), and the alpha-hemolysin, encoded by the hlyA gene (26).
Among the virulence factors, the presence of various combinations of type III effector genes, toxin-producing genes, or adhesin-producing genes in pathogenicity islands (OI-122, OI-43/48, OI-57, OI-71, and the high-pathogenicity island [HPI]), on plasmids, or on chromosomes was used to distinguish EHEC from STEC strains and to perform molecular risk assessment based on the presence of several virulence genes in STEC strains (27–31).
Among pathogenic E. coli strains, there are also EPEC strains. These are involved in the majority of infantile watery diarrheas in low-income countries but are rarely involved in adult diarrhea (32). EPEC strains are characterized by the presence of the LEE, containing the eae gene, as described above. They are classified into typical EPEC and atypical EPEC strains on the basis of the presence of the EPEC adherence factor (EAF) plasmid. The plasmid harbors the bfp operon, encoding the bundle-forming pilus, which is involved in the initial adherence of strains to intestinal epithelial cells (32). Twelve serogroups have been recognized as EPEC by the World Health Organization: O26, O55, O86, O111, O114, O119, O125, O126, O127, O128, O142, and O158 (33).
EPEC strains could possess the majority of the virulence genes described above other than stx genes. Furthermore, EPEC strains could be lysogenized by stx-converting bacteriophages and consequently could become EHEC strains. Conversely, STEC strains possessing the eae gene could lose the stx-converting bacteriophage and become EPEC or EHEC-like strains (34).
The main reservoir of STEC is cattle (11). However, other animals, such as sheep, goats, swine, birds, wild animals, and humans, can also harbor STEC strains in their digestive tracts (35–37). For typical EPEC, the only reservoir is humans, whereas for atypical EPEC, both humans and other animals can be reservoirs (37). The environment may be contaminated with STEC and EPEC through the spreading of livestock manure on pastures, via wastewaters from slaughterhouses or treatment plant effluents, or by wildlife (38, 39). Only a few studies have focused on the prevalence and description of STEC and EPEC strains in the environment and particularly on the virulence gene profiles of such strains (40–43). Thus, we were prompted to characterize STEC and EPEC strains isolated from French shellfish-harvesting areas.
In the present study, high-throughput microfluidic real-time PCR methods, which had been developed previously and used to investigate the pathogenic potential of E. coli strains isolated mainly from animal feces (10), from the carcasses of cattle (44), and from food and animals (45, 46), were used. During a 2-year study, STEC and EPEC strains were isolated from three French shellfish-harvesting areas (shellfish, sediment, and seawater samples) and their watersheds (river water samples), (47). In addition to the stx and eae genes already investigated, 75 E. coli virulence-associated gene targets were examined in these strains using high-throughput microfluidic PCR.
MATERIALS AND METHODS
Bacterial strains and DNA extraction.
Most of the strains used in this study were isolated between February 2013 and January 2015 from three French shellfish-harvesting areas and their watersheds, located on the English Channel coast (47). Three strains were isolated from other French shellfish-harvesting areas in 2006 (48). A total of 28 STEC and 75 EPEC strains belonging to 68 distinguishable serotypes isolated from three types of shellfish (oysters, mussels, and cockles) and from freshwater, seawater, and surface sediment samples were investigated. The determination of their serotypes, phylogroups and MLST (multilocus sequence typing) sequence types (STs) has been described previously (47, 48). After cultivation of bacteria on Tryptone Bile X–glucuronide agar (TBX) (AES Chemunex, Bruz, France) at 37°C for 24 h, DNA was extracted with the InstaGene Matrix kit (Bio-Rad, Nanterre, France) according to the manufacturer's instructions.
Characterization of the stx subtypes.
STEC strains (n = 28) were previously identified by Balière et al. (47) by PCR using the stx primers and probes described by Perelle et al. (49), and stx subtyping was performed here using the PCR tests described by Scheutz et al. (7) for targeting the stx1a, stx1c, and stx1d subtypes and the stx2a, stx2b, stx2c, stx2d, stx2e, stx2f, and stx2g subtypes. The stx2 subtypes were also determined by sequencing using primers F4, R1, F4-f, and R1-e/f (see Table 1).
TABLE 1.
PCR primers used in this study for stx and eae subtyping by sequencing
| Gene | Primer designation | Primer sequence (5′–3′) | Fragment size (bp) | Reference or source |
|---|---|---|---|---|
| stx2 | F4 | GGCACTGTCTGAAACTGCTCCTGT | 627 | 7 |
| R1 | ATTAAACTGCACTTCAGCAAATCC | |||
| F4-f | CGCTGTCTGAGGCATCTCCGCT | 625 | ||
| R1-e/f | TAAACTTCACCTGGGCAAAGCC | |||
| eaea | EAE-F | ATTACTGAGATTAAGGCTGAT | 682 | 51 |
| EAE-RB | ATTTATTTGCAGCCCCCCAT | |||
| eae-ε | EAE-F | ATTACTGAGATTAAGGCTGAT | 722 | 51 |
| LP5 | AGCTCACTCGTAGATGACGGCAAGCG | |||
| eae-η | EAE-F | ATTACTGAGATTAAGGCTGAT | 712 | 51 |
| LP8 | TAGATGACGGTAAGCGAC | |||
| eae-ξ | EAE-F | ATTACTGAGATTAAGGCTGAT | 468 | 51 |
| B49R | ACCACCTTTAGCAGTCAATTTG | |||
| eae-μ | FV373F | CAACGGTAAGTCTCAGACAC | 443 | 51 |
| FV373R | CATAATAAGCTTTTTGGCCTACC | |||
| eae-ν | IH1229aF | CACAGCTTACAATTGATAACA | 311 | 51 |
| IH1229aR | CTCACTATAAGTCATACGACT | |||
| eaeb | eae-F1 | ACTCCGATTCCTCTGGTGAC | ∼1,800–2,100, depending on the allele | 75 |
| escD-R1 | GTATCAACATCTCCCGCCCA | |||
| eaeb | cesT-F3 | CAGGAGCACAATCGCTGTTG | 1,727 | This study |
| eae-R3 | CAGACGATACGATCCAGACC |
Universal primers targeting the 3′ variable regions of the eae-α1, -α2, -β1, -β2, -γ2, -θ, -δ, -κ, -ζ, and -ι subtypes.
Primers targeting overlapping DNA fragments of the eae gene.
Characterization of the eae subtypes.
E. coli eae-positive strains (n = 76) were identified by Balière et al. previously (47) with the eae primers and probe described by Nielsen and Andersen (50). The eae subtypes were determined here by sequencing 311- to 722-bp amplicons of the 3′ variable region of the eae gene. Amplicons were obtained with the universal EAE-F and EAE-RB primers, which target the 3′ variable regions of the eae-α1, -α2, -β1, -β2, -γ2, -θ, -δ, -κ, -ζ, and -ι subtypes or with primers targeting the 3′ variable regions of the eae-ε, -η, -ξ, -μ, and -ν subtypes (Table 1). The amplicons obtained by following the PCR program described by Blanco et al. (51) were subjected to gel electrophoresis and were then sequenced in both directions with the corresponding primers using the fluorescent dye terminator Sanger method (ABI 3730 system; Applied Biosystems) by Eurofins Genomics (Ebersberg, Germany). DNA sequences were edited using the BioEdit program (52) and were compared with the GenBank database. The first valid published sequence with 100% similarity was chosen to identify the eae variant.
Nine E. coli strains, which were PCR negative for these primer combinations, were chosen for sequencing of the entire eae gene. Two overlapping DNA fragments of the eae gene were PCR amplified using primers eae-F1 and escD-R1 or primers cesT-F3 and eae-R3, respectively (Table 1). The PCR program included a 10-min initial denaturing step at 94°C, followed by 35 cycles of amplification (94°C for 20 s, 56°C for 20 s, and 72°C for 90 s) and a final extension step at 72°C for 5 min. The PCR amplicons were then subjected to gel electrophoresis and were purified using the NucleoSpin Extract II kit (Macherey-Nagel, Düren, Germany) before being sequenced as described above.
High-throughput real-time PCR system.
The BioMark real-time PCR system (Fluidigm, San Francisco, CA) was used for high-throughput microfluidic real-time PCR amplification using the 96.96 dynamic arrays (Fluidigm). Amplifications were performed in accordance with the recommendations of the manufacturer, using the EvaGreen DNA binding dye (Biotium Inc., Hayward, CA) followed by melting curve analysis. The BioMark real-time PCR system was used with the following thermal profile: 95°C for 10 min (enzyme activation), followed by 35 cycles of 95°C for 15 s and 60°C for 1 min (annealing of primers and amplification step).
Seventy-five E. coli virulence-associated gene targets were selected according to their roles in pathogenesis, their association with human and nonhuman animal illness, and their usefulness, as demonstrated previously, for the characterization of STEC and EPEC strains, isolated mainly from finishing swine (10), human patient and animal feces, food, and animals (45, 46), the carcasses of cattle originating from different farms and food (44), and the feces of adults and children (28, 30, 31, 43).
Virulence gene targets were classified into five groups according to function: the adhesion group (eibG, iha, saa, toxB, orfA, orfB, paa, stcE, sab, efa1 or lifA, bfpA, espP, the F6/F987P [fasA], F18/F107 [fedA], and F41 [fimF41a] genes, lpfAO157, lpfAO26, lpfAO113, ehaA, and epeA [n = 20]), the type III secretion system group (espB, espD, espA, tir, espZ, escC, espG, escD, escN, escV, escJ, nleB, nleE, nleF, nleG, nleH1-2, nleA, espM1, nleC, nleD, nleH1-1, nleG2, nleG5-1, nleG5-2, nleG6-2, espV, espK, espN, espJ, and espM2 [n = 30]), the toxin group (subA, ent, ehxA, cdt-I, cdt-III, cdt-V, sta, lt, hlyA, cnf1, cnf2, astA [n = 12]), the resistance and persistence group (katP, ecf1, pagC, terE, and ureD [n = 5]), and the “other function” group (aggR, pic, irp2, fyuA, Z2098, ECs1763, ECs1822, and etpD [n = 8]). The wecA gene was used as an E. coli reference genetic marker.
RESULTS
STEC and EHEC virulence gene profiles.
A collection of E. coli strains, comprising 27 STEC (stx-positive, eae-negative) strains and 1 EHEC (stx-positive, eae-positive) strain collected from French coastal areas, was investigated. Eleven STEC strains were positive for the stx1 gene only, and 13 were positive for the stx2 gene only, while 4 strains harbored a combination of the stx1 and stx2 genes. The most commonly identified stx1 subtype was stx1d (23% of stx genes detected), followed by stx1a (17%) (Fig. 1A; Table 2). The most common stx2 subtype was stx2a (17%) (Fig. 1A). Five strains were shown to possess several stx subtypes: three possessed stx1a and stx2a and belonged to serotypes O185:H28, ONT:H11, and O130:H11; one possessed stx2a and stx2d, and one possessed stx1a, stx2a, stx2c, and stx2d, corresponding to serotypes O8:H19 and O91:H21, respectively (Table 2). Three strains harbored either the stx1c, stx2e, or stx2g variant. Six strains harbored at least one of the stx2a, stx2c, and stx2d variants, which had been reported previously (8, 9) as being often isolated from patients with HUS (Table 2).
