ABSTRACT
Hepatitis A virus (HAV) is the main causative agent of hepatitis infection associated with waterborne outbreaks worldwide. In Tunisia, there is no specific surveillance system for HAV and current secondary wastewater treatment processes are unable to remove viral particles, which present a potential public health problem. Qualitative and quantitative analysis of HAV in 271 raw and treated wastewater samples from five sewage treatment plants (STPs) during 13 months was performed. Moreover, the efficiency of three secondary wastewater treatment processes (conventional activated sludge, extended aeration, and oxidation ditch activated sludge) was evaluated. Data obtained demonstrated that HAV is endemic in Tunisia and circulates with high prevalence in both raw (66.9%) and treated (40.7%) wastewater. HAV circulates throughout the year in the coastal areas, with the highest rates found during summer and autumn, whereas in central Tunisia, high levels were shown in autumn and winter. Total virus removal was not achieved, since no difference in mean HAV loads was observed in effluents (6.0 × 103 genome copies [GC]/ml) and influents (2.7 × 103 GC/ml). The comparison of the HAV removal values of the three different wastewater treatment methods indicates that extended aeration and oxidation ditch activated sludge had better efficiency in removing viruses than conventional activated sludge did. Molecular characterization revealed that the vast majority of HAV strains belonged to subgenotype IA, with the cocirculation of subgenotype IB in wastewater treatment plants that collect tourism wastewater.
IMPORTANCE This report provides important data on the incidence, behavior, seasonality, and genotype distribution of HAV in the environment in Tunisia, as well as the risk of infection derived from its occurrence in effluents due to inadequate wastewater treatment. In addition, these findings seem to confirm that the prevalence of HAV depends on socioeconomic level, sanitary conditions in the communities, sewage facilities, the locality, and the climate. The wide dispersion of HAV in effluents proves the inefficacity of the current wastewater treatment processes used in Tunisia to remove virus; therefore, establishment of tertiary treatment processes or replacement of the medium-charge activated sludge (conventional activated sludge) by the low-charge version (oxidation ditch activated sludge) is absolutely needed. Rapid detection of the HAV genome in wastewater may provide a timely warning sign to health authorities to implement population protection measures.
INTRODUCTION
Hepatitis A virus (HAV) is one of the most important human waterborne viruses and constitutes the main cause of human enteric hepatitis transmitted via the fecal-oral route (1). It is widely prevalent in the world, as millions of new cases of HAV infections occur worldwide every year (2). It was previously demonstrated that areas with inadequate water supply and poor wastewater facilities and hygienic conditions generally have very high HAV prevalence (3, 4). Thus, the geographic distribution pattern of HAV is highly correlated to the socioeconomic level and sanitary conditions (5–7). In fact, HAV infection is highly endemic in developing regions, e.g., in South Mediterranean regions (6, 8), where it is still frequently detected in wastewaters (9), while it is much less frequently detected in industrialized countries. However, HAV can easily contaminate the environment due to the large quantities of viral particles excreted by infected people, symptomatic or not, which pass through the inefficient sewage treatment plants (STPs) and reach water environments such as rivers, lakes, and seas. Due to its nonenveloped nature, HAV is very stable in the environment for long periods and is particularly resistant to the current wastewater treatment processes (10, 11). Even though Tunisia has developed its secondary sewage treatment plants (12), the quality of treated wastewater is still poor (13). Unfortunately, Tunisia, among other Mediterranean countries with high endemicity of HAV infections, is using contaminated treated wastewater for irrigation and to refill aquifers to palliate the lack of water resources (14), which may pose a real threat for public health. Indeed, the lack of a specific system in Tunisia for surveillance of HAV or other enteric viruses in wastewaters (15) and the changing epidemiology of HAV (16) are among the many risk factors (3, 5, 12, 17) that increase the potential human risk associated with viral contamination in wastewater.
HAV, a member of the genus Hepatovirus of the Picornaviridae family, is classified into six genotypes (18, 19). Subgenotype IA appears to be the most prevalent variant worldwide, whereas in Europe and the Mediterranean region, a more heterogeneous pattern has been found, with cocirculation of the subgenotypes IA and IB (17, 20, 21). It has been demonstrated that the subgenotype IA strain was predominant in Tunisia, while subgenotype IB was rarely detected (4, 22).
The aim of the present study was to extend the previous knowledge on the prevalence of HAV in wastewater in Tunisia (4, 23) through the monitoring of five sewage treatment plants with different characteristics during 13 months. The seasonal distribution of HAV is described on the basis of the geographical location of the studied areas and also on the basis of the origin of influents, using quantitative and qualitative data which may give more information about HAV circulation in our country. Indeed, the efficiency of three different activated sludge processes (secondary biological wastewater treatment) in removing HAV was calculated in order to evaluate the risk associated with the wastewater and to demonstrate the need of improvement of the conventional sewage treatment systems currently used in Tunisia and other developing countries. In addition, we aimed to analyze the distribution of emergent HAV subgenotypes, introducing the origin of effluents as one of key factors that may provide a better understanding of their circulation in Tunisia.
MATERIALS AND METHODS
Sampling.
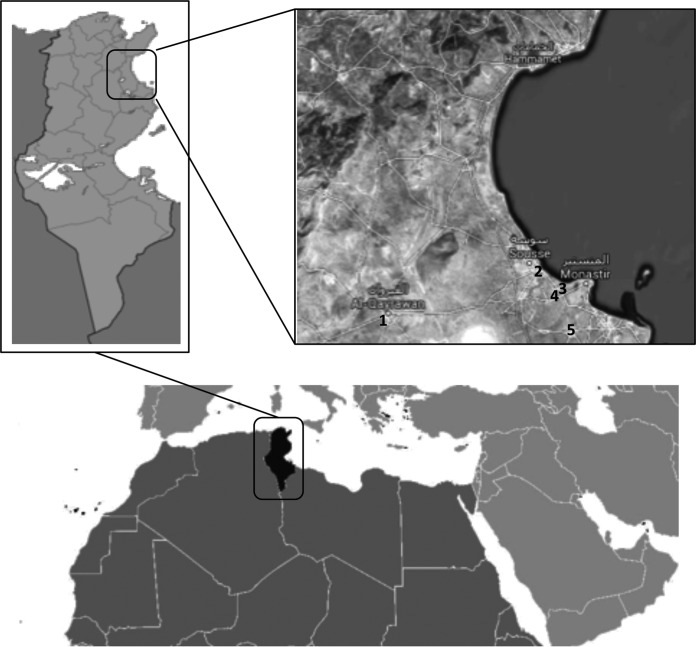
A total of 271 samples of wastewater, 136 at entry points (raw) and 135 at exit points (treated), were collected from five sewage treatment plants (STPs) located in different Tunisian urban regions. The STP of Kairouan belongs to the Kairouan governorate, located in west-central Tunisia, characterized by a semiarid hot climate. The Sousse-South STP belongs to the Sousse governorate and the Dekhila, Sahline, and Jammel STPs to the Monastir governorate. These four STPs are located in the east-central coast of Tunisia, which is influenced by the Mediterranean climate (Fig. 1). Samples were collected twice per month between December 2009 and December 2010. Each sample was collected at entry and exit points in 2-liter plastic container that had been cleaned and kept at 4°C. Wastewater treatment processes, physicochemical parameters, total numbers of inhabitants served by STP (expressed as population equivalents), origins of influents (domestic, touristic, and industrial), and discharge points of treated wastewater data were obtained from the National Office of Sanitation (ONAS) and are listed in Table 1.
