ABSTRACT
Using microorganisms to remove waste and/or neutralize pollutants from contaminated water is attracting much attention due to the environmentally friendly nature of this methodology. However, cell recovery remains a bottleneck and a considerable challenge for the development of this process. Magnetotactic bacteria are a unique group of organisms that can be manipulated by an external magnetic field due to the presence of biogenic magnetite crystals formed within their cells. In this study, we demonstrated an account of accumulation and precipitation of amorphous elemental selenium nanoparticles within magnetotactic bacteria alongside and independent of magnetite crystal biomineralization when grown in a medium containing selenium oxyanion (SeO32−). Quantitative analysis shows that magnetotactic bacteria accumulate the largest amount of target molecules (Se) per cell compared with any other previously reported nonferrous metal/metalloid. For example, 2.4 and 174 times more Se is accumulated than Te taken up into cells and Cd2+ adsorbed onto the cell surface, respectively. Crucially, the bacteria with high levels of Se accumulation were successfully recovered with an external magnetic field. The biomagnetic recovery and the effective accumulation of target elements demonstrate the potential for application in bioremediation of polluted water.
IMPORTANCE The development of a technique for effective environmental water remediation is urgently required across the globe. A biological remediation process of waste removal and/or neutralization of pollutant from contaminated water using microorganisms has great potential, but cell recovery remains a bottleneck. Magnetotactic bacteria synthesize magnetic particles within their cells, which can be recovered by a magnetic field. Herein, we report an example of accumulation and precipitation of amorphous elemental selenium nanoparticles within magnetotactic bacteria independent of magnetic particle synthesis. The cells were able to accumulate the largest amount of Se compared to other foreign elements. More importantly, the Se-accumulating bacteria were successfully recovered with an external magnetic field. We believe magnetotactic bacteria confer unique advantages of biomagnetic cell recovery and of Se accumulation, providing a new and effective methodology for bioremediation of polluted water.
INTRODUCTION
Environmental remediation, a technique of waste removal and/or neutralization of pollutant from a contaminated site, is an attractive field because of the increasing difficulty and importance of pure water acquisition in both developing and industrial countries. Among the various technologies for environmental water remediation, biorecovery of waste using microorganisms has great potential and is an environmentally friendly alternative to conventional techniques, such as reclamation treatment (1–3). Studies of the waste biosorption onto microorganisms and uptake into cells have been well demonstrated, but cell recovery remains a bottleneck in this approach because scale-up of collection methods, such as centrifugation and filtration, provides a huge logistical and monetary challenge.
Magnetotactic bacteria are unique prokaryotes, recognized by their response to a magnetic field. This is due to the presence of magnetic nanoparticles of Fe3O4 or Fe3S2 within the cells (4–6). The particle formation occurs within an organelle, called a magnetosome, which is formed along the intracellular filamentous structure (7–9). The magnetosomes confer a magnetic moment to the cells, allowing them to migrate in aquatic environments under the influence of the Earth's geomagnetic field. We have already investigated the use of magnetotactic bacteria for the biomagnetic recovery of toxic and/or valuable metals and metalloid such as Cd (10, 11), Au (12), and Te (13). In these studies, Cd2+ and AuCl4− were mainly adsorbed onto the cell surface (10, 12), while the Te oxyanion (TeO32−) was reduced and biomineralized as discrete independent elemental Te nanocrystals within the cells, with no incorporation into the magnetite crystals (13). The dual crystallization of tellurium and magnetite by magnetotactic bacteria enabled approximately 70 times more bioaccumulation of the pollutant per cell than cell surface adsorption. Therefore, intracellular accumulation of target elements within magnetotactic bacteria offers the most promising system for bioremediation due to the unique advantages of magnetic manipulation with external magnetic field and of effective target molecule accumulation.
