ABSTRACT
The blue wavelengths within the visible light spectrum are intrinisically antimicrobial and can photodynamically inactivate the cells of a wide spectrum of bacteria (Gram positive and negative) and fungi. Furthermore, blue light is equally effective against both drug-sensitive and -resistant members of target species and is less detrimental to mammalian cells than is UV radiation. Blue light is currently used for treating acnes vulgaris and Helicobacter pylori infections; the utility for decontamination and treatment of wound infections is in its infancy. Furthermore, limited studies have been performed on bacterial biofilms, the key growth mode of bacteria involved in clinical infections. Here we report the findings of a multicenter in vitro study performed to assess the antimicrobial activity of 400-nm blue light against bacteria in both planktonic and biofilm growth modes. Blue light was tested against a panel of 34 bacterial isolates (clinical and type strains) comprising Acinetobacter baumannii, Enterobacter cloacae, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Enterococcus faecium, Klebsiella pneumoniae, and Elizabethkingia meningoseptica. All planktonic-phase bacteria were susceptible to blue light treatment, with the majority (71%) demonstrating a ≥5-log10 decrease in viability after 15 to 30 min of exposure (54 J/cm2 to 108 J/cm2). Bacterial biofilms were also highly susceptible to blue light, with significant reduction in seeding observed for all isolates at all levels of exposure. These results warrant further investigation of blue light as a novel decontamination strategy for the nosocomial environment, as well as additional wider decontamination applications.
IMPORTANCE Blue light shows great promise as a novel decontamination strategy for the nosocomial environment, as well as additional wider decontamination applications (e.g., wound closure during surgery). This warrants further investigation.
INTRODUCTION
Antimicrobial resistance (AMR) is rapidly evolving and emerging to be a large threat to modern medicine. Although affecting only a minority of admissions, health care-associated infections are associated with increased mortality, prolonged hospital stays, and increased treatment costs (1). With the rise in resistance to the carbapenem class of antibiotics in Gram-negative organisms (2), there is a significant threat of infections becoming wholly untreatable with current treatment regimens (3, 4).
Much research is now focused on alternatives to the conventional antimicrobial agents. These mostly involve topical agents (with the aim to reduce surface contamination and therefore lower the risks of sepsis and infection progression) with research to date on a large number of agents. Since the environment is a key source of nosocomial pathogens (5), there has also been renewed focus on hospital cleaning and disinfection, especially via antimicrobial chemicals delivered in a novel way, including antimicrobial light sources (1, 6). These novel strategies, capable of decontaminating both the patient's wound and the environment, have the potential to be highly beneficial in the fight against AMR and nosocomial infections.
The blue wavelengths within the visible light spectrum (especially wavelengths between 400 and 470 nm) are intrinsically antimicrobial and do not require additional exogenous photosensitizers to exert an antimicrobial effect (4). Photodynamic inactivation of both bacterial and fungal cells occurs as a result of photoexcitation of intracellular porphyrins (1) by blue light, leading to energy transfer and the production of highly cytotoxic reactive oxygen species (ROS), primarily singlet oxygen (1O2) (4, 7–9). All wavelengths from 400 to 425 nm can be used for microbial inactivation; however, the optimal antimicrobial activity occurs at 405 nm, since this is the point in the electromagnetic spectrum where maximum porphyrin excitation occurs (10). Although blue light is less germicidal than UV light (1), pathogens can be selectively inactivated without damaging human cells, and consequently, blue light is considered much less detrimental to mammalian cells than UV light (11, 12). One potential benefit of light-based antimicrobial therapies is equal efficacies against drug-sensitive and resistant members of target species (13, 14).
Blue light has been shown to exhibit a broad spectrum of antimicrobial effect against bacteria and fungi, although generally the Gram-positive bacteria are considered more susceptible to blue light than the Gram-negative bacteria (15, 16). Successful inactivation has been demonstrated in vitro against Staphylococcus aureus (including methicillin-resistant S. aureus [MRSA]), Clostridium difficile (both spores and vegetative cells), Acinetobacter baumannii, Escherichia coli, Staphylococcus epidermidis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Streptococcus pyogenes, and Mycobacterium spp. (14, 15, 17, 18). In addition to the key nosocomial pathogens, blue light is also effective against Propionibacterium acnes and has been used topically to treat acne vulgaris (19, 20) and Helicobacter pylori, in which case blue light is used internally as a “light string” to treat stomach infections (21). Owing to the mechanism of action of blue light, it is unlikely that viruses will be susceptible unless photosensitizers are added to enhance virucidal activity (22).
The use of blue light for treatment of wound infections in vivo is an emerging technology. To date, blue light therapy has been shown to significantly reduce the bacterial burden of wounds infected with P. aeruginosa (23), MRSA (24), and A. baumannii (25), and it saved the lives of mice subjected to potentially lethal burns contaminated with P. aeruginosa and A. baumannii (23, 25).
As well as having clinical application for patient treatment, blue light is also a promising candidate for the control of problematic microorganisms in the clinical setting (e.g., the disinfection of air and exposed surfaces). In this regard, Bache et al. (26) and Maclean et al. (1) have performed studies with a new disinfection technology termed the HINS-light environmental decontamination system (EDS) which delivers low-irradiance 405-nm light continuously and is suitable for use in patient-occupied settings. Evaluation studies performed by the latter authors showed that there was a statistically significant 90% reduction in numbers of culturable Staphylococci spp. following 24 h of use in an unoccupied room (5) and reductions of 56 to 86% when used in burn isolation rooms occupied by MRSA-positive patients. Furthermore, when the system was no longer used, the room became recontaminated to levels similar to those pretreatment.
The vast majority of research on blue light has been carried out on bacteria in the planktonic phase, dispersed evenly in a liquid medium. In nature this is rarely the case, since most bacteria aggregate to form complex communities within a matrix of extracellular polymeric substances termed a biofilm. There are many advantages for this compared to planktonic growth, including increased resistance to killing via antimicrobials, immune cells, chemicals, and environmental stresses (27). Furthermore, once a biofilm has become established on a surface, it is extremely hard to eradicate. Medically, biofilms have been associated with a myriad of chronic infections, acute infections, colonization of indwelling medical devices, and wound infections (27–29).
Since we know that the majority of clinical infections and environmental contamination involve microbial biofilms (30), this multicenter in vitro study was performed to assess the antibacterial activity of blue light against biofilms of a range of important nosocomial pathogens.
MATERIALS AND METHODS
A series of in vitro experiments were conducted with a panel of organisms (Table 1) to determine the efficacy of blue light (400 nm) against bacteria in planktonic (free-floating in broth) and biofilm (attached to a surface) modes of growth. The panel comprised well-characterized control and clinical isolates (in terms of their antibiograms and abilities to form biofilms in vitro) and concentrated mostly on A. baumannii strains from a protracted outbreak at the Queen Elizabeth Hospital in Birmingham (QEHB) (31). A. baumannii is a key nosocomial pathogen which survives in hospital and health care environments despite conditions such as desiccation, nutrient starvation, and antimicrobial chemicals (e.g., disinfectants) (32, 33). Despite stringent infection control practices, a large outbreak of A. baumannii occurred at QEHB, where 65 patients tested positive during the outbreak period (July 2011 to February 2013). The strains from this outbreak demonstrated a high degree of resilience in the hospital environment, and there was also evolution among the isolates over time to increase desiccation resistance and biofilm formation capacity. Additional A. baumannii isolates (representing genetically diverse strains) were tested in this panel to add some diversity to the strains, including strains ACI_AYE (a representative of international clone I, a major globally relevant lineage), ACI_C60 (a control strain of a unique pulsed-field gel electrophoresis [PFGE] type), and ACI_19606 (a control strain of a further unique PFGE type) (typing data not shown).
