ABSTRACT
The Pacific white shrimp (Litopenaeus vannamei) is widely used in aquaculture, where it is reared at high stocking densities, temperatures, and nutrient concentrations. Here we report that adult L. vannamei shrimp emit the greenhouse gas nitrous oxide (N2O) at an average rate of 4.3 nmol N2O/individual × h, which is 1 to 2 orders of magnitude higher than previously measured N2O emission rates for free-living aquatic invertebrates. Dissection, incubation, and inhibitor experiments with specimens from a shrimp farm in Germany indicated that N2O is mainly produced in the animal's gut by microbial denitrification. Microsensor measurements demonstrated that the gut interior is anoxic and nearly neutral and thus is favorable for denitrification by ingested bacteria. Dinitrogen (N2) and N2O accounted for 64% and 36%, respectively, of the nitrogen gas flux from the gut, suggesting that the gut passage is too fast for complete denitrification to be fully established. Indeed, shifting the rearing water bacterial community, a diet component of shrimp, from oxic to anoxic conditions induced N2O accumulation that outlasted the gut passage time. Shrimp-associated N2O production was estimated to account for 6.5% of total N2O production in the shrimp farm studied here and to contribute to the very high N2O supersaturation measured in the rearing tanks (2,099%). Microbial N2O production directly associated with aquacultured animals should be implemented into life cycle assessments of seafood production.
IMPORTANCE The most widely used shrimp species in global aquaculture, Litopenaeus vannamei, is shown to emit the potent greenhouse gas nitrous oxide (N2O) at a particularly high rate. Detailed experiments reveal that N2O is produced in the oxygen-depleted gut of the animal by bacteria that are part of the shrimp diet. Upon ingestion, these bacteria experience a shift from oxic to anoxic conditions and therefore switch their metabolism to the anaerobic denitrification process, which produces N2O as an intermediate and dinitrogen (N2) gas as an end product. The N2O/N2 production ratio is unusually high in the shrimp gut, because denitrification cannot be fully established during the short gut passage time of food-associated bacteria. Nitrous oxide emission directly mediated by L. vannamei contributes significantly to the overall N2O emission from aquaculture facilities.
INTRODUCTION
Aquaculture facilities are characterized by large loads of nutrients, especially nitrogen compounds originating from the protein-rich feed. Accordingly, they are sites of intense nitrogen turnover, including the microbial processes of nitrification and denitrification (1, 2). Both processes produce nitrous oxide (N2O), which is a potent greenhouse gas and ozone-depleting substance (3, 4). Aquaculture was recently discussed as an important source of atmospheric N2O (2, 5). It was estimated that total N2O emissions from aquaculture currently account for 0.09 to 0.12 Tg N/year and will rise to 0.38 to 1.01 Tg N/year by 2030 due to the rapidly growing aquaculture industry (2, 5, 6). The maximum projection of N2O emissions from aquaculture for 2030 would correspond to 5.7% of the current estimate of global N2O emissions (2). Unfortunately, direct measurements of N2O emissions from aquaculture are missing, and the current estimates are based on the overall nitrogen load of aquaculture facilities and N2O emission factors that were derived from wastewater treatment plants (2, 5, 7). It is highly uncertain whether these emission factors reflect the true N2O yield of nitrogen cycling processes in aquaculture facilities, since little is known about the mechanisms and controlling factors of microbial N2O production in aquaculture facilities (7).
Established compartments for microbial nitrogen turnover in aquaculture facilities are the water column, suspended organic particles, and the biofilm-covered walls of the rearing tanks. In recirculating aquaculture systems (RASs), the rearing water is biologically treated in filter units to prevent accumulation of toxic nitrogen compounds, such as ammonia and nitrite, in the rearing tanks. Just as in wastewater treatments plants, one of the main purposes of these biofilters is to eliminate inorganic nitrogen compounds through microbial conversion to dinitrogen (N2) through denitrification and/or anammox (8). However, the production and emission of N2O are often undesired side effects during the microbial processing of wastewater (9).
In this study, an additional compartment of microbial N2O production in aquaculture facilities, namely, the guts of the reared animals, is proposed and investigated. In many free-living terrestrial, freshwater, and marine invertebrates, the gut is a distinct compartment of microbial nitrogen turnover and, in particular, a site of significant N2O production (10–12). The guts of earthworms, freshwater insect larvae, and marine copepods are characterized by the absence of oxygen and/or the presence of nitrate (NO3−) and labile organic carbon, which together stimulate the denitrification activity of ingested bacteria (13–15). During the feeding processes of these invertebrates, facultative denitrifying bacteria from the ambient oxic environment are abruptly transferred into the anoxic gut (11, 16). Such oxic-anoxic shifts cause transiently high N2O yields of the denitrification process (i.e., a high ratio of N2O production to N2O plus N2 production), due to delayed induction of the N2O reductase (17, 18).
The most important invertebrate used in aquaculture worldwide is the Pacific white shrimp Litopenaeus vannamei (Crustacea, Penaeidae). In 2008, this species made up 2.3 of the 5.0 million tons of crustaceans globally produced in aquaculture (19). L. vannamei is often reared in superintensive aquaculture systems with high stocking densities, temperatures, and nutrient concentrations (19–21). It follows that shrimp guts are potential compartments of N2O production at very high abundance in the rearing tanks (up to 100 adult shrimp/m2 [21, 22]). Therefore, we investigated the rate and mechanism of N2O production directly associated with L. vannamei and its gut. The aims were (i) to measure the overall N2O emission rate of L. vannamei under nearly in situ conditions and the N2O saturation level in the rearing water, (ii) to establish the shrimp gut as a compartment of N2O production in aquaculture systems, by combined dissection, incubation, and inhibitor experiments, (iii) to characterize the gut microenvironment (O2 and pH) with microsensor measurements, and (iv) to test the hypothesis that a sudden shift from oxic to anoxic conditions induces unbalanced denitrification in the bacterial community in the rearing water and thus high N2O yields of gut denitrification.