Selenium (Se) is a rare element of high use in industry for production of various valuable materials because of its unusual semiconducting and photo-optical physical properties (14). The increased use of Se has led to its rising price and its increase in water contamination, which is in danger of presenting both ecological and human health risks (15, 16). Therefore, the growing demand for Se in industrial technologies and the increased pollution effects of its byproducts into aquatic environments are rendering the recovery and recycling of this valuable element a very attractive global proposition. In aqueous environments, selenium is generally found as the toxic oxyanions selenate (SeO42−, +VI) and selenite (SeO32−, +IV). The selenium oxide ions can adsorb extracellularly to the cell surfaces of microorganisms (1, 17). In addition, some microorganisms in the environment possess various strategies of detoxification, such as methylation, assimilation as selenoamino acid, and reduction, that could provide the potential to effectively accumulate Se within the cell (18, 19).
In this study, we investigated the MIC of selenium oxyanion (SeO32−) for the magnetotactic bacterium Magnetospirillum magneticum AMB-1; the effect of this anion on magnetite crystal synthesis; and, if taken up, whether the Se dopes into the magnetite crystals (similar to Co and Mn in previously reported studies) (20, 21) or forms discrete crystals/inclusions within the cells (similar to the Te study) (13). Finally, we investigated the magnetic recovery of Se using magnetotactic bacteria.
MATERIALS AND METHODS
Determination of the MIC of selenite ion for M. magneticum AMB-1 growth.
M. magneticum AMB-1 (ATCC 700264) (22) was microaerobically cultured in magnetic spirillum growth medium (MSGM) at 28°C, as previously described (23). Microaerobic conditions were established by purging the cultures with argon gas. The MIC of selenium for M. magneticum AMB-1 in MSGM was determined by growing the cells in various initial concentrations of selenite salt (Na2SeO3): 0 (control), 5, 10, 20, 40, 60, 80, 100, and 250 μM. The cells were directly counted with a hemacytometer under an optical microscope (Leica DML) after 7 days of culture. Additionally the optical density at 600 nm (OD600) was recorded.
Transmission electron microscopy and energy-dispersive X-ray spectrometry analyses of M. magneticum AMB-1 grown in the presence of SeO32−.
Cultured bacterial cells harvested from medium were washed with MilliQ three times and spotted onto 300-mesh Formvar-carbon-coated copper grids (Agar Scientific, Ltd.). The samples were analyzed by transmission electron microscopy (TEM) operated at an accelerating voltage of 100 kV (Philips, CM10). High-resolution TEM imaging and analysis were conducted on an FEI CM200 field emission gun TEM running at 200 kV equipped with an Oxford Instruments energy-dispersive X-ray spectroscopy (EDX) spectrometer and a Gatan imaging filter. EDX analysis was conducted for at least six crystals in different cells under the same experimental conditions, with representative spot data shown.
Se accumulation in M. magneticum AMB-1.
To evaluate the amount of uptake into and adsorption onto cells by SeO32−, an atomic absorption spectrophotometer (Shimadzu, AA-6600G) was used. After the cells were collected by centrifugation (or, in the case of the magnetic recovery assay, collection by magnetic trap in a glass test tube), the precipitates were washed three times with HEPES buffer (pH 7.4), dried, and then dissolved with nitric acid solution (0.1 N) with heating in an oil bath. After the supernatant was discarded, the cells were dissolved by the same procedure described above. The dissolved solutions were quantitatively analyzed by atomic absorption spectrophotometry, using a calibration curve derived from standard solutions. All assays were performed three times.
Magnetic recovery assay of magnetotactic bacteria grown in the presence of selenite ions.
To verify the ability of biomagnetic recovery of M. magneticum AMB-1 in the presence of SeO32− using magnetic force, a magnetic cell recovery assay was conducted. The M. magneticum AMB-1 wild-type strain was harvested at the late logarithmic phase of growth, and cells were counted and adjusted to 1.0 × 108 cells/ml of MSGM in the presence of the SeO32− at different concentrations (0, 25, 50, and 100 μM). Three milliliters of each sample was then transferred to separate glass test tubes (diameter, 7 mm; height, 7.5 cm), each of which was sealed with a rubber cork. Cylindrical neodymium-boron magnets (diameter, 15 mm; height, 1 cm) were placed on the exterior of the horizontal center of each test tube to allow cell recovery to take place. At the designated times (1, 2, 4, 6, 8, 10, 15, and 20 h), culture medium was collected by inserting a syringe through the rubber cork and extracting culture medium (20 μl) from around the water surface. A cell count was performed against the extracted culture medium samples. After the magnetic separation, the amounts of SeO32− uptake into and adsorbed SeO32− onto magnetically manipulated cells were evaluated using an atomic absorption spectrophotometer (Shimadzu, AA-6600G). In addition, the magnetically collected cells and the Se concentration were measured at the endpoint for further verification.