TABLE 1.
Clinical and control isolates used in this studya
| Study identifier | Organism | Description |
|---|---|---|
| ACI_616 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_618 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_642 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_648 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_659 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_665 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_671 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_672 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_698 | Acinetobacter baumannii | QEHB clinical outbreak isolate |
| ACI_AYE | Acinetobacter baumannii | MPR clinical isolate (unique) |
| ACI_C60 | Acinetobacter baumannii | NCTC 13424 (unique) |
| ACI_19606 | Acinetobacter baumannii | ATCC 19606 (unique) |
| ENTCL_525 | Enterobacter cloacae complex | QEHB clinical isolate |
| ENTCL_801 | Enterobacter cloacae complex | QEHB clinical isolate |
| ENTCL_804 | Enterobacter cloacae complex | QEHB clinical isolate |
| STEMA_529 | Stenotrophomonas maltophilia | QEHB clinical isolate |
| STEMA_551 | Stenotrophomonas maltophilia | QEHB clinical isolate |
| STEMA_558 | Stenotrophomonas maltophilia | QEHB clinical isolate |
| PSE_568 | Pseudomonas aeruginosa | QEHB clinical isolate |
| PSE_PA01 | Pseudomonas aeruginosa | ATCC 15692 |
| PSE_6749 | Pseudomonas aeruginosa | NCTC 6749 |
| PSE_1054 | Pseudomonas aeruginosa | QEHB clinical burn isolate |
| PSE_1586 | Pseudomonas aeruginosa | QEHB clinical burn isolate |
| EKIN_502 | Elizabethkingia meningoseptica | QEHB clinical isolate |
| EC_073 | Escherichia coli | EPEC CFT_073 |
| EC_042 | Escherichia coli | EAEC_042 |
| MDR_A | CPE Klebsiella pneumoniae (NDM-1 positive) | QEHB clinical isolate |
| MDR_B | CRE Klebsiella pneumoniae (ESBL positive with additional permeability changes) | QEHB clinical isolate |
| MRSA_508 | Staphylococcus aureus | QEHB clinical isolate |
| MRSA_520 | Staphylococcus aureus | QEHB clinical isolate |
| MRSA_531 | Staphylococcus aureus | QEHB clinical isolate |
| MSSA_10788 | Staphylococcus aureus | NCTC 10788 |
| MSSA_F77 | Staphylococcus aureus | NCTC 8532 |
| EFM_513 | Enterococcus faecium | QEHB clinical isolate |
| MSSA_29213 | Staphylococcus aureus | ATCC 29213 |
| MSSA_10442 | Staphylococcus aureus | NCTC 10442 |
| MSSA_33807 | Staphylococcus aureus | ATCC 33807 |
| MSSA_4163 | Staphylococcus aureus | NCTC 4163 |
MPR, Ministry of Research, Paris; CPE, carbapenemase-producing Enterobacteriaceae; NDM-1, New Delhi metallo-β-lactamase; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended-spectrum β-lactamase.
We also tested a small range of other comparator organisms commonly causing hospital-acquired infections, including Enterobacter cloacae, Stenotrophomonas maltophilia, P. aeruginosa, E. coli, S. aureus, and Enterococcus faecium, and we included control strains (PS_6749 and MSSA_10788) recognized in the EN standards for assessing the efficacy of chemical disinfectants (e.g., EN 13727 [34]). The panel comprised isolates that in previous tests had demonstrated ability to form relevant quantities of biofilm in vitro and furthermore included two carbapenem (multidrug)-resistant isolates of K. pneumoniae and a single isolate of Elizabethkingia meningoseptica from a wound swab. This is an intrinsically highly resistant organism, usually resistant to extended-spectrum β-lactam agents (due to production by most strains of two β-lactamases: one extended-spectrum β-lactamase [ESBL] and one class B carbapenem-hydrolyzing metallolactamase), aminogylcosides, tetracycline, and chloramphenicol (35).
All isolates were stored at −80°C on Protect beads and were routinely cultured on cysteine lactose electrolyte-deficient (CLED) agar or blood agar prior to each experiment.
Experiments were designed to assess the antibacterial activity of blue light against planktonic and biofilm growth forms of the panel of bacteria described above. Testing was performed at the Defense Science Technology Laboratory (Dstl) (planktonic growth) and the Surgical Reconstruction and Microbiology Research Centre (biofilms), and blue light of a 400-nm wavelength was used for all experiments.
Blue light equipment.
High-intensity blue light was provided by a LED flood array (Henkel-Loctite, Hemel Hempstead, United Kingdom). This array utilizes 144 reflectorized LEDs which produce a homogeneous illuminated area of 10 cm by 10 cm. The emission spectrum of the LED array was determined using a USB2000 spectrophotometer (Ocean Optics, Oxford, United Kingdom). Two identical platforms were used for the testing, both of which were calibrated at Dstl using a PM100D radiant power meter (Thorlabs, Newton, NJ) prior to in vitro testing to ensure a reproducible irradiance of 60 mW/cm2 when the LED array is positioned 15.5 cm above the test area. All of the experimental conditions (except wavelength) adhere to the optimal criteria outlined by Coohill and Sagripanti (36) for the assessment of bacterial sensitivity to UV-C radiation.
Impact of blue light on planktonic bacteria.
Bacterial isolates were grown overnight in Luria broth (LB; Sigma-Aldrich, United Kingdom) and then diluted in sterile phosphate-buffered saline (PBS) to produce a starting concentration of approximately 1 × 106 bacteria per ml. Test samples (2 ml) were inoculated into a 12-well microtiter plate (Corning, NY), sealed with an optically clear ABsolute quantitative PCR (qPCR) sealer (Thermo Fisher Scientific, Paisley, Scotland) to prevent evaporation, and then exposed to blue light for 30 min (samples were taken for viable cell counting at 5-min intervals). If the strains still showed viability after 30 min of blue light exposure, the test was repeated over 180 min, with samples taken at 20-min intervals. An identical dark control plate was set up, wrapped in aluminum foil, and placed in the flood array adjacent to the blue light-irradiated samples.
At time increments during the experiment, samples exposed to blue light and incubated in the dark were removed and viable bacteria enumerated by serial dilution and growth on LB agar plates. The blue light sensitivity for each strain was determined from the mean of three independent biological replicates, with two technical replicates within each experiment.
The blue light dose (joules per square centimeter) received by the bacteria was calculated by multiplying the irradiance of light (watts per square centimeter) to which the sample was exposed by the exposure time (seconds).
Impact of blue light on preformed biofilms.
The antibacterial activity of blue light against preformed biofilms was assessed by conducting minimum biofilm eradication concentration (MBEC) experiments (37) on each isolate. Overnight LB cultures of the test strains (made by inoculating approximately three to five colonies into 5 ml of fresh LB and incubating them at 37°C overnight) were diluted in fresh antibiotic-free Mueller-Hinton (MH) broth to an optical density at 600 nm (OD600) of 0.1, and then 200 μl was seeded into wells of a 96-well microtiter tray (MTT). Positive (200 μl of organisms diluted to an OD600 of 0.1) and negative (200 μl of MH broth) controls were included per blue light time point to be tested.
To produce a “transferable biofilm,” a 96-well polypropylene plate (Starlabs, United Kingdom) was then placed into the MTT so that each well contained a “peg” on which biofilms could form, before the plates were sealed, and statically incubated at 33°C for 72 h. After 72 h, the pegs (±biofilm) were removed and washed in an MTT containing sterile water (to remove any unbound cells). The negative control (sterile broth only) “peg plate” was then placed in a clean, empty MTT and wrapped in foil. Following this, both the negative control and the positive test peg plate (bacteria only) were placed in the test area (15.5.cm beneath the light source) and exposed to the blue light for periods of 15, 30, 45, or 60 min (corresponding to a blue light dose of 54, 108, 162, and 216 J/cm2, respectively). The foil around the control plate prevented the pegs from receiving any blue light treatment (and hence these positive-control biofilms were not exposed to the blue light), but the control plate biofilms would have most likely been exposed to the same amount of heating and drying as the blue light-exposed test plate.