FIG 1.
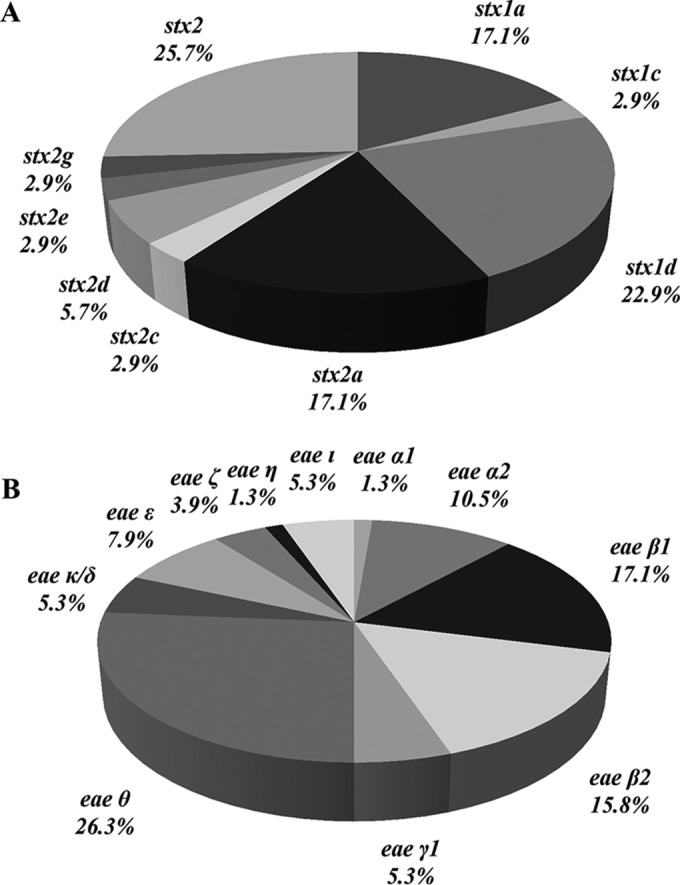
Subtyping of genes encoding Shiga toxin and intimin. (A) Distribution of stx gene subtypes found in STEC strains. (B) Distribution of eae gene subtypes found in E. coli strains.
TABLE 2.
| Strain no. | Serotypea | Sampleb |
E. coli pathogroup | Virulence gene subtype |
Profile no. | Total no. of genes detected | No. of genetic markers in the following groupc: |
Phylogroupa,d | STa (no. of strains) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Site | Date | Origin | stx1 | stx2 | eae | Adhesion (n = 20) | Type III secretion system (n = 30) | Toxin (n = 12) | Other function (n = 13) | OI-71 (n = 5) | OI-122 (n = 5) | OI-57 (n = 6) | OI-43/48 (n = 3) | HPI (n = 2) | EHEC markers (n = 4) | nle genes (n = 13) | |||||||
| 78 | O26:H11 | Shellfish | 1 | November 2013 | M1.2 | STEC | 1a | − | β1 | 1 | 47 | 8 | 25 | 3 | 9 | 5 | 4 | 6 | 3 | 2 | 4 | 12 | B1 | 21 |
| 95 | O91:H21 | Freshwater | 3 | May 2013 | W3.2 | STEC | 1a | 2a, 2c, 2d | − | 2 | 15 | 8 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | B1 | 442 |
| 59 | O130:H11 | Freshwater | 1 | December 2013 | W1.1 | STEC | 1a | 2a | − | 3 | 12 | 7 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B1 | ND |
| 14 | ONT:H11 | Freshwater | 1 | May 2013 | W1.1 | STEC | 1a | 2a | − | 4 | 11 | 7 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B1 | 295 |
| 163 | O63:H6 | Freshwater | 1 | November 2014 | W1.1 | STEC | − | 2a | − | 5 | 10 | 7 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B1 | 2105 |
| 124 | ONT:H10 | Freshwater | 2 | April 2014 | W2.1 | STEC | 1a | − | − | 6 | 9 | 4 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B1 | 1258 |
| 88 | O185:H28 | Freshwater | 2 | April 2013 | W2.1 | STEC | 1a | 2a | − | 7 | 8 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B2 | 658 |
| 72 | O2:H32 | Seawater | 1 | February 2014 | W1.5 | STEC | − | 2e | − | 8 | 6 | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | A | 10 |
| 126 | O8:H19 | Freshwater | 3 | July 2014 | W3.1 | STEC | − | 2a, 2d | − | 9 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B1 | 162 |
| 16 | O154:H31 | Shellfish | 1 | May 2013 | O1.1 | STEC | 1d | − | − | 10 | 5 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | D | 1892 |
| 19 | O15:H16 | Shellfish | 1 | June 2013 | M1.1 | STEC | − | 2g | − | 11 | 5 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | A | 325 |
| 127 | O76:H19 | Freshwater | 2 | August 2014 | W2.3 | STEC | 1d | − | − | 12 | 5 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B1 | 675 |
| LR2 | O100:H21 | Shellfish | 6 | January 2003 | M6.1 | STEC | 1d | − | − | 13 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| 15 | O154:H31 | Freshwater | 1 | May 2013 | W1.1 | STEC | 1d | − | − | 14 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | D | 1892 |
| 44 | O154:HNM | Shellfish | 1 | November 2013 | M1.1 | STEC | 1d | − | − | 14 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | D | 1892 |
| 20 | O100:HNM | Shellfish | 2 | May 2013 | M2.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 24 | O100:HNM | Freshwater | 3 | May 2013 | W3.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 31 | O100:HNM | Shellfish | 3 | May 2013 | O3.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 32 | O100:HNM | Shellfish | 3 | May 2013 | M3.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 33 | O100:HNM | Shellfish | 2 | May 2013 | C2.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 35 | O100:HNM | Shellfish | 3 | June 2013 | M3.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 36 | O100:HNM | Sediment | 3 | June 2013 | S3.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 122 | O100:HNM | Shellfish | 2 | March 2014 | M2.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| 125 | O100:HNM | Shellfish | 3 | June 2014 | O3.1 | STEC | − | + | − | 15 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | A | 993 |
| PB4 | O38:H26 | Shellfish | 5 | July 2003 | M5.1 | STEC | 1c | − | − | 16 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND | ND |
| 1 | O149:H1 | Freshwater | 1 | April 2013 | W1.1 | STEC | 1d | − | − | 17 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | D | 132 |
| 3 | O149:HNM | Freshwater | 1 | April 2013 | W1.1 | STEC | 1d | − | − | 17 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | D | 132 |
| 73 | O28:H1 | Shellfish | 1 | March 2014 | C1.1 | STEC | 1d | − | − | 17 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | D | 132 |
| SP5 | O157:H7 | Shellfish | 4 | September 2003 | O4.1 | EPEC | − | − | γ1 | 1 | 50 | 8 | 30 | 3 | 8 | 5 | 5 | 5 | 3 | 0 | 3 | 13 | ND | ND |
| 77 | O145:H28 | Shellfish | 2 | June 2013 | C2.1 | EPEC | − | − | γ1 | 2 | 39 | 4 | 24 | 3 | 7 | 3 | 5 | 2 | 3 | 0 | 3 | 7 | D | ND |
| 79 | O145:H28 | Shellfish | 2 | June 2013 | M2.1 | EPEC | − | − | γ1 | 2 | 39 | 4 | 24 | 3 | 7 | 3 | 5 | 2 | 3 | 0 | 3 | 7 | D | ND |
| 121 | O103:H25 | Freshwater | 3 | March 2014 | W3.1 | EPEC | − | − | θ | 2 | 35 | 5 | 19 | 3 | 7 | 3 | 5 | 3 | 2 | 0 | 4 | 7 | B1 | 343 |
| 96 | O103:H2 | Freshwater | 2 | June 2013 | W2.3 | EPEC | − | − | θ | 3 | 35 | 5 | 19 | 3 | 7 | 3 | 5 | 3 | 2 | 0 | 4 | 7 | B1 | 343 |
| 120 | O153:H2 | Freshwater | 2 | March 2014 | W2.3 | EPEC | − | − | ε | 4 | 34 | 5 | 19 | 2 | 7 | 1 | 5 | 2 | 2 | 2 | 2 | 8 | B1 | 27 |
| 123 | O153:H2 | Shellfish | 3 | March 2014 | M3.1 | EPEC | − | − | ε | 4 | 34 | 5 | 19 | 2 | 7 | 1 | 5 | 2 | 2 | 2 | 2 | 8 | B1 | 27 |
| 81 | O26:H11 | Shellfish | 1 | November 2013 | M1.2 | EPEC | − | − | β1 | 5 | 34 | 5 | 20 | 3 | 5 | 5 | 4 | 3 | 1 | 2 | 0 | 10 | B1 | 29 |
| 84 | O26:H11 | Freshwater | 1 | November 2013 | W1.3 | EPEC | − | − | β1 | 5 | 34 | 5 | 20 | 3 | 5 | 5 | 4 | 3 | 1 | 2 | 0 | 10 | B1 | 29 |
| 85 | O26:H11 | Shellfish | 1 | November 2013 | C1.1 | EPEC | − | − | β1 | 5 | 34 | 5 | 20 | 3 | 5 | 5 | 4 | 3 | 1 | 2 | 0 | 10 | B1 | 29 |
| 102 | O23:H8 | Freshwater | 2 | August 2013 | W2.1 | EPEC | − | − | θ | 6 | 33 | 5 | 22 | 2 | 3 | 5 | 5 | 5 | 0 | 0 | 0 | 11 | B1 | 327 |
| 129 | O23:H8 | Shellfish | 2 | August 2014 | C2.1 | EPEC | − | − | θ | 7 | 32 | 5 | 22 | 2 | 2 | 5 | 4 | 5 | 0 | 0 | 0 | 11 | B1 | 327 |
| 80 | O26:H11 | Freshwater | 2 | August 2013 | W2.1 | EPEC | − | − | β1 | 8 | 27 | 4 | 17 | 2 | 3 | 2 | 4 | 2 | 1 | 2 | 0 | 8 | B1 | 29 |
| 64 | O39:HNM | Freshwater | 1 | January 2014 | W1.3 | EPEC | − | − | ι | 9 | 25 | 3 | 19 | 1 | 1 | 3 | 0 | 5 | 0 | 0 | 0 | 10 | B1 | 39 |
| 111 | ONT:H31 | Freshwater | 1 | October 2014 | W1.3 | EPEC | − | − | θ | 10 | 24 | 3 | 16 | 3 | 1 | 1 | 3 | 2 | 0 | 0 | 0 | 6 | B1 | 40 |
| 131 | O15:H2 | Freshwater | 3 | August 2014 | W3.2 | EPEC | − | − | β1 | 11 | 23 | 3 | 17 | 1 | 1 | 2 | 5 | 2 | 0 | 0 | 0 | 8 | B1 | 20 |
| 153 | O28:HNM | Freshwater | 1 | June 2014 | W1.1 | EPEC | − | − | γ1 | 12 | 23 | 0 | 15 | 2 | 5 | 1 | 0 | 2 | 2 | 0 | 3 | 2 | D | 137 |
| 61 | O153:H21 | Freshwater | 1 | January 2014 | W1.1 | EPEC | − | − | θ | 13 | 22 | 3 | 17 | 1 | 0 | 2 | 3 | 1 | 0 | 0 | 0 | 7 | B1 | 40 |
| 115 | O108:H21 | Freshwater | 3 | November 2014 | W3.1 | EPEC | − | − | θ | 13 | 22 | 3 | 17 | 1 | 0 | 2 | 3 | 1 | 0 | 0 | 0 | 7 | B1 | 337 |
| 117 | O108:H21 | Shellfish | 3 | November 2014 | W3.2 | EPEC | − | − | θ | 13 | 22 | 3 | 17 | 1 | 0 | 2 | 3 | 1 | 0 | 0 | 0 | 7 | B1 | 337 |
| 118 | O108:H21 | Shellfish | 2 | January 2014 | C2.1 | EPEC | − | − | θ | 13 | 22 | 3 | 17 | 1 | 0 | 2 | 3 | 1 | 0 | 0 | 0 | 7 | B1 | 337 |
| 134 | O146:H21 | Shellfish | 3 | August 2014 | M3.1 | EPEC | − | − | θ | 14 | 22 | 3 | 17 | 1 | 0 | 2 | 3 | 1 | 0 | 0 | 0 | 7 | B1 | 442 |
| 65 | O40:HNM | Freshwater | 1 | February 2014 | W1.1 | EPEC | − | − | β1 | 15 | 22 | 0 | 16 | 1 | 4 | 3 | 0 | 1 | 1 | 2 | 2 | 6 | A | 10 |
| 162 | ONT:H8 | Freshwater | 1 | August 2014 | W1.2 | EPEC | − | − | θ | 16 | 21 | 4 | 15 | 1 | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 5 | B1 | 327 |
| 49 | O98:H8 | Shellfish | 1 | November 2014 | M1.1 | EPEC | − | − | ι | 17 | 21 | 5 | 15 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 5 | B1 | 590 |