RESULTS AND DISCUSSION
Effect of SeO32− on cell growth and on magnetite biomineralization in M. magneticum AMB-1.
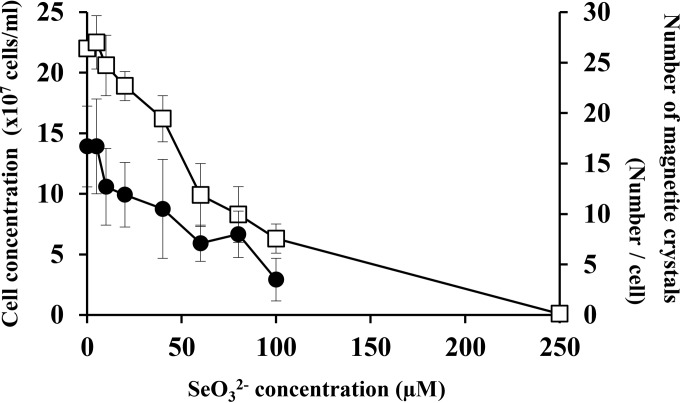
The effect of selenium oxyanion (SeO32−) on the growth of M. magneticum AMB-1 was investigated at various concentrations (Fig. 1). Cells cultured in MSGM containing 0 and 5 μM SeO32− showed similar growth rates, with stationary-phase cell densities of approximately 2.2 × 108 cells/ml. Cell growth was negatively affected by the increase of SeO32− concentration, and no cell growth was found at ≥250 μM. The MIC of selenium oxyanion for M. magneticum AMB-1 was determined to be 250 μM under these experimental conditions. The result indicated that SeO32− is mildly toxic to this bacterium compared with the other chalcogen, tellurium oxyanion (e.g., MIC of 60 μM) (13). As the MIC of SeO32− for Escherichia coli is 400 mM (24), M. magneticum AMB-1 is less resistant to this element. Similar observations have been previously found for other ions, including Co2+, Ni2+, and Cu2+, with M. magneticum AMB-1 showing approximately 90% less resistance than E. coli (20). It is noteworthy that light-orange colors developed during the cell growth in the presence of SeO32−. Similar observations were reported in various selenite-reducing bacteria (24, 25). The effect of the chalcogen on magnetite crystal formation in magnetotactic bacteria was also investigated (Fig. 1). The result showed a gradual decrease of magnetosomes with the increase of the SeO32− concentration, but magnetite formation was observed even in the presence of high concentrations (100 μM) of SeO32−. In addition, optical microscopy showed that approximately 100% and 70% of cells grown in the presence of 25 μM and 100 μM SeO32−, respectively, responded to the external magnetic field.
FIG 1.
Tolerance of M. magneticum AMB-1 to SeO32− and magnetite nanoparticle synthesis. The numbers of cells (□) and of magnetite crystals (●) grown in different concentrations (0, 5, 10, 20, 40, 60, 80, 100, and 250 μM) of SeO32− were directly counted. To evaluate the number of magnetite crystals within the cells, over 50 cells randomly selected were manually counted. Error bars show standard deviations.
Observation of discrete formation of magnetite crystals and Se granules in M. magneticum AMB-1 grown in the presence of SeO32−.