MATERIALS AND METHODS
Nitrous oxide emission from complete animals and dissected guts.
L. vannamei shrimp were obtained from an indoor RAS in Affinghausen (Germany), designed and supervised by Polyplan GmbH (Bremen, Germany). Rearing conditions are summarized in Table 1. Shrimp, with a wet weight of 20.4 ± 7.3 g (mean ± standard deviation [n = 36]), were kept in original rearing water until used for experiments. The animals were killed in ice water immediately prior to incubation experiments or gut dissection. Killing was necessary because living animals immediately evacuated their guts when transferred to incubation bottles (probably as a stress response), which would have compromised the study of gut denitrification. To avoid artifacts due to incipient decay processes, incubations of freshly killed shrimp were generally limited to 2 h, during which the production of N2O was linear. Complete animals were incubated in 100-ml glass bottles that contained 30 ml of aerated, 0.2-μm-filtered, rearing water and an air-filled headspace. The bottles were sealed gastight with butyl rubber stoppers.
TABLE 1.
In situ conditions in rearing tank
| Variable | Mean ± SD |
|---|---|
| Temperature (°C) | 29.5 ± 0.5 |
| Salinitya | 19 ± 2 |
| pH | 8.08 ± 0.14 |
| NO3− concentration (mmol/liter) | 9.13 ± 3.73 |
| NH4+ concentration (mmol/liter) | 0.024 ± 0.007 |
| NO2− concentration (mmol/liter) | 0.008 ± 0.003 |
| N2O concentration (nmol/liter) | 140 ± 59 |
| N2O atmospheric saturation (%) | 2,099 ± 877 |
| Adult L. vannamei stocking density | |
| Shrimp/m2 | 100 |
| Shrimp/liter | 0.5 |
Salinity is a dimensionless variable (65).
For incubation of intact guts, freshly killed animals were dissected along their dorsal side with scissors and the guts were carefully removed with sterile forceps. Dissected complete guts (i.e., gut contents and gut wall but excluding digestive glands) were incubated in 6-ml Exetainer vials (Labco, High Wycombe, United Kingdom) that contained 1 ml of aerated, 0.2-μm-filtered, rearing water and an air-filled headspace.
In addition to these oxic incubations, dissected complete guts and gut walls were incubated under anoxic conditions in 6-ml Exetainer vials that contained 1 ml of N2-flushed, 0.2-μm-filtered, rearing water. After the vials were closed for anoxic incubation, the headspace was flushed again with N2 for 5 to 10 min. As a negative control, N2-flushed, 0.2-μm-filtered, rearing water was incubated in gastight 100-ml glass bottles; the minute rate of the negative control was subtracted from the rate obtained for the gut samples.
For each incubation assay, 4 to 15 replicates were run with 1 shrimp or 1 dissected gut per incubation vial. Incubations were conducted at a mean temperature of 28 ± 2°C for up to 3 h. The incubation vials were placed on a shaker to enforce the equilibration of N2O between the water and the headspace. N2O accumulation was monitored by regularly taking 1-ml headspace samples through the rubber stopper (every 15 to 60 min). The analysis of N2O production by gas chromatography (GC 7890; Agilent Technologies) and the calculation of N2O emission rates were as described previously (12).
The potential rate of total denitrification (i.e., the rate of production of N2O plus N2) of dissected complete guts was measured with the acetylene inhibition technique (23). Freshly dissected guts were incubated in 6-ml Exetainers with 1 ml of N2-flushed, 0.2-μm-filtered, rearing water and a headspace containing a 1:10 mixture of acetylene and N2. Sampling, N2O analysis, and calculation of N2O production rates were performed as described above.
The in situ concentration of N2O in the rearing water was determined by adding 100 ml unfiltered rearing water to 125-ml gastight bottles (n = 10) that contained 4 ml saturated HgCl2 to stop any biological activity. The bottles were shaken for several hours for equilibration of N2O between the water and the headspace, and the N2O concentration in the headspace was measured as described above. The N2O concentration in the water was calculated as described previously (24).
Gut microenvironment.
Microsensors for O2 and pH measurements in dissected guts were constructed as described previously (25, 26). The tip diameters of the sensors were 10 to 20 μm. The sensors were calibrated before, during, and after the measurements. Oxygen microsensors were calibrated in Ringer's solution (Merck, Germany) at 0 and 100% air saturation by N2 and air flushing, respectively. The pH sensors were calibrated in standard solutions of pH 7 and pH 9. Freshly dissected guts were fixed on an agarose bottom in a flow cell, which was continuously flushed with aerated Ringer's solution (14). Aided by a dissection microscope, the microsensor tip was positioned at the outer surface of the gut wall, which was then defined as depth zero. Radial concentration profiles through the gut were started 1 mm above the upper gut wall in the aerated Ringer's solution and continued in increments of 0.1 mm to 3 mm below the lower gut wall, into the agarose bottom. Flattening of the gut due to insertion of the thin-tipped microsensors was negligible. Profiles were determined in the foregut, midgut, and hindgut at different degrees of gut filling. All measurements and calibrations were performed at 28°C.
Oxic-anoxic shift imposed on bacteria in rearing water.
Unfiltered rearing water containing free-living and particle-attached bacteria was aerated and added to 100-ml glass bottles (n = 3) that were sealed with butyl rubber stoppers. The water was continuously stirred with a glass-coated magnetic stirring bar. Water samples (3 ml) were taken every 20 min through the stopper and transferred to N2-flushed 6-ml Exetainers. After 1 h of oxic incubation, the water in the bottles was flushed with N2 for 10 min, the bottles were sealed again, and the remaining headspace was flushed with N2 for 5 min. After this oxic-anoxic shift, water samples (3 ml) were taken at intervals for a total incubation period of 16 h. The N2O concentration in the headspace was measured after forced equilibration of N2O between the water and the gas phase. Calculation of N2O production rates was performed as described above.