| 159 | O91:H10 | Shellfish | 1 | July 2014 | C1.1 | EPEC | − | − | β2 | 18 | 21 | 3 | 14 | 1 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 4 | B2 | 641 |
| 139 | O88:H25 | Shellfish | 3 | September 2014 | M3.1 | EPEC | − | − | β1 | 19 | 21 | 4 | 15 | 1 | 0 | 1 | 4 | 1 | 0 | 0 | 0 | 6 | B1 | 328 |
| 154 | ONT:H8 | Freshwater | 1 | April 2014 | W1.2 | EPEC | − | − | θ | 20 | 20 | 3 | 15 | 1 | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 5 | B1 | 327 |
| 105 | O71:HNM | Freshwater | 1 | August 2014 | W1.2 | EPEC | − | − | η | 21 | 20 | 5 | 14 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 5 | A | 517 |
| 133 | O88:H8 | Shellfish | 3 | August 2014 | O3.1 | EPEC | − | − | ι | 22 | 20 | 2 | 17 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 1 | 7 | B1 | 590 |
| 136 | O146:H6 | Shellfish | 2 | September 2014 | C2.1 | EPEC | − | − | θ | 23 | 20 | 3 | 15 | 1 | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 5 | B1 | 442 |
| 37 | O167:H3 | Freshwater | 1 | August 2013 | W1.1 | EPEC | − | − | β1 | 24 | 20 | 0 | 17 | 0 | 2 | 5 | 0 | 2 | 0 | 0 | 0 | 7 | D | 2558 |
| 113 | O128:H2 | Freshwater | 2 | November 2014 | W2.3 | EPEC | − | − | β1 | 25 | 19 | 3 | 13 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 3 | B1 | 20 |
| 132 | O113:H6 | Shellfish | 3 | August 2014 | O3.1 | EPEC | − | − | β2 | 26 | 19 | 0 | 14 | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 4 | B2 | 121 |
| 160 | ONT:H6 | Freshwater | 1 | August 2014 | W1.1 | EPEC | − | − | β2 | 27 | 19 | 1 | 15 | 0 | 2 | 2 | 0 | 1 | 0 | 2 | 0 | 5 | B2 | 28 |
| 86 | O5:H40 | Shellfish | 2 | February 2014 | M2.1 | EPEC | − | − | θ | 28 | 19 | 1 | 17 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 7 | A | 10 |
| 128 | O86:H31 | Shellfish | 2 | August 2014 | C2.1 | EPEC | − | − | θ | 29 | 19 | 3 | 14 | 1 | 0 | 2 | 3 | 0 | 0 | 0 | 1 | 4 | D | 2569 |
| 135 | O125:H6 | Freshwater | 2 | September 2014 | W2.3 | EPEC | − | − | α2 | 30 | 19 | 1 | 15 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 4 | B2 | 583 |
| 152 | ONT:H6 | Seawater | 1 | December 2014 | W1.5 | EPEC | − | − | β2 | 31 | 18 | 1 | 13 | 0 | 3 | 1 | 0 | 1 | 1 | 2 | 0 | 4 | B2 | 28 |
| 54 | O125:H6 | Freshwater | 1 | November 2014 | W1.3 | EPEC | − | − | α2 | 32 | 18 | 1 | 15 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 4 | B2 | 583 |
| 116 | O113:H6 | Freshwater | 3 | November 2014 | W3.2 | EPEC | − | − | β2 | 33 | 18 | 0 | 14 | 1 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 4 | B2 | 121 |
| 138 | ONT:H6 | Freshwater | 3 | September 2014 | W3.2 | EPEC | − | − | β2 | 34 | 18 | 1 | 14 | 0 | 2 | 2 | 0 | 1 | 0 | 2 | 0 | 5 | B2 | 28 |
| 149 | O159:H7 | Freshwater | 1 | November 2014 | W1.3 | EPEC | − | − | ε | 35 | 18 | 3 | 14 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 4 | B1 | ND |
| 104 | O157:H16 | Freshwater | 1 | July 2014 | W1.4 | EPEC | − | − | ε | 36 | 18 | 0 | 17 | 0 | 0 | 3 | 0 | 4 | 0 | 0 | 0 | 10 | A | 10 |
| 58 | O63:H6 | Freshwater | 2 | November 2014 | W2.1 | EPEC | − | − | α2 | 37 | 17 | 1 | 14 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | B2 | 122 |
| 62 | O63:H6 | Freshwater | 1 | January 2014 | W1.2 | EPEC | − | − | α2 | 37 | 17 | 1 | 14 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | B2 | 122 |
| 142 | O63:H6 | Freshwater | 3 | October 2014 | W3.2 | EPEC | − | − | α2 | 37 | 17 | 1 | 14 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | B2 | 122 |
| 144 | O63:HNM | Freshwater | 3 | October 2014 | W3.2 | EPEC | − | − | α2 | 37 | 17 | 1 | 14 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | B2 | 122 |
| 141 | ONT:H6 | Freshwater | 3 | October 2014 | W3.2 | EPEC | − | − | α2 | 37 | 17 | 1 | 14 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | B2 | 122 |
| 43 | O51:HNM | Freshwater | 2 | October 2013 | W2.2 | EPEC | − | − | α1 | 38 | 17 | 1 | 14 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 4 | B2 | 589 |
| 51 | O113:H6 | Shellfish | 1 | November 2013 | M1.2 | EPEC | − | − | β2 | 39 | 17 | 0 | 14 | 0 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 4 | B2 | 121 |
| 60 | O2:H45 | Freshwater | 2 | December 2013 | W2.1 | EPEC | − | − | κ/δ | 40 | 17 | 2 | 13 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | B2 | 1907 |
| 63 | O179:H31 | Freshwater | 1 | January 2014 | W1.2 | EPEC | − | − | ζ | 41 | 17 | 1 | 13 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 4 | B2 | 1092 |
| 107 | O25:H2 | Freshwater | 1 | September 2014 | W1.1 | EPEC | − | − | β1 | 42 | 17 | 2 | 14 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 5 | B1 | 20 |
| 38 | ONT:H2 | Freshwater | 1 | August 2013 | W1.2 | EPEC | − | − | β1 | 42 | 17 | 2 | 14 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 5 | B1 | 20 |
| 47 | O63:H6 | Freshwater | 1 | November 2013 | W1.4 | EPEC | − | − | α2 | 43 | 17 | 1 | 13 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | B2 | 583 |
| 42 | O29:H19 | Freshwater | 2 | October 2013 | W2.1 | EPEC | − | − | ε | 44 | 16 | 3 | 12 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | B1 | 517 |
| 46 | O116:H20 | Freshwater | 1 | November 2013 | W1.3 | EPEC | − | − | β1 | 45 | 16 | 1 | 14 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 | A | 10 |
| 50 | O28:H16 | Shellfish | 1 | November 2013 | M1.1 | EPEC | − | − | β1 | 45 | 16 | 1 | 14 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 | A | 10 |
| 52 | O113:H6 | Shellfish | 1 | November 2013 | C1.1 | EPEC | − | − | β2 | 46 | 16 | 0 | 13 | 0 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 3 | B2 | 1583 |
| 161 | O137:H6 | Freshwater | 1 | August 2014 | W1.1 | EPEC | − | − | β2 | 47 | 16 | 1 | 12 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 4 | B2 | 2678 |
| 39 | ONT:H6 | Freshwater | 1 | September 2013 | W1.3 | EPEC | − | − | β2 | 48 | 16 | 1 | 12 | 0 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 4 | B2 | 28 |
| 67 | O33:H6 | Freshwater | 1 | February 2014 | W1.3 | EPEC | − | − | β2 | 49 | 15 | 1 | 10 | 0 | 3 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | B2 | 28 |
| 112 | O85:H31 | Freshwater | 1 | October 2014 | W1.3 | EPEC | − | − | ζ | 50 | 13 | 0 | 10 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | B2 | 803 |
| 71 | O33:H6 | Freshwater | 1 | February 2014 | W1.3 | EPEC | − | − | β2 | 51 | 13 | 0 | 10 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | B2 | 28 |
| 158 | O20:HNT | Seawater | 1 | April 2014 | W1.5 | EPEC | − | − | θ | 52 | 12 | 0 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | A | 3747 |
| 164 | O98:H56 | Freshwater | 1 | December 2014 | W1.4 | EPEC | − | − | θ | 52 | 12 | 0 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | A | ND |
| 70 | O98:HNM | Seawater | 1 | February 2014 | W1.5 | EPEC | − | − | θ | 52 | 12 | 0 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | A | 3747 |
| 150 | O98:HNT | Freshwater | 1 | December 2014 | W1.4 | EPEC | − | − | θ | 52 | 12 | 0 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | A | 3747 |
| 40 | O71:H49 | Shellfish | 1 | October 2013 | C1.1 | EPEC | − | − | κ/δ | 53 | 12 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | B2 | 2813 |
| 41 | O71:H49 | Shellfish | 1 | October 2013 | M1.2 | EPEC | − | − | κ/δ | 53 | 12 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | B2 | 2813 |
| 48 | O8:H14 | Freshwater | 1 | November 2013 | W1.4 | EPEC | − | − | κ/δ | 53 | 12 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | B2 | 4178 |
| 106 | O85:HNM | Freshwater | 1 | August 2014 | W1.4 | EPEC | − | − | ζ | 54 | 12 | 0 | 9 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | B2 | 803 |
| 74 | O98:HNM | Freshwater | 1 | March 2014 | W1.4 | EPEC | − | − | θ | 55 | 11 | 0 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | A | 3747 |
| 103 | O145:H34 | Freshwater | 1 | July 2014 | W1.3 | EPEC | − | − | ι | 56 | 11 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | B2 | 526 |
The serotype, phylogroup, and ST (MLST sequence type) were determined previously by Balière et al. (47) and Gourmelon et al. (48).