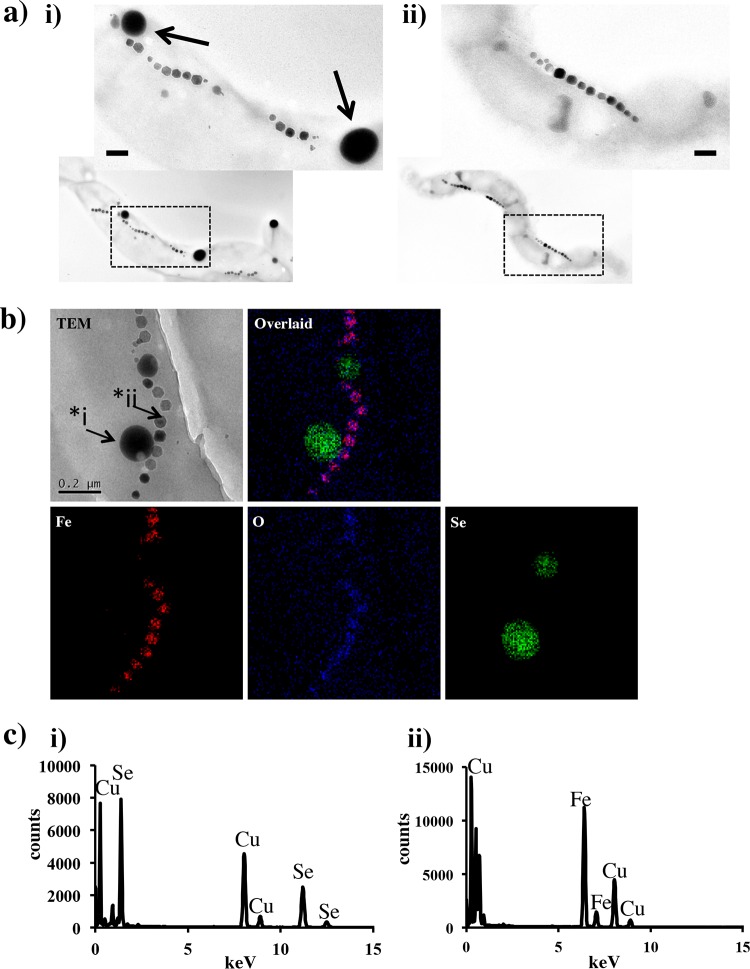
Fig. 2a shows representative TEM images of M. magneticum AMB-1 grown in the presence (100 μM) and absence of SeO32− in the MSGM medium. Approximately 10 independent spherical granules (30 to 300 nm diameter) were observed in the cell grown in the presence of SeO32− (Fig. 2a), while all cells revealed the presence of the magnetite crystals in a chain structure. The number and size of Se inclusions within the cell increased with increasing initial concentration of SeO32− in the medium. In a previous study, we observed the doping of some metals (Cu, Mn, and Co) into bacterial magnetite crystal under laboratory-controlled conditions (20). However, in this study, the elemental mapping showed no signal from Se in magnetite crystals (Fig. 2b). To verify the elemental components in these Se particles, scanning transmission electron microscope (STEM)-EDX spot spectra were recorded and showed that Se was the only element present (the Cu was from the TEM grid) (Fig. 2b and c). No oxygen was detected, suggesting that the inclusions are composed of pure elemental Se (0), which seems to be reduced and precipitated from SeO32− in the cell. Selenium is a group-16 nonmetal (chalcogens) neighbored by sulfur and the metalloid tellurium. Thiosulfate (S2O32−), tellurite (TeO32−), and selenite (SeO32−) are proposed to be taken up by bacteria and reduced to elemental S, Te, and Se, respectively (24, 26, 27). This is supported by the fact that S globules are present in many microbes, including magnetotactic bacteria (28, 29), and we have also reported the formation of Te nanocrystals in magnetotactic bacteria independent from the magnetosome (13). Here, we showed that magnetotactic bacteria take up, reduce, and intracellularly form discrete Se granules independently of magnetosomes, similar to Te crystal precipitation in the same organism (13). The granules were examined by high-resolution TEM with selected area electron diffraction which showed a diffuse pattern, revealing the amorphous Se structure.
FIG 2.