FIG 1.
Location of the five Tunisian STPs included in the study. 1, Kairouan (KR); 2, Sousse-South (SS); 3, Dekhila (D); 4, Sahline (SH); 5, Jammel (J). Maps from http://mapamundial.co/a/mapadeTunez#mapa; serial view map obtained from Google Maps (Imagery@2016 Landsat, Data SIO, NOAA, U.S. Navy, NGA, GEBCO, map data @Google) under the Fair Use terms.
TABLE 1.
Data from wastewater treatment plants (sewage treatment plants) involved in the study: wastewater treatment processes, numbers of population equivalents (domestic, touristic, and industrial), discharge points, and physicochemical characteristicsa
| STP | Wastewater treatment process | Population equivalentb |
Discharge point | Physicochemical characteristic (mean value) |
Temp range (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Domestic | Touristic | Industrial | pH (mean value) | TSS (mean value) | BOD (mg/liter) [mean value] | COD (mg/liter) | Cl (mg/liter) | ||||
| Kairouan (KR) | Oxidation ditch extended aeration activated sludge (low charge) | 250,570 | 238,540 | 530 | 11,500 | River Oued Laataf to Lake Sabkhet el Kalbia | 15−26.4 | |||||
| Influents | 7.0 ± 1.0 | 440 | 557 | 1,408 | 497 | |||||||
| Effluents | 8.0 ± 1.0 | 25 | 26 | 84 | 426 | |||||||
| Sousse-South (SS) | Conventional activated sludge (medium charge) | 325,150 | 270,968 | 31,000 | 23,182 | River Oued el Hallouf to sea | 15.8−30.1 | |||||
| Influents | 7.0 ± 1.0 | 409 | 406 | 800 | 710 | |||||||
| Effluents | 8.0 ± 1.0 | 43 | 48 | 117 | 639 | |||||||
| Dekhila (D) | Conventional activated sludge (medium charge) | 44,615 | 31,345 | 3,080 | 10,190 | River Oued Hamdoun to sea | NAc | |||||
| Influents | 7.0 ± 1.0 | 348 | 335 | 1,040 | 1,597 | |||||||
| Effluents | 8.0 ± 1.0 | 26 | 23 | 71 | 1,216 | |||||||
| Sahline (SH) | Oxidation ditch extended aeration activated sludge (low charge) | 11,350 | 0 | 11,350 | 0 | River Oued Hamdoun to sea | 17−29.2 | |||||
| Influents | 7.0 ± 1.0 | 322 | 369 | 1,268 | 587 | |||||||
| Effluents | 8.0 ± 1.0 | 13 | 21 | 70 | 489 | |||||||
| Jammel (J) | Extended aeration activated sludge (low charge) | 83,610 | 80,430 | 0 | 3,180 | River Oued el Melah to sea | 15.9−29.4 | |||||
| Influents | 7.0 ± 1.0 | 240 | 270 | 795 | 604 | |||||||
| Effluents | 8.0 ± 1.0 | 25 | 30 | 106 | 639 | |||||||
STP, sewage treatment plants; TSS, total suspended solids; BOD, biochemical oxygen demand; COD, chemical oxygen demand.
Population equivalents are expressed in numbers of subjects.
NA, data not available.
STPs.
Wastewater at all the sewage treatment plants (STPs) is subjected to secondary biological treatment with activated sludge (Table 1). However, the following three types of activated sludge treatment were involved in the study: conventional activated sludge (medium charge), used by the Sousse-South and Dekhila STPs; extended aeration oxidation ditch activated sludge (low charge), used by the Sahline and Kairouan STPs; and extended aeration activated sludge (low charge), used by Jammel STP. All the STPs follow the legal requirements of the Tunisian law included in decree no. 2005-1991 (11-07-2005) (24) on environmental impact assessment and the regulations in NT 106.02 (20-07-1989) (25) on the quality of discharges according to the nature and particularities of the receiving environment (physicochemical, bacteriological, and micropollutants) and in NT 106.03 (06-1989) (26) on the reuse of treated wastewater.
Virus concentration.
Viruses were concentrated from wastewater samples by the adsorption-elution method recommended by the U.S. Environmental Protection Agency (27), with minor modifications (4). Briefly, 100 ml of wastewater sample was supplemented with aluminum chloride (AlCl3) and adjusted to pH 3.5 with HCl. Viruses were then eluted by using beef extract (pH 9) and concentrated by precipitation with polyethylene glycol (PEG) 6000. The pellet was resuspended in 3 ml of phosphate-buffered saline (PBS) and stored at −20°C until use.
Total RNA was extracted from 150 μl of the concentrates with a NucleoSpin RNA Virus kit (Macherey-Nagel, Germany) and resuspended in a final volume of 50 μl of RNase-free H2O.
RT-qPCR assay.
One-step real-time TaqMan reverse transcription-PCR (RT-PCR) was employed for the detection and the quantification of HAV. Each reaction was performed using 5 μl of RNA solution with a Platinum quantitative RT-PCR ThermoScript One-Step System kit (Invitrogen, France) using primers HAV240 and HAV68 and TaqMan probe HAV150 targeting the noncoding region at the 5′ end (5′-NCR) (4, 28). Amplification conditions were as follows: reverse transcription at 55°C for 1 h and denaturation at 95°C for 5 min followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 1 min, and extension at 65°C for 1 min. Only samples showing cycle threshold (CT) values of ≤39 were considered positive and quantifiable. Samples showing CT values between 39 and 41 were considered positive but nonquantifiable. To reduce the effect of potential quantitative reverse transcription-PCR (RT-qPCR) inhibitors, extracted viral RNA samples (undiluted and 10-fold dilution) were tested.
Viral RNA extraction and PCR efficiencies were controlled as previously described (4, 28). Thus, a known amount of mengovirus clone MC0 was spiked into concentrates before the RNA extraction process was performed and was detected under the same conditions used for HAV. To calculate extraction efficiency, the CT value of a mengovirus-positive amplification control and the CT value of each sample for mengovirus were compared. RT-qPCR efficiency and the presence of RT-qPCR inhibitors were evaluated by comparing the CT value of an internal control amplification, containing 2.5 μl of positive control for HAV and 2.5 μl of extracted RNA, with the CT value of the HAV-positive amplification control (28, 29).
HAV typing.
To genotype the detected HAV strains, seminested RT-PCR was performed to amplify a 222-bp fragment of the VP3/VP1 junction, employing specific primers for HAV as described previously (29). Briefly, reverse transcription of extracted viral RNA was conducted at 42°C for 45 min using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen, France) in a 20-μl reaction mixture. Both the first and second rounds of PCR were performed using Immolase DNA polymerase (Bioline, Germany). Amplification conditions for the first round of PCR were as follows: an initial denaturation of 94°C for 5 min, 40 cycles of denaturation at 94°C for 45 s, annealing at 57°C for 1 min, and elongation at 72°C for 1 min, and a final elongation at 72°C for 10 min. The seminested PCR was performed under the same conditions as the first PCR round except for the annealing temperature being set at 55°C. Amplification products were analyzed by electrophoresis using a 2% agarose gel and visualized after ethidium bromide staining using a Gel-Doc apparatus (Bio-Rad). PCR products were purified from the gel using a QIAquick gel extraction kit (Qiagen) and sequenced.
Phylogenetic analysis of HAV strains.