Sampling sites are coded as follows: 1, La Fresnaye, Brittany; 2, Regnéville sur mer, Normandy; 3, La Vanlée, Normandy; 4, Saint-Pol-de-Léon, Brittany; 5, Pirou, Normandy; 6, Sèvre, Poitou-Charente region. Freshwater samples are coded as follows: W1.1, Frémur (lat 48.3711280, long −2.208756); W1.2, Le Rat (lat 48.650571, long −2.1912390); W1.3, Le Clos (lat 48.3728867, long −2.1736788); W1.4, Kermiton (lat 48.3738516, long −2.1716267); W2.1, La Sienne (lat 49.0195333, long −1.5055472); W2.2, La Soulles (lat 49.0234806, long −1.4890194); W2.3, Regnéville sur mer (lat 48.9989694, long −1.5585417); W3.1, La Vanlée (lat 48.9147833, long −1.5498417); W3.2, Les Hardes (lat 48.9309028, long −1.5350306). Seawater samples are coded as W1.5 (Kermiton [lat 48.627192, long −2.291158]). Samples from cockles are coded as C1.1 (Cockles La Fresnaye [lat 48.3750614, long −2.1716026]) or C2.1 (Cockles Regnéville sur mer [lat 48.9716028, long −1.5648194]). Samples from mussels are coded as follows: M1.1, Mussels1 La Fresnaye (lat 48.3825912, long −2.1731444); M1.2, Mussels2 La Fresnaye (lat 48.3812179, long −2.175184); M2.1, Mussels Regnéville sur mer (lat 48.9716444, long −1.5748083); M3.1, Mussels La Vanlée (lat 48.9303583, long −1.5939472); M5.1, Mussels Pirou (lat 49.182884, long −1.6146958); M6.1, Mussels Sèvre (lat 46.29665, long −1.1457804). Samples from oysters are coded as follows: O1.1, Oysters 1 La Fresnaye (lat 48.3820423, long −2.1738512); O1.2, Oysters 2 La Fresnaye (lat 48.388116, long −2.1713438); O3.1, Oysters La Vanlée (lat 48.9303583, long −1.5939472); O4.1, Oysters Saint-Pol-de-Léon (lat 48.674128, long −3.970621). Sediment samples are coded as S3.1 (Sediment La Vanlée [lat 48.9147833, long −1.5498417]).
Genetic marker groups are as follows. The adhesion group includes eibG, iha, saa, toxB, orfA, orfB, paa, stcE, sab, efa1 or lifA, bfpA, espP, the F6/F987P gene (fasA), the F18/F107 gene (fedA), the F41 gene (fimF41a), lpfAO157, lpfAO26, lpfAO113, ehaA, and epeA. The type III secretion system group includes espB, espD, espA, tir, espZ, escC, espG, escD, escN, escV, escJ, nleB, nleE, nleF, nleG, nleH1-2, nleA, espM1, nleC, nleD, nleH1-1, nleG2, nleG5-1, nleG5-2, nleG6-2, espV, espK, espN, espJ, and espM2. The toxin group includes subA, ent, ehxA, cdt-I, cdt-III, cdt-V, sta (estla), lt (elt), hlyA, cnf1, cnf2, and astA. The “other function” group includes katP, ecf1, pagC, terE, ureD, aggR, pic, irp2, fyuA, Z2098, ECs1763, ECs1822, and etpD. The OI-71 pathogenicity island includes nleF, nleG, nleH1-2, nleA, and espM1; OI-122 includes efa1 or lifA, nleB, nleE, ent, and pagC; OI-57 includes nleG2, nleG5-1, nleG5-2, nleG6-2, Z2098, and ECs1763; OI-43/48 includes iha, terE, and ureD; the HPI includes irp2 and fyuA. EHEC markers include espV, espK, ureD, and Z2098. The nle gene group includes nleB, nleE, nleF, nleG, nleH1-2, nleA, nleC, nleD, nleH1-1, nleG2, nleG5-1, nleG5-2, and nleG6-2.
ND, not done.
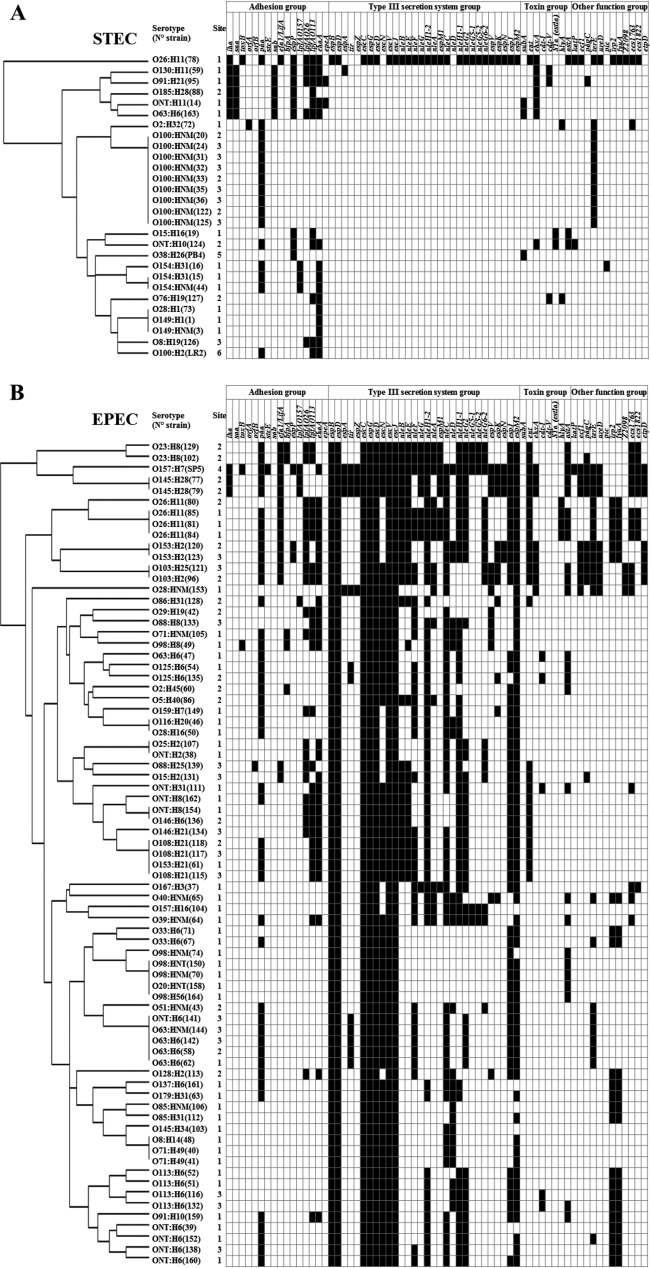
Among the STEC strains investigated, 17 unique virulence gene profiles were identified, based on virulence gene detection (Table 2). The EHEC O26:H11 strain harbored the profile with the highest number of E. coli virulence-associated gene targets, with 47 genes detected (profile 1). In addition to the genes carried on the LEE and linked to the type III secretion system and to the eae gene (and specifically the eae-β1 subtype [Table 2]), this strain harbors genes associated with pathogenicity islands such as OI-122 (nleB, nleE, and ent), OI-71 (espM1), OI-43/48 (ureD and terE), OI-50 (espK and espN), OI-57 (Z2098), and HPI (irp2 and fuyA). One strain of serotype O91:H21 (profile 2) was isolated and was found to possess genes associated with certain pathogenicity islands, such as OI-122 (pagC), OI-43/48 (iha), and OI-15 (ehaA), and with plasmids (pO113 [sab and epeA] and pO157 [espP and ehxA]). For the other STEC strains, 2 to 12 genes were detected, corresponding to 15 additional virulence gene profiles. These strains did not possess genes from the LEE, or genes associated with pathogenicity island OI-122, OI-71, OI-50, or HPI. Serotype O100:HNM, the serotype most commonly represented in isolated STEC strains (n = 9), was associated with only two additional virulence genes (Table 2).