Transmission electron micrographs and STEM-EDX analyses for magnetite and Se within magnetotactic bacteria. (a) TEM micrographs of magnetotactic bacteria grown in the presence of SeO32− (100 μM) (i) and in its absence (ii). Characteristic intracellular granules were indicated with arrows. Scale bar indicates 100 nm. (b) TEM image and STEM-EDX maps of Se, Fe, and O taken using a probe size of approximately 5 nm. (c) Spot EDX spectra of i and ii in panel b as a representation of Se and magnetite. The Cu signal is from the copper TEM grid.
Time course measurements of Se accumulation in M. magneticum AMB-1.
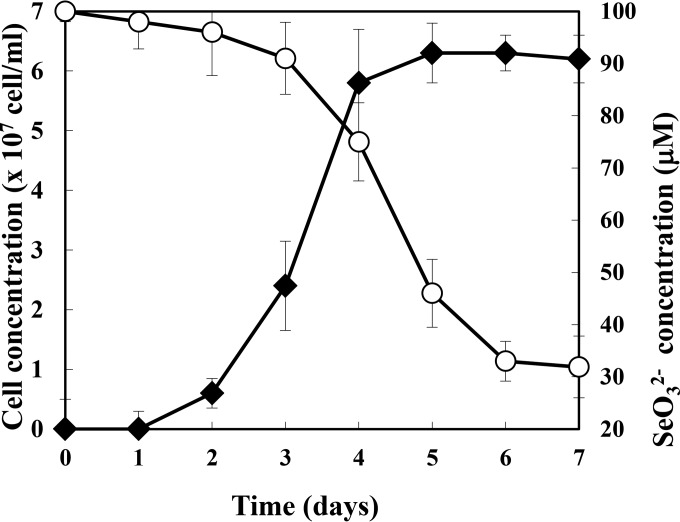
The time course of Se accumulation in magnetotactic bacteria was measured (Fig. 3). The cell growth and Se accumulation were saturated within 7 days, and the Se uptake in cells mainly occurred in the stationary phase (for cells grown in 100 μM SeO32−). Under this condition, 68.1% of the initial Se (100 μM) was accumulated by the cells, which resulted in 6.6 × 108 Se atoms per cell. In the case of Te accumulation found in the previous study, the most effective condition revealed that 2.7 × 108 Te atoms were accumulated per cell, which indicated that 2.4 times more Se was accumulated than Te. Furthermore, surface hexahistidine-expressing modified AMB-1 cells have previously been shown to adsorb Cd2+ onto these sites on the cell surface, showing the adsorption of 3.8 × 106 metal ions. Therefore, 2.4 and 174 times more Se was accumulated than Te uptake into the cell and than Cd2+ adsorption onto the cell surface, respectively. These results highlight the greater loading of elemental Se into AMB-1 cells than any other metalloid or nonferrous metal.
FIG 3.
SeO32− removal from medium during magnetotactic bacterial cell growth. SeO32− concentrations (○) and cell growth (◆) were evaluated for 7 days. The average values from three independent experiments were obtained. Error bars show standard deviations.
Biomagnetic recovery of SeO32− using M. magneticum AMB-1.