The obtained sequences, including 14 reference sequences retrieved from GenBank, were corrected using the DNASTAR Lasergene SeqMan program (DNASTAR, USA) and then aligned using MEGA6 and the Clustal W algorithm (30). In addition, three subgenotype IA sequences, two corresponding to clinical strains TunS-01-01and TunS-05-01, identified in serum samples collected in 2001 from Jammel and Monastir hospitals, and the third corresponding to environmental strain TunEU-05-01, collected from raw wastewater in the Monastir region (2001) (31), were also included in the analysis. Inter- and intragenotype nucleotide identity percentages were calculated using the DNASTAR Lasergene MegAlign program. A phylogenetic tree was constructed using the neighbor-joining algorithm (32) with calculation of distance matrices performed using the Kimura two-parameter model and a reliability test of the tree performed by bootstrap analysis (1,000 replications) with the MEGA6 program (30).
Nucleotide sequence accession numbers.
Sequences obtained in the present study were deposited in the GenBank database under accession numbers LN898274 to LN898414.
RESULTS
HAV detection and quantification in wastewater samples.
The HAV genome was detected in 146 (53.9%) of 271 analyzed wastewater samples collected from five STPs between December 2009 and December 2010. HAV rates in raw wastewater (66.9%) were higher than those detected in treated wastewater (40.7%). However, HAV percentages in the studied STPs varied widely in both raw and treated wastewater (Table 2) and ranged between 32.1% and 96.3% in inflows and between 18.5% and 77.8% in outflows. In raw wastewater, the highest rate (96.3%) was detected in the Jammel STP, whereas the Sahline STP showed the lowest HAV contamination rate (32.1%). Rates of positive samples decreased after primary and secondary treatments in all STPs except in the Dekhila STP, where treated wastewater was found to be slightly more contaminated than raw sewage (77.8% versus 74.1%) (Table 2).
TABLE 2.
Number and percentage of HAV-positive samples detected in raw and treated wastewater from five STPs during 13-month collection period
| Mo and sample category | % HAV-positive samples (no. of positive samples/total no. of samples) |
|||||
|---|---|---|---|---|---|---|
| Kairouan | Sousse-South | Dekhila | Sahline | Jammel | Total | |
| December 2009 | ||||||
| Influents | 100 (1/1) | 0 (0/2) | 100 (2/2) | 0 (0/2) | 100 (1/1) | 50 (4/8) |
| Effluents | 0 (0/1) | 0 (0/2) | 0 (0/2) | 0 (0/2) | 0 (0/1) | 0 (0/8) |
| January 2010 | ||||||
| Influents | 100 (1/1) | 0 (0/2) | 100 (1/1) | 0 (0/2) | 100 (2/2) | 50 (4/8) |
| Effluents | 0 (0/1) | 50 (1/2) | 0 (0/1) | 50 (1/2) | 0 (0/2) | 25 (2/8) |
| February 2010 | ||||||
| Influents | 100 (2/2) | 50 (1/2) | 50 (1/2) | 0 (0/2) | 100 (2/2) | 60 (6/10) |
| Effluents | 0 (0/2) | 0 (0/2) | 100 (2/2) | 0 (0/2) | 0 (0/2) | 20 (2/10) |
| March 2010 | ||||||
| Influents | 100 (3/3) | 33.3 (1/3) | 66.7 (2/3) | 33.3 (1/3) | 66.7 (2/3) | 60 (9/15) |
| Effluents | 66.7 (2/3) | 33.3 (1/3) | 66.7 (2/3) | 33.3 (1/3) | 33.3 (1/3) | 47 (7/15) |
| April 2010 | ||||||
| Influents | 0 (0/2) | 50 (1/2) | 100 (2/2) | 50 (1/2) | 100 (2/2) | 60 (6/10) |
| Effluents | 50 (1/2) | 50 (1/2) | 100 (2/2) | 50 (1/2) | 0 (0/2) | 50 (5/10) |
| May 2010 | ||||||
| Influents | 50 (1/2) | 50 (1/2) | 0 (0/2) | 0 (0/2) | 100 (2/2) | 40 (4/10) |
| Effluents | 100 (2/2) | 0 (0/2) | 100 (2/2) | 0 (0/2) | 0 (0/2) | 40 (4/10) |
| June 2010 | ||||||
| Influents | 100 (2/2) | 50 (1/2) | 50 (1/2) | 50 (1/2) | 100 (2/2) | 70 (7/10) |
| Effluents | 50 (1/2) | 100 (2/2) | 50 (1/2) | 0 (0/2) | 0 (0/2) | 40 (4/10) |
| July 2010 | ||||||
| Influents | 0 (0/2) | 100 (2/2) | 50 (1/2) | 50 (1/2) | 100 (2/2) | 60 (6/10) |
| Effluents | 0 (0/2) | 100 (2/2) | 100 (2/2) | 50 (1/2) | 0 (0/2) | 50 (5/10) |
| August 2010 | ||||||
| Influents | 66.7 (2/3) | 100 (3/3) | 100 (3/3) | 100 (3/3) | 100 (3/3) | 93 (14/15) |
| Effluents | 66.7 (2/3) | 100 (3/3) | 66.7 (2/3) | 33.3 (1/3) | 33.3 (1/3) | 60 (9/15) |
| September 2010 | ||||||
| Influents | 100 (2/2) | 100 (2/2) | 50 (1/2) | 50 (1/2) | 100 (2/2) | 80 (8/10) |
| Effluents | 50 (1/2) | 50 (1/2) | 100 (2/2) | 0 (0/2) | 50 (1/2) | 50 (5/10) |
| October 2010 | ||||||
| Influents | 50 (1/2) | 100 (2/2) | 100 (2/2) | 0 (0/2) | 100 (2/2) | 70 (7/10) |
| Effluents | 0 (0/2) | 50 (1/2) | 100 (2/2) | 0 (0/1) | 100 (2/2) | 56 (5/9) |
| November 2010 | ||||||
| Influents | 50 (1/2) | 100 (2/2) | 100 (2/2) | 50 (1/2) | 100 (2/2) | 80 (8/10) |
| Effluents | 0 (0/2) | 50 (1/2) | 100 (2/2) | 0 (0/2) | 0 (0/2) | 30 (3/10) |
| December 2010 | ||||||
| Influents | 100 (2/2) | 100 (2/2) | 100 (2/2) | 0 (0/2) | 100 (2/2) | 80 (8/10) |
| Effluents | 0 (0/2) | 50 (1/2) | 100 (2/2) | 0 (0/2) | 50 (1/2) | 40 (4/10) |
| Total influents | 69.2 (18/26) | 64.3 (18/28) | 74.1 (20/27) | 32.1 (9/28) | 96.3 (26/27) | 66.9 (91/136) |
| Total effluents | 34.6 (9/26) | 50 (14/28) | 77.8 (21/27) | 18.5 (5/27) | 22.2 (6/27) | 40.7 (55/135) |
HAV was also quantified by RT-qPCR in raw and treated wastewater samples of the 5 STPs. The results corresponding to calculations of the mean, minimum, and maximum numbers of HAV genome copies per milliliter in inflows and outflows are detailed by region in Table 3. Considering all the STPs together, no reduction in HAV loads was observed in effluents (6.0 × 103 GC/ml) compared to influents (2.7 × 103 GC/ml). The highest virus titer detected in raw wastewater samples was observed in the STP of Sousse-South (5.6 × 107 GC/ml), while the lowest concentration was detected in the Kairouan STP (6.7 × 101 GC/ml). In treated wastewater, the highest and lowest HAV titers were also observed in the Sousse-South (1.4 × 108 GC/ml) and Kairouan (7.2 × 101 GC/ml) STPs, respectively. However, HAV titers found in the Sahline STP in outflows (1.6 × 103 to 5.7 × 106 GC/ml) were similar to those observed in inflows (1.2 × 103 to 4.4 × 106 GC/ml). Interestingly, in the Sousse-South and Dekhila STPs, the number of HAV copies detected in treated wastewater was higher than that detected in raw sewage, ranging from 1.7 × 105 to 1.4 × 108 GC/ml and from 1.7 × 102 to 2.9 × 107 GC/ml, respectively.