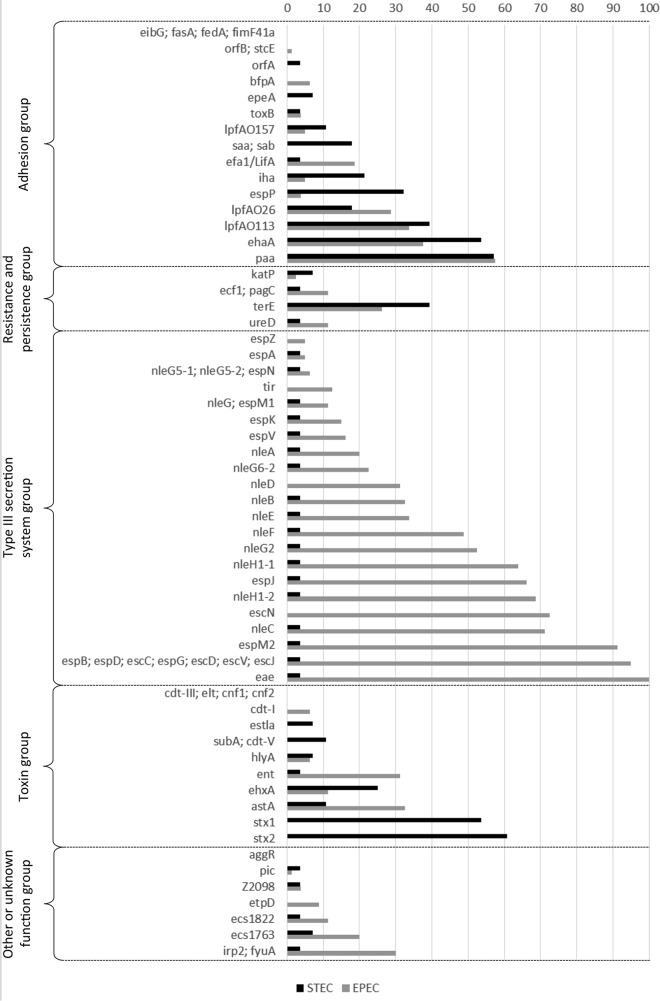
In those STEC strains without the LEE pathogenicity island, other genes involved in adhesion were detected. Of these, the paa gene, which encodes the porcine A/E-associated gene, was detected most frequently (in 57% of STEC strains) (Fig. 2 and 3A). This was followed by the ehaA gene, which was detected in 54% of the strains. The other genes involved in adhesion that were detected were mainly those encoding long polar fimbriae (lpfAO113 [in 39% of strains] and lpfAO26 [18%]), extracellular serine protease (espP) (32%), the IrgA homologue adhesin (iha) (21%), an autoagglutinating adhesin (saa) (18%), and an autotransporter (sab) (18%).
FIG 2.
Prevalence of virulence gene targets among STEC and EPEC strains. Each result is expressed as the percentage of strains bearing the indicated gene.
FIG 3.
Tree and presence/absence matrix for virulence genes target detected in STEC (A) and EPEC (B) strains. The trees were generated using the heat map hierarchical clustering method with the R software (GitHub). Black or white squares in matrices indicate the presence or absence of a virulence gene target, respectively. The virulence gene targets eibG, F6/F987P gene (fasA), F18/F107 gene (fedA), F41 gene (fimF41a), cdt-III, lt (elt), cnf1, cnf2, and aggR are not listed in this figure.
In addition to the stx genes, genes that could lead to the production of other toxins or hemolysins were present in 11 different STEC strains (corresponding to 11 different virulence profiles). The most frequent toxin-associated gene detected was ehxA (in 25% of strains) (Fig. 2). The subA, cdt-V, and astA genes, encoding the subtilase cytotoxin, a cytolethal distending toxin, and an EAEC heat-stable enterotoxin, respectively, were each detected in 11% of the strains. The sta gene, encoding a heat-stable toxin, and the hlyA gene, encoding the alpha hemolysin, were both detected in 7% of the strains.
EPEC virulence gene profiles.
Seventy-five EPEC strains (i.e., eae-positive and stx-negative strains) from the French coastal areas were also investigated. The EPEC strains isolated were mainly atypical EPEC strains (93%), since the typical EPEC marker bfpA was detected in only 7% of these strains (Fig. 3B).
The most commonly identified eae subtype was eae-θ (26% of strains), followed by eae-β1 (17%), eae-β2 (16%), and eae-α2 (11%) (Fig. 1B). All four O26:H11 EPEC strains isolated from shellfish and freshwater harbored eae-β1 (Table 2). All the strains of serotypes O63:H6/HNM (n = 5) and O125:H6 (n = 2) harbored eae-α2, even though they belonged to four different profiles (profiles 30, 32, 37, and 43). The same observation was made for strains of serotypes O33:H6 (n = 2) and O113:H6 (n = 4), which belonged to six different profiles but all harbored the eae-β2 subtype, and for strains of serotypes O23:H8 (n = 2) and ONT:H8 (n = 2), which belonged to four different profiles but all harbored the eae-θ subtype. The other 21 strains harbored one of the following eae subtypes: ε, γ1, κ/δ, ι, ζ, or η (Table 2).
Among the 75 EPEC strains, 56 unique virulence gene profiles were identified, based on the virulence genes detected, suggesting a high diversity of virulence genes in the EPEC strains isolated from the environment (Table 2; Fig. 3B). The number of virulence genes detected ranged from 11 to 50. Most of the profiles were represented by one serotype, except for seven EPEC profiles (profiles 3, 13, 37, 42, 45, 52, and 53) that were represented by two to four different serotypes. EPEC profile 37, corresponding to serotypes O63:H6/HNM and ONT:H6, with 17 virulence genes, was the profile most commonly identified among EPEC strains (n = 5 [Table 2]). The profiles with the highest numbers of E. coli virulence-associated genes were represented by 10 strains that belonged to the top five EHEC serotypes (i.e., O26:H11, O103:H2, O103:H25, O145:H28, and O157:H7) and to serotype O153:H2, harboring 34 to 50 virulence genes. These strains possess genes carried by some pathogenicity islands, such as OI-122, OI-71, and OI-43/48 (Table 2), and at least one of the genes espM1, espK, espV, espN, ureD, and Z2098, which are highly associated with typical EHEC (Fig. 3B). The latter genes were also detected in 13 additional EPEC strains corresponding to serotypes O125:H6, O128:H2, O159:H7, O167:H3, O23:H8, O28:HNM, O29:H19, O40:HNM, O71:HNM, O86:H31, O88:H8, and O98:H8 (Fig. 3B).
In addition to eae, seven genes (espB, espD, escC, espG, escD, escV, and escJ), all of which were carried by the LEE, were detected in 100% of EPEC strains. The other genes that were detected most frequently (i.e., espM2, escN, nleC, nleH1-2, espJ, and nleH1-1) were observed in >60% of strains and are associated with the type III secretion system (Fig. 2). Among the 20 genes of the adhesion group that were investigated, paa was detected most frequently (in 60% of strains), followed by ehaA, lpfAO113, and lpfAO26, found in 39%, 33%, and 29% of strains, respectively, and by the efa1 or lifA gene, encoding an EHEC factor for adherence (20%) (Fig. 2). A number of genes that encode toxins and hemolysins were present in EPEC strains. For example, 33% of strains carried the astA gene, which encodes the EAEC heat-stable toxin. The ent gene, which encodes an ankyrin repeat, was also detected in 33% of the EPEC strains. The ehxA gene, which encodes an enterohemolysin, and cdt-I, which encodes a cytolethal distending toxin, were detected in 11% and 7% of EPEC strains, respectively. Finally, the hlyA gene, which encodes alpha-hemolysin, was present in 5% of EPEC strains.
Genes not detected in STEC and EPEC strains.
Nine virulence genes were never detected in STEC and EPEC strains. Four of these are linked to the adhesion function (eibG and the F6/F987P [fasA], F18/F107 [fedA], and F41 [fimF41a] genes), four to the toxin production function (cdt-III, lt [elt], cnf1, and cnf2), and one (aggR) to the enteroaggregative function. No obvious association was observed between the virulence profiles and the types of samples from which the strains were isolated (shellfish, water, or sediment).
DISCUSSION
This study presents a molecular risk assessment of STEC and EPEC strains isolated in France from shellfish, seawater, and sediment samples collected in shellfish-harvesting areas and from freshwater samples in their upstream watersheds. These strains derived from a larger collection of E. coli strains (12,016 isolates) isolated during a recent study (47). The molecular risk assessment of 28 STEC and 75 EPEC strains was conducted by testing a large panel of virulence genetic markers (i.e., a total of 75 markers) associated with human and animal infections using a high-throughput real-time PCR approach and by identifying stx and eae subtypes.
Some STEC and EPEC strains characterized in this study were found to display a large number or a particular combination of virulence genetic markers and the presence of stx and/or eae variants, suggesting their potential pathogenicity for humans. The identification of E. coli strains that pose a significant threat to human health is still challenging and requires the screening of more genetic markers than only stx and eae genes. The stx and eae genes are, respectively, hallmarks of STEC (including pathogenic and nonpathogenic strains) and EPEC strains, but the genetic basis of E. coli pathogenicity is much more complex than the presence or absence of one or both of these genes. The literature reports many genetic markers that may play roles in the virulence of E. coli and some variants of the eae and/or stx genes (for example) that are closely associated with human-pathogenic E. coli strains. In this study, we took this information into account to define criteria that, according to the current literature, could best recapitulate the most virulent strains isolated from human patients.
Among the stx-positive strains, only the EHEC O26:H11 stx1a eae-β1 strain was associated with a large number of virulence-associated gene targets (60% of genes). Furthermore, this strain, isolated from shellfish, was the only one to harbor the two EHEC gene markers stx and eae. The combined presence of stx, eae, and the 45 supplementary virulence genes has been associated with enhanced virulence. Similar O26:H11 stx1a eae-β1 strains (sequence type 21; phylogroup B1) have been isolated previously from human patients with HUS or diarrhea in Europe (53, 54).
Although they lack the eae gene, most of the STEC strains isolated here have the genetic potential to adhere to host cells through other structures. For example, 18% of eae-negative STEC strains displayed the saa gene, encoding the STEC autoagglutinating adhesion factor. This gene was observed only in LEE-negative STEC strains, in agreement with previous findings from strains of human and bovine origins (55). Other genes encoding proteins associated with attachment were detected in some strains. These include paa, detected here in 15 eae-negative STEC strains, as well as ehaA, lpfAO113, lpfAO26, espP, iha, and sab. Many of these genes have been detected in eae-negative strains isolated previously, in other studies, and could play a role in adhesion to host cells and consequently in the virulence potential of the isolated strains (19–22, 56).
Among the strains that were negative for the eae gene, a STEC strain of serotype O91:H21, isolated from freshwater, could potentially be pathogenic for humans as a result of (i) the presence of seven alternative adhesion factors (saa, ehaA, lpfAO113, lpfAO26, espP, iha, and sab), some of which are included in pathogenicity islands such as OI-15 or OI-43/48, or (ii) the presence of the three stx2 variants stx2a, stx2c, and stx2d. Furthermore, this strain also possesses the cdt-V gene, encoding a toxin, which has been found in STEC strains involved in serious diseases (57). Finally, this strain presented sequence type ST442, which has been found to be the unique ST associated with hemolytic-uremic syndrome among the 10 STs identified in 100 STEC O91 strains isolated from different patients (58).
In addition to the O91:H21 strain, five other STEC strains harbored at least one of the stx2a, stx2c, or stx2d variants, and four of them (i.e., O8:H19, O185:H28, ONT:H11, and O130:H11) were found to combine several distinct stx1 and/or stx2 subtypes. The presence of a combination of stx genes has been observed previously among strains of similar serotypes isolated from humans (18). Furthermore, in the study of Bertin et al. (59), strains harboring two or three stx subtypes were found to be highly cytotoxic toward Vero cells more frequently than other strains. We can hypothesize that strains with a combination of stx1 and/or stx2 subtypes are more virulent than others. Furthermore, strains of serotypes O8:H19 and O130:H11 were isolated from human patients previously (60). The latter strain, like the O91:H21 strain described above, harbors the iha, lpfAO113, ehxA, and cdt-V genes, suggesting a potential virulence trait for humans. Indeed, these genetic markers have already been identified in human LEE-negative STEC strains associated with diseases (18, 61).