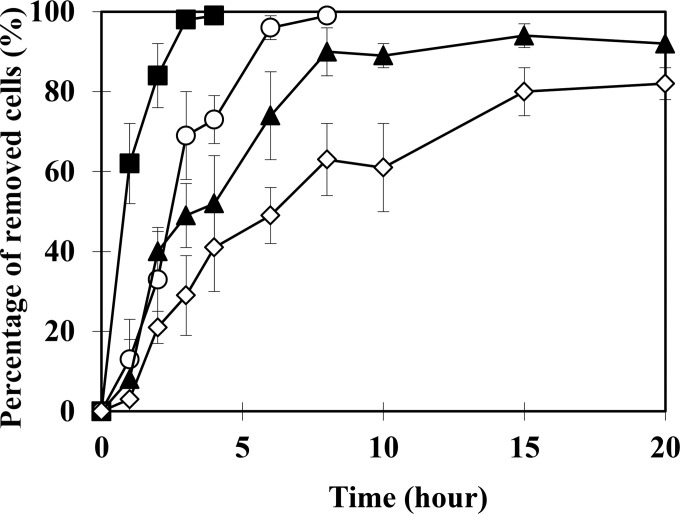
Magnetotactic bacteria harboring our target element (Se) for recovery can be manipulated and isolated by an external magnetic field, significantly magnifying the bioremediation potential of these cells for targeted recovery from polluted water environments. Herein, biomagnetic recovery of magnetotactic bacteria grown in the presence of SeO32− was described. The results shown in Fig. 4 revealed that almost all cells grown in 25 μM SeO32− were successfully recovered within 8 h. The time for magnetic recovery of cells gradually increased with increasing concentration of SeO32−. This seemed to be the result of the decreasing quantities of magnetite under higher Se concentration conditions (Fig. 1). However, even in the presence of 100 μM SeO32−, approximately 80% of magnetotactic bacteria was magnetically recovered within 20 h. To confirm the biomagnetic recovery of Se, the amount of Se from magnetically recovered harvested cells was measured and revealed 3.6 × 108 Se atoms per cell recovery. Though some Se was lost during the recovery process (3.0 × 108 Se atoms after recovery), the result clearly showed that magnetotactic bacteria could be applied in biomagnetic recovery of Se from SeO32−-containing water. We note that a more effective recovery could be established by process optimization (e.g., cell number, vessel size, and magnetic force enhancement).
FIG 4.
Magnetic recovery assay of Se granule-containing M. magneticum AMB-1. The percentage of recovered cells is calculated from the initial cell numbers (1.0 × 108/ml) by counting the number of dispersed cells left within the culture medium. In addition, the number of cells recovered by magnetic force was also verified by counting the cells recovered at the endpoints. M. magneticum AMB-1 was cultured and assayed with the following respective SeO32− concentrations: 0 μM (control), ■; 25 μM, ○; 50 μM, ▲; and 100 μM, ◇. The average values from three independent experiments were obtained. Error bars show standard deviations.
Current genetic and environmental microbiological research shows that magnetic particle production within bacteria occurs across a diverse group of bacterial species. In fact, the genetic region corresponding to magnetosome formation, called the magnetosome island (MAI), is found within microbes spread across the phylogenetic tree. As M. magneticum AMB-1 does not show strong resistance to SeO32− (Fig. 1), a magnetotactic bacterial species with higher tolerance and effective accumulation of target molecule could be found and used to improve the biomagnetic recovery, identified either from environments local to the bioremediation site or through evolving conditions similar to those in the polluted environment for a range of candidate magnetotactic bacteria. In addition, recently, magnetosome formation was enabled in another bacterial species by artificially transferring key genetic regions of the MAI into the host organism (30). Therefore, the induction of magnetosome formation within known bacteria showing high resistance to a target element is another promising approach to improve the biomagnetic recovery efficiency.
In conclusion, in this study, we showed an account of amorphous elemental Se particle formation from the reduction of SeO32− within the magnetotactic bacterial cell, completely independent of the crystallization of magnetite within the cell magnetosomes. The cells accumulated the largest amount of Se compared to any other foreign elements. For example, 2.4 and 174 times more Se was accumulated than Te into cells and Cd2+ adsorption onto cell surfaces, respectively. Importantly, the Se-accumulating bacteria were successfully recovered with an external magnetic field. Therefore, we believe that magnetotactic bacteria have the unique advantage of biomagnetic cell recovery, providing a new effective methodology for bioremediation of polluted water and an additional potential to utilize the pollutant product for further material applications (31).
ACKNOWLEDGMENTS
We thank Liane Benning and Jean Ingram (University of Leeds) for supporting bacterial culture works.
M.T., A.A., S.B., S.S., and T.M. conceived and designed the experiments. M.T., W.K., R.B., N.H., and S.S. performed the experiments. All authors analyzed the data. M.T., A.A., S.S., and T.M. wrote the paper.
We have no conflict of interest directly relevant to the content of this article.
