ABSTRACT
A novel bacterial aldehyde dehydrogenase (ALDH) that converts retinal to retinoic acid was first identified in Bacillus cereus. The amino acid sequence of ALDH from B. cereus (BcALDH) was more closely related to mammalian ALDHs than to bacterial ALDHs. This enzyme converted not only small aldehydes to carboxylic acids but also the large aldehyde all-trans-retinal to all-trans-retinoic acid with NAD(P)+. We newly found that BcALDH and human ALDH (ALDH1A1) could reduce all-trans-retinal to all-trans-retinol with NADPH. The catalytic residues in BcALDH were Glu266 and Cys300, and the cofactor-binding residues were Glu194 and Glu457. The E266A and C300A variants showed no oxidation activity. The E194S and E457V variants showed 15- and 7.5-fold higher catalytic efficiency (kcat/Km) for the reduction of all-trans-retinal than the wild-type enzyme, respectively. The wild-type, E194S variant, and E457V variant enzymes with NAD+ converted 400 μM all-trans-retinal to 210 μM all-trans-retinoic acid at the same amount for 240 min, while with NADPH, they converted 400 μM all-trans-retinal to 20, 90, and 40 μM all-trans-retinol, respectively. These results indicate that BcALDH and its variants are efficient biocatalysts not only in the conversion of retinal to retinoic acid but also in its conversion to retinol with a cofactor switch and that retinol production can be increased by the variant enzymes. Therefore, BcALDH is a novel bacterial enzyme for the alternative production of retinoic acid and retinol.
IMPORTANCE Although mammalian ALDHs have catalyzed the conversion of retinal to retinoic acid with NAD(P)+ as a cofactor, a bacterial ALDH involved in the conversion is first characterized. The biotransformation of all-trans-retinal to all-trans-retinoic acid by BcALDH and human ALDH was altered to the biotransformation to all-trans-retinol by a cofactor switch using NADPH. Moreover, the production of all-trans-retinal to all-trans-retinol was changed by mutations at positions 194 and 457 in BcALDH. The alternative biotransformation of retinoids was first performed in the present study. These results will contribute to the biotechnological production of retinoids, including retinoic acid and retinol.
INTRODUCTION
Retinoic acid can be formed in the body by two sequential oxidation steps which convert retinol to retinaldehyde to retinoic acid. Retinol has been utilized in the manufacturing of food products, cosmetics, pharmaceuticals, nutraceuticals, and animal feed additives (1–3). Retinoic acid, a metabolite of vitamin A (retinol), is essential for a wide range of biological processes in mammals, including cell proliferation, differentiation, and morphogenesis (4, 5). Retinoic acid deficiency impairs vestibular functions (6, 7) and is associated with Parkinson's disease (8). Retinoic acid is also one of the most important ingredients of skin care products in cosmetics because it is effective against UV radiation-induced skin damage (9). Therefore, high demands for retinoic acid have arisen from the cosmetic and pharmaceutical industries. However, retinoic acid is commercially produced only by chemical synthesis. Biotechnological production of retinoic acid is limited, however, because a selective production route has not been developed.
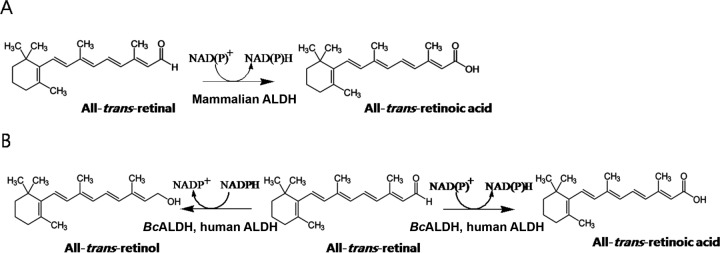
In mammals, the biosynthesis of retinoic acid from retinol occurs by two sequential enzyme reactions: retinol is converted to retinal by the oxidation reaction of retinol dehydrogenase, and retinal is then converted to retinoic acid by the additional oxidation of retinal dehydrogenase (RALDH), which belongs to the aldehyde dehydrogenase (ALDH) family. ALDHs (EC 1.2.1) have been well studied in mammals for their biochemical properties and catalytic mechanisms (10–12). The oxidation of retinal to retinoic acid with NAD(P)+ has been catalyzed by mammalian ALDHs (Fig. 1A); however, the reduction of retinal to retinol by these enzymes has not been reported (Fig. 1B) thus far. In spite of numerous studies on mammalian ALDHs, there are no reports of bacterial enzymes involved in the conversion of retinal to retinoic acid. Since the bacterial ALDHs showed diverse and versatile catalytic power for oxidation, we searched bacterial homologs of human ALDH (ALDH1A1) involved in the oxidation of retinal to retinoic acid. Among bacterial ALDHs, ALDH from Bacillus cereus (BcALDH) was selected because the enzyme exhibited the highest sequence identity with human ALDH.
FIG 1.
Schematic diagrams of reactions catalyzed by aldehyde dehydrogenase. (A) Oxidation reaction of all-trans-retinal to all-trans-retinoic acid reported by mammalian ALDH. (B) Oxidation reaction of all-trans-retinal to all-trans-retinoic acid as well as reduction reaction of all-trans-retinal to all-trans-retinol identified by BcALDH and human ALDH in this study.
In this study, the mammalian-gene-like aldH gene from B. cereus was cloned, and its purified enzyme was characterized. Mutations of the conserved catalytic and cofactor-binding residues involved in the oxidation and reduction with cofactor selectivity of BcALDH were performed, and the variants with increased activity for oxidation and reduction were obtained. The alternative biotransformation of all-trans-retinal to all-trans-retinol or all-trans-retinoic acid by the wild-type and variant enzymes was first attempted with a cofactor switch.