RESULTS AND DISCUSSION
Nitrous oxide emission from complete animals and dissected guts.
Under simulated in situ conditions of the rearing tanks (Table 1), freshly killed aquacultured shrimp (L. vannamei) emitted N2O with a rate of 4.3 ± 1.5 nmol/individual × h (mean ± standard deviation [n = 15]), which is equivalent to a biomass-specific rate of 0.20 ± 0.07 nmol/g (wet weight) × h (Fig. 1). On an individual basis, this is the highest N2O emission rate recorded to date for any aquatic invertebrate species. In fact, this rate is 14 to 62 times higher than the mean rates of free-living freshwater (0.07 nmol/individual × h) or marine (0.32 nmol/individual × h) invertebrates (11, 12). Conversely, the biomass-specific N2O emission rate of L. vannamei is 3 to 4 times lower than the mean biomass-specific rates of free-living freshwater (0.85 nmol/g [wet weight] × h) or marine (0.60 nmol/g [wet weight] × h) invertebrates (11, 12).
FIG 1.
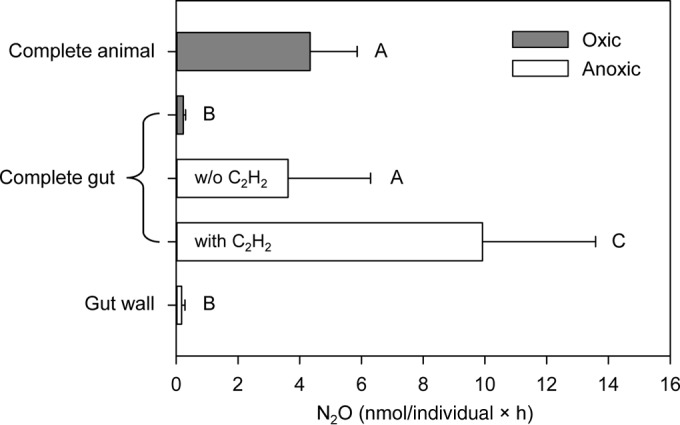
Rates of N2O emission from the shrimp Litopenaeus vannamei and its dissected guts incubated in filtered rearing water at 28°C. Complete animals were incubated under oxic conditions, dissected complete guts (i.e., gut contents plus gut wall) were incubated under oxic and anoxic conditions, and gut walls were incubated under anoxic conditions. Complete guts were also incubated under anoxic conditions with 10% acetylene (C2H2), which inhibits the last step of denitrification; the resulting N2O production indicates total denitrification. Means ± standard deviations are shown (n = 4 to 15). Treatments that share the same letter are not significantly different (see the text for details on statistics).
The N2O emitted from L. vannamei was mainly produced inside the animal's gut (Fig. 1). Dissected complete guts (i.e., gut contents plus gut wall) incubated under anoxic conditions produced N2O at 3.6 ± 2.7 nmol/gut × h (mean ± standard deviation [n = 13]), which was not significantly different from the N2O emission rate for the complete animal (P = 0.666, one-way analysis of variance [ANOVA] followed by the Holm-Šidák post hoc test). In contrast, dissected gut walls produced significantly less N2O than complete guts under anoxic conditions (P < 0.001). Hence, N2O production mainly took place in the gut contents rather than in the gut wall, which strongly suggests that N2O production is mediated by ingested microbes rather than by microbes colonizing the gut wall or by specific symbionts. Complete guts incubated under oxic conditions produced significantly less N2O than complete guts incubated under anoxic conditions (P = 0.020). This observation indicates that, in the shrimp gut, N2O production is due to anaerobic denitrification rather than aerobic nitrification. To test for denitrification, acetylene (C2H2) was added to complete guts incubated anoxically, to inhibit the last reduction step of denitrification (23). Nitrous oxide was then produced at a rate of 9.9 ± 3.7 nmol/gut × h (mean ± standard deviation [n = 9]), which was significantly higher than the N2O production rate for complete guts incubated anoxically but without C2H2 (P < 0.001). The N2O production rate in the presence of C2H2 is interpreted as the total denitrification rate in the shrimp gut. For comparison, total denitrification rates measured in dissected guts of freshwater invertebrates and earthworms are approximately 0.5 and 1.2 to 6.6 nmol/gut × h, respectively (11, 27). Total denitrification in the shrimp gut produced 64% N2 and 36% N2O, as calculated from N2O production rates measured in the presence and absence of C2H2.
Gut microenvironment.
Microsensor measurements through dissected L. vannamei guts (still filled with food particles) revealed anoxic conditions through almost the entire gut diameter (Fig. 2A). Thus, O2 diffusing from the air-saturated Ringer's solution into the gut was efficiently consumed inside the gut. Even empty guts were not fully oxygenated, which suggests that the gut walls, the epithelium itself, and/or some residential gut bacteria contribute to O2 consumption (Fig. 2B). No obvious variation in the radial O2 profiles along the length axis of filled or empty guts was observed (Fig. 2A and B). It should be kept in mind, however, that the transport of food particles is interrupted due to dissection, which may obscure axial concentration gradients. Under in vivo conditions, the O2 flux into the gut is probably much lower than after dissection. The hemolymph surrounding the gut typically has a much lower O2 concentration (approximately 2 to 3 μmol/liter) than the air-saturated Ringer's solution (28, 29). Therefore, it can be safely assumed that, under in vivo conditions, the entire gut is anoxic even when not completely filled. The largely anoxic conditions in the shrimp gut seem to be common for invertebrates, since previous microsensor measurements in the guts of diverse species all showed similar results (13–15, 30, 31).
FIG 2.