After the treatment, the peg plates were carefully placed into an MTT containing 200 μl of sterile MH broth (herein referred to as reporter broth) for overnight incubation. After 18 h, the OD of the reporter broth was measured to assess the viability (seeding) of the biofilms following blue light exposure.
To demonstrate the presence of biofilms on the pegs, crystal violet (CV) assays were additionally performed on the pegs after the OD of the reporter broth had been measured. This involved placing the pegs into MTTs containing 200 μl of 1% CV (which binds to any present microbial biomass of biofilm), followed by washing (to remove unbound CV) and solubilization of the CV in 200 μl of 70% ethanol. The peg biofilm biomass could then be measured using OD readings as done previously and the presence of the biofilm confirmed. Two biological and 10 technical replicates were performed for each strain and blue light exposure duration, respectively.
Statistical analysis. (i) Planktonic tests.
For the planktonic data, the surviving fraction was determined from the quotient N/N0, with N being the number of colony formers of the irradiated sample and N0 that of the nonirradiated controls. Plotting the logarithm of N/N0 as a function of dose (blue light fluence in joules per square centimeter) allowed survival curves to be obtained.
To determine the curve parameters, the following relationship was used: ln N/N0 = IC × F + n, where N is the number of colony formers after blue light irradiation, N0 is the number of colony formers without irradiation, IC is the inactivation constant (in joules per square centimeter), F is fluence, and n is the extrapolation number (i.e., the intercept with the ordinate of the extrapolated semilog straight line). The inactivation constant and the reciprocal lethal dose (LD) values were determined from the slope of the dose-effect curves (linear portion of the curve).
To allow for comparison with other bactericidal radiation sources in the literature, blue light-mediated killing was calculated in terms of the inactivation constant slope (IC) and the kill kinetics, shown as the doses required to kill 37% and 90% of the bacterial cells (LD37 and LD90, respectively). The significance of the difference of the dose-effect curves was statistically analyzed using Student's t test. Differences with P values of <0.05 were considered statistically significant.
LD90 values were analyzed using the statistical software package IBM SPSS V21.0, were found to be log normal by QQ plot (data not shown), and were consequently transformed to the logarithm of 10 prior to parametric analysis. Differences between bacterial species was investigated using a 1-way analysis of variance (ANOVA), and the suitability of the data for parametric analysis was further established with the use of Levene's test for unequal variance (P = 0.165). Where only one bacterial strain of a species was available, this species was taken out of the analysis. Multiple comparisons were made using Bonferroni's correction. Similarly, the effect of pigmentation of S. aureus strains on susceptibility to blue light was tested by using t tests without Welch's correction, and suitability was further tested using Levene's test for unequal variance (P = 0.984).
(ii) Biofilm tests.
The ability of biofilms to seed new growth following exposure to blue light was assessed by comparing the OD values at each blue light time point versus that of the untreated (positive) control, and significance was determined using Student's t test. In order to investigate any possible link between biofilm size and depth (colorimetry) and blue light sensitivity, these two parameters were investigated through QQ plots in SPSS (SPSS Statistics for Windows, version 21.0, IBM Corp., Armonk, NY). Initial analysis suggested that a transformation of both parameters by the logarithm of 10 was needed to render the data suitable for parametric analysis data not shown). Very little difference between the technical replicates was observed with regard to either parameter, and therefore, the median of the log10 of the technical replicates was used for analysis. The capacity for each strain to form a biofilm was taken from the average of the OD values over the 4 time points for the positive control. Comparisons of biofilm values were made using 1-way ANOVA and Student's t tests (without Welch's correction), and comparisons of variances were made with F tests and Brown-Forsythe tests. The viability of each strain of bacteria in biofilm was analyzed by Bonferroni's posttests across each time point (SPSS). Where significant differences between the positive control and the blue light occurred, the blue light was regarded as having an effect from there on, leading to an ordinal score for each strain of 15 min (54 J/cm2), 30 min (108 J/cm2), 45 min (162 J/cm2), 60 min (216 J/cm2), or >60 min. Comparisons of biofilm sensitivity scores were made using Kruskal-Wallis tests.
In order to characterize whether correlation existed between measured parameters, Spearman's method was used. In order to determine the statistical power of the correlations, the computer program SPSS sample power V3.0 (IBM) was used, and power was calculated for one sample correlations using the derived R value and the sample size (n = 34).
RESULTS
Blue light was tested against 34 bacterial isolates, including clinical isolates from QEHB and culture collection type strains. The results of the spectral output testing of the blue light platform (with an Ocean Optics USB2000 spectrometer) determined that the emission peak of the blue light produced was at 400 nm, with a full-width half-maximum value of ±8.5 nm (see Fig. S1 in the supplemental material).
Sensitivity of isolates to blue light when grown in planktonic culture.
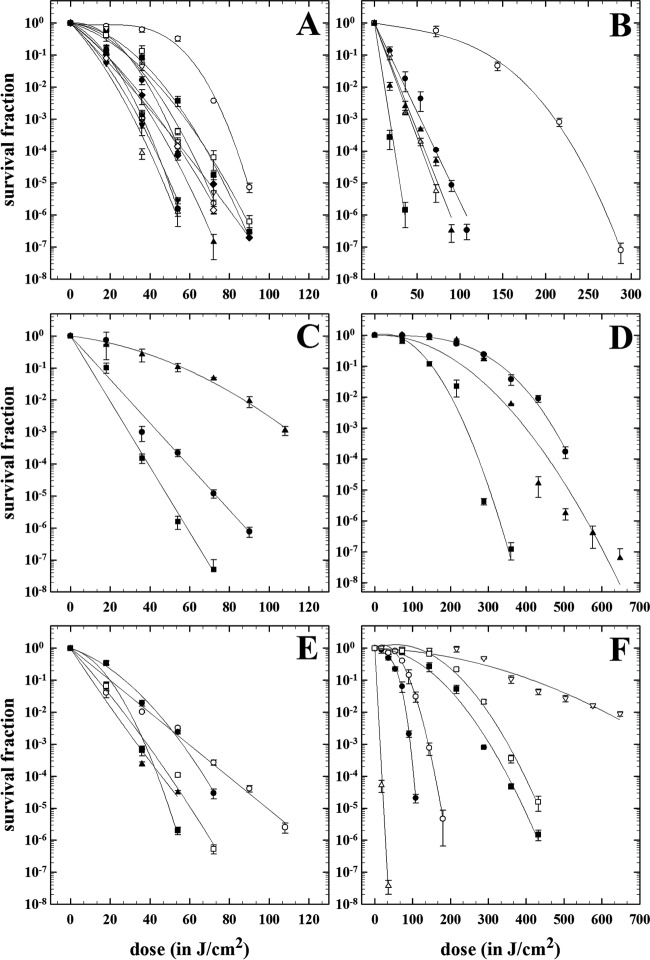
All 34 isolates were sensitive to blue light treatment, and there was no significant decay in the dark incubated controls. In contrast, rapid and substantive loss of viability was observed where all test bacteria were exposed to blue light (Fig. 1).
FIG 1.