Subtypes stx1a and stx2a were each found in 17% of the STEC strains (essentially from freshwater samples) and have been associated with only six to eight supplementary virulence genes. These subtypes have frequently been identified in STEC strains from human, animal, environmental, and food samples (62–65). STEC strains with stx2a have been found to be associated with several clinical symptoms, such as HUS and HC, whereas STEC strains with stx1a have been associated mainly with diarrhea without HUS (8).
On the other hand, the simultaneous detection of subtype stx2e and the paa, orfA, hlyA, and ECs1763 genes in the serotype O2:H32 strain (from a seawater sample) suggests that this strain could potentially be associated with swine edema disease (10) or could derive from a pig source (64). In the same way, strain O15:H16 (from a shellfish sample), associated with the stx2g subtype and four supplementary virulence genes (two toxins and two adhesion factors), could potentially be linked with cattle sources, as has been shown previously for similar strains (41, 66). We can hypothesize that this strain is associated with a low human risk. Similarly, the O38:H26 STEC strain isolated from shellfish was associated with a low human risk; indeed it was associated with the stx1c subtype and only one other toxin gene (subA) and one adhesion gene (espP) and may potentially derive from a sheep source, as shown previously (64, 67). Finally, the stx1d subtype dominates (23% of detected stx genes) in STEC strains isolated in our study (from shellfish and freshwater samples). This subtype does not appear to be common in STEC strains (62–64). However, a proportion of stx1d variants similar to those observed here has been observed in STEC strains from ruminant stools in India (18.7%) (68). Little is known of the clinical significance of this subtype, but it seems to be associated with a low risk (69). In agreement with this suspected low virulence, STEC strains with stx1d were found to be associated with only one to four additional virulence genes from the panel of 75 genes investigated.
Among the EPEC strains isolated in this study, some appear potentially pathogenic for humans as a result of the high number and the composition of virulence genes they possess. First, the E. coli O157:H7 strain isolated from shellfish possessed the highest number of virulence genes (50 genes), and especially the ehxA, astA, lpfA, katP, etpD, espP, terE, and ureD genes, identified by using whole-genome sequencing analysis for STEC O157:H7 strains and the EPEC O157:H7 strains isolated from patients with gastrointestinal complaints in the Netherlands (70). These EPEC strains were mostly related to the STEC group and might be referred to as EHEC strains that have lost the Shiga toxin (EHEC-LST [70]). Another category of such strains could be “EHEC-like,” as proposed previously for strains of the O26:H11 serotype (71). In addition, strains with non-O157 serotypes (such as O145:H28, O103:H25, O103:H2, and O26:H11) isolated in this study were also found to possess a high number of genes (27 to 39 virulence genes) and could also be regarded as EHEC strains that have lost the Shiga toxin. The large number of virulence genes in these strains is consistent with the fact that members of the top five serotypes are the strains most frequently associated with human diseases (5). Among the strains with the highest number of virulence genes (>27 virulence genes), there are also strains of serotypes O153:H2 and O23:H8. STEC strains of the O153:H2 serotype have already been isolated from human patients (60), whereas EPEC strains that belong to serotype O23:H8 and show similarities to strains in the present study (with the same ST [ST327], genes encoding the same adhesins [lpfAO26, lpfAO113, and paa], and genes of OI-122) were previously associated with nonbloody diarrhea (72).
Among the strains with the highest numbers of virulence genes, several could be potentially pathogenic, since specific virulence gene associations were found. For example, detection of the four genetic markers espK, espV, ureD, and Z2098 in strains belonging to the O103:H2 and O103:H25 serotypes suggests that these strains, isolated from freshwater samples, could be highly virulent for humans, as proposed previously by studies in the detection of EHEC strains and their stx-negative derivative strains (30) and in the prediction of strain virulence (29).
Furthermore, 10 strains displayed more than 60% of the genetic markers related to the pathogenicity islands OI-122 (efa1, pagC, nleB, nleE, and ent), OI-57 (nleG2, nleG5-1, nleG5-2, nleG6-2, Z2098, and ECs1763), OI-71 (espM1, nleA, nleF, nleG, and nleH1-2), and OI-43/48 (iha, terE, and ureD) and to the high-pathogenicity island (HPI) (irp2 and fuyA), which are used to identify strains with the ability to cause severe disease outbreaks (22, 43). These strains were isolated from shellfish and freshwater samples and belonged to serotypes O23:H8, O26:H11, O103:H25, O103:H2, O145:H28, and O157:H7. They are among the strains described above as having the largest number of virulence genes and represent a group of strains with a high virulence potential for humans. This is also corroborated by their serotype, which is associated with the classical EHEC serotype.
The presence of nle (non-LEE effector) genes and the number of genes carried by an E. coli strain are important criteria for estimating its virulence potential (27, 73). Eight strains carried at least 10 of the 13 nle genes analyzed. Most of these strains belong to the O26:H11, O23:H8, and O157:H7 serotypes described above, while two strains of serotypes O157:H16 and O39:HNM were isolated from shellfish and freshwater samples.
The majority of the EPEC strains isolated in this study were found to possess the four main intimin subtypes, which could be highly related to pathogenic serotypes (eae subtypes β, γ, ε, and θ, found in 72% of the EPEC strains [17]). The eae-γ1 subtype was found in the serotype O157:H7 strain and the two serotype O145:H28 strains, while eae-β1 was found in the four strains of serotype O26:H11 (isolated from shellfish and freshwater samples). These correlations are consistent with results obtained previously for strains belonging to the top five EHEC serotypes that were isolated from slaughtered adult cattle (11) and for strains linked to human diseases (51). These data are also consistent with publications that showed that the eae-β and eae-γ subtypes were the two subtypes most frequently detected in clinical isolates associated with human infections (15, 51, 74).
Some correlations were found between the eae subtype and specific virulence genes or phylogroups. All EPEC strains with the eae-γ1 subtype, and only those, harbored the espA and espZ genes. Although from different serotypes, the three eae-γ1-containing strains assigned to phylogroups were from phylogroup D and harbored the tir gene, which was also detected in seven of the nine eae-α2 variants. Similarly, all eae-β2 strains (n = 12) were from phylogroup B2 and contained the fyuA and irp2 genes (HPI), while only 18% of other EPEC variant strains harbored these genes. This resulted in a greater proportion of strains carrying these fyuA and irp2 genes among members of phylogroup B2. Conversely, strains from phylogroup B1 preferentially carried the ehaA (89.2%), lpfAO113 (83.8%), lpfAO26 (70.3%), nleE (62.2%), nleB (59.5%), and ent (59.5%) genes, in contrast to strains of phylogroups A, B1, and D (<15%).
In this study, STEC and EPEC strains were found together in some samples, suggesting that a mixture of STEC and EPEC strains could be present in an environmental sample, thus providing the opportunity for horizontal gene transfer of multiple virulence factors, including gain or loss of stx genes. Furthermore, in a selection of water samples, stx1- or stx2-converting bacteriophages were searched for by real-time PCR and were found to be present at the same time as STEC or EPEC strains in the samples tested (M. Muniesa, personal communication). These results suggest that new pathogens could emerge as a result of the simultaneous presence and recombination of STEC, EPEC, and stx-converting bacteriophages.
In conclusion, this molecular risk assessment study of STEC and EPEC strains isolated from the coastal environment used genetic markers associated with human or nonhuman animal diseases to evaluate the potential risk for shellfish consumers. Seven STEC strains (corresponding to profiles 1 to 5, 7, and 9) were associated with a probable virulence potential for humans either because they displayed a large number of virulence genetic markers or because they harbored stx2a, whether in addition to the stx2c and/or stx2d variant or not. Fifteen EPEC strains (corresponding to profiles 1 to 9 and 36), which could be “EHEC-like,” displayed a large number or a particular combination of virulence genetic markers (i.e., >60% of genetic markers related to pathogenicity islands OI-122, OI-57, OI-71, OI-43-48, and HPI or at least 10 of the 13 nle genes tested), suggesting an association with human or nonhuman animal infections. This study clearly highlights the ubiquitous presence of potentially pathogenic STEC and EPEC strains in coastal environments (shellfish, water, and sediment samples), even if these strains are less prevalent in such environments than in upstream watersheds as a result of the distance from the source and the negative impact of a saline environment. Risk of a human infection by STEC caused by shellfish consumption seems to be limited because a depuration step or relaying step has to be performed before shellfish from category B and C areas, respectively, reach market (1, 47). To date, no shellfish outbreak involving STEC or EPEC strains has been described. The sanitary classification of shellfish-harvesting areas in Europe is probably an important measure that helps to prevent shellfish food-borne outbreaks caused by these bacteria. However, the absence of a case description could also be linked to an underestimation of the hazard associated with potentially pathogenic STEC and EPEC strains. Therefore, the acquisition of data on circulating strains in the environment is crucial for preventing the risk of human infection and improving our understanding of STEC and EPEC.
ACKNOWLEDGMENTS
We thank Huw Taylor (University of Brighton, Brighton, United Kingdom) for critical review of the manuscript, Maite Muniesa (University of Barcelona, Barcelona, Spain) for providing the results of stx1- or stx2-converting bacteriophage detection, and Eliette Riboulet (Université de Caen-Normandie, Caen, France) for help in producing the dendrograms shown in Fig. 3 using the R software.
Funding Statement
This work was funded by the European Regional Development Fund Interreg IVA Programme, as part of the collaborative project RiskManche. The thesis of Charlotte Balière was supported by a grant from Institut Français de Recherche pour l'Exploitation de la Mer (Ifremer) and the Agence de l'Eau Loire-Bretagne.