MATERIALS AND METHODS
Gene cloning and site-directed mutagenesis.
The bcere0002_25940 gene (1,485 bp), encoding an ALDH from B. cereus, and the aldH1a1 gene (1,506 bp), encoding an ALDH1A1 from humans, were amplified by PCR using the genomic DNA isolated from B. cereus and human cDNA as a template, respectively. The primer sequences used for gene cloning were based on the DNA sequence of BcALDH (GenBank accession no. ACLT01000061.1), and forward (5′-GGATCCGATGTTAAAGACAAATATTGAGCTG-3′) and reverse (5′-GCGGCCGCTTACTTTATTACTTTATATTTACCCAAACA-3′) primers were designed to introduce the BamHI and NotI restriction sites (underlined), respectively. The sequences of the oligonucleotide primers used for gene cloning were based on the cDNA sequence of human ALDH (GenBank accession no. AAP35567.1), and forward (5′- GTCGACATGTCATCCTCAGGCACGCCA-3′) and reverse (5′-CTCGAGTGAGTTCTTCTGAGAGATTTTCAC-3′) primers were designed to introduce the SalI and XhoI restriction sites (underlined), respectively. The amplified DNA fragments were purified using a QIAquick gel extraction kit (Qiagen, Germany), and the purified DNA fragments were ligated into the pRSFDuet-1 vector (Novagen, San Diego, CA) digested with the same restriction enzymes. The resulting plasmid was transformed into Escherichia coli ER2566 (New England BioLabs, Hertfordshire, United Kingdom). Site-directed mutagenesis was performed using a QuikChange kit (Stratagene, La Jolla, CA).
Protein purification and molecular mass determination.
The BcALDH protein and human ALDH were expressed and purified as described by Ngo et al. (13) and Morgan and Hurley (14), respectively. The subunit molecular mass of BcALDH was determined by SDS-PAGE under denaturing conditions, using prestained protein makers as reference proteins. The molecular mass of the native enzyme was determined using Sephacryl S-300 high-resolution gel filtration chromatography (GE Healthcare, Pittsburgh, PA). The purified enzyme solution was applied to the gel filtration column and eluted at a flow rate of 0.3 ml min−1 with 50 mM PIPES [N,N′-bis(2-ethanesulfonic acid)] buffer (pH 7.0) containing 150 mM NaCl. The column was calibrated using thyroglobulin (689 kDa), ferritin (453 kDa), aldolase (158 kDa), and conalbumin (75 kDa) as reference proteins. The mass of the native enzyme was calculated by comparing the migration time of the enzyme with that of the reference proteins.
Enzyme assay.
Unless otherwise stated, the enzymatic reaction of BcALDH for the conversion of all-trans-retinal to all-trans-retinoic acid or all-trans-retinol was performed at 37°C in 50 mM PIPES buffer (pH 7.0) or at 40°C in 50 mM EPPS [4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid] buffer (pH 7.5), respectively, containing 0.2 mM all-trans-retinal, 0.4 unit ml−1 (1 mg ml−1) enzyme, 5% (vol/vol) methanol, 4 mM 2-mercaptoethanol (2-ME), and 1 mM NAD+ or NADPH, respectively, for 30 min. Methanol at 5% (vol/vol) was used to solubilize all-trans-retinal (15). One unit was defined as the amount of enzyme required to produce 1 pmol of all-trans-retinoic acid or all-trans-retinol per min from all-trans-retinal at 37°C and pH 7.0 or at 40°C and pH 7.5, respectively, for BcALDH activity, and at 25°C and pH 7.0 for human ALDH. The Km (micromolar) and kcat (per minute) were determined by the Lineweaver-Burk plot derived from the Michaelis-Menten equation. To determine the kinetic parameters for the aldehyde substrates, the concentrations of retinoids were in the range of 2 to 40 μM, and those of acetaldehyde and benzaldehyde were in the range of 20 to 1,000 μM with 1 mM NAD+. To determine the kinetic parameters for cofactors, the concentrations of cofactors were in the range of 1 to 200 μM with 200 μM all-trans-retinal.
Conversion of all-trans-retinal into all-trans-retinoic acid or all-trans-retinol.
The conversion of all-trans-retinal to all-trans-retinoic acid or all-trans-retinol by the wild-type and variant enzymes of BcALDH was performed under the same conditions as were used in the enzyme assay except that 0.4 mM all-trans-retinal and 4 mM NAD+ or NADPH, respectively, were used for 240 min. The conversion by human ALDH was performed in 50 mM EPPS buffer (pH 8.0) containing 0.4 mM all-trans-retinal, 0.4 unit/ml of enzyme, 1 mM dithiothreitol (DTT), and 4 mM NAD+ or NADPH at 25°C for 80 min.
Analytical methods.
The same volume of methanol was added to the reaction solution, and the resulting solution was mixed with hexane and kept on ice for 5 min. After centrifugation at 10,000 × g for 30 min at 4°C, the substrates and products in the supernatant were analyzed by a high-performance liquid chromatography (HPLC) system (Agilent 1200; Agilent, Santa Clara, CA) equipped with an Atlantis difunctional C18 column (4.6 by 150 mm; Waters, Milford, MA) and a UV detector at 350 nm for retinal and retinoic acid, at 210 nm for acetaldehyde and acetic acid, and at 250 nm for benzaldehyde and benzoic acid. The column was eluted with a mixture of acetonitrile and 1% ammonium acetate in water, with 80:20 (vol/vol) as the mobile phase, at a flow rate of 1.2 ml min−1 at 30°C.
RESULTS
Sequence alignment of ALDHs.