Survival of planktonic bacteria after exposure to 400-nm blue light. (A) Acinetobacter baumannii strains are represented as follows: ACI_616, closed circles; ACI_618, open circles; ACI_AYE, closed triangles; ACI_665, open triangles; ACI_19606, closed inverted triangles; ACI_648, open inverted triangles; ACI_659, closed diamonds; ACI_C60, open diamonds; ACI_671, closed squares; ACI_672, open squares; ACI_698, closed hexagons; and ACI_642, open hexagons. (Note that the hexagons are behind the other symbols.) (B) Staphylococcus aureus strains are represented as follows: MSSA _10788, open circles; MSSA_F77, closed triangles; MRSA_520, closed squares; MRSA_531, open triangles; and MRSA_508, closed circles. (C) Stenotrophomonas maltophilia strains are represented as follows: STEMA_ 558, triangles; STEMA_551, circles; and STEMA_529, squares. (D) Enterobacter cloacae strains are represented as follows: ENTCL_804, circles; ENTCL_801, triangles; and ENTCL_525, squares. (E) Pseudomonas aeruginosa strains are represented as follows: PSE_1586, closed circles; PSE_PAO1, open circles; PSE_568, closed triangles; PSE_1054, closed squares; and PSE_6479, open squares. (F) Other strains are represented as follows: E. coli EC_042, open circles; E. coli EC_073, closed circles; K. pneumoniae MDR-A, open squares; K. pneumoniae MDR-B, closed squares; Elizabethkingia meningoseptica EKIN_502, open triangles; and Enterococcus faecium EFM_513, open inverted triangles. Data are averages ± standard deviations (n = 3).
Twenty-four of the isolates (71%) demonstrated at least a 5-log10 decrease in viability following 15 min (54 J/cm2) to 30 min (108 J/cm2) of blue light exposure (Table 2), and for the majority of these isolates (A. baumannii [12/12], S. aureus [4/5], S. maltophilia [2/3], and E. meningoseptica [1/1]), there was a >6-log10 decrease in viability. Ten of the 34 isolates showed a <5-log10 decrease in viability. The isolates concerned included E. cloacae (ENTCL_525, ENTCL_801, and ENTCL_804), E. coli (EC_073 and EC_042), K. pneumoniae (MDR_A and MDR_B), S. aureus (MSSA_10788), S. maltophilia (STEMA_551), and E. faecium (EFM_513). Four of the 34 isolates (E. cloacae ENTCL_525, ENTCL_801, and ENTCL_804 and E. faecium EFM_513) took longer to kill than the majority of isolates, requiring extended periods up to 120 min (432 J/cm2) to obtain 2- to 3-log10 decrease in viability.
TABLE 2.
Antimicrobial effects of blue light on planktonic cells
| Isolate | Exposure time (min) | Irradiance (mW/cm2) | Dose (J/cm2) | Log10 reduction | P value | LD37 valuea (J/cm2) | LD90 valuea (J/cm2) |
|---|---|---|---|---|---|---|---|
| ACI_616 | 30 | 60 | 108 | 7.06 | 0.006 | 21 ± 2 | 27 ± 2 |
| ACI_618 | 30 | 60 | 108 | 5.78 | 0.007 | 55 ± 4 | 59 ± 3 |
| ACI_642 | 30 | 60 | 108 | 6.73 | 0.006 | 9 ± 1 | 16 ± 2 |
| ACI_648 | 30 | 60 | 108 | 6.14 | 0.007 | 21 ± 2 | 29 ± 3 |
| ACI_659 | 30 | 60 | 108 | 6.55 | 0.006 | 8 ± 1 | 16 ± 2 |
| ACI_665 | 30 | 60 | 108 | 6.14 | 0.006 | 7 ± 1 | 12 ± 1 |
| ACI_671 | 30 | 60 | 108 | 6.34 | 0.006 | 25 ± 2 | 32 ± 4 |
| ACI_672 | 30 | 60 | 108 | 6.22 | 0.006 | 16 ± 2 | 24 ± 2 |
| ACI_698 | 30 | 60 | 108 | 6.39 | 0.008 | 9 ± 1 | 14 ± 2 |
| ACI_AYE | 30 | 60 | 108 | 6.70 | 0.006 | 10 ± 1 | 16 ± 2 |
| ACI_C60 | 30 | 60 | 108 | 6.76 | 0.007 | 7 ± 1 | 14 ± 1 |
| ACI_19606 | 30 | 60 | 108 | 6.81 | 0.006 | 7 ± 1 | 13 ± 1 |
| ENTCL_525 | 100 | 60 | 360 | 6.76 | 0.006 | 113 ± 12 | 136 ± 19 |
| ENTCL_801 | 180 | 60 | 648 | 6.61 | 0.009 | 212 ± 20 | 246 ± 25 |
| ENTCL_804 | 160 | 60 | 576 | 6.24 | 0.007 | 258 ± 18 | 306 ± 24 |
| STEMA_529 | 30 | 60 | 108 | 7.21 | 0.006 | 7 ± 1 | 12 ± 2 |
| STEMA_551 | 30 | 60 | 108 | 2.97 | 0.006 | 26 ± 3 | 48 ± 5 |
| STEMA_558 | 30 | 60 | 108 | 7.33 | 0.006 | 8 ± 1 | 18 ± 2 |
| PSE_568 | 30 | 60 | 108 | 6.48 | 0.002 | 6 ± 1 | 12 ± 2 |
| PSE_PA01 | 30 | 60 | 108 | 5.59 | 0.001 | 6 ± 1 | 17 ± 3 |
| PSE_6749 | 30 | 60 | 108 | 6.55 | 0.009 | 7 ± 1 | 13 ± 2 |
| PSE_1054 | 30 | 60 | 108 | 6.01 | 0.002 | 9 ± 1 | 15 ± 2 |
| PSE_1586 | 30 | 60 | 108 | 6.07 | 0.002 | 13 ± 2 | 22 ± 2 |
| EKIN_502 | 15 | 60 | 54 | 6.79 | 0.006 | 1 ± 0.5 | 4 ± 3 |
| EC_073 | 30 | 60 | 108 | 4.71 | 0.006 | 56 ± 4 | 64 ± 7 |
| EC_042 | 30 | 60 | 108 | 1.55 | 0.006 | 74 ± 8 | 85 ± 9 |
| MDR_A | 140 | 60 | 504 | 6.88 | 0.002 | 124 ± 18 | 159 ± 25 |
| MDR_B | 140 | 60 | 504 | 6.61 | 0.007 | 185 ± 16 | 219 ± 22 |
| MRSA_508b | 30 | 60 | 108 | 6.17 | 0.002 | 12 ± 1 | 21 ± 3 |
| MRSA_520b | 15 | 60 | 54 | 6.82 | 0.002 | 1 ± 0.5 | 5 ± 1 |
| MRSA_531b | 30 | 60 | 108 | 6.41 | 0.001 | 7 ± 1 | 15 ± 2 |
| MSSA_10788c | 80 | 60 | 288 | 7.07 | 0.001 | 99 ± 12 | 118 ± 15 |
| MSSA_ F77b | 30 | 60 | 108 | 6.76 | 0.006 | 3 ± 1 | 12 ± 2 |
| EFM_513 | 180 | 60 | 648 | 1.86 | 0.007 | 277 ± 16 | 393 ± 20 |
| Additional S. aureus isolates (for pigmentation investigation) | |||||||
| ATCC 29213b | 30 | 60 | 108 | 6.76 | 0.002 | 5 ± 1 | 15 ± 2 |
| NCTC 10442b | 30 | 60 | 108 | 6.69 | 0.002 | 8 ± 1 | 20 ± 2 |
| ATCC 33807c | 80 | 60 | 288 | 7.01 | 0.002 | 15 ± 2 | 40 ± 5 |
| NCTC 4163c | 80 | 60 | 288 | 6.07 | 0.003 | 38 ± 5 | 71 ± 6 |
Values are means ± standard deviations.
Yellow pigmentation.
Orange pigmentation.