TABLE 3.
Quantification of HAV in raw and treated wastewater collected from five STPs during 13-month collection period as determined by qRT-PCRa
| Mo and sample category | Quantification range of wastewater HAV (GC/ml) |
||||
|---|---|---|---|---|---|
| Kairouan | Sousse-South | Dekhila | Sahline | Jammel | |
| December 2009 | |||||
| Influents | 3.2 × 102 | 2.8 × 106 | |||
| Effluents | |||||
| January 2010 | |||||
| Influents | 2.9 × 102–2.9 × 103 | ||||
| Effluents | 1.6 × 103 | ||||
| February 2010 | |||||
| Influents | 2.7 × 102–4.3 × 102 | 2.7 × 102 | 2.9 × 103 | 1.6 × 103–7.7 × 103 | |
| Effluents | |||||
| March 2010 | |||||
| Influents | 6.7 × 101–3.7 × 102 | ||||
| Effluents | 1.5 × 102– 5.0 × 102 | 5.7 × 106 | |||
| April 2010 | |||||
| Influents | 5.4 × 102 | 3.4 × 102 | |||
| Effluents | 8.4 × 101 | ||||
| May 2010 | |||||
| Influents | 1.5 × 103 | 7.2 × 102 | 1.9 × 103–4.1 × 103 | ||
| Effluents | 7.2 × 101 | 2.9 × 107 | |||
| June 2010 | |||||
| Influents | 2.8 × 103–6.6 × 103 | 1.4 × 103 | 9.5 × 105 | 1.7 × 103–2.2 × 103 | |
| Effluents | 1.7 × 105 | ||||
| July 2010 | |||||
| Influents | 7.3 × 104 | 2.2 × 102–7.6 × 103 | |||
| Effluents | 1.4 × 108 | ||||
| August 2010 | |||||
| Influents | 6.2 × 102–5.6 × 107 | 1.3 × 105 | 9.7 × 101–1.6 × 103 | ||
| Effluents | 1.8 × 102–1.8 × 102 | 1.1 × 102 | |||
| September 2010 | |||||
| Influents | 9.9 × 102 | 1.1 × 103 | 1.2 × 103 | 8.9 × 102–2.2 × 103 | |
| Effluents | 9.1 × 101 | 7.4 × 103–7.6 × 104 | |||
| October 2010 | |||||
| Influents | 3.3 × 102 | 4.0 × 102 | |||
| Effluents | 9.6 × 103–1.0 × 105 | 8.3 × 102 | |||
| November 2010 | |||||
| Influents | 1.9 × 103–2.3 × 103 | 4.4 × 106 | 5.3 × 102–2.0 × 103 | ||
| Effluents | 1.7 × 102–9.6 × 103 | ||||
| December 2010 | |||||
| Influents | 5.8 × 103 | 1.6 × 105–8.4 × 105 | 4.0 × 102–4.3 × 102 | ||
| Effluents | 1.4 × 105 | 1.7 × 103 | |||
| Mean influents | 7.1 × 102 | 6.3 × 103 | 9.7 × 103 | 1.7 × 105 | 1.7 × 103 |
| Mean effluents | 1.3 × 102 | 4.8 × 106 | 1.3 × 104 | 9.4 × 104 | 5.5 × 102 |
qRT-PCR, quantitative reverse transcription-PCR; GC/ml, genome copies per milliliter.
Seasonal variations.
Results obtained for HAV detection were analyzed to determine if there was any seasonal effect on the viral circulation. Table 2 shows the occurrence of HAV during the 13-month collection period in all detected HAV-positive wastewater samples in influents and effluents. In raw or treated wastewater, HAV was detected throughout the year, with certain seasonal variations. The largest percentages of raw wastewater samples with positive results were detected in summer (77.1%), with a peak in the month of August (93.3%), and in autumn (76.7%). In treated wastewater samples, HAV was detected mainly in the summer (51.4%), and a peak in the month of August (60%) was also observed. However, as a different seasonal pattern for HAV was seen in each STP, data were classified by the origin of influents (Table 1) and pooled by month (Tables 2 and 3).
In the Sahline STP, which collects only tourism waters corresponding to 11,350 inhabitants, HAV was detected mainly in summer (71.4%), also showing a peak in August (100%). However, the highest viral concentrations were found during June (9.5 × 105 GC/ml) and November (4.4 × 106 GC/ml). In the STP of the Sousse-South region, which collects mainly domestic waters (270,968 inhabitants) but also waters from areas of tourism (31,000 inhabitants) and industrial waters (23,182 population equivalent), the HAV prevalence was low in winter (37.5%), increased slightly during the spring (42.9%), and peaked in summer (85.7%) and autumn (100%). High viral concentrations were noted in summer, with a maximum of 5.6 × 107 GC/ml obtained in the third week of August.
The Kairouan and Dekhila STPs collect mostly domestic water (238,540 and 31,345 inhabitants, respectively), followed by industrial waters (11,500 and 10,190 population equivalents) and a smaller volume of water in areas of tourism (530 and 3,080 inhabitants), and had a similar pattern of HAV seasonal distribution, with predominance during winter (100% and 85.7%, respectively) and autumn (66.7% and 83.3%). Maximum numbers of viral genomes have been detected in December in the Dekhila STP (8.4 × 105 GC/ml). However, in the Kairouan STP, highest concentrations were found in June (6.6 × 103 GC/ml) and December (5.8 × 103 GC/ml). Qualitative data showed that all influent samples taken in the month of June from this site were positive for HAV.
Interestingly, in the Jammel STP, HAV was not affected by any seasonal effect and was detected throughout the year at high percentages (100%) except in the month of March, in which the HAV rate decreased to 66.7%. In this STP, the highest viral load (2.8 × 106 GC/ml) was obtained in December 2009. It is noteworthy that the STP of Jammel collects mainly domestic waters (80,430 inhabitants) and a small volume of industrial waters (3,180 population equivalent).
HAV removal by biological wastewater treatment.
In order to evaluate the degree of viral contamination in effluents and the efficiency of wastewater treatment processes to remove viruses and to assess the possible seasonal effect on HAV circulation in effluents, viral rates was calculated and categorized by type of wastewater treatment process and by month. HAV removal efficiency after wastewater treatment is quantified in Table 4, and the data clearly show a high level of resistance of HAV to the three biological activated sludge treatment processes: conventional, oxidation ditch, and extended aeration activated sludge. The HAV removal rate, considering all samples regardless the wastewater treatment process, reached 39.1%.
TABLE 4.