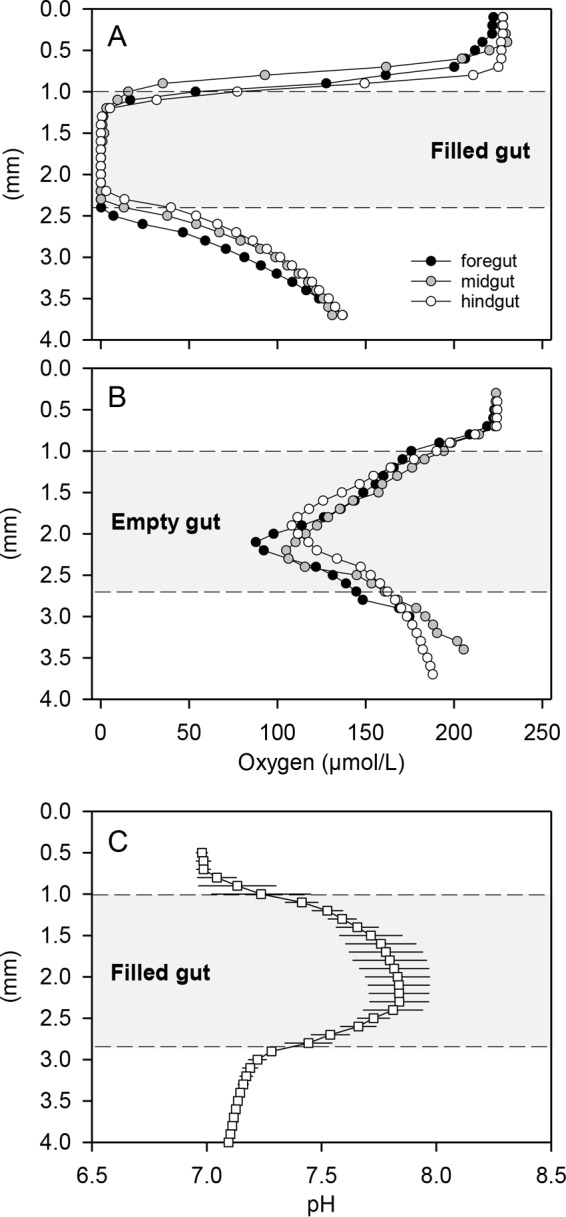
Radial microprofiles of O2 and pH through dissected guts of L. vannamei. Guts were fixed on agarose in a flow cell that was continuously supplied with air-saturated Ringer's solution. Gray area, gut interior; dashed lines, gut walls; upper white area, Ringer's solution; lower white area, agarose. (A) Oxygen concentration profiles through filled foreguts, midguts, and hindguts. (B) Oxygen concentration profiles through empty foreguts, midguts, and hindguts. In panels A and B, single representative profiles are shown. (C) pH profile (mean ± standard deviation [n = 6]) through filled guts, calculated from pH profiles measured in foreguts, midguts, and hindguts.
The pH in the gut lumen was 7.6 to 7.8 and thus slightly lower than that of the rearing water (pH 8.1) and higher than that of the Ringer's solution (pH 7.0 to 7.1) (Fig. 2C). No significant variation of the radial pH profiles along the length axis of the gut was observed (data not shown). The gut pH in L. vannamei is thus similar to that of other marine detritivores (30). Extreme values that might enable the digestion of refractory organic matter or create exclusive environments for gut symbionts, as in many terrestrial invertebrates, were not observed (32, 33). Gut pH might also be influenced by the metabolism of ingested or residential microorganisms (30). In fact, microbial denitrification activity increases the pH by alkalinity production, which may explain the higher gut pH relative to the Ringer's solution.
The O2 and pH microenvironment of the L. vannamei gut is favorable for microbial denitrification. Complete anoxia and nearly neutral pH values in the center of the gut should favor complete denitrification to N2 (which accounted for 64% of the nitrogen gas flux), rather than incomplete denitrification to N2O (which accounted for 36% of the nitrogen gas flux). The activity of the N2O reductase is partially inhibited by low pH values (34, 35), which were not observed in the L. vannamei gut. Therefore, the best explanation for the large fraction of N2O in the total nitrogen flux from the gut is that the gut passage time is too short to allow full establishment of complete denitrification to N2. Partial inhibition of N2O reductase by low O2 concentrations (36) might occur close to the gut wall, however, where O2 is present in trace amounts (Fig. 2A).
Due to the largely anoxic conditions inside the L. vannamei gut, it is unlikely that nitrification contributes to N2O production directly in the gut, where the bulk of N2O emitted by L. vannamei has its origin. The microoxic conditions close to the gut wall, however, may allow nitrifier denitrification, a process known to produce N2O in the near absence of O2 (37). Nitrification might contribute to the total N2O emission by L. vannamei if oxygenated biofilms are present on the body surface of the animal, as was observed for other marine and freshwater invertebrate species (12, 38–40). For L. vannamei, however, exoskeletal N2O production must be very low or even absent, because the anoxic gut alone emits N2O at the same rate as the complete animal (Fig. 1).
Oxic-anoxic shift imposed on bacteria in rearing water.