Loss of bacterial viability associated with blue light was calculated as previously described to give LD90 values in terms of joules per square centimeter. Investigation of these LD90 values indicated that differences between the values were very likely driven by differences in the blue light susceptibilities of different bacterial species (P < 0.001). We found that the highest LD90 values belonged to E. cloacae and K. pneumoniae strains, which had values statistically higher than all other species included in the analysis (where more than one representative strain was tested) (P < 0.05 in all cases) (Fig. 2). The exception to this was E. coli, which had moderate blue light tolerance, but no statistical differences were seen between the strains tested. A. baumannii, S. aureus, P. aeruginosa, and S. maltophilia all had similar and low levels of resistance to the blue light exposure.
FIG 2.
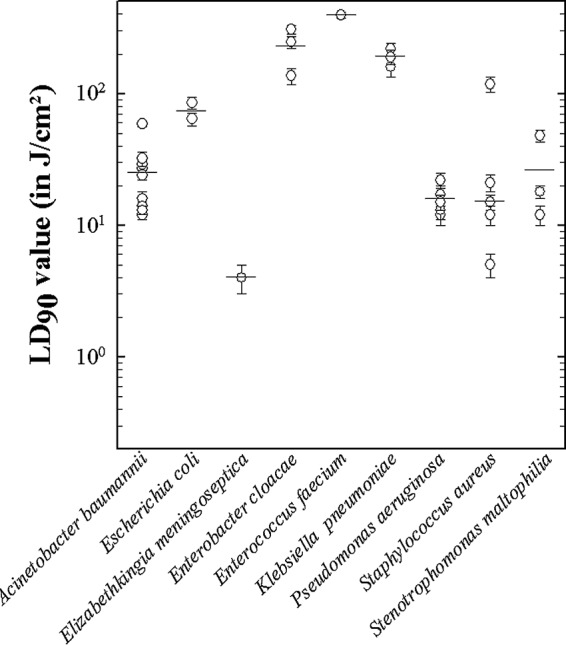
Comparison of blue light LD90 values between strains and species. Each individual circle represents the average LD90 for each strain ± the standard deviations (n = 3). The average LD90 values for the species are shown by horizontal lines.
In the initial assay of planktonic cell resistance to blue light, we observed that the S. aureus strains demonstrated different colony pigmentations, appearing as either pale yellow (MRSA_508, MRSA_520, MRSA_531, and MSSA_F77) or orange (MSSA_10788) when grown on LB agar. We hypothesized that this pigmentation may be responsible for the variability seen in both the survival fraction curves and LD90 values when exposed to blue light (Fig. 1B and 2). Four additional culture collection strains of S. aureus were assessed for blue light sensitivity, including two pale yellow (MSSA_29213 and MSSA_10442) and two orange (MSSA_33807 and MSSA_4163) strains. In total, nine strains of S. aureus were tested, six yellow and three orange. We determined that the orange pigmentation correlated with increased resistance to blue light in both the survival fraction curves and in LD90 values (Fig. 3). The LD90 values were statistically significantly higher in the orange-pigmented strains than in their yellow counterparts (P = 0.003).
FIG 3.
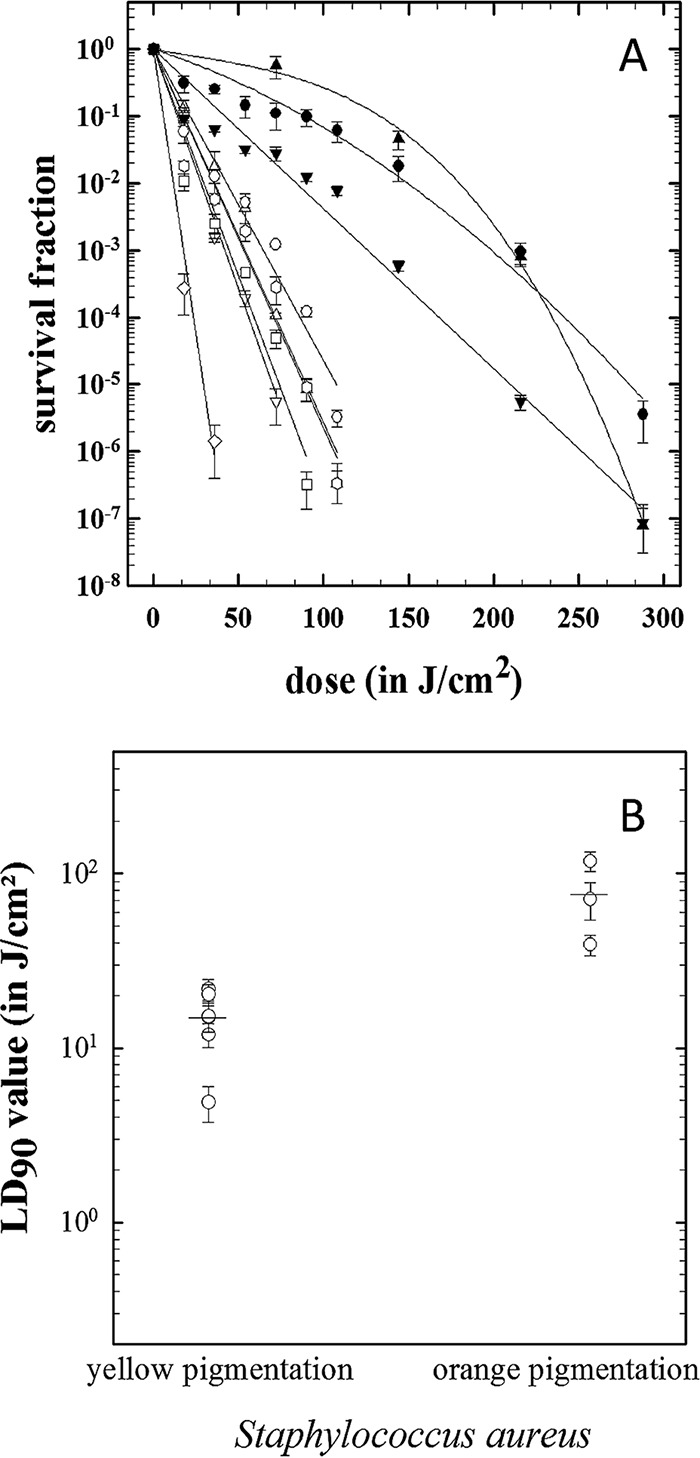
(A) Correlation between survival of planktonic S. aureus strains following blue light exposure and cell pigmentation. Orange carotenoid-producing strains are represented as follows: MSSA_4163, closed circles; MSSA_33807, closed inverted triangles; and MSSA_10788, closed triangles. Yellow non-carotenoid-producing strains are represented as follows: MSSA_10442, open circles; MSSA_F77, open squares; MRSA_520, open diamonds; MRSA_531, open inverted triangles; MRSA_508, open triangles; and MSSA_29213, open hexagons. Data are averages ± standard deviations (n = 3). (B) Comparison of blue light LD90 values between yellow- and orange-pigmented S. aureus strains. Each individual circle represents the average LD90 for each strain ± the standard deviation (n = 3). The average LD90 values for yellow- and orange-pigmented strains are shown by horizontal lines.
Sensitivities of isolates to blue light when grown in biofilms.