Percentage of HAV-positive wastewater samples in influents and effluents and percentage of virus removal by wastewater treatment process
| Season | Wastewater treatment process |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Conventional activated sludge (medium charge)a |
Extended aeration oxidation ditch activated sludge (low charge)b |
Extended aeration activated sludge (low charge)c |
|||||||
| % HAV-positive influent samples | % HAV-positive effluent samples | % virus removal | % HAV-positive influent samples | % HAV-positive effluent samples | % virus removal | % HAV-positive influent samples | % HAV-positive effluent samples | % virus removal | |
| Winter | 60 | 40 | 33.3 | 42.9 | 7.1 | 83.3 | 100 | 14.3 | 85.7 |
| Spring | 50 | 57.1 | 0 | 42.9 | 50 | 0 | 85.7 | 14.3 | 83.3 |
| Summer | 78.6 | 85.7 | 0 | 64.3 | 35.7 | 44.4 | 100 | 14.3 | 85.7 |
| Autumn | 91.7 | 75 | 18.2 | 50 | 9.1 | 81.8 | 100 | 50 | 50 |
| Total | 69.1 | 63.6 | 7.9 | 50 | 26.4 | 47.2 | 96.3 | 22.2 | 76.9 |
Treatment used by Sousse-South and Dekhila STPs.
Treatment used by Sahline and Kairouan STPs.
Treatment used by Jammel STP.
Analysis of HAV data categorized by wastewater treatment process showed a total failure of virus removal in the Dekhila and Sousse-South plants using the conventional activated sludge process, with a viral reduction rate of 7.9%. In those two plants, the HAV level slightly decreased from 69.1% in raw wastewater samples to 63.6% in treated wastewater samples. The mean viral loads in effluent samples were higher (Sousse-South, 4.8 × 106 GC/ml; Dekhila, 1.3 × 104 GC/ml) than those detected in influent samples (Sousse-South, 6.3 × 103 GC/ml; Dekhila, 9.7 × 103 GC/ml) (Table 3). Using the conventional activated sludge process, effluents were found to be more contaminated than influents during spring (57.1%) and summer (85.7%). In autumn, HAV was resistant to treatment, with a viral reduction rate of 18.2%, while in winter, the HAV reduction level achieved 33.3%. The highest HAV concentrations found in effluents were observed in July (up to 1.4 × 108 GC/ml for Sousse-South) and in May (up to 2.9 × 107 GC/ml for Dekhila) (Table 3).
The Sahline and Kairouan STPs, using oxidation ditch activated sludge, showed a moderate efficiency rate of 47.2% for HAV removal. No differences were observed in mean viral titer reduction rates, and HAV was detected at the exit points with mean loads of 9.4 × 104 GC/ml and 1.3 × 102 GC/ml in Sahline and Kairouan, respectively (Table 3). HAV was partially removed in summer (44.4%) and efficiently cleared in winter (83.3%) and autumn (81.8%). However, total ineffectiveness of the treatment against HAV was observed in spring, when the HAV rate was slightly higher in effluents (50%) than in influents (42.9%). The highest concentrations of HAV in outflows were also detected in spring (March) for both the Sahline (5.7 × 106 GC/ml) and Kairouan (5.0 × 102 GC/ml) STPs, confirming resistance to the treatment.
In contrast, in the Jammel plant, using extended aeration activated sludge treatment, the HAV removal rate reached 76.9% and the viral titer reduction was 1 log unit (1.7 × 103 to 5.5 × 102 GC/ml). Extended aeration activated sludge treatment effectively removed 85.7% of the HAV genome in winter and summer and 83.3% in spring and partially (50%) cleared HAV in autumn. Effluent samples showed HAV loads in October (up to 8.3 × 102 GC/ml) that were slightly higher than those of influent samples (up to 4.0 × 102 GC/ml) as well as in the first week of December 2010, in which HAV concentrations reached 1.7 × 103 GC/ml in outflows, in contrast to 4.0 × 102 GC/ml in inflows (Table 3).
Molecular epidemiology of HAV in wastewater.
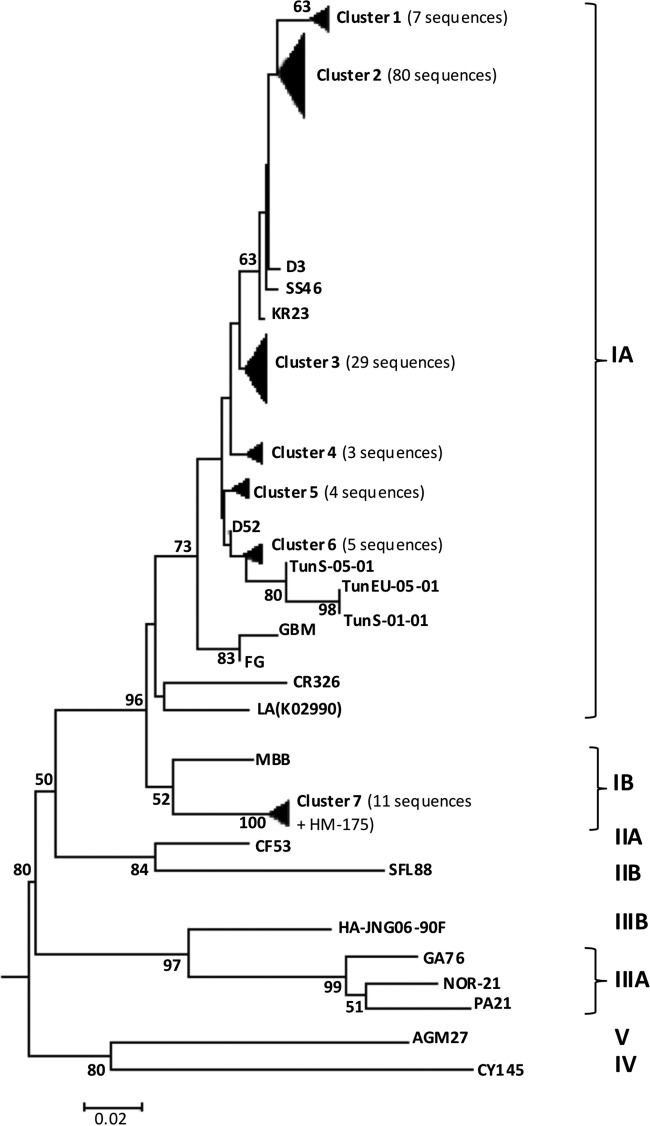
Among the 146 samples positive for HAV, 141 sequences were obtained. Analysis of the 225 bp of the VP3-VP1 region revealed that the vast majority of sequences obtained (130 sequences) showed 94.7% to 96.9% similarity to the sequence of the GBM reference strain, and those sequences were thus classified as representing subgenotype IA, while 11 sequences belonged to subgenotype IB, showing 99.1% to 100% identity with the HM-175 reference strain. Phylogenetic analysis of sequences obtained within subgenotype IA showed four unique sequences (D3, SS46, KR23, and D52) and six clusters in the neighbor-joining tree (Fig. 2). Furthermore, subgenotype IA strains showed percentages of identity of 96% to 98.7% with clinical strain TunS-05-01 and 94.2 to 96.9% with both the clinical TunS-01-01 strain and the wastewater TunEU-05-01 strain. The 11 subgenotype IB strains clustered with the HM-175 strain (cluster 7) and were detected in Sousse-South (5 strains), Sahline (3 strains), Dekhila (2 strains), and Kairouan (1 strain).
FIG 2.
Phylogenetic analysis of the HAV strains isolated in raw and treated wastewater samples from five STPs in Tunisia during 13 months of study. Wastewater sample codes: KR, Kairouan; SS, Sousse-South; D, Dekhila; SH, Sahline; J, Jammel. The phylogenetic tree was constructed using 223-bp sequences of the VP3-VP1 region and the NJ algorithm with distance calculation by Kimua-2-parameter correction (MEGA6). Only bootstrap values of >50% are shown on the phylogenetic tree. Bar, 0.02 substitutions per nucleotide position. Sequences obtained in the present study were deposited in the GenBank database.