REFERENCES
- 1.Anonymous. 30 April 2004. Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Official Journal of the European Union, L 139, p 206–320. [Google Scholar]
- 2.Mathusa EC, Chen Y, Enache E, Hontz L. 2010. Non-O157 Shiga toxin-producing Escherichia coli in foods. J Food Prot 73:1721–1736. [DOI] [PubMed] [Google Scholar]
- 3.Luna-Gierke RE, Griffin PM, Gould LH, Herman K, Bopp CA, Strockbine N, Mody RK. 2014. Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol Infect 142:2270–2280. doi: 10.1017/S0950268813003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.USDA Food Safety and Inspection Service. 2011. Risk profile for pathogenic non-O157 Shiga toxin-producing Escherichia coli (non-O157 STEC). USDA Food Safety and Inspection Service, Washington, DC. [Google Scholar]
- 5.EFSA Panel on Biological Hazards (BIOHAZ). 2013. Scientific opinion on VTEC-seropathotype and scientific criteria regarding pathogenicity assessment. EFSA J 11:3138. [Google Scholar]
- 6.O'Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 7.Scheutz F, Teel LD, Beutin L, Piérard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR, Sanchez M, Persson S, O'Brien AD. 2012. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol 50:2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis 185:74–84. doi: 10.1086/338115. [DOI] [PubMed] [Google Scholar]
- 9.Bielaszewska M, Friedrich AW, Aldick T, Schürk-Bulgrin R, Karch H. 2006. Shiga toxin activatable by intestinal mucus in Escherichia coli isolated from humans: predictor for a severe clinical outcome. Clin Infect Dis 43:1160–1167. doi: 10.1086/508195. [DOI] [PubMed] [Google Scholar]
- 10.Tseng M, Fratamico PM, Bagi L, Delannoy S, Fach P, Manning SD, Funk JA. 2014. Diverse virulence gene content of Shiga toxin-producing Escherichia coli from finishing swine. Appl Environ Microbiol 80:6395–6402. doi: 10.1128/AEM.01761-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bibbal D, Loukiadis E, Kerouredan M, Ferre F, Dilasser F, de Garam CP, Cartier P, Oswald E, Gay E, Auvray F, Brugere H. 2015. Prevalence of carriage of Shiga toxin-producing Escherichia coli serotypes O157:H7, O26:H11, O103:H2, O111:H8, and O145:H28 among slaughtered adult cattle in France. Appl Environ Microbiol 81:1397–1405. doi: 10.1128/AEM.03315-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt H, Scheef J, Morabito S, Caprioli A, Wieler LH, Karch H. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl Environ Microbiol 66:1205–1208. doi: 10.1128/AEM.66.3.1205-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paton JC, Paton AW. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev 11:450–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito K, Iida M, Yamazaki M, Moriya K, Moroishi S, Yatsuyanagi J, Kurazono T, Hiruta N, Ratchtrachenchai OA. 2007. Intimin types determined by heteroduplex mobility assay of intimin gene (eae)-positive Escherichia coli strains. J Clin Microbiol 45:1038–1041. doi: 10.1128/JCM.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang WL, Köhler B, Oswald E, Beutin L, Karch H, Morabito S, Caprioli A, Suerbaum S, Schmidt H. 2002. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J Clin Microbiol 40:4486–4492. doi: 10.1128/JCM.40.12.4486-4492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K, Pagaling E, Yan T. 2014. Estimating the prevalence of potential enteropathogenic Escherichia coli and intimin gene diversity in a human community by monitoring sanitary sewage. Appl Environ Microbiol 80:119–127. doi: 10.1128/AEM.02747-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bibbal D, Loukiadis E, Kérourédan M, Peytavin de Garam C, Ferré F, Cartier P, Gay E, Oswald E, Auvray F, Brugère H. 2014. Intimin gene (eae) subtype-based real-time PCR strategy for specific detection of Shiga toxin-producing Escherichia coli serotypes O157:H7, O26:H11, O103:H2, O111:H8, and O145:H28 in cattle feces. Appl Environ Microbiol 80:1177–1184. doi: 10.1128/AEM.03161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galli L, Miliwebsky E, Irino K, Leotta G, Rivas M. 2010. Virulence profile comparison between LEE-negative Shiga toxin-producing Escherichia coli (STEC) strains isolated from cattle and humans. Vet Microbiol 143:307–313. doi: 10.1016/j.vetmic.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Vidotto MC, Florian ECT, Ono MA. 2013. Prevalence of the paa gene (porcine attaching and effacing associated) in porcine enteropathogenic Escherichia coli (PEPEC) associated with postweaning diarrhea in south Brazil. Braz J Microbiol 44:515–517. doi: 10.1590/S1517-83822013000200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells TJ, Sherlock O, Rivas L, Mahajan A, Beatson SA, Torpdahl M, Webb RI, Allsopp LP, Gobius KS, Gally DL, Schembri MA. 2008. EhaA is a novel autotransporter protein of enterohemorrhagic Escherichia coli O157:H7 that contributes to adhesion and biofilm formation. Environ Microbiol 10:589–604. doi: 10.1111/j.1462-2920.2007.01479.x. [DOI] [PubMed] [Google Scholar]
- 21.Paton AW, Srimanote P, Woodrow MC, Paton JC. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect Immun 69:6999–7009. doi: 10.1128/IAI.69.11.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doughty S, Sloan J, Bennett-Wood V, Robertson M, Robins-Browne RM, Hartland EL. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect Immun 70:6761–6769. doi: 10.1128/IAI.70.12.6761-6769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Angulo VA, Martinez-Santos VI, Villasenor T, Santana FJ, Huerta-Saquero A, Martinez LC, Jimenez R, Lara-Ochoa C, Tellez-Sosa J, Bustamante VH, Puente JL. 2012. A distinct regulatory sequence is essential for the expression of a subset of nle genes in attaching and effacing Escherichia coli. J Bacteriol 194:5589–5603. doi: 10.1128/JB.00190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, An T, Wang S, Wang G, Si W, Tu Y, Liu Y, Wu J, Liu S, Cai X. 2015. Role of the ehxA gene from Escherichia coli serotype O82 in hemolysis, biofilm formation, and in vivo virulence. Can J Microbiol 61:335–341. doi: 10.1139/cjm-2014-0824. [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa Y, Zhou Z, Hase A, Ogasawara J, Kitase T, Abe N, Nakamura H, Wada T, Ishii E, Haruki K. 2002. Diarrheagenic Escherichia coli isolated from stools of sporadic cases of diarrheal illness in Osaka City, Japan between 1997 and 2000: prevalence of enteroaggregative E coli heat-stable enterotoxin 1 gene-possessing E coli. Jpn J Infect Dis 55:183–190. [PubMed] [Google Scholar]
- 26.Lorenz SC, Son I, Maounounen-Laasri A, Lin A, Fischer M, Kase JA. 2013. Prevalence of hemolysin genes and comparison of ehxA subtype patterns in Shiga toxin-producing Escherichia coli (STEC) and non-STEC strains from clinical, food, and animal sources. Appl Environ Microbiol 79:6301–6311. doi: 10.1128/AEM.02200-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coombes BK, Wickham ME, Mascarenhas M, Gruenheid S, Finlay BB, Karmali MA. 2008. Molecular analysis as an aid to assess the public health risk of non-O157 Shiga toxin-producing Escherichia coli strains. Appl Environ Microbiol 74:2153–2160. doi: 10.1128/AEM.02566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bugarel M, Martin A, Fach P, Beutin L. 2011. Virulence gene profiling of enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli strains: a basis for molecular risk assessment of typical and atypical EPEC strains. BMC Microbiol 11:142. doi: 10.1186/1471-2180-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju W, Shen J, Toro M, Zhao S, Meng J. 2013. Distribution of pathogenicity islands OI-122, OI-43/48, and OI-57 and a high-pathogenicity island in Shiga toxin-producing Escherichia coli. Appl Environ Microbiol 79:3406–3412. doi: 10.1128/AEM.03661-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delannoy S, Beutin L, Fach P. 2013. Discrimination of enterohemorrhagic Escherichia coli (EHEC) from non-EHEC strains based on detection of various combinations of type III effector genes. J Clin Microbiol 51:3257–3262. doi: 10.1128/JCM.01471-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delannoy S, Beutin L, Fach P. 2013. Towards a molecular definition of enterohemorrhagic Escherichia coli (EHEC): detection of genes located on O island 57 as markers to distinguish EHEC from closely related enteropathogenic E. coli strains. J Clin Microbiol 51:1083–1088. doi: 10.1128/JCM.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization 1987. Program for control of diarrhoeal diseases. Manual for laboratory investigation of acute enteric infections. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 34.Bielaszewska M, Prager R, Köck R, Mellmann A, Zhang W, Tschäpe H, Tarr PI, Karch H. 2007. Shiga toxin gene loss and transfer in vitro and in vivo during enterohemorrhagic Escherichia coli O26 infection in humans. Appl Environ Microbiol 73:3144–3150. doi: 10.1128/AEM.02937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mora A, López C, Dhabi G, López-Beceiro AM, Fidalgo LE, Díaz EA, Martínez-Carrasco C, Mamani R, Herrera A, Blanco JE, Blanco M, Blanco J. 2012. Seropathotypes, phylogroups, Stx subtypes, and intimin types of wildlife-carried, Shiga toxin-producing Escherichia coli strains with the same characteristics as human-pathogenic isolates. Appl Environ Microbiol 78:2578–2585. doi: 10.1128/AEM.07520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandran A, Mazumder A. 2013. Prevalence of diarrhea-associated virulence genes and genetic diversity in Escherichia coli isolates from fecal material of various animal hosts. Appl Environ Microbiol 79:7371–7380. doi: 10.1128/AEM.02653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh P, Sha Q, Lacher DW, Del Valle J, Mosci RE, Moore JA, Scribner KT, Manning SD. 2015. Characterization of enteropathogenic and Shiga toxin-producing Escherichia coli in cattle and deer in a shared agroecosystem. Front Cell Infect Microbiol 5:29. doi: 10.3389/fcimb.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muniesa M, Jofre J, García-Aljaro C, Blanch AR. 2006. Occurrence of Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environ Sci Technol 40:7141–7149. doi: 10.1021/es060927k. [DOI] [PubMed] [Google Scholar]
- 39.Loukiadis E, Kérourédan M, Beutin L, Oswald E, Brugère H. 2006. Characterization of Shiga toxin gene (stx)-positive and intimin gene (eae)-positive Escherichia coli isolates from wastewater of slaughterhouses in France. Appl Environ Microbiol 72:3245–3251. doi: 10.1128/AEM.72.5.3245-3251.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrido P, Blanco M, Moreno-Paz M, Briones C, Dahbi G, Blanco J, Parro V. 2006. STEC-EPEC oligonucleotide microarray: a new tool for typing genetic variants of the LEE pathogenicity island of human and animal Shiga toxin–producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) strains. Clin Chem 52:192–201. doi: 10.1373/clinchem.2005.059766. [DOI] [PubMed] [Google Scholar]