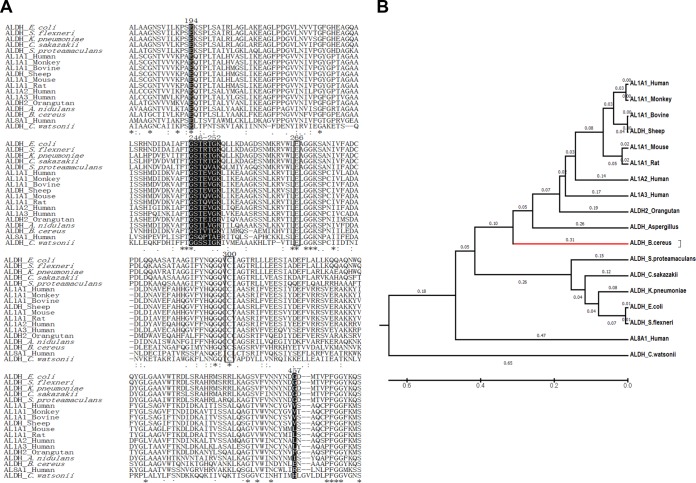
The amino acid sequence of BcALDH was compared with those of mammalian and bacterial ALDHs using ClustalX2 (16). The proposed catalytic residues Glu266 and Cys300 were completely conserved for all of the ALDHs. The Glu194, Glu246, Ser247, and Glu457 residues were conserved for all of the ALDHs among the proposed cofactor-binding residues Glu194, Gly246-Lys252, and Glu457 (17) (Fig. 2A). The amino acid sequence of BcALDH had 54.2, 54.0, 51.1, 50.9, 49.9, 49.7, 48.9, and 48.6% identity with ALDHs from eukaryotes, including orangutan, Aspergillus nidulans, human, sheep, monkey, bovine, rat, and mouse, respectively, and had 42.2, 41.7, 41.6, 41.2, 40.4, and 22.1% identity with ALDHs from bacteria, including Cronobacter sakazakii, E. coli, Shigella flexneri, Serratia proteamaculans, Klebsiella pneumoniae, and Crocosphaera watsonii, respectively. A phylogenic tree based on genetic characteristics was constructed using ClustalW and MEGA6 (18), and BcALDH showed a closer relationship to mammalian ALDHs than to bacterial ALDHs in the phylogenic tree (Fig. 2B).
FIG 2.
(A) Sequence alignment of bacterial and mammalian ALDHs. The amino acid sequences of ALDHs from B. cereus, E. coli, Shigella flexneri, Klebsiella pneumoniae, Cronobacter sakazakii, Serratia proteamaculans, and Crocosphaera watsonii and of bovine ALDH1A1, sheep ALDH, human ALDH1A1, mouse ALDH1A1, rat ALDH1A1, human ALDH1A2, human ALDH1A3, and human ALDH8A1 are aligned. The GenBank accession numbers of these ALDHs are KFL74159.1, BAA14869.1, ABF03518.1, ABR76453.1, ABU77332.1, ABV41168.1, CCQ55365.1, AAI05194.1, AAA85435.1, AAP35567.1, AAH54386.1, AAC53305.1, BAG54714.1, AAH69274.1, and BAD96568.1, respectively. The proposed catalytic and active-site residues are highlighted with a black box. Cofactor-binding residues are presented with white letters in black backgrounds. The asterisks, colons, and dots represent identity, conserved substitutions, and semiconserved substitutions, respectively. (B) Phylogenetic tree of mammalian and bacterial ALDHs. The evolutionary history was inferred using the unweighted pair group method using average linkages (UPGMA) method (35). The optimal tree, with the sum of branch lengths equal to 3.73188906, is shown. The tree is drawn to scale, with branch lengths (next to the branches) measured in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. The analysis involved 18 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 421 positions in the final data set. Evolutionary analyses were conducted in MEGA6 (18).
Molecular mass of BcALDH and identification of all-trans-retinoic acid.
The purified BcALDH showed a single band by SDS-PAGE, with a molecular mass of approximately 54 kDa (see Fig. S1A in the supplemental material), which is consistent with the calculated value of 54 kDa based on the 494 amino acids plus the hexa histidine tag. The native protein existed as a tetramer with a molecular mass of 216 kDa (Fig. S1B), as determined by gel filtration chromatography based on the masses of the reference proteins. Liquid chromatography-mass spectrometry (LC-MS) analysis was performed to identify the product obtained from all-trans-retinal by the reaction of BcALDH (Fig. S6A). The total molecular mass of the product was shown as a peak at m/z 301.19, corresponding to exactly the same molecular mass of the all-trans-retinoic acid standard (Fig. S6B) (19), indicating that the peak was all-trans-retinoic acid.
Effects of metal ions, temperature, pH, and antioxidants on BcALDH's activity.
The effects of metal ions on the activity of BcALDH were evaluated in the presence of metal ions at 1 mM. The oxidation activity of BcALDH that produces all-trans-retinoic acid (see Fig. S2A in the supplemental material) and the reduction activity (see Fig. S3A in the supplemental material) that produces all-trans-retinol from all-trans-retinal were not activated by divalent cations. The maximum activity was observed at 37°C and pH 7.0 for oxidation (see Fig. S2B and C in the supplemental material) and 40°C and pH 7.5 for reduction (see Fig. S3B and C in the supplemental material). The effects of antioxidants in preventing the disruption of retinal and restoring the catalytic residue cysteine (20, 21), such as butylated hydroxytoluene (BHT), DTT, hydroquinone (HQ), and 2-ME, on the activity of BcALDH were investigated. The enzyme activity for both oxidation and reduction was the highest when 2-ME was used as the antioxidant (see Fig. S4A in the supplemental material), and its optimal concentration was 4 mM (see Fig. S4B in the supplemental material). Thus, 4 mM 2-ME was used for the enzymatic reactions. A cysteine residue in ALDH with a thiol group as a functional group has been involved in the catalytic activity. The addition of DTT or 2-ME reduces the thiol group of cysteine to a thiolate group by changing the antioxidant to the oxidized form (20, 21). 2-ME has a reducing power and increased enzyme solubility, which may result in the increased activity for ALDH by 2-ME.