Funding Statement
M.T. thanks the Royal Society UK for the funds under the Newton international fellowships scheme. This work was supported by Leeds EPSRC Nanoscience and Nanotechnology Facility (LENNF) and the University of Leeds, School of Physics, summer program as well as by contributions from a grant-in-aid for scientific research (no. 23226016) provided by the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Adams GO, Fufeyin PT, Okoro SE, Ehinomen I. 2015. Bioremediation, biostimulation, and bioaugmention: a review. Int J Environ Bioremediation Biodegrad 3:28–39. doi: 10.12691/ijebb-3-1-5. [DOI] [Google Scholar]
- 2.Tordoff G, Baker AJ, Willis A. 2000. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228. doi: 10.1016/S0045-6535(99)00414-2. [DOI] [PubMed] [Google Scholar]
- 3.Kleindienst S, Paul JH, Joye SB. 2015. Using dispersants after oil spills: impacts on the composition and activity of microbial communities. Nat Rev Microbiol 13:388–396. doi: 10.1038/nrmicro3452. [DOI] [PubMed] [Google Scholar]
- 4.Jogler C, Schüler D. 2009. Genomics, genetics, and cell biology of magnetosome formation. Annu Rev Microbiol 63:501–521. doi: 10.1146/annurev.micro.62.081307.162908. [DOI] [PubMed] [Google Scholar]
- 5.Matsunaga T, Suzuki T, Tanaka M, Arakaki A. 2007. Molecular analysis of magnetotactic bacteria and development of functional bacterial magnetic particles for nano-biotechnology. Trends Biotechnol 25:182–188. doi: 10.1016/j.tibtech.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Rahn-Lee L, Komeili A. 2013. The magnetosome model: insights into the mechanisms of bacterial biomineralization. Front Microbiol 4:352. doi: 10.3389/fmicb.2013.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komeili A, Li Z, Newman DK, Jensen GJ. 2006. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311:242–245. doi: 10.1126/science.1123231. [DOI] [PubMed] [Google Scholar]
- 8.Scheffel A, Schüler D. 2007. The acidic repetitive domain of the Magnetospirillum gryphiswaldense MamJ protein displays hypervariability but is not required for magnetosome chain assembly. J Bacteriol 189:6437–6446. doi: 10.1128/JB.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blakemore RP. 1975. Magnetotactic bacteria. Science 190:377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- 10.Arakaki A, Takeyama H, Tanaka T, Matsunaga T. 2002. Cadmium recovery by a sulfate-reducing magnetotactic bacterium, Desulfovibrio magneticus RS-1, using magnetic separation. Appl Biochem Biotechnol 98-100:833–840. doi: 10.1385/ABAB:98-100:1-9:833. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Nakata Y, Mori T, Okamura Y, Miyasaka H, Takeyama H, Matsunaga T. 2008. Development of a cell surface display system in a magnetotactic bacterium, “Magnetospirillum magneticum” AMB-1. Appl Environ Microbiol 74:3342–3348. doi: 10.1128/AEM.02276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka M, Kawase M, Tanaka T, Matsunaga T. 2011. Gold biorecovery from plating waste by magnetotactic bacterium, Magnetospirillum magneticum AMB-1. MRS Proc 1169:1169–Q03–12. doi: 10.1557/PROC-1169-Q03-12. [DOI] [Google Scholar]
- 13.Tanaka M, Arakaki A, Staniland SS, Matsunaga T. 2010. Simultaneously discrete biomineralization of magnetite and tellurium nanocrystals in magnetotactic bacteria. Appl Environ Microbiol 76:5526–5532. doi: 10.1128/AEM.00589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C-P, Yin Y-X, Guo Y-G. 2015. Elemental selenium for electrochemical energy storage. J Phys Chem Lett 6:256–266. doi: 10.1021/jz502405h. [DOI] [PubMed] [Google Scholar]