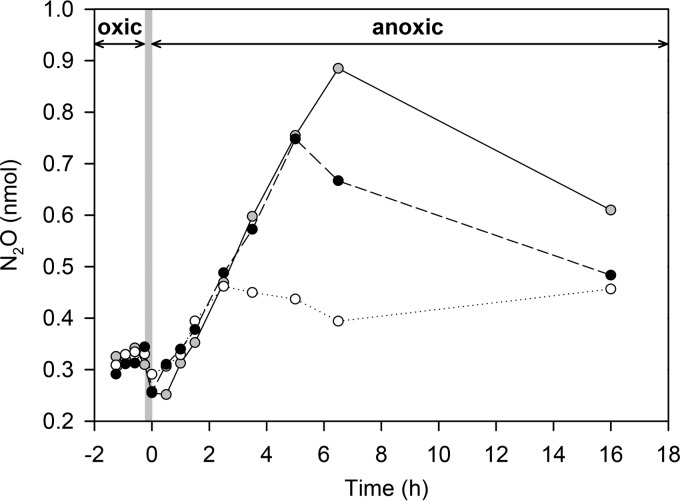
When the bacterial community of the rearing water was experimentally transferred from oxic to anoxic conditions, N2O production immediately increased from 0.20 ± 0.28 nmol/liter × h to 0.92 ± 0.18 nmol/liter × h (Fig. 3). This indicates that the oxic-anoxic shift prompted facultative anaerobic bacteria to switch rapidly from aerobic respiration to denitrification, with initially unbalanced enzyme activities. The accumulation of N2O in the incubation vials continued for 2.5 to 6.5 h, after which the N2O concentration started to decrease (Fig. 3). The gut passage time in L. vannamei is approximately 1 h (41, 42); therefore, the food bolus would have moved through the first quarter of the gut (i.e., the foregut) during the time it took to complete the oxic-anoxic shift of the rearing water (i.e., 15 min). The microsensor profiles revealed anoxic conditions in the foregut, and thus the experimentally induced oxic-anoxic shift was not unrealistically fast. Notably, the total gut passage time is considerably shorter than the time required for the bacterial inoculum from the rearing water to reach the phase of net N2O consumption. Thus, it is likely that many bacteria in the gut contents of L. vannamei have low N2O reductase activities, which is consistent with a high N2O yield for gut denitrification. The N2O yield of 36% for gut denitrification in L. vannamei is in the same range as observed for gut denitrification in earthworms and freshwater insect larvae (11, 43). Similar N2O yields were also measured when complete animals were incubated, which argues against a dissection artifact (11, 44). Nitrous oxide yields for gut denitrification are thus much higher than the <1% typically measured in the water column and sediments of aquatic ecosystems under undisturbed conditions (45, 46).
FIG 3.
Induction of N2O production in unfiltered rearing water by an oxic-anoxic shift. White areas with arrows, oxic and anoxic periods of the incubation experiment; gray area, transition period. Results from three replicate incubations are shown.
Transient N2O accumulation after shifts from oxic to anoxic conditions is also known from pure cultures of denitrifiers (17, 18, 47, 48). The time required to balance enzyme activities and then perform complete denitrification after the oxic-anoxic shift varies with the species and culture conditions. Thus, the community composition of denitrifiers ingested by L. vannamei might influence the temporal patterns of N2O production and consumption and eventually the N2O yield of gut denitrification. Furthermore, knowing the community composition of gut denitrifiers could be revealing, because N2O-respiring taxa constitute only 10 to 15% of all known denitrifying taxa (49). In the highly NO3−-enriched RAS, N2O-respiring taxa may in fact be underrepresented, since N2O reduction is inhibited by high NO3− (50, 51).
In summary, N2O production associated with L. vannamei is likely due to denitrification by ingested bacteria in the anoxic gut of the animal. This interpretation is in accordance with observations on terrestrial and aquatic invertebrates, for which ingested denitrifiers are the key players in N2O production (11, 52, 53). The bacterial abundance in aquaculture water is generally high, due to the copious supplies of inorganic and organic nutrients, and can reach up to 3.9 × 108 cells/ml in RASs (21, 54, 55). L. vannamei mainly takes up particle-attached microorganisms by feeding on suspended particulate organic matter, and it uses these microorganisms as an additional food source (56, 57). This study uncovered an additional role of microorganisms ingested by L. vannamei, i.e., the production of N2O through denitrification by microorganisms that survive and remain or become metabolically active during the gut passage.
Nitrous oxide produced in the L. vannamei gut is first released into the rearing water and then expelled into the atmosphere, due to the intense aeration of the rearing tanks. Nevertheless, the steady-state N2O concentration in the rearing water of the shrimp farm studied here corresponded to 2,099% atmospheric saturation (Table 1). The oxic conditions in the rearing tanks exclude the possibility that this N2O is converted by free-living bacteria capable of anaerobic denitrification. Similarly, N2O cannot be metabolized by aerobic nitrifying bacteria and archaea, because it is only a by-product and not a true intermediate of ammonia oxidation. To date, assimilation of N2O into the microbial biomass has been reported only for N2-fixing cyanobacteria (58), which are not abundant in the nitrogen-rich rearing water of shrimp farms. Thus, the most likely fate of N2O produced by L. vannamei is emission into the atmosphere.
Significance of direct N2O emission from L. vannamei.
In light of the fast-growing aquaculture industry, especially for penaeid shrimp species such as L. vannamei, direct N2O emissions from reared animals need to be constrained and integrated into whole-aquaculture emission budgets (2). For the RAS from which the tested shrimp were obtained, the relative contribution of N2O production directly associated with shrimp (RN2O-shrimp) to the total N2O production in the rearing tank (RN2O-tank) was estimated. Assumptions for this estimate were that the gas flux between the rearing water and the air was at steady state and that the rearing tank formed one well-mixed unit with the biofilter operating in recirculation. According to reference 59, RN2O-tank can be described as follows: RN2O-tank = Vtank × kN2O × (CN2O-tank – CN2O-sat), where Vtank is the volume of the rearing tank (53 m3), kN2O is the volumetric mass transfer coefficient for N2O (see below), CN2O-tank is the N2O concentration measured in the rearing tank (140 ± 59 nmol/liter) (Table 1), and CN2O-sat is the N2O equilibrium concentration at the given temperature and salinity (6.7 nmol/liter). The parameter kN2O was estimated as follows. Based on the aeration rate (40 m3/h) and the bottom area (265 m2) of the rearing tank, the superficial gas velocity (vGAS) was calculated to be 0.000042 m3/m2 × s. This value was used in an empirical equation derived in reference 59 to calculate kN2O as 34,525 × vGAS0.86, i.e., 5.9 day−1. RN2O-tank was thus estimated to be 42.0 ± 17.5 mmol/day (mean ± standard deviation [n = 10 replicate measurements of CN2O-tank]). RN2O-shrimp amounted to 4.3 nmol/individual × h × 100 individuals/m2 × 265 m2, i.e., 2.75 ± 0.95 mmol/day (n = 15 replicate measurements of the individual-specific N2O emission rate). Thus, RN2O-shrimp was estimated to be 6.5 ± 3.5% of RN2O-tank. This value may be different in other RASs with superintensive shrimp production but, to our knowledge, has not been quantified in any other shrimp farm.