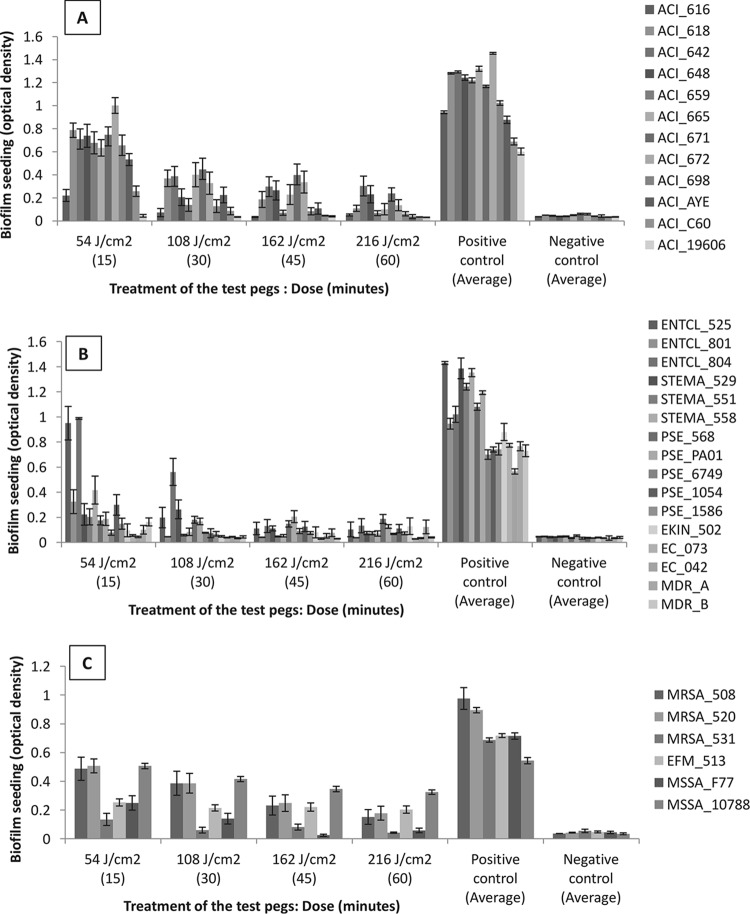
Blue light treatment resulted in reductions in biofilm seeding for all isolates tested (Fig. 4), and the majority of these reductions (apart from the time point of 15 min for MSSA_10788) were statistically significant (P < 0.05 in Student's t tests compared to the positive control). The percentage reductions are shown in Table 3, with the single nonsignificant result indicated by a caret.
FIG 4.
Graphs showing the biofilm seeding results for all isolates. Optical density on the y axis refers to the average biofilm seeding for the isolates tested after exposure to blue light at the range of durations tested (in minutes) on the x axis. The positive control was to the average biofilm seeding of the dark-incubated, non-blue-light-exposed isolates. The negative control was to a negative (broth-only) control. The error bars represent the standard errors.
TABLE 3.
Average percent change in biofilm seeding in isolates exposed to blue light compared to nonexposed dark-incubated controlsa
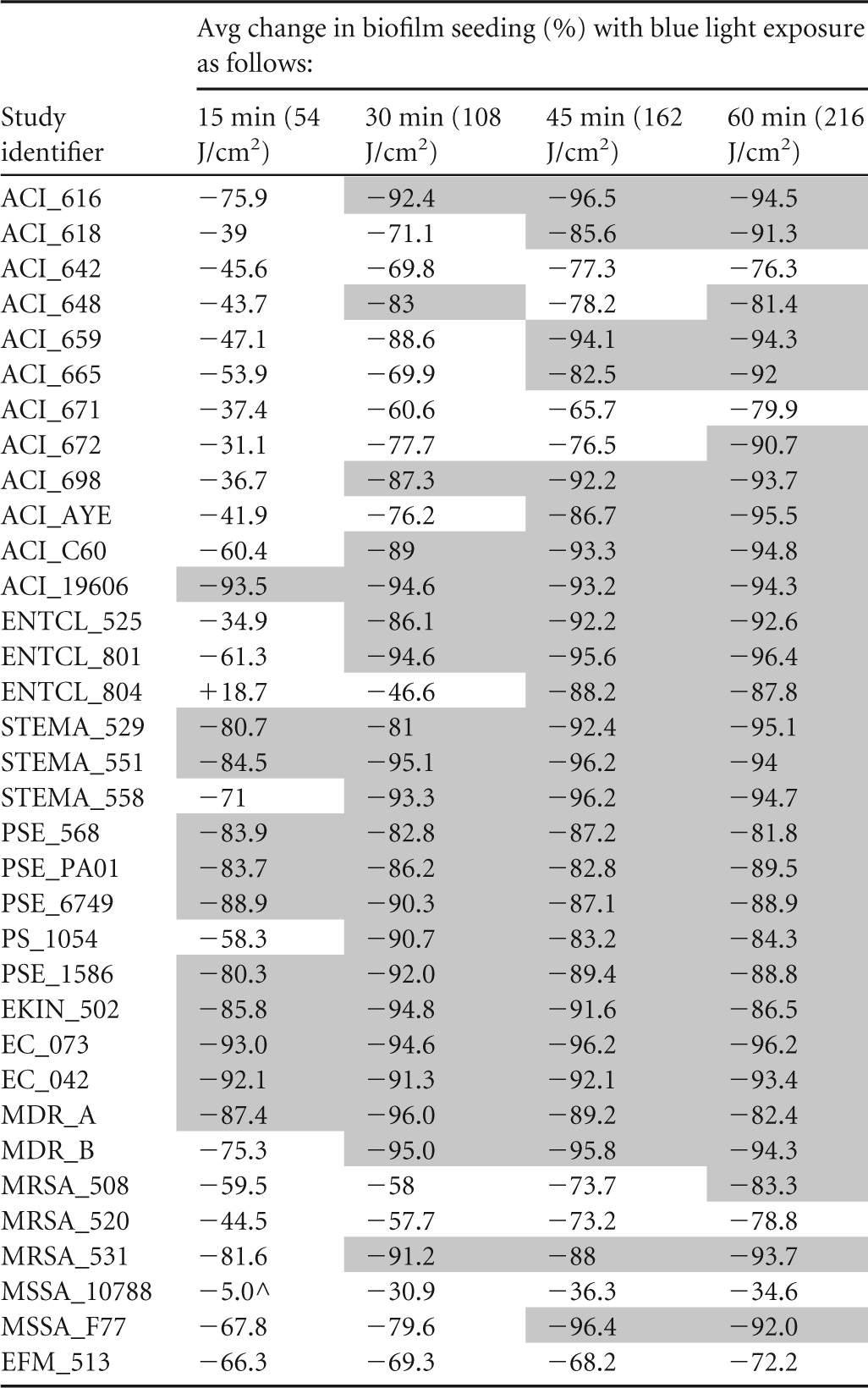
Shading denotes reductions of at least 80% in biofilm seeding compared to the positive control. ^, P value = 0.15.
The most susceptible isolates were the Gram-negative organisms, in particular ACI_19606, for which there was a 93.5% reduction in biofilm seeding (P < 0.001) after 15 min (54 J/cm2) of blue light exposure. As a group, the other Gram-negative comparator organisms were the most susceptible, with 10/16 (63%) showing greater than 80% reductions in biofilm seeding (average = 86%) at 15 min, compared to 1/12 for A. baumannii and 1/6 for the Gram-positive organisms. Although ENTCL_804 responded well to blue light treatment for 30 min at 108 J/cm2 (46.6% reduction), 45 min at 162 J/cm2 (88.2% reduction), and 60 min at 216 J/cm2 (87.8% reduction), the treatment actually resulted in increased biofilm seeding at 15 min of 18.7%. This result was repeatable and was seen in a number of replicates.
As mentioned above, the Gram-positive biofilms were less susceptible to blue light treatment, with only two isolates (33%) achieving at least 90% reductions in seeding. It is important to note, however, the small sample size. One isolate of S. aureus (MSSA_10788), which is recognized in the EN standards for assessing the efficacy of chemical disinfectants, was the least sensitive to blue light, achieving a maximum reduction in biofilm seeding of 36% at 45 min (162 J/cm2). This result was again repeatable and was seen in 48 replicate pegs. This is further evidence toward the hypothesis that bacterial pigmentation attenuates the sensitivity to blue light in biofilms as well as in planktonic cells.