Substrate and cofactor specificities of BcALDH.
The oxidation activities for aldehyde substrates were measured in the presence of 1 mM NAD+. The specific activities occurred in the order benzaldehyde, acetaldehyde, and all-trans-retinal, and no activity was observed for 9-cis-retinal and 13-cis-retinal (Table 1). All-trans-retinal showed the lowest Km, which was 14- and 29-fold lower than the Km values of acetaldehyde and benzaldehyde, respectively. The kinetic parameters of BcALDH with a cofactor were determined using all-trans-retinal (Table 2). All-trans-retinal was oxidized to all-trans-retinoic acid with NADP+, as well as NAD+. The Km and kcat of NAD+ for the oxidation of all-trans-retinal were similar to those of NADP+. BcALDH reduced all-trans-retinal to all-trans-retinol with NADPH, although the reduction activity was much lower than the typical oxidation activity. However, the reduction activity for all-trans-retinal was not detected with NADH. For other substrates, including benzaldehyde, acetaldehyde, 9-cis-retinal, and 13-cis-retinal, the atypical reduction activity of BcALDH was not found with NADH or NADPH.
TABLE 1.
Kinetic parameters for aldehyde substrates of BcALDH in oxidation activity using NAD+ as a cosubstratea
| Substrate | Product | Sp act (U/mg) | Km (μM) | kcat (min−1) | kcat/Km (mM−1 min−1) |
|---|---|---|---|---|---|
| All-trans-retinal | All-trans-retinoic acid | 461 ± 10 | 7 ± 0.1 | 0.28 ± 0.03 | 40 ± 0.2 |
| 9-cis-Retinal | 9-cis-Retinoic acid | NC | NC | NC | NC |
| 13-cis-Retinal | 13-cis-Retinoic acid | NC | ND | ND | ND |
| Acetaldehyde | Acetic acid | 2,550 ± 10 | 95 ± 2 | 11 ± 0.4 | 111 ± 5 |
| Benzaldehyde | Benzoic acid | 3,370 ± 16 | 200 ± 5 | 13 ± 0.8 | 65 ± 4 |
Data represent the means ± standard deviations of the results from three experiments. NAD+ at 1 mM was used as the cosubstrate in each reaction mixture. NC, not calculated by the analytical methods used in this study due to limits of sensitivity; ND, not detected by the analytical methods used in this study.
TABLE 2.
Kinetic parameters for cofactors of BcALDH in oxidation and reduction reactions using all-trans-retinal as a cosubstratea
| Substrate | Reaction | Km (μM) | kcat (min−1) | kcat/Km (mM−1 min−1) |
|---|---|---|---|---|
| NAD+ | Oxidation | 4.0 ± 0.2 | 0.10 ± 0.01 | 25 ± 0.1 |
| NADP+ | Oxidation | 4.2 ± 0.3 | 0.11 ± 0.02 | 26 ± 0.2 |
| NADPH | Reduction | 40 ± 1.0 | 0.033 ± 0.00 | 0.83 ± 0.05 |
| NADH | Reduction | ND | ND | ND |
Data represent the means ± standard deviations of the results from three experiments. All-trans-retinal at 0.2 mM was used as the cosubstrate in each reaction mixture. ND, not detected by the analytical methods used in this study.
The production of all-trans-retinoic acid and all-trans-retinol was investigated by supplementing NADH with NAD+ or NADP+ with NADPH at ratios ranging from 0:4 to 4:0 mM to make up a total concentration of 4 mM. All-trans-retinoic acid increased as the ratio of NAD+ to NADH increased. However, all-trans-retinoic acid decreased and all-trans-retinol increased as the ratio of NADPH to NADP+ increased (see Fig. S7A and B in the supplemental material). When NADH was added to 4 mM NAD+, all-trans-retinoic acid decreased as the concentration of additional NADH increased (Fig. S7C). However, all-trans-retinoic acid increased and all-trans-retinol decreased as the concentration of additional NADP+, which was supplemented with 4 mM NADPH, increased (Fig. S7D). Thus, all-trans-retinoic acid production is stimulated by decreasing the ratio of NADPH to NADP+, whereas all-trans-retinol production is stimulated by increasing the ratio.
The effect of cofactor concentration on the conversion of all-trans-retinoic acid or all-trans-retinol from all-trans-retinal by BcALDH was performed by varying the concentration of NAD+ or NADPH, respectively (Fig. 3). The conversion of all-trans-retinal to all-trans-retinoic acid or all-trans-retinol was increased up to approximately 5 mM cofactor. However, above this concentration, the formation of products did not increase. Thus, the optimal concentration for the conversion of all-trans-retinal to all-trans-retinoic acid or all-trans-retinol was 5 mM.
FIG 3.