The findings of this study should be implemented into life cycle assessments of shrimp production, which currently lack the aspect of N2O emissions (60). Mitigation strategies for animal-associated N2O emissions should aim at reducing the extremely high NO3− concentrations in the rearing tanks, as gut denitrification is significantly reduced with depletion of ambient NO3−, as shown for free-living aquatic invertebrates (61). Shrimp production accounts for only 6.35% of global aquaculture production (2), and thus the questions of whether other aquacultured animal species emit N2O directly and how much must be raised. The guts of fish and mollusks are potentially anoxic compartments in aquaculture systems in which anaerobic microbial processes such as denitrification might occur (62). For these animals, however, the availability of NO3− inside the gut and the fate of gut-produced N2O are currently not known. For mollusks, including Mytilus edulis, significant N2O production also proceeds in microbial biofilms growing on the shells of the animals (40). This phenomenon can also be expected for the richly sculptured shells of oysters, which have been recognized as keystone species for coastal nitrogen management (63), but to date has not been noted for N2O emission, which may be a disadvantage of nitrogen removal stimulated by benthic macrofauna (39, 64). The current report on N2O emission from L. vannamei should thus inspire further research on N2O production directly associated with a larger variety of aquacultured and free-living animals.
ACKNOWLEDGMENTS
Our thanks are due to Christina Peppler (Polyplan GmbH, Bremen, Germany) and Marco Schäfer for providing access to the shrimp farm in Affinghausen (Germany), L. vannamei shrimp, and water samples. We are grateful to the technicians of the Microsensor Group at the Max Planck Institute for Marine Microbiology for the construction of microsensors.
Funding Statement
This work, including the efforts of Peter Stief, was funded by German Research Foundation (STI202/6-1) and by the Max Planck Society.
REFERENCES
- 1.Crab R, Avnimelech Y, Defoirdt T, Bossier P, Verstraete W. 2007. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270:1–14. doi: 10.1016/j.aquaculture.2007.05.006. [DOI] [Google Scholar]
- 2.Hu Z, Lee JW, Chandran K, Kim S, Khanal SK. 2012. Nitrous oxide (N2O) emission from aquaculture: a review. Environ Sci Technol 46:6470–6480. doi: 10.1021/es300110x. [DOI] [PubMed] [Google Scholar]
- 3.Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga G, Schulz M, Van Dorland R. 2007. Changes in atmospheric constituents and in radiative forcing. In Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (ed), Climate change 2007: the physical science basis: contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 4.Ravishankara AR, Daniel JS, Portmann RW. 2009. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- 5.Williams J, Crutzen PJ. 2010. Nitrous oxide from aquaculture. Nat Geosci 3:143. doi: 10.1038/ngeo804. [DOI] [Google Scholar]
- 6.Food and Agricultural Organization of the United Nations. 2014. The state of world fisheries and aquaculture. Food and Agricultural Organization of the United Nations, Rome, Italy. [Google Scholar]
- 7.Paudel SR, Choi O, Khanal SK, Chandran K, Kim S, Lee JW. 2015. Effects of temperature on nitrous oxide (N2O) emission from intensive aquaculture system. Sci Total Environ 518-519:16–23. [DOI] [PubMed] [Google Scholar]
- 8.van Rijn J, Tal Y, Schreier HJ. 2006. Denitrification in recirculating systems: theory and applications. Aquacult Eng 34:364–376. doi: 10.1016/j.aquaeng.2005.04.004. [DOI] [Google Scholar]
- 9.Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, Van Loosdrecht MCM. 2009. Nitrous oxide emission during wastewater treatment. Water Res 43:4093–4103. doi: 10.1016/j.watres.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Karsten GR, Drake HL. 1997. Denitrifying bacteria in the earthworm gastrointestinal tract and in vivo emission of nitrous oxide (N2O) by earthworms. Appl Environ Microbiol 63:1878–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stief P, Poulsen M, Nielsen LP, Brix H, Schramm A. 2009. Nitrous oxide emission by aquatic macrofauna. Proc Natl Acad Sci U S A 106:4296–4300. doi: 10.1073/pnas.0808228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heisterkamp IM, Schramm A, de Beer D, Stief P. 2010. Nitrous oxide production associated with coastal marine invertebrates. Mar Ecol Prog Ser 415:1–9. doi: 10.3354/meps08727. [DOI] [Google Scholar]
- 13.Horn MA, Schramm A, Drake HL. 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl Environ Microbiol 69:1662–1669. doi: 10.1128/AEM.69.3.1662-1669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stief P, Eller G. 2006. The gut microenvironment of sediment-dwelling Chironomus plumosus larvae as characterised with O2, pH, and redox microsensors. J Comp Physiol B 176:673–683. doi: 10.1007/s00360-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 15.Tang KW, Glud RN, Glud A, Rysgaard S, Nielsen TG. 2011. Copepod guts as biogeochemical hotspots in the sea: evidence from microelectrode profiling of Calanus spp. Limnol Oceanogr 56:666–672. doi: 10.4319/lo.2011.56.2.0666. [DOI] [Google Scholar]
- 16.Drake HL, Schramm A, Horn MA. 2006. Earthworm gut microbial biomes: their importance to soil microorganisms, denitrification, and the terrestrial production of the greenhouse gas N2O, p 65–87. In König H, Varma A (ed), Intestinal microorganisms of soil invertebrates. Springer-Verlag, Heidelberg, Germany. [Google Scholar]