The seasonal occurrence of subgenotype IB was also studied. In the Sousse-South STP, collecting mixed influents, subgenotype IB was detected mainly during spring (2 strains) and autumn (2 strains) but was also detected in winter (1 strain). In the Dekhila STP, which also collected mixed influents, it was present in spring (1 strain) and summer (1 strain). In the Kairouan STP, with major influence of domestic and industrial water, the only strain belonging to subgenotype IB was detected in February. Finally, in the Sahline STP, collecting only tourism influents, the presence of this subgenotype was observed only during spring (1 strain) and summer (2 strains). Cocirculation of subgenotype IA and subgenotype IB was observed in our study during April and August in the Sahline STP and during April in Sousse-South STP.
DISCUSSION
Contaminated treated wastewater discharged in the environment is one of the main sources of viral diseases and is considered the major vehicle for viral transmission (5), since enteric viruses entering the STP are released to the environment and can reinfect susceptible populations. HAV is shed in high numbers in the feces of infected individuals, whether symptomatic or not, and ends up in large quantities in raw sewage (1). Due to its high infectivity with low doses and greater resistance to usual wastewater treatments and the ability to survive for long periods in several environments (33), HAV can contaminate soil, food, shellfish, and natural water courses (34) and can then be transmitted to humans by fecal-oral routes (35). Therefore, detection of HAV in sewage would provide an important overview of its spread in an entire population, revealing the appearance of asymptomatic infections, and of its circulation between the environment and the human population in a given area.
HAV is still endemic in Tunisia, with a high/intermediate ranking (4, 12, 31, 36, 37), and in the Mediterranean region (8, 38, 39). Despite the potential public health hazard linked to the use of contaminated treated sewage, few studies have been carried out on the prevalence of HAV in wastewater and its seasonal and geographical distribution in Tunisia. The present work describes a large-scale environmental HAV surveillance, taking into account several factors and different characteristics of the studied STPs which complete our previous report (4) and offering a more extensive vision with respect to the occurrence and behavior of this virus within the whole of central Tunisia. On the basis of the use of molecular tools such as RT-qPCR and seminested PCR, high levels of HAV were detected in raw wastewater samples, reflecting the common presence of this virus in Tunisia. Despite of the improvement of hygiene conditions in many urban areas, the incidence of hepatitis has been not reduced in those areas. This result is in accordance with environmental studies conducted in the country, including analysis of wastewater samples, where HAV rates ranged between 46% and 68.3% (4, 31, 40). Seroprevalence data in Tunisia and in some regions of the Middle East and North Africa showed high HAV endemicity also, with levels ranging between 83% and 96.4% (12, 31, 36, 37).
Analysis of the epidemiological pattern of HAV infection, taking into account the socioeconomic and sanitary conditions, geographic localization, and climate, showed that the viral rate detected in the central Tunisia was slightly higher (69.2%) than that detected in the central coast (65.7%). These results are in line with those found in our previous report of a study performed using environmental samples (4) and in other seroprevalence studies demonstrating that higher HAV rates were found in the inland region and in the south of Tunisia (37, 41) than in big coastal cities (36, 37). It has been reported that HAV infection is still highly endemic in developing countries, with differences in epidemiological patterns that are mainly associated with the development of socioeconomic and hygienic conditions (3, 6, 7, 12).
Considering the four STPs located on the Mediterranean coast, the HAV genome was found frequently throughout the year, with high prevalence in summer and autumn. This finding is in agreement with those from previous seroprevalence studies (36, 42). However, present data from the west-central region showed the same HAV pattern found in our previous study (4) conducted in different STPs from the same area which showed the highest number of positive samples in winter and autumn also. Thus, geographic localization and climate may play an important role in the seasonal variation of HAV circulation. The quantitative results obtained were coherent with the qualitative data, showing high (107 to 108 GC/ml) HAV rates during summer, which might suggest the occurrence of outbreaks in this period.
Differences in seasonal prevalences were observed within the coastal region, where the origin of the influents was the parameter that best explained such variability. The highest level of viral contamination was observed during the summer in the STP that collects only tourism sewage (Sahline), while higher prevalences were displayed during the summer and autumn in the STPs collecting mixed domestic and tourism waters (Sousse-South and Dekhila). Interestingly, a regular increase in the level of viral contamination during summer was noted in these three areas, reaching the maximum in August. These results are in accordance with those found previously (4) and have been explained by the fact of the proximity of these areas to the coast and the consequent condensation of incoming tourism, mainly from neighboring countries such as Algeria and Libya but also from the European Union.
Little is known about the efficiency of the wastewater treatment processes used in Tunisia to HAV removal. Therefore, in the present study, a detailed comparative analysis of the levels of viral contamination in outflows based on the origin of influents and the wastewater treatment process has been described. Note that, as in other developing countries such as Algeria, Egypt, Morocco, and Syria, Tunisia utilizes both primary and secondary treatment plants where a range of wastewater treatment options are used, and treatment levels differ between urban and rural areas (43). In all these countries, with the increase in urban populations, some sewage treatment plants do not have the capacity to process such large volumes; consequently, retention times for wastewater treatment become too short to be effective. Currently, Tunisian efforts are focused on the upgrade to tertiary-level treatment (12). In contrast to previous studies showing viral reduction rates of 20% to 80% at STPs (4, 11, 44, 45), our qualitative and quantitative data showed HAV levels in the outflows that were similar to or slightly lower than those in the inflows. These results point to a potential health risk if final effluents are discharged into fresh or marine water systems.
According to ONAS, several factors may affect the efficiency and reliability of the treatment process in the STPs, namely, hydraulic and organic overloads of the STP, an antiquated state of equipment and structures, lack of operating staff, and the inflow of industrial wastewaters. In the present study, the influence of the latter parameter on the treatment efficiency was demonstrated, since the lowest HAV removal rates were found in STPs collecting large volume of industrial wastewaters. Interestingly, in those STPs and in a few other cases, the numbers of HAV copies in treated wastewater were higher than those found in raw sewage. These findings could be partially explained by the fact that raw wastewater, especially industrial wastewater, contains high loads of both organic and chemical pollutants as well as of heavy metals that may inhibit the PCR (46, 47). The activated sludge process is widely used by cities and communities where large volumes of sewage should be treated in a cost-saving manner. The comparison of three secondary treatments showed a total failure of HAV removal when conventional activated sludge treatment (medium charge) was used. However, the extended aeration and oxidation ditch activated sludge processes (low charge) used to overcome some of limitations associated with the conventional activated sludge process (40) showed high/moderate efficiency in elimination of HAV. These results are in accordance with previous studies performed by Jamwal et al. (48).
Results of the phylogenetic analysis of HAV sequences obtained from wastewater samples were in accordance with several epidemiological studies showing the predominance of subgenotype IA in Tunisia over several years (4, 31, 42). Interestingly, some strains belonging to this subgenotype were closely related to European strains, confirming the circulation of this subgenotype variant in the Mediterranean region (49). The presence of subgenotype IB is still infrequent in Tunisia (4, 22, 23). A slight rise in the prevalence of subgenotype IB was observed in the present study compared to results reported by other authors, who found only 2% of this subgenotype in sewage or clinical samples (22, 23). However, this could be explained by the large number of wastewater samples included in this monitoring, which may increase the possibility of detecting this rare subgenotype. Our results also demonstrated that subgenotype IB was more prevalent in the coastal area, especially in STPs collecting large volumes of tourism wastewaters, with high numbers detected during spring and summer. Hence, this finding could validate the hypothesis that subgenotype IB, circulating in Europe, is imported by travelers during Easter or summer vacations.