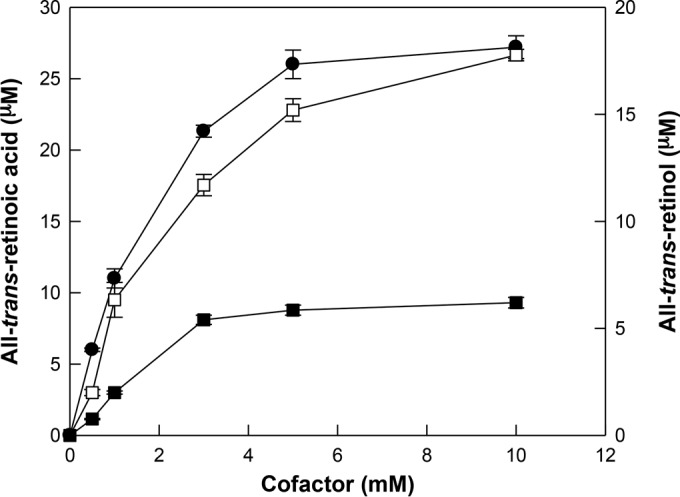
Effect of cofactor concentration on the conversion of all-trans-retinal to all-trans-retinoic acid or all-trans-retinol. The cofactor concentrations used for investigating the effect were significantly higher than the Km values. The conversion of all-trans-retinal to all-trans-retinoic acid using NAD+ (●) or NADP+ (□) was performed in 50 mM PIPES buffer (pH 7.0) containing 0.4 mM all-trans-retinal, 0.4 unit/ml of enzyme, and 1 mM 2-ME at 37°C for 30 min. The conversion of all-trans-retinal to all-trans-retinol using NADPH (■) was performed in 50 mM EPPS buffer (pH 7.5) containing 0.4 mM all-trans-retinal, 0.4 unit/ml enzyme, and 1 mM 2-ME at 40°C for 30 min. The data represent the means of the results from three experiments and the error bars represent standard deviations.
Catalytic and cofactor-binding residues of BcALDH are involved in the oxidation and reduction of all-trans-retinal.
Site-directed mutagenesis for the catalytic and cofactor-binding residues involved in the oxidation and reduction of all-trans-retinal was performed, and the kinetic parameters of the wild-type and variant enzymes were determined (Table 3). The E266A and C300A variants for the catalytic residues abolished the oxidation activity, and the catalytic efficiency (kcat/Km) of the C300S variant for the oxidation of all-trans-retinal with NAD+ was 23.5% of that of the wild-type enzyme. The catalytic efficiencies of the E194S and E457V variants for the cofactor-binding residues for the reduction of all-trans-retinal with NADPH were 15- and 6.2-fold higher than the catalytic efficiency of the wild-type enzyme, respectively. The catalytic efficiency of the E194S variant for the oxidation with NAD+ was 1.3-fold lower than that of the wild-type enzyme, whereas the catalytic efficiency of the E457V variant for the oxidation was 7.5-fold higher.
TABLE 3.
Kinetic parameters of the wild-type and variant enzymes of BcALDH related to catalytic and cofactor-binding residues for all-trans-retinal in oxidation and reduction activitiesa
| Enzyme | Cofactor | Sp act (U mg−1) | Km (μM) | kcat (min−1) | kcat/Km (mM−1 min−1) |
|---|---|---|---|---|---|
| Wild-type | NAD+ | 461 ± 10 | 7.0 ± 0.13 | 0.28 ± 0.03 | 40 ± 0.2 |
| NADPH | 136 ± 6 | 130 ± 5 | 0.12 ± 0.01 | 0.90 ± 0.08 | |
| E194S variant | NAD+ | 493 ± 3 | 10 ± 0.5 | 0.30 ± 0.01 | 30 ± 0.1 |
| NADPH | 408 ± 11 | 29 ± 0.9 | 0.39 ± 0.01 | 14 ± 0.1 | |
| E266A variant | NAD+ | ND | ND | ND | ND |
| NADPH | 80 ± 2.0 | 920 ± 5 | 0.07 ± 0.003 | 0.07 ± 0.005 | |
| C300A variant | NAD+ | ND | ND | ND | ND |
| NADPH | 68 ± 2.0 | 38 ± 2.0 | 0.04 ± 0.003 | 1.1 ± 0.05 | |
| C300S variant | NAD+ | 38.6 ± 2 | 3.70 ± 0.05 | 0.04 ± 0.002 | 9.4 ± 0.05 |
| NADPH | 12 ± 0.5 | 420 ± 3 | 0.04 ± 0.002 | 0.08 ± 0.01 | |
| E457V variant | NAD+ | 2,536 ± 11 | 6.25 ± 0.5 | 1.54 ± 0.1 | 246 ± 6 |
| NADPH | 260 ± 2 | 34 ± 0.5 | 0.23 ± 0.02 | 6.76 ± 0.2 |
Data represent the means ± standard deviations of the results from three experiments. ND, not detected by the analytical methods used in this study.
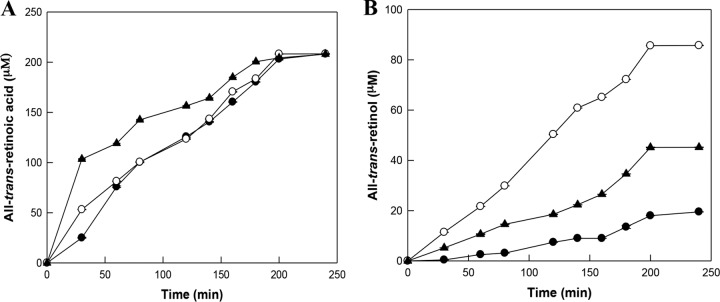
Conversion of all-trans-retinal to all-trans-retinoic acid or all-trans-retinol by the wild-type, E194S variant, and E457V variant enzymes of BcALDH with cofactor switching.