- 17.Baumann B, Snozzi M, Zehnder AJB, van der Meer JR. 1996. Dynamics of denitrification activity of Paracoccus denitrificans in continuous culture during aerobic-anaerobic changes. J Bacteriol 178:4367–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kester RA, deBoer W, Laanbroek HJ. 1997. Production of NO and N2O by pure cultures of nitrifying and denitrifying bacteria during changes in aeration. Appl Environ Microbiol 63:3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Agricultural Organization of the United Nations. 2010. The state of world fisheries and aquaculture. Food and Agricultural Organization of the United Nations, Rome, Italy. [Google Scholar]
- 20.Cuzon G, Lawrence A, Gaxiola G, Rosas C, Guillaume J. 2004. Nutrition of Litopenaeus vannamei reared in tanks or in ponds. Aquaculture 235:513–551. doi: 10.1016/j.aquaculture.2003.12.022. [DOI] [Google Scholar]
- 21.Browdy CL, Jory DE. 2009. The rising tide: proceedings of the special session on sustainable shrimp farming. World Aquaculture Society, Baton Rouge, LA. [Google Scholar]
- 22.Krummenauer D, Peixoto S, Cavalli RO, Poersch LH, Wasielesky W. 2011. Superintensive culture of white shrimp Litopenaeus vannamei in a biofloc technology system in southern Brazil at different stocking densities. J World Aquacult Soc 42:726–733. doi: 10.1111/j.1749-7345.2011.00507.x. [DOI] [Google Scholar]
- 23.Sørensen J. 1978. Denitrification rates in a marine sediment as measured by the acetylene inhibition technique. Appl Environ Microbiol 36:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss RF, Price BA. 1980. Nitrous oxide solubility in water and seawater. Mar Chem 8:347–359. doi: 10.1016/0304-4203(80)90024-9. [DOI] [Google Scholar]
- 25.Revsbech NP. 1989. An oxygen microsensor with a guard cathode. Limnol Oceanogr 34:474–478. doi: 10.4319/lo.1989.34.2.0474. [DOI] [Google Scholar]
- 26.de Beer D, Schramm A, Santegoeds CM, Kuhl M. 1997. A nitrite microsensor for profiling environmental biofilms. Appl Environ Microbiol 63:973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn MA, Mertel R, Gehre M, Kastner M, Drake HL. 2006. In vivo emission of dinitrogen by earthworms via denitrifying bacteria in the gut. Appl Environ Microbiol 72:1013–1018. doi: 10.1128/AEM.72.2.1013-1018.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JC, Cheng SY. 1995. Hemolymph oxygen content, oxyhemocyanin, protein levels and ammonia excretion in the shrimp Penaeus monodon exposed to ambient nitrite. J Comp Physiol B 164:530–535. [Google Scholar]
- 29.Lin CH, Chen JC. 2001. Hemolymph oxyhemocyanin and protein levels and acid-base balance in the tiger shrimp Penaeus monodon exposed to copper sulfate. J World Aquacult Soc 32:335–341. doi: 10.1111/j.1749-7345.2001.tb00457.x. [DOI] [Google Scholar]
- 30.Plante CJ, Jumars P. 1992. The microbial environment of marine deposit-feeder guts characterized via microelectrodes. Microb Ecol 23:257–277. doi: 10.1007/BF00164100. [DOI] [PubMed] [Google Scholar]
- 31.Brune A, Emerson D, Breznak JA. 1995. The termite gut microflora as an oxygen sink: microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl Environ Microbiol 61:2681–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brune A. 1998. Termite guts: the world's smallest bioreactors. Trends Biotechnol 16:16–21. doi: 10.1016/S0167-7799(97)01151-7. [DOI] [Google Scholar]
- 33.Charrier M, Brune A. 2003. The gut microenvironment of helicid snails (Gastropoda: Pulmonata): in-situ profiles of pH, oxygen, and hydrogen determined by microsensors. Can J Zool 81:928–935. doi: 10.1139/z03-071. [DOI] [Google Scholar]
- 34.Baumann B, van der Meer JR, Snozzi M, Zehnder AJB. 1997. Inhibition of denitrification activity but not of mRNA induction in Paracoccus denitrificans by nitrite at a suboptimal pH. Antonie Van Leeuwenhoek 72:183–189. doi: 10.1023/A:1000342125891. [DOI] [PubMed] [Google Scholar]
- 35.Bergaust L, Mao YJ, Bakken LR, Frostegard A. 2010. Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrogen oxide reductase in Paracoccus denitrificans. Appl Environ Microbiol 76:6387–6396. doi: 10.1128/AEM.00608-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zumft WG. 1997. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poth M, Focht DD. 1985. 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl Environ Microbiol 49:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svenningsen NB, Heisterkamp IM, Sigby-Clausen M, Larsen LH, Nielsen LP, Stief P, Schramm A. 2012. Shell biofilm nitrification and gut denitrification contribute to emission of nitrous oxide by the invasive freshwater mussel Dreissena polymorpha (zebra mussel). Appl Environ Microbiol 78:4505–4509. doi: 10.1128/AEM.00401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsh DT, Nizzoli D, Fano EA, Viaroli P. 2015. Direct contribution of clams (Ruditapes philippinarum) to benthic fluxes, nitrification, denitrification and nitrous oxide emission in a farmed sediment. Estuar Coast Shelf Sci 154:84–93. doi: 10.1016/j.ecss.2014.12.021. [DOI] [Google Scholar]
- 40.Heisterkamp IM, Schramm A, Larsen LH, Svenningsen NB, Lavik G, de Beer D, Stief P. 2013. Shell biofilm-associated nitrous oxide production in marine molluscs: processes, precursors, and relative importance. Environ Microbiol 15:1943–1955. doi: 10.1111/j.1462-2920.2012.02823.x. [DOI] [PubMed] [Google Scholar]
- 41.Beseres JJ, Lawrence AL, Feller RJ. 2005. Variation in fiber protein and lipid content of shrimp feed: effects on gut passage times measured in the field. J Shellfish Res 24:301–308. doi: 10.2983/0730-8000(2005)24[301:VIFPAL]2.0.CO;2. [DOI] [Google Scholar]