Rapid detection of the HAV genome in wastewater may provide an early warning tool for use by health authorities to manage and control the operation of STPs to avoid viral spread (50). This report provides important data on the incidence, behavior, seasonality, and genotype distribution of HAV in the environment in Tunisia, as well as on the risk of infection derived from its occurrence in effluents due to inadequate wastewater treatment. These findings seem to confirm that the prevalence of HAV depends on the socioeconomic level, sanitary conditions in the communities, sewage facilities, the locality, and the climate. The wide dispersion of HAV in effluents proves the inefficacity of the current wastewater treatment processes used in Tunisia to remove viral particles, whether infectious or not. Consequently, treated wastewaters discharged in the environment, mainly in the sea, or reused in irrigation or for refilling aquifers present a real public health risk. Hence, establishment of tertiary treatment processes or replacement of the activated sludge at medium charge by the sludge at low charge is absolutely needed. Finally, in this study, we observed a correlation between the incidence of subgenotype IB and the origin of influents, suggesting that this subgenotype variant could be imported by travelers.
ACKNOWLEDGMENTS
This work was supported in part by grant 2014–PG110 from the Xunta de Galicia (Spain). I.O. thanks the Erasmus Mundus (EMMAG) program for a research fellowship.
We thank the workers of the National Office of Sanitation (ONAS) of Tunisia for their help in collecting the wastewater samples and in providing the environmental data.
REFERENCES
- 1.Bosch A, Guix S, Sano D, Pinto RM. 2008. New tools for the study and direct surveillance of viral pathogens in water. Curr Opin Biotechnol 19:295–301. doi: 10.1016/j.copbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2012. WHO position paper on hepatitis A vaccines—June 2012. Wkly Epidemiol Rec 87:261–276.22905367 [Google Scholar]
- 3.Jacobsen KH, Koopman JS. 2005. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol 34:600–609. [DOI] [PubMed] [Google Scholar]
- 4.Ouardani I, Manso CF, Aouni M, Romalde JL. 2015. Efficiency of hepatitis A virus removal in six sewage treatment plants from central Tunisia. Appl Microbiol Biotechnol 99:10759–10769. doi: 10.1007/s00253-015-6902-9. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Lázaro D, Cook N, Ruggeri FM, Sellwood J, Nasser A, Nascimento MS, D'Agostino M, Santos R, Saiz JC, Rzeżutka A, Bosch A, Gironés R, Carducci A, Muscillo M, Kovač K, Diez-Valcarce M, Vantarakis A, von Bonsdorff CH, de Roda Husman AM, Hernández M, van der Poel WH. 2012. Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev 36:786–814. doi: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itani T, Jacobsen KH, Nguyen T, Wiktor SZ. 2014. A new method for imputing country-level estimates of hepatitis A virus endemicity levels in the Eastern Mediterranean region. Vaccine 32:6067–6074. doi: 10.1016/j.vaccine.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Karimi A, Mortazaei S, Moradi MT. 2015. High prevalence of symptomatic hepatitis A infection in rural area of Chaharmahal VA Bakhtiari Province, Iran. J Clin Diagn Res 9:DC01–DC03. doi: 10.7860/JCDR/2015/9798.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formiga-Cruz M, Tofiño-Quesada G, Bofill-Mas S, Lees DN, Henshilwood K, Allard AK, Conden-Hansson AC, Hernroth BE, Vantarakis A, Tsibouxi A, Papapetropoulou M, Furones MD, Girones R. 2002. Distribution of human virus contamination in shellfish from different growing areas in Greece, Spain, Sweden, and the United Kingdom. Appl Environ Microbiol 68:5990–5998. doi: 10.1128/AEM.68.12.5990-5998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pina S, Buti M, Jardi R, Clemente-Casares P, Jofre J, Girones R. 2001. Genetic analysis of hepatitis A virus strains recovered from the environment and from patients with acute hepatitis. J Gen Virol 82:2955–2963. doi: 10.1099/0022-1317-82-12-2955. [DOI] [PubMed] [Google Scholar]
- 10.Hollinger FB, Emerson SU. 2007. Hepatitis A virus, p 911–947. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 11.La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. 2010. Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Ann Ist Super Sanita 46:266–273. doi: 10.4415/ANN_10_03_07. [DOI] [PubMed] [Google Scholar]
- 12.Melhem NM, Talhouk R, Rachidi H, Ramia S. 2014. Hepatitis A virus in the Middle East and North Africa region: a new challenge. J Viral Hepat 21:605–615. doi: 10.1111/jvh.12282. [DOI] [PubMed] [Google Scholar]
- 13.El Ayni F, Manoli E, Cherif S, Jrad A, Assimacopoulos D, Trabelsi-Ayadi M. 2013. Deterioration of a Tunisian coastal aquifer due to agricultural activities and possible approaches for better water management. Water Environ J 27:348–361. doi: 10.1111/j.1747-6593.2012.00354.x. [DOI] [Google Scholar]
- 14.Jebri S, Jofre J, Barkallah I, Saidi M, Hmaied F. 2012. Presence and fate of coliphages and enteric viruses in three wastewater treatment plants effluents and activated sludge from Tunisia. Environ Sci Pollut Res Int 19:2195–2201. doi: 10.1007/s11356-011-0722-y. [DOI] [PubMed] [Google Scholar]
- 15.Sdiri-Loulizi K, Hassine M, Aouni Z, Gharbi-Khelifi H, Chouchane S, Sakly N, Neji-Guédiche M, Pothier P, Aouni M, Ambert-Balay K. 2010. Detection and molecular characterization of enteric viruses in environmental samples in Monastir, Tunisia between January 2003 and April 2007. J Appl Microbiol 109:1093–1104. doi: 10.1111/j.1365-2672.2010.04772.x. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickx G, Van Herck K, Vorsters A, Wiersma S, Shapiro C, Andrus JK, Ropero AM, Shouval D, Ward W, Van Damme P. 2008. Has the time come to control hepatitis A globally? Matching prevention to the changing epidemiology. J Viral Hepat 15:1–15. [DOI] [PubMed] [Google Scholar]
- 17.La Rosa G, Libera SD, Iaconelli M, Ciccaglione AR, Bruni R, Taffon S, Equestre M, Alfonsi V, Rizzo C, Tosti ME, Chironna M, Romanò L, Zanetti AR, Muscillo M. 2014. Surveillance of hepatitis A virus in urban sewages and comparison with cases notified in the course of an outbreak, Italy 2013. BMC Infect Dis 14:419. doi: 10.1186/1471-2334-14-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Ching KZ, de Paula VS, Nakano T, Siegl G, Weitz M, Robertson BH. 2004. Characterization of the complete genomic sequence of genotype II hepatitis A virus (CF53/Berne isolate). J Gen Virol 85:2943–2952. doi: 10.1099/vir.0.80304-0. [DOI] [PubMed] [Google Scholar]
- 19.Cristina J, Costa-Mattioli M. 2007. Genetic variability and molecular evolution of hepatitis A virus. Virus Res 127:151–157. doi: 10.1016/j.virusres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Robertson BH, Jansen RW, Khanna B, Totsuka A, Nainan OV, Siegl G, Widell A, Margolis HS, Isomura S, Ito K, Ishizu T, Moritsugu Y, Lemon SM. 1992. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions. J Gen Virol 73:1365–1377. doi: 10.1099/0022-1317-73-6-1365. [DOI] [PubMed] [Google Scholar]