In order to characterize how the different biofilm forming properties (seeding ability and biofilm size) of each bacterial species relate to each other, a series of correlations were performed. We found no evidence for significant correlations existing between (i) median biofilm size (CV assay) and median sensitivity of biofilm to blue light (P = 0.133) or (ii) median biofilm size and LD90 (P = 0.912). For these reasons, we feel that any differences between species in biofilm resistance to blue light are likely to be intrinsic differences rather than a function of the biofilm. However, we are not able to dismiss the alternative hypothesis that correlations do exist, as these analyses were insufficiently powered. We found the statistical power to be 21% when considering the potential correlation between planktonic and biofilm killing (R = 0.200) and 33% when considering the correlation between biofilm killing and biofilm formation (R = −0.263). In this respect, we must actually conclude that real correlations between these parameters might exist; however, if they do exist, they are likely to less apparent than the correlation observed between biofilm formation and planktonic killing.
We found a significant correlation between the sensitivity of strains to blue light in the planktonic state and their ability to form biofilms (Spearman's coefficient = 0.369; P = 0.032). This indicated that strains that demonstrated greater resistance to blue light in the planktonic state were more likely to produce thicker biofilm.
DISCUSSION
In this study, we have shown blue light (400 nm) to be effective at inactivating both planktonic cells and biofilms of important nosocomial wound pathogens. Contrary to published research (1, 15, 16), we found Gram-negative organisms to be more susceptible to blue light. There are a number of differences between these published studies and our study which may contribute to these conflicting findings. First, we tested a number of isolates per species (most of the studies test one strain of each species) comprising both clinical and control strains (most of the published studies use control strains which may have been passaged many times), and our light box exposed the bacteria to higher doses (60 mW/cm2) than the 10 mW/cm2 used by Maclean et al. (15). Although we only tested a small number of isolates, the Gram-positive biofilms appeared less sensitive to blue light treatment, with one strain (MSSA_10788) consistently resisting the effects of blue light.
Analyzing blue light susceptibility against multiple clinical strains from the same species has permitted us to assess the heterogeneity of intraspecies kill rates. In some species, such as A. baumannii, the rate of blue light-mediated killing was extremely homogeneous; however, S. aureus strains display a much more heterogeneous response to blue light stress.
It has long been recognized that bacterial cells have utilized pigmentation as a virulence factor (38). Among the most easily recognizable bacterial pigments are the triterpenoid carotenoids, which impart the eponymous golden color to S. aureus strains. Various authors have identified a correlation between strains containing the carotenoid pigment staphyloxanthin and the ability to survive on surfaces exposed to natural sunlight (39), and it is well known that carotenoids function as antioxidants. Furthermore, staphyloxanthin has been shown to provide protection for pigmented S. aureus strains against ROS produced by phagocytes (40, 41).
Augmentation of the clinical isolates of S. aureus with a series of well-characterized strains from culture collections allowed us to correlate increased blue light killing times (as seen with MSSA_10788) with colony pigmentation. Planktonic testing and assessment of the LD90 values per color group showed that the light sensitivity of the strongly orange-pigmented strains is significantly different from that of the standard pale yellow strains (P value < 0.003) (Fig. 3B). Therefore, although all species of S. aureus tested were susceptible to blue light, it is important to consider the effects of bacterial pigmentation when determining the required blue light exposure for effective decontamination.
As well as differences in sensitivity, there were also several instances where blue light treatment increased the planktonic growth and biofilm seeding. For example, with ENTCL_804, there was an increase of 18.7% in seeding after 15 min of blue light treatment. Light has been shown to facilitate growth when the wavelengths and dose are not appropriate (42), and Nussbaum et al. (43) found that wavelengths of 810 nm and 905 nm improved the growth of E. coli and S. aureus, respectively. Furthermore, Mussi et al. (44) reported that blue light treatment decreased motility and biofilm formation in A. baumannii and increased pathogenesis when cocultured with Candida albicans (a model for apoptosis in human alveolar macrophages) (45). However, there are several important differences between the studies; the fluence was considerably lower than in this study (1.32 to 1.89 mW/cm2 versus 60 mW/cm2) and the light wavelength peaked at 460 nm versus 400 nm, while the exposure was measured over 4 days versus 30 min. However, it does raise interesting questions on the effects of suboptimal light exposure on bacterial cells, which should be looked into in future studies. Furthermore, the enhanced growth in our study warrants further investigation.
We found that a correlation existed between the strains which were sensitive to blue light in the planktonic state and those which produced larger amounts of biofilm in the CV assay. The reason for this observation is not clear; however, we hypothesize that this indicates that blue light selective pressure may exist in environmental niches where protection as a biofilm might provide benefits against other stimuli that are likely to coexist with blue light. The fact that we were unable to observe a correlation between biofilm and planktonic resistance to blue light might indicate that these mechanisms are functionally independent. Planktonic cells need to rely on their intrinsic transparency, pigmentation, and repair to protect against blue light. In biofilm, bacteria can rely on more extracellular exudate and neighbors to protect against blue light.
To the best of our knowledge, our work is one of the first to show the antibacterial activity of 400-nm light against a range of clinically relevant bacterial strains (as well as control strains) and one of just a handful to look at nondental biofilms. The inclusion of multiple isolates is an additional strength of our study, as it allows correlations between phenotypic characteristics and blue light resistance to be explored. Although there are several limitations (monomicrobial biofilms tested instead of polymicrobial, the lack of formal assessment of the potential for the development for resistance, the relatively small number of isolates tested, and the potential biasing effect of the included resistant isolates), our work nonetheless provides valuable insights into this technology and especially how it relates to the eradication of biofilms for environmental decontamination.
The findings in this paper demonstrate that high-intensity blue light can be used to inactivate a wide range of clinical pathogens, not only in the planktonic state but also as mature biofilms. This technology has many practical applications within health care settings, as blue light may ameliorate opportunistic infections indirectly by reducing the bacterial load on environmental surfaces and directly within wounds. Future studies are warranted to investigate this further, especially whether the exposure times of the 400-nm blue light can be reduced for a range of different clinical applications. As blue light is equally efficacious against antibiotic-resistant pathogens, this technology may prove an important weapon in the future fight against antimicrobial resistance.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIHR Surgical Reconstruction and Microbiology Research Centre, the Institute of Microbiology at the University of Birmingham, and Aston University (R.B. was a student on secondment during the project). The Loctite LED Flood System was provided free of charge by Dstl to the SRMRC for the duration of the project.
This paper presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
M.R. and R.M. were supported in parts by the DLR grant DLR-FuE-Projekt ISS-Nutzung in der Biodiagnostik (Use of the ISS for biodiagonstics), Programm RF-FuW, Teilprogramm 475, and the German Research Foundation (DFG) Paketantrag (PlasmaDecon PAK 728) grant (MO 2023/2-1).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00756-16.