Time course reactions for the production of all-trans-retinoic acid or all-trans-retinol were performed from 400 μM all-trans-retinal in the presence of 5 mM NAD+ using the wild-type, E194S variant, and E457V variant enzymes of BcALDH. After 240 min, the concentrations of all-trans-retinoic acid produced from 400 μM all-trans-retinal by the wild-type E194S and E457V variant enzymes were the same as those produced at 210 μM, with a molar conversion yield of 53% (Fig. 4A). The concentrations of all-trans-retinol produced from 400 μM all-trans-retinal by the wild-type, E194S variant, and E457V variant enzymes were 20, 90, and 40 μM, respectively, with molar conversion yields of 5, 24, and 11%, respectively (Fig. 4B). Glu194 of BcALDH corresponded to Glu196 of human ALDH. The production of all-trans-retinoic acid or all-trans-retinol from all-trans-retinal by the wild-type and E196S variant enzymes of human ALDH was performed. After 80 min, the concentration of all-trans-retinoic acid produced by the wild-type enzyme was almost the same (5 μM) as that produced by the E196S variant (see Fig. S5A in the supplemental material). However, the concentration of all-trans-retinol produced by the E196S variant (13 μM) was 4.6-fold higher than that produced by the wild-type enzyme (3 μM) (Fig. S5B). Therefore, the effect of the E194S variant of BcALDH on the production of all-trans-retinol or all-trans-retinoic acid was similar to that of the E196S variant of human ALDH.
FIG 4.
Biotransformation of all-trans-retinal to retinoic acid or retinol by the wild-type, E194S variant, and E457V variant enzymes of BcALDH with cofactor switching. (A) Time course reactions for the conversion of all-trans-retinal to all-trans-retinoic acid by the wild-type (●), E194S variant (○), and E457V variant (▲) enzymes of BcALDH using NAD+. The reactions were performed in 50 mM PIPES buffer (pH 7.0) containing 0.4 mM all-trans-retinal, 0.4 unit/ml enzyme, 4 mM 2-ME, and 5 mM NAD+ at 37°C for 240 min. (B) Time course reactions for the conversion of all-trans-retinal to all-trans-retinol by the wild-type (●), E194S variant (○), and E457V variant (▲) enzymes of BcALDH using NADPH. The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 0.4 mM all-trans-retinal, 0.4 unit/ml enzyme, 4 mM 2-ME, and 5 mM NADPH at 40°C for 240 min.
DISCUSSION
ALDHs catalyze the oxidation of aldehydes to carboxylic acids, which are given from EC 1.2.1.1 to EC 1.2.1.97. EC 1.2.1.1 and EC 1.2.1.2 are S-(hydroxymethyl)glutathione dehydrogenase and formate dehydrogenase, respectively. EC 1.2.1.3, EC 1.2.1.4, and EC 1.2.1.5 are dependent on NAD+, NADP+, and NAD(P)+, respectively. Other fourth EC digits are specified by preferred aldehydes. Among them, retinal dehydrogenase (RALDH) (EC 1.2.1.36) catalyzes the oxidation of retinal to retinoic acid. BcALDH was identified as EC 1.2.1.5 (22) because it converted aldehydes to carboxylic acids using both NAD+ and NADP+. BcALDH also exhibited RALDH activity, with a high sequence identity to RALDH. Although retinol dehydrogenase (EC 1.1.1.300) catalyzes the conversion of retinal to retinol using NAD(P)H, the enzyme belongs to subclass groups (EC 1.1) different from alcohol dehydrogenases.
Retinoic acid has been synthesized by the oxidation activity of mammalian ALDHs from retinal (Fig. 1A). Retinoic acid plays a key role in embryonic development, and the formation of retinoic acid is generally considered to be a mammal-specific reaction (23). Bacterial ALDHs are known for being enzymes that transform small aldehydes, such as acetaldehyde and benzaldehyde, into their corresponding carboxylic acids for detoxification (24). Thus, so far, no evidence for the formation retinoic acid by bacterial ALDHs has been provided. To find a bacterial ALDH that converts retinal to retinoic acid, the sequences of bacterial ALDHs were aligned with those of mammalian ALDHs showing all-trans-retinal conversion (Fig. 2A), and a phylogenic tree with bacterial and mammalian ALDHs was constructed (Fig. 2B). As a result, human ALDH1 and B. cereus ALDHs were grouped in the same branch of the ALDH1 family in the phylogenic tree. The ALDH1 family has been known to be involved in the conversion of the large aldehyde all-trans-retinal to all-trans-retinoic acid (17), suggesting that BcALDH can transform retinal to retinoic acid. This suggestion was verified by determining the converting activity of BcALDH for retinal. Therefore, this enzyme is the first retinoic acid-producing enzyme among bacterial ALDHs.
Glu266, Cys300, Glu194, and Glu457 of BcALDH were selected based on sequence alignment with mammalian ALDHs (Fig. 2A), because the Glu266 and Cys300 residues are known to be catalytic residues for the oxidation activity for all-trans-retinal (25, 26), and the Glu194 and Glu457 residues are the cofactor-binding residues in mammalian ALDHs (13, 26–28). The E266A and C300A variants of BcALDH completely lost their oxidative activity (Table 3). ALDHs favor NADPH if the residue at position 194 has a short polar side chain, like Ser or Thr, and prefer NAD+ if the residue at position 194 has a longer side chain, like Glu (29). The substitution of a smaller residue at position 194 might help the reduction of BcALDH by the higher usage of NADPH. Thus, the reduction activity of the E194S variant with NADPH was significantly increased compared to that of the wild-type enzyme (Table 3). The acidic residue of Glu at position 457 of BcALDH is uncommon in mammalian ALDHs, which have the hydrophobic residue Val or Leu at the corresponding position (Fig. 2A). The residue at position 457 is located in the substrate-binding channel for the long hydrophobic chain of all-trans-retinal (17). The activity of the E457V variant increased in both the oxidation and reduction of all-trans-retinal (Table 3). Thus, we speculate that the increased hydrophobicity via the substitution of Glu to Val at position 457 may facilitate the substrate binding.