- 21.Nainan OV, Xia G, Vaughan G, Margolis HS. 2006. Diagnosis of hepatitis a virus infection: a molecular approach. Clin Microbiol Rev 19:63–79. doi: 10.1128/CMR.19.1.63-79.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gharbi-Khelifi H, Ferre V, Sdiri K, Berthome M, Fki L, Harrath R, Billaudel S, Aouni M. 2006. Hepatitis A in Tunisia: phylogenetic analysis of hepatitis A virus from 2001 to 2004. J Virol Methods 138:109–116. doi: 10.1016/j.jviromet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Béji-Hamza A, Khélifi-Gharbi H, Hassine-Zaafrane M, Della Libera S, Iaconelli M, Muscillo M, Petricca S, Ciccaglione AR, Bruni R, Taffon S, Equestre M, Aouni M, La Rosa G. 2014. Qualitative and quantitative assessment of hepatitis A virus in wastewaters in Tunisia. Food Environ Virol 6:246–252. doi: 10.1007/s12560-014-9163-3. [DOI] [PubMed] [Google Scholar]
- 24.Journal Officiel de la République Tunisienne. 2005. Décret n° 2005-1991 du 11 juillet 2005, relativ à l'étude d'impact sur l'environnement et fixant les catégories d'unités soumises à l'étude d'impact sur l'environnement et les catégories d'unités soumises aux cahiers des charges. J Off Repub Tunis 57:1834–1840. http://faolex.fao.org/docs/pdf/tun55061.pdf. [Google Scholar]
- 25.INNORPI (Institut National de la Normalisation et de la Propriété Industrielle). 1989. Normes de rejet dans un milieu hydrique, NT 106.002. INNORPI, Tunis, Tunisia. [Google Scholar]
- 26.INNORPI (Institut National de la Normalisation et de la Propriété Industrielle). 1989. Normes de réutilisation des eaux traitées en Agriculture, NT 106.03. INNORPI, Tunis, Tunisia. [Google Scholar]
- 27.Federal Register. 1993. Part 503. Standards for the disposal of sewage sludge. Fed Regist 58:9387–9404. [Google Scholar]
- 28.Costafreda MI, Bosch A, Pintó RM. 2006. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Appl Environ Microbiol 72:3846–3855. doi: 10.1128/AEM.02660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manso CF, Polo D, Vilariño ML, Romalde JL. 2010. Genotyping of hepatitis A virus detected in bivalve shellfish in Galicia (NW Spain). Water Sci Technol 61:15–24. doi: 10.2166/wst.2010.768. [DOI] [PubMed] [Google Scholar]
- 30.Yun H, Kim S, Soon Byun K, Kwon SY, Yim HJ, Lim Y, Jeong S, Jee Y. 2008. Genetic analysis of HAV strains isolated from patients with acute hepatitis in Korea, 2005–2006. J Med Virol 80:777–784. doi: 10.1002/jmv.21127. [DOI] [PubMed] [Google Scholar]
- 31.Gharbi-Khelifi H, Sdiri K, Ferre V, Harrath R, Berthome M, Billaudel S, Aouni M. 2007. A 1-year study of the epidemiology of hepatitis A virus in Tunisia. Clin Microbiol Infect 13:25–32. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rzezutka A, Cook N. 2004. Survival of human enteric viruses in the environment and food. FEMS Microbiol Rev 28:441–453. doi: 10.1016/j.femsre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Cook N, Rzezutka A. 2006. Hepatitis viruses, p 282–308. In Motarjemi Y, Adams M (ed), Emerging foodborne pathogens. Woodhead Publishing Limited, Cambridge, United Kingdom. [Google Scholar]
- 35.Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, Khudyakov YE. 2014. Hepatitis A virus: host interactions, molecular epidemiology and evolution. Infect Genet Evol 21:227–243. doi: 10.1016/j.meegid.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Letaief A, Kaabia N, Gaha R, Bousaadia A, Lazrag F, Trabelsi H, Ghannem H, Letaief J. 2005. Age-specific seroprevalence of hepatitis A among school children in central Tunisia. Am J Trop Med Hyg 73:40–43. [PubMed] [Google Scholar]
- 37.Rezig D, Ouneissa R, Mhiri L, Mejri S, Haddad-Boubaker S, Ben Alaya N, Triki H. 2008. Seroprevalences of hepatitis A and E infections in Tunisia. Pathol Biol 56:148–153. doi: 10.1016/j.patbio.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Pintó RM, Alegre D, Domínguez A, El-Senousy WM, Sánchez G, Villena C, Costafreda MI, Aragonés L, Bosch A. 2007. Hepatitis A virus in urban sewage from two Mediterranean countries. Epidemiol Infect 135:270–273. doi: 10.1017/S0950268806006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobsen KH, Wiersma ST. 2010. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 28:6653–6657. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Benjes HH., Jr 1976. Small community wastewater treatment facilities—biological treatment systems. United States Environmental Protection Agency (EPA), Washington, DC. [Google Scholar]
- 41.Louati N, Feki L, Rekik H, Feki H, Chaabouni M, Hammami A, Gargouri J, Karray-Hakim H. 2009. Comparison of hepatitis A seroprevalence in blood donors in South Tunisia between 2000 and 2007. Arch Inst Pasteur Tunis 86:69–74. [PubMed] [Google Scholar]
- 42.Gharbi-Khelifi H, Abid NB, Beji A, Bhiri L, Harrath R, Sdiri K, Billaudel S, Ferre V, Aouni M. 2012. Seroprevalence and molecular characterisation of human hepatitis A virus in serum samples of Tunisian patients with clinical symptoms of viral hepatitis. Indian J Virol 23:29–35. doi: 10.1007/s13337-012-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qadir M, Bahri A, Sato T, Al-Karadsheh E. 2010. Wastewater production, treatment, and irrigation in Middle East and North Africa. Irrig Drainage Syst 24:37–51. doi: 10.1007/s10795-009-9081-y. [DOI] [Google Scholar]
- 44.Payment P, Fortin S, Trudel M. 1986. Elimination of human enteric viruses during conventional wastewater treatment by activated sludge. Can J Microbiol 32:922–925. doi: 10.1139/m86-170. [DOI] [PubMed] [Google Scholar]
- 45.Prado T, Silva DM, Guilayn WC, Rose TL, Gaspar AM, Miagostovich MP. 2011. Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res 45:1287–1297. doi: 10.1016/j.watres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Sobsey MD, Hickey AR. 1985. Effects of humic and fulvic acids on poliovirus concentration from water by microporous filtration. Appl Environ Microbiol 49:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai YL, Olson BH. 1992. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol 58:2292–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jamwal P, Mittal AK, Mouchel JM. 2009. Efficiency evaluation of sewage treatment plants with different technologies in Delhi (India). Environ Monit Assess 153:293–305. doi: 10.1007/s10661-008-0356-9. [DOI] [PubMed] [Google Scholar]
- 49.Chironna M, Grottola A, Lanave C, Villa E, Barbuti S, Quarto M. 2003. Genetic analysis of HAV strains recovered from patients with acute hepatitis from Southern Italy. J Med Virol 70:343–349. doi: 10.1002/jmv.10402. [DOI] [PubMed] [Google Scholar]
- 50.Elkana Y, Manaa A, Marzouk Y, Manor J, Halmut T, Herscovici M, Barak S. 1983. Detection of hepatitis A virus in sewage. J Virol Methods 7:259–262. doi: 10.1016/0166-0934(83)90077-0. [DOI] [PubMed] [Google Scholar]