- 41.Martínez-Castillo A, Allué-Guardia A, Dahbi G, Blanco J, Creuzburg K, Schmidt H, Muniesa M. 2012. Type III effector genes and other virulence factors of Shiga toxin-encoding Escherichia coli isolated from wastewater. Environ Microbiol Rep 4:147–155. doi: 10.1111/j.1758-2229.2011.00317.x. [DOI] [PubMed] [Google Scholar]
- 42.Tseng M, Fratamico PM, Bagi L, Manzinger D, Funk JA. 2015. Shiga toxin-producing E. coli (STEC) in swine: prevalence over the finishing period and characteristics of the STEC isolates. Epidemiol Infect 143:505–514. doi: 10.1017/S0950268814001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chui L, Li V, Fach P, Delannoy S, Malejczyk K, Patterson-Fortin L, Poon A, King R, Simmonds K, Scott AN, Lee MC. 2015. Molecular profiling of Escherichia coli O157:H7 and non-O157 strains isolated from humans and cattle in Alberta, Canada. J Clin Microbiol 53:986–990. doi: 10.1128/JCM.03321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miko A, Rivas M, Bentancor A, Delannoy S, Fach P, Beutin L. 2014. Emerging types of Shiga toxin-producing E. coli (STEC) O178 present in cattle, deer, and humans from Argentina and Germany. Front Cell Infect Microbiol 4:78. doi: 10.3389/fcimb.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miko A, Delannoy S, Fach P, Strockbine NA, Lindstedt BA, Mariani-Kurkdjian P, Reetz J, Beutin L. 2013. Genotypes and virulence characteristics of Shiga toxin-producing Escherichia coli O104 strains from different origins and sources. Int J Med Microbiol 303:410–421. doi: 10.1016/j.ijmm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Feng P. 2014. Shiga toxin-producing Escherichia coli (STEC) in fresh produce—a food safety dilemma. Microbiol Spectr 2(4):EHEC-0010–2013. doi: 10.1128/microbiolspec.EHEC-0010-2013. [DOI] [PubMed] [Google Scholar]
- 47.Balière C, Rincé A, Blanco J, Dahbi G, Harel J, Vogeleer P, Giard JC, Mariani-Kurkdjian P, Gourmelon M. 2015. Prevalence and characterization of Shiga toxin-producing and enteropathogenic Escherichia coli in shellfish-harvesting areas and their watersheds. Front Microbiol 6:1356. doi: 10.3389/fmicb.2015.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gourmelon M, Montet M, Lozach S, Le Mennec C, Pommepuy M, Beutin L, Vernozy-Rozand C. 2006. First isolation of Shiga toxin 1d producing Escherichia coli variant strains in shellfish from coastal areas in France. J Appl Microbiol 100:85–97. doi: 10.1111/j.1365-2672.2005.02753.x. [DOI] [PubMed] [Google Scholar]
- 49.Perelle S, Dilasser F, Grout J, Fach P. 2004. Detection by 5′-nuclease PCR of Shiga-toxin producing Escherichia coli O26, O55, O103, O111, O113, O145 and O157:H7, associated with the world's most frequent clinical cases. Mol Cell Probes 18:185–192. doi: 10.1016/j.mcp.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen EM, Andersen MT. 2003. Detection and characterization of verocytotoxin-producing Escherichia coli by automated 5′ nuclease PCR assay. J Clin Microbiol 41:2884–2893. doi: 10.1128/JCM.41.7.2884-2893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanco M, Padola NL, Krüger A, Sanz ME, Blanco JE, González EA, Dahbi G, Mora A, Bernárdez MI, Etcheverría AI, Arroyo GH, Lucchesi PM, Parma AE, Blanco J. 2004. Virulence genes and intimin types of Shiga-toxin-producing Escherichia coli isolated from cattle and beef products in Argentina. Int Microbiol 7:269–276. [PubMed] [Google Scholar]
- 52.Hall TA. 2013. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. [Google Scholar]
- 53.Bielaszewska M, Mellmann A, Bletz S, Zhang W, Koeck R, Kossow A, Prager R, Fruth A, Orth-Hoeller D, Marejkova M, Morabito S, Caprioli A, Pierard D, Smith G, Jenkins C, Curova K, Karch H. 2013. Enterohemorrhagic Escherichia coli O26:H11/H−: a new virulent clone emerges in Europe. Clin Infect Dis 56:1373–1381. doi: 10.1093/cid/cit055. [DOI] [PubMed] [Google Scholar]
- 54.Bonanno L, Loukiadis E, Mariani-Kurkdjian P, Oswald E, Garnier L, Michel V, Auvray F. 2015. Diversity of Shiga toxin-producing Escherichia coli (STEC) O26:H11 strains examined via stx subtypes and insertion sites of Stx and EspK bacteriophages. Appl Environ Microbiol 81:3712–3721. doi: 10.1128/AEM.00077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenkins C, Perry NT, Cheasty T, Shaw DJ, Frankel G, Dougan G, Gunn GJ, Smith HR, Paton AW, Paton JC. 2003. Distribution of the saa gene in strains of Shiga toxin-producing Escherichia coli of human and bovine origins. J Clin Microbiol 41:1775–1778. doi: 10.1128/JCM.41.4.1775-1778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarr PI, Bilge SS, Vary JC Jr, Jelacic S, Habeeb RL, Ward TR, Baylor MR, Besser TE. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect Immun 68:1400–1407. doi: 10.1128/IAI.68.3.1400-1407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bielaszewska M, Fell M, Greune L, Prager R, Fruth A, Tschape H, Schmidt MA, Karch H. 2004. Characterization of cytolethal distending toxin genes and expression in Shiga toxin-producing Escherichia coli strains of non-O157 serogroups. Infect Immun 72:1812–1816. doi: 10.1128/IAI.72.3.1812-1816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mellmann A, Fruth A, Friedrich AW, Wieler LH, Harmsen D, Werber D, Middendorf B, Bielaszewska M, Karch H. 2009. Phylogeny and disease association of Shiga toxin-producing Escherichia coli O91. Emerg Infect Dis 15:1474–1477. doi: 10.3201/eid1509.090161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertin Y, Boukhors K, Pradel N, Livrelli V, Martin C. 2001. Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France: detection of a new Stx2 subtype and correlation with additional virulence factors. J Clin Microbiol 39:3060–3065. doi: 10.1128/JCM.39.9.3060-3065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beutin L, Fach P. 2014. Detection of Shiga toxin-producing Escherichia coli from nonhuman sources and strain typing. Microbiol Spectr 2(3):EHEC-0001-2013. doi: 10.1128/microbiolspec.EHEC-0001-2013. [DOI] [PubMed] [Google Scholar]
- 61.Hauser E, Mellmann A, Semmler T, Stoeber H, Wieler LH, Karch H, Kuebler N, Fruth A, Harmsen D, Weniger T, Tietze E, Schmidt H. 2013. Phylogenetic and molecular analysis of food-borne Shiga toxin-producing Escherichia coli. Appl Environ Microbiol 79:2731–2740. doi: 10.1128/AEM.03552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng PCH, Reddy S. 2013. Prevalences of Shiga toxin subtypes and selected other virulence factors among Shiga-toxigenic Escherichia coli strains isolated from fresh produce. Appl Environ Microbiol 79:6917–6923. doi: 10.1128/AEM.02455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monaghan A, Byrne B, Fanning S, Sweeney T, McDowell D, Bolton DJ. 2011. Serotypes and virulence profiles of non-O157 Shiga toxin-producing Escherichia coli isolates from bovine farms. Appl Environ Microbiol 77:8662–8668. doi: 10.1128/AEM.06190-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin A, Beutin L. 2011. Characteristics of Shiga toxin-producing Escherichia coli from meat and milk products of different origins and association with food producing animals as main contamination sources. Int J Food Microbiol 146:99–104. doi: 10.1016/j.ijfoodmicro.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 65.Shen J, Rump L, Ju W, Shao J, Zhao S, Brown E, Meng J. 2015. Virulence characterization of non-O157 Shiga toxin-producing Escherichia coli isolates from food, humans and animals. Food Microbiol 50:20–27. doi: 10.1016/j.fm.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Zweifel C, Schumacher S, Blanco M, Blanco JE, Tasara T, Blanco J, Stephan R. 2005. Phenotypic and genotypic characteristics of non-O157 Shiga toxin-producing Escherichia coli (STEC) from Swiss cattle. Vet Microbiol 105:37–45. doi: 10.1016/j.vetmic.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Brett KN, Ramachandran V, Hornitzky MA, Bettelheim KA, Walker MJ, Djordjevic SP. 2003. stx1c is the most common Shiga toxin 1 subtype among Shiga toxin-producing Escherichia coli isolates from sheep but not among isolates from cattle. J Clin Microbiol 41:926–936. doi: 10.1128/JCM.41.3.926-936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar A, Taneja N, Kumar Y, Sharma M. 2012. Detection of Shiga toxin variants among Shiga toxin-forming Escherichia coli isolates from animal stool, meat and human stool samples in India. J Appl Microbiol 113:1208–1216. doi: 10.1111/j.1365-2672.2012.05415.x. [DOI] [PubMed] [Google Scholar]
- 69.Beutin L, Miko A, Krause G, Pries K, Haby S, Steege K, Albrecht N. 2007. Identification of human-pathogenic strains of Shiga toxin-producing Escherichia coli from food by a combination of serotyping and molecular typing of Shiga toxin genes. Appl Environ Microbiol 73:4769–4775. doi: 10.1128/AEM.00873-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferdous M, Zhou K, Mellmann A, Morabito S, Croughs PD, de Boer RF, Kooistra-Smid AM, Rossen JW, Friedrich AW. 2015. Is Shiga toxin-negative Escherichia coli O157:H7 enteropathogenic or enterohemorrhagic Escherichia coli? Comprehensive molecular analysis using whole-genome sequencing. J Clin Microbiol 53:3530–3538. doi: 10.1128/JCM.01899-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bugarel M, Beutin L, Scheutz F, Loukiadis E, Fach P. 2011. Identification of genetic markers for differentiation of Shiga toxin-producing, enteropathogenic, and avirulent strains of Escherichia coli O26. Appl Environ Microbiol 77:2275–2281. doi: 10.1128/AEM.02832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bielaszewska M, Middendorf B, Köck R, Friedrich AW, Fruth A, Karch H, Schmidt MA, Mellmann A. 2008. Shiga toxin-negative attaching and effacing Escherichia coli: distinct clinical associations with bacterial phylogeny and virulence traits and inferred in-host pathogen evolution. Clin Infect Dis 47:208–217. doi: 10.1086/589245. [DOI] [PubMed] [Google Scholar]
- 73.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. 2013. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jenkins C, Lawson AJ, Cheasty T, Willshaw GA, Wright P, Dougan G, Frankel G, Smith HR. 2003. Subtyping intimin genes from enteropathogenic Escherichia coli associated with outbreaks and sporadic cases in the United Kingdom and Eire. Mol Cell Probes 17:149–156. doi: 10.1016/S0890-8508(03)00046-X. [DOI] [PubMed] [Google Scholar]
- 75.Lacher DW, Steinsland H, Blank TE, Donnenberg MS, Thomas S, Whittam TS. 2007. Molecular evolution of typical enteropathogenic Escherichia coli: clonal analysis by multilocus sequence typing and virulence gene allelic profiling. J Bacteriol 189:342–350. doi: 10.1128/JB.01472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]