- 42.Beardsley C, Moss S, Malfatti F, Azam F. 2011. Quantitative role of shrimp fecal bacteria in organic matter fluxes in a recirculating shrimp aquaculture system. FEMS Microbiol Ecol 77:134–145. doi: 10.1111/j.1574-6941.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- 43.Drake HL, Horn MA. 2007. As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Annu Rev Microbiol 61:169–189. doi: 10.1146/annurev.micro.61.080706.093139. [DOI] [PubMed] [Google Scholar]
- 44.Stief P, Schramm A. 2010. Regulation of nitrous oxide emission associated with benthic invertebrates. Freshw Biol 55:1647–1657. [Google Scholar]
- 45.Seitzinger SP. 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33:702–724. doi: 10.4319/lo.1988.33.4_part_2.0702. [DOI] [Google Scholar]
- 46.Bange HW. 2008. Gaseous nitrogen compounds (NO, N2, NH3) in the ocean, p 51–94. In Capone DG, Bronk DA, Mulholland MR, Carpenter EJ (ed), Nitrogen in the marine environment, 2nd ed Academic Press, Burlington, MA. [Google Scholar]
- 47.Otte S, Grobben NG, Robertson LA, Jetten MSM, Kuenen JG. 1996. Nitrous oxide production by Alcaligenes faecalis under transient and dynamic aerobic and anaerobic conditions. Appl Environ Microbiol 62:2421–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergaust L, Shapleigh J, Frostegard A, Bakken L. 2008. Transcription and activities of NOx reductases in Agrobacterium tumefaciens: the influence of nitrate, nitrite and oxygen availability. Environ Microbiol 10:3070–3081. doi: 10.1111/j.1462-2920.2007.01557.x. [DOI] [PubMed] [Google Scholar]
- 49.Zumft WG, Kroneck PMH. 2007. Respiratory transformation of nitrous oxide (N2O) to dinitrogen by bacteria and archaea. Adv Microb Physiol 52:107–227. [DOI] [PubMed] [Google Scholar]
- 50.Blackmer AM, Bremner JM. 1978. Inhibitory effect of nitrate on reduction of N2O to N2 by soil microorganisms. Soil Biol Biochem 10:187–191. doi: 10.1016/0038-0717(78)90095-0. [DOI] [Google Scholar]
- 51.Richardson D, Felgate H, Watmough N, Thomson A, Baggs E. 2009. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle: could enzymic regulation hold the key? Trends Biotechnol 27:388–397. doi: 10.1016/j.tibtech.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Ihssen J, Horn MA, Matthies C, Gossner A, Schramm A, Drake HL. 2003. N2O-producing microorganisms in the gut of the earthworm Aporrectodea caliginosa are indicative of ingested soil bacteria. Appl Environ Microbiol 69:1655–1661. doi: 10.1128/AEM.69.3.1655-1661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horn MA, Drake HL, Schramm A. 2006. Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Appl Environ Microbiol 72:1019–1026. doi: 10.1128/AEM.72.2.1019-1026.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burford MA, Thompson PJ, McIntosh RP, Bauman RH, Pearson DC. 2003. Nutrient and microbial dynamics in high-intensity zero-exchange shrimp ponds in Belize. Aquaculture 219:393–411. doi: 10.1016/S0044-8486(02)00575-6. [DOI] [Google Scholar]
- 55.Holl CM, Glazer CT, Moss SM. 2011. Nitrogen stable isotopes in recirculating aquaculture for super-intensive shrimp production: tracing the effects of water filtration on microbial nitrogen cycling. Aquaculture 311:146–154. doi: 10.1016/j.aquaculture.2010.11.028. [DOI] [Google Scholar]
- 56.Avnimelech Y. 1999. Carbon nitrogen ratio as a control element in aquaculture systems. Aquaculture 176:227–235. doi: 10.1016/S0044-8486(99)00085-X. [DOI] [Google Scholar]
- 57.de Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W. 2008. The basics of bio-flocs technology: the added value for aquaculture. Aquaculture 277:125–137. doi: 10.1016/j.aquaculture.2008.02.019. [DOI] [Google Scholar]
- 58.Farias L, Faundez J, Fernandez C, Cornejo M, Sanhueza S, Carrasco C. 2013. Biological N2O fixation in the eastern South Pacific Ocean and marine cyanobacterial cultures. PLoS One 8:e63956. doi: 10.1371/journal.pone.0063956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foley J, de Haas D, Yuan Z, Lant P. 2010. Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res 44:831–844. doi: 10.1016/j.watres.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 60.Cao L, Diana JS, Keoleian GA, Lai QM. 2011. Life cycle assessment of Chinese shrimp farming systems targeted for export and domestic sales. Environ Sci Technol 45:6531–6538. doi: 10.1021/es104058z. [DOI] [PubMed] [Google Scholar]
- 61.Stief P, Polerecky L, Poulsen M, Schramm A. 2010. Control of nitrous oxide emission from Chironomus plumosus larvae by nitrate and temperature. Limnol Oceanogr 55:872–884. doi: 10.4319/lo.2009.55.2.0872. [DOI] [Google Scholar]
- 62.Pond MJ, Stone DM, Alderman DJ. 2006. Comparison of conventional and molecular techniques to investigate the intestinal microflora of rainbow trout (Oncorhynchus mykiss). Aquaculture 261:194–203. doi: 10.1016/j.aquaculture.2006.06.037. [DOI] [Google Scholar]
- 63.Rose JM, Bricker SB, Tedesco MA, Wikfors GH. 2014. A role for shellfish aquaculture in coastal nitrogen management. Environ Sci Technol 48:2519–2525. doi: 10.1021/es4041336. [DOI] [PubMed] [Google Scholar]
- 64.Stief P. 2013. Stimulation of microbial nitrogen cycling in aquatic ecosystems by benthic macrofauna: mechanisms and environmental implications. Biogeosciences 10:7829–7846. doi: 10.5194/bg-10-7829-2013. [DOI] [Google Scholar]
- 65.UNESCO. 1985. The international system of units (SI) in oceanography. UNESCO Tech Pap Mar Sci 45:1–124. [Google Scholar]