Mammalian-ALDH-like BcALDH catalyzes not only the oxidation of all-trans-retinal to all-trans-retinoic acid but also the reduction of all-trans-retinal to all-trans-retinol with a cofactor switch. Although the production of all-trans-retinoic acid by the E457V and E194S variants of BcALDH was the same as that by the wild-type enzyme (Fig. 4A), the production of all-trans-retinol by the E457V and E194S variants was higher (Fig. 4B). The results imply that we can engineer BcALDH to be more suitable for the specific production of all-trans-retinol. All-trans-retinal has been produced from β-carotene by β-carotene 15,15′-oxygenases (30–32) and from glycerol by metabolically engineered E. coli expressing carotenoid-synthesizing enzymes and carotenoid oxygenase (33, 34). However, the biotechnological production of retinoic acid from retinal has not been attempted yet, because a convincing metabolic pathway or specific enzyme related to retinoic acid production in prokaryotes has not been identified. We first found BcALDH to be an effective enzyme for the conversion of retinal to retinoic acid. BcALDH can be used with carotenoid oxygenase to produce all-trans-retinoic acid from β-carotene by enzymatic reactions or in whole-cell production with a cofactor regeneration system. Its gene can be introduced into all-trans-retinal-producing, metabolically engineered cells for the production of all-trans-retinoic acid from a relevant substrate, such as glucose or glycerol, for cost-effective industrial production.
In summary, BcALDH, which was found to be a mammalian-ALDH-like ALDH in the phylogenic tree of mammalian and bacterial ALDHs, was first identified as a bacterial retinoic acid-producing enzyme from retinal. We newly found that BcALDH and human ALDH also could reduce retinal to retinol with a cofactor switch. The reduction activity of BcALDH was increased by its E457V and E194S variants. Wild-type BcALDH and its variants can be used for the alternative biotransformation of retinal to retinoic acid or retinol, and the genes can be introduced in retinal-producing metabolically engineered cells for retinoic acid or retinol production. Our work is an initiative for the biotechnological production of retinoids, including retinoic acid and retinol, using a specific bacterial enzyme. Our approach can also contribute to finding a novel biocatalyst that has not yet been discovered from prokaryotic origins based on the information of eukaryotic biocatalysts.
Supplementary Material
ACKNOWLEDGMENT
We declare no conflicts of interest.
Funding Statement
This research was supported by a research grant from the 2015 KU Brain Pool of Konkuk University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00848-16.
REFERENCES
- 1.De Luca LM. 1991. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J 5:2924–2933. [PubMed] [Google Scholar]
- 2.Fisher GJ, Voorhees JJ. 1996. Molecular mechanisms of retinoid actions in skin. FASEB J 10:1002–1013. [DOI] [PubMed] [Google Scholar]
- 3.Semba RD. 1999. Vitamin A as “anti-infective” therapy, 1920–1940. J Nutr 129:783–791. [DOI] [PubMed] [Google Scholar]
- 4.Chytil F, Riaz-ul-Hag . 1990. Vitamin A mediated gene expression. Crit Rev Eukaryot Gene Expr 1:61–73. [PubMed] [Google Scholar]
- 5.Raifen R, Altman Y, Zadik Z. 1996. Vitamin A levels and growth hormone axis. Horm Res 46:279–281. doi: 10.1159/000185101. [DOI] [PubMed] [Google Scholar]
- 6.Chanda B, Ditadi A, Iscove NN, Keller G. 2013. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 155:215–227. doi: 10.1016/j.cell.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 7.Romand R, Krezel W, Beraneck M, Cammas L, Fraulob V, Messaddeq N, Kessler P, Hashino E, Dollé P. 2013. Retinoic acid deficiency impairs the vestibular function. J Neurosci 33:5856–5866. doi: 10.1523/JNEUROSCI.4618-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs FM, Smits SM, Noorlander CW, von Oerthel L, van der Linden AJ, Burbach JP, Smidt MP. 2007. Retinoic acid counteracts developmental defects in the substantia nigra caused by Pitx3 deficiency. Development 134:2673–2684. doi: 10.1242/dev.02865. [DOI] [PubMed] [Google Scholar]
- 9.Ascenso A, Ribeiro H, Marques HC, Oliveira H, Santos C, Simões S. 2014. Is tretinoin still a key agent for photoaging management? Mini Rev Med Chem 14:629–641. doi: 10.2174/1389557514666140820102735. [DOI] [PubMed] [Google Scholar]
- 10.Bhat PV, Poissant L, Wang XL. 1996. Purification and partial characterization of bovine kidney aldehyde dehydrogenase able to oxidize retinal to retinoic acid. Biochem Cell Biol 74:695–700. doi: 10.1139/o96-076. [DOI] [PubMed] [Google Scholar]
- 11.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penzes P, Wang X, Napoli JL. 1997. Enzymatic characteristics of retinal dehydrogenase type I expressed in Escherichia coli. Biochim Biophys Acta 1342:175–181. doi: 10.1016/S0167-4838(97)00102-7. [DOI] [PubMed] [Google Scholar]