- 15.Lemly AD. 2002. Symptoms and implications of selenium toxicity in fish: the Belews Lake case example. Aquat Toxicol 57:39–49. doi: 10.1016/S0166-445X(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 16.Lavu RV, Van De Wiele T, Pratti VL, Tack F, Du Laing G. 2016. Selenium bioaccessibility in stomach, small intestine and colon: comparison between pure Se compounds, Se-enriched food crops and food supplements. Food Chem 197:382–387. doi: 10.1016/j.foodchem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Johansson CL, Paul NA, de Nys R, Roberts DA. 2016. Simultaneous biosorption of selenium, arsenic, and molybdenum with modified algal-based biochars. J Environ Manage 165:117–123. doi: 10.1016/j.jenvman.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Silva IR, Serrão VHB, Manzine LR, Faim LM, da Silva MTA, Makki R, Saidemberg DM, Cornélio ML, Palma MS, Thiemann OH. 2015. Formation of a ternary complex for selenocysteine biosynthesis in bacteria. J Biol Chem 290:29178–29188. doi: 10.1074/jbc.M114.613406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nancharaiah YV, Lens PNL. 2015. Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev 79:61–80. doi: 10.1128/MMBR.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka M, Brown R, Hondow N, Arakaki A, Matsunaga T, Staniland S. 2012. Highest levels of Cu, Mn, and Co doped into nanomagnetic magnetosomes through optimized biomineralization. J Mater Chem 22:11919. doi: 10.1039/c2jm31520c. [DOI] [Google Scholar]
- 21.Staniland S, Williams W, Telling N, Van Der Laan G, Harrison A, Ward B. 2008. Controlled cobalt doping of magnetosomes in vivo. Nat Nanotechnol 3:158–162. doi: 10.1038/nnano.2008.35. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga T, Sakaguchi T, Tadakoro F. 1991. Magnetite formation by a magnetic bacterium capable of growing aerobically. Appl Microbiol Biotechnol 35:651–655. doi: 10.1007/BF00169632. [DOI] [Google Scholar]
- 23.Tanaka M, Okamura Y, Arakaki A, Tanaka T, Takeyama H, Matsunaga T. 2006. Origin of magnetosome membrane: proteomic analysis of magnetosome membrane and comparison with cytoplasmic membrane. Proteomics 6:5234–5247. doi: 10.1002/pmic.200500887. [DOI] [PubMed] [Google Scholar]
- 24.Klonowska A, Heulin T, Vermeglio A. 2005. Selenite and tellurite reduction by Shewanella oneidensis. Appl Environ Microbiol 71:5607–5609. doi: 10.1128/AEM.71.9.5607-5609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruta L, Paraschivescu C, Matache M, Avramescu S, Farcasanu IC. 2010. Removing heavy metals from synthetic effluents using kamikaze Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol 85:763–771. doi: 10.1007/s00253-009-2266-3. [DOI] [PubMed] [Google Scholar]
- 26.Baesman SM, Bullen TD, Dewald J, Zhang D, Curran S, Islam FS, Beveridge TJ, Oremland RS. 2007. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl Environ Microbiol 73:2135–2143. doi: 10.1128/AEM.02558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathgeber C, Yurkova N, Stackebrandt E, Beatty JT, Yurkov V. 2002. Isolation of tellurite- and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca Ridge in the Pacific Ocean. Appl Environ Microbiol 68:4613–4622. doi: 10.1128/AEM.68.9.4613-4622.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazylinski DA, Dean AJ, Williams TJ, Long LK, Middleton SL, Dubbels BL. 2004. Chemolithoautotrophy in the marine, magnetotactic bacterial strains MV-1 and MV-2. Arch Microbiol 182:373–387. doi: 10.1007/s00203-004-0716-y. [DOI] [PubMed] [Google Scholar]
- 29.Spring S, Amann R, Ludwig W, Schleifer KH, van Gemerden H, Petersen N. 1993. Dominating role of an unusual magnetotactic bacterium in the microaerobic zone of a freshwater sediment. Appl Environ Microbiol 59:2397–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolinko I, Lohße A, Borg S, Raschdorf O, Jogler C, Tu Q, Pósfai M, Tompa E, Plitzko JM, Brachmann A, Wanner G, Müller R, Zhang Y, Schüler D. 2014. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat Nanotechnol 9:193–197. doi: 10.1038/nnano.2014.13. [DOI] [PubMed] [Google Scholar]
- 31.Nies DH. 1999. Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]