REFERENCES
- 1.Maclean M, McKenzie K, Anderson JG, Gettinby G, MacGregor SJ. 2014. 405 nm light technology for the inactivation of pathogens and its potential role for environmental disinfection and infection control. J Hosp Infect 88:1–11. doi: 10.1016/j.jhin.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Mochon AB, Garner OB, Hindler JA, Krogstad P, Ward KW, Lewinski MA, Rasheed JK, Anderson KF, Limbago BM, Humphries RM. 2011. New Delhi metallo-beta-lactamase (NDM-1)-producing Klebsiella pneumoniae: case report and laboratory detection strategies. J Clin Microbiol 49:1667–1670. doi: 10.1128/JCM.00183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Poirel L, Toleman MA, Walsh TR. 2011. Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J Antimicrob Chemother 66:689–692. doi: 10.1093/jac/dkq520. [DOI] [PubMed] [Google Scholar]
- 4.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. 2012. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat 15:223–236. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA, Coia JE, Hamilton K, Taggart I, Watson SB, Thakker B, Gettinby G. 2010. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. J Hosp Infect 76:247–251. doi: 10.1016/j.jhin.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Dancer SJ. 2014. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev 27:665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashkenazi H, Malik Z, Harth Y, Nitzan Y. 2003. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol 35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin MR, Hasan T. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA. 2008. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B 92:180–184. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Goldoni A. 2002. Porphyrins: fascinating molecules with biological significance, p 64–65. In ELETTRA highlights 2001–2002. Sincrotrone Trieste SCpA, Trieste, Italy. [Google Scholar]
- 11.McDonald R, Macgregor SJ, Anderson JG, Maclean M, Grant MH. 2011. Effect of 405-nm high-intensity narrow-spectrum light on fibroblast-populated collagen lattices: an in vitro model of wound healing. J Biomed Opt 16(4):048003. doi: 10.1117/1.3561903. [DOI] [PubMed] [Google Scholar]
- 12.Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC, Gerritsen RM. 2010. Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed 26:16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 13.Wainwright M. 1998. Photodynamic antimicrobial chemotherapy. J Antimicrob Chemother 42:13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 14.Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. 2008. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med 40:734–737. doi: 10.1002/lsm.20724. [DOI] [PubMed] [Google Scholar]
- 15.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. 2009. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol 75:1932–1937. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murdoch LE, McKenzie K, Maclean M, Macgregor SJ, Anderson JG. 2013. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger. Fungal Biol 117(7–8):519–527. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch LE, Maclean M, Endarko E, MacGregor SJ, Anderson JG. 2012. Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. ScientificWorldJournal 2012:137805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maclean M, Murdoch LE, MacGregor SJ, Anderson JG. 2013. Sporicidal effects of high-intensity 405 nm Visible Light on endospore-forming bacteria. Photochem Photobiol 89:120–126. doi: 10.1111/j.1751-1097.2012.01202.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawada A, Aragane Y, Kameyama H, Sangen Y, Tezuka T. 2002. Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation. J Dermatological Sci 30:129–135. doi: 10.1016/S0923-1811(02)00068-3. [DOI] [PubMed] [Google Scholar]
- 20.Noborio R, Nishida E, Kurokawa M, Morita A. 2007. A new targeted blue light phototherapy for the treatment of acne. Photodermatol Photoimmunol Photomed 23:32–34. doi: 10.1111/j.1600-0781.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- 21.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. 2005. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med 36:260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin R, Dai T, Avci P, Jorge AE, de Melo WC, Vecchio D, Huang YY, Gupta A, Hamblin MR. 2013. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol 13:731–762. doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP, Hamblin MR. 2013. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother 57:1238–1245. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai T, Gupta A, Huang YY, Sherwood ME, Murray CK, Vrahas MS, Kielian T, Hamblin MR. 2013. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed Laser Surg 31:531–538. doi: 10.1089/pho.2012.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR, Dai T. 2014. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis 209:1963–1971. doi: 10.1093/infdis/jit842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bache SE, Maclean M, MacGregor SJ, Anderson JG, Gettinby G, Coia JE, Taggart I. 2012. Clinical studies of the high-intensity narrow-spectrum light environmental decontamination system (HINS-light EDS), for continuous disinfection in the burn unit inpatient and outpatient settings. Burns 38:69–76. doi: 10.1016/j.burns.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW, Costerton JW. 2012. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen 20:647–657. doi: 10.1111/j.1524-475X.2012.00836.x. [DOI] [PubMed] [Google Scholar]
- 28.Percival SL, Kite P. 2007. Intravascular catheters and biofilm control. J Vasc Access 8:69–80. [PubMed] [Google Scholar]
- 29.Burmølle M, Thomsen TR, Fazli M, Dige I, Christensen L, Homoe P, Tvede M, Nyvad B, Tolker-Nielsen T, Givskov M, Moser C, Kirketerp-Moller K, Johansen HK, Hoiby N, Jensen PO, Sorensen SJ, Bjarnsholt T. 2010. Biofilms in chronic infections—a matter of opportunity—monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol 59:324–336. doi: 10.1111/j.1574-695X.2010.00714.x. [DOI] [PubMed] [Google Scholar]
- 30.Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Hola V, Imbert C, Lebeaux D, Oliver A, Ullmann AJ, Williams C. 2015. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21(Suppl 1):S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Halachev MR, Chan JZ, Constantinidou CI, Cumley N, Bradley C, Smith-Banks M, Oppenheim B, Pallen MJ. 2014. Genomic epidemiology of a protracted hospital outbreak caused by multidrug-resistant Acinetobacter baumannii in Birmingham, England. Genome Med 6(11):70. doi: 10.1186/s13073-014-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villegas MV, Hartstein AI. 2003. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol 24:284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 33.Borer A, Gilad J, Smolyakov R, Eskira S, Peled N, Porat N, Hyam E, Trefler R, Riesenberg K, Schlaeffer F. 2005. Cell phones and Acinetobacter transmission. Emerg Infect Dis 11:1160–1161. doi: 10.3201/eid1107.050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.British Standards Institution. 2012. Chemical disinfectants and antiseptics—quantitative suspension test for the evaluation of bactericidal activity in the medical area—test methods and requirements (phase 2, step 1). Standards Publication BSI, BS EN13727. British Standards Institution, London, United Kingdom. [Google Scholar]
- 35.Torok EME, Cooke F. 2009. Oxford handbook of infectious diseases and microbiology. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 36.Coohill TP, Sagripanti J-L. 2008. Overview of the inactivaton by 254 nm ultraviolet radiation of bacteria with particular reference to biodefence. Photochem Photobiol 84:1084–1090. [DOI] [PubMed] [Google Scholar]
- 37.Ceri H, Olson M, Morck D, Storey D, Read R, Buret A, Olson B. 2001. The MBEC assay system: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol 337:377–385. doi: 10.1016/S0076-6879(01)37026-X. [DOI] [PubMed] [Google Scholar]
- 38.Liu GY, Nizet V. 2009. Color me bad: microbial pigments as virulence factors. Trends Microbiol 17:406–413. doi: 10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beard-Pegler MA, Stubbs E, Vickery AM. 1988. Observations on the resistance to drying of staphylococcal strains. J Med Microbiol 26:251–255. doi: 10.1099/00222615-26-4-251. [DOI] [PubMed] [Google Scholar]
- 40.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. 2005. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clauditz A, Resch A, Wieland K-P, Peschel A, Götz F. 2006. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74:4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guffey JS, Payne W, Jones T, Martin K. 2013. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 m, light from a supraluminous diode array. Photomed Laser Surg 31:179–182. doi: 10.1089/pho.2012.3450. [DOI] [PubMed] [Google Scholar]
- 43.Nussbaum EL, Lilge L, Mazzulli T. 2002. Effects of 630-, 660-, 810-, and 905-nm laser irradiation delivering radiant exposure of 1–50 Jcm−2 on three species of bacteria in vitro. J Clin Laser Med Surg 20:325–333. doi: 10.1089/104454702320901116. [DOI] [PubMed] [Google Scholar]
- 44.Mussi MA, Gaddy JA, Cabruja M, Arivett BA, Viale AM, Rasia R, Actis LA. 2010. The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. J Bacteriol 192:6336–6345. doi: 10.1128/JB.00917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaddy JA, Actis LA. 2009. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 4:273–278. doi: 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.