- 13.Ngo HP, Hong SH, Oh DK, Kang LW. 2013. Expression, crystallization and preliminary X-ray crystallographic analysis of aldehyde dehydrogenase (ALDH) from Bacillus cereus. Acta Crystallogr Sect F Struct Biol Cryst Commun 69:528–531. doi: 10.1107/S1744309113007288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan CA, Hurley TD. 2015. Characterization of two distinct structural classes of selective aldehyde dehydrogenase 1A1 inhibitors. J Med Chem 58:1964–1975. doi: 10.1021/jm501900s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong SH, Nam HK, Kim KR, Kim SW, Oh DK. 2013. Molecular characterization of an aldo-keto reductase from Marivirga tractuosa that converts retinal to retinol. J Biotechnol 169:23–33. [DOI] [PubMed] [Google Scholar]
- 16.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 17.Sobreira TJ, Marlétaz F, Simões-Costa M, Schechtman D, Pereira AC, Brunet F, Sweeney S, Pani A, Aronowicz J, Lowe CJ, Davidson B, Laudet V, Bronner M, de Oliveira PS, Schubert M, Xavier-Neto J. 2010. Structural shifts of aldehyde dehydrogenase enzymes were instrumental for the early evolution of retinoid-dependent axial patterning in metazoans. Proc Natl Acad Sci U S A 108:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Oh DG, Kim GR, Lee JS, Son YK, Bae CH, Yeo J, Lee CH. 2015. Chemotaxonomic metabolite profiling of 62 indigenous plant species and its correlation with bioactivities. Molecules 20:19719–19734. doi: 10.3390/molecules201119652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daiber A, Oelze M, Coldewey M, Bachschmid M, Wenzel P, Sydow K, Wendt M, Kleschyov AL, Stalleicken D, Ullrich V, Mulsch A, Münzel T. 2004. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol 66:1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- 21.Mukerjee N, Pietruszko R. 1994. Inactivation of human aldehyde dehydrogenase by isosorbide dinitrate. J Biol Chem 269:21664–21669. [PubMed] [Google Scholar]
- 22.Barthelmes J, Ebeling C, Chang A, Schomburg I, Schomburg D. 2007. BRENDA, AMENDA and FRENDA: the enzyme information system in 2007. Nucleic Acids Res 35:D511–D514. doi: 10.1093/nar/gkl972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janesick A, Wu SC, Blumberg B. 2015. Retinoic acid signaling and neuronal differentiation. Cell Mol Life Sci 72:1559–1576. doi: 10.1007/s00018-014-1815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo JE, Mohan Raj S, Rathnasingh C, Selvakumar E, Jung WC, Park S. 2008. Cloning, expression, and characterization of an aldehyde dehydrogenase from Escherichia coli K-12 that utilizes 3-hydroxypropionaldehyde as a substrate. Appl Microbiol Biotechnol 81:51–60. doi: 10.1007/s00253-008-1608-x. [DOI] [PubMed] [Google Scholar]
- 25.Farres J, Wang TT, Cunningham SJ, Weiner H. 1995. Investigation of the active site cysteine residue of rat liver mitochondrial aldehyde dehydrogenase by site-directed mutagenesis. Biochemistry 34:2592–2598. doi: 10.1021/bi00008a025. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Weiner H. 1995. Involvement of glutamate 268 in the active site of human liver mitochondrial (class 2) aldehyde dehydrogenase as probed by site-directed mutagenesis. Biochemistry 34:237–243. doi: 10.1021/bi00001a028. [DOI] [PubMed] [Google Scholar]
- 27.Abriola DP, Fields R, Stein S, MacKerell AD Jr, Pietruszko R. 1987. Active site of human liver aldehyde dehydrogenase. Biochemistry 26:5679–5684. doi: 10.1021/bi00392a015. [DOI] [PubMed] [Google Scholar]
- 28.Moore SA, Baker HM, Blythe TJ, Kitson KE, Kitson TM, Baker EN. 1998. Sheep liver cytosolic aldehyde dehydrogenase: the structure reveals the basis for the retinal specificity of class 1 aldehyde dehydrogenases. Structure 6:1541–1551. doi: 10.1016/S0969-2126(98)00152-X. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Z, Yin B, Wei D, Yuan YR. 2013. Structural basis for cofactor and substrate selection by cyanobacterium succinic semialdehyde dehydrogenase. J Struct Biol 182:125–135. doi: 10.1016/j.jsb.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Kim NH, Kim YS, Kim HJ, Oh DK. 2008. Optimized formation of detergent micelles of beta-carotene and retinal production using recombinant human beta,beta-carotene 15,15′-monooxygenase. Biotechnol Prog 24:227–231. doi: 10.1021/bp070239k. [DOI] [PubMed] [Google Scholar]
- 31.Kim YS, Kim NH, Kim HJ, Lee JK, Kim SW, Oh DK. 2007. Effective production of retinal from beta-carotene using recombinant mouse beta-carotene 15,15′-monooxygenase. Appl Microbiol Biotechnol 76:1339–1345. doi: 10.1007/s00253-007-1118-2. [DOI] [PubMed] [Google Scholar]
- 32.Kim YS, Park CS, Oh DK. 2010. Retinal production from beta-carotene by beta-carotene 15,15′-dioxygenase from an unculturable marine bacterium. Biotechnol Lett 32:957–961. doi: 10.1007/s10529-010-0239-3. [DOI] [PubMed] [Google Scholar]
- 33.Jang HJ, Ha BK, Zhou J, Ahn J, Yoon SH, Kim SW. 2015. Selective retinol production by modulating the composition of retinoids from metabolically engineered E. coli. Biotechnol Bioeng 112:1604–1612. doi: 10.1002/bit.25577. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Choi JG, Kim YS, Kim KR, Kim SW, Oh DK. 2012. Enhancement of retinal production by supplementing the surfactant Span 80 using metabolically engineered Escherichia coli. J Biosci Bioeng 113:461–466. doi: 10.1016/j.jbiosc.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Jones JH, Lennard-Jones JE, Morson BC, Chapman M, Sackin MJ, Sneath PH, Spicer CC, Card WI. 1973. Numerical taxonomy and discriminant analysis applied to non-specific colitis. Q J Med 42:715–732. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.