Abstract
The aims of the present study were to investigate the impact of rapamycin (RAPA) on the endometriosis (EMS) lesions in severe combined immunodeficiency (SCID) mice, and to examine the possible mechanism involved in a novel therapy in EMS. Following the successful establishment of an EMS-SCID mouse model, the mice were randomly assigned into the RAPA, control and saline treatment groups. Subsequent to treatment for 2 weeks, the serum hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) were detected using ELISA. The levels of HIF-1α and VEGF, as well as the size of EMS lesions, were compared among the three groups. In addition, the HIF-1α, VEGF and CD34 protein expression levels, and the microvessel density (MVD) of the lesions were determined by immunohistochemical analysis. Compared with the control and saline groups, the volume of EMS lesions in the RAPA-treated SCID mice was significantly reduced. Furthermore, the serum level and protein expression of VEGF, and the MVD in the lesions of the RAPA-treated group were significantly reduced when compared with the other two groups. These parameters were comparable in the control and saline groups. In conclusion, RAPA may inhibit the growth of endometriotic lesions, most possibly through the inhibition of the expression of VEGF in lesions, thereby inhibiting angiogenesis.
Keywords: rapamycin, endometriosis, hypoxia-inducible factor-1α, vascular endothelial growth factor, microvessel density
Introduction
Endometriosis (EMS) is a common benign gynecological disorder, which is invasive and prone to metastasis (1). It has been estimated that endometriosis is present in 6–10% of reproductive age women and in up to 20–40% of infertile women (2). The mechanism underlying the etiology of EMS remains unclear and no efficient treatment is available. Seeking an effective therapeutic approach has been the focus of numerous studies in recent years.
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that integrates a wide range of signaling pathways, leading to its involvement in divergent physiological processes, including transcription, translation, ribosome biogenesis and apoptosis (3). mTOR has been demonstrated to be a crucial regulator in certain types of malignant cancer (4,5) and has been shown to be tightly associated with the occurrence and development of EMS (6). Rapamycin (RAPA), an inhibitor of mTOR, was found to be involved in the induction of cell apoptosis, suppression of cancer cell proliferation, invasion and angiogenesis in tumors (7,8). In addition, Leconte et al (9) revealed that blocking the mTOR signaling pathway inhibits the formation of deep infiltrating EMS nodules in mice. However, the overall effects of mTOR inhibitors on EMS remain unclear and the possible underlying mechanism is unknown.
Hypoxia-inducible factor-1α (HIF-1α), which is a general regulatory factor in the response to hypoxia, regulates the expression of vascular endothelial growth factor (VEGF) and various upstream enzymes, resulting in the regulation of the process of angiogenesis (10). The occurrence and development of angiogenesis in EMS has been closely associated with ischemia and hypoxia of the ectopic endometrium (11–13). In our previous studies, it was demonstrated that HIF-1α is involved in the process of angiogenesis in EMS (14,15). Furthermore, previous studies reported that mTOR was able to enhance the expression of HIF-1α, thereby promoting the expression of VEGF (16).
In the present study, the effect of RAPA on EMS and its possible mechanism were investigated in order to provide new evidence for a targeted EMS therapy. The study investigated the effect of RAPA on the development of EMS lesions and microvessel density (MVD), as well as the expression of HIF-1α and VEGF.
Materials and methods
Animals and reagents
A total of 30 female specific pathogen free (SPF) CB17 severe combined immunodeficiency (SCID) mice (weight, 17.02±0.75 g; age, 6–7 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). RAPA was purchased from LC Laboratories (Woburn, MA, USA). Mouse HIF-1α and VEGF ELISA kits (cat. nos. CSB-E08541m and CSB-E04756m, respectively) were obtained from Sino-American Biotechnology Co., Ltd. (Wuhan, China). Mouse anti-human HIF-1α polyclonal antibody (1:100; cat. no. BM0912) was purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Mouse anti-human VEGF (1:200; cat. no. RB-9031) and CD34 (1:100; cat. no. MS-363) monoclonal antibodies, streptomycin avidin-peroxidase (SP) immunochemistry (cat. no. Kit-9701) and DAB (cat. no. Kit-0017) kits were from Maxim Biotech, Inc. (Fuzhou, China). Lidocaine and pentobarbital were obtained from Sigma-Aldrich (St. Louis, MO, USA). Formalin, paraffin and bovine serum albumin (BSA) Sangon Biotech, Co., Ltd. (Shanghai, China). Citric acid and sodium citrate were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China).
Animal model
Tissue samples were acquired from 15 female patients during laparoscopic resection of EMS at the Departments of Obstetrics and Gynecology at the Guangzhou Red Cross Hospital (Guangzhou, China) between January 2014 and February 2014. The average age of the participants was 32±8 years. Informed consent was obtained from all the participants prior to the procedure. The samples were confirmed to be eutopic secretory endometrial tissues by a pathologist who was blinded to the research design. The tissues were sectioned into 1-mm3 fragments and stored temporarily in a cold sterile bottle with an appropriate volume of Dulbecco's modified Eagle's medium (GE Healthcare Life Sciences, Logan, UT, USA) for <1 h. This study was approved by the Ethics Committee of Zhujiang Hospital of Southern Medical University.
In order to establish an EMS-SCID animal model, 2 ml of the tissue suspension obtained from the EMS patients was injected into the abdominal cavity of 30 female SPF CB17 SCID mice within 60 min after the harvest of the tissue sample. The mice were maintained in cages (5 mice/cage), under SPF conditions at 22–24°C and 50–70% relative humidity, and under a 12-h light/dark cycle. All mice were fed a normal laboratory diet. After 4 weeks, the EMS-SCID mouse model was successfully established, as confirmed by endometrial tissue investigation during laparotomy, which showed a cyst on the internal abdominal wall. For laparotomy, 0.1 ml 2% lidocaine was injected subcutaneously. The volume of EMS lesions prior to treatment (V1) were measured, according to the method of Katsuki (17), the 30 EMS-SCID mice were then randomly assigned into three groups (n=10 each), as follows: i) RAPA group, in which the lesions were injected with 250 µg/mouse RAPA once per week; ii) control group, receiving no treatment; and iii) saline group, receiving lesions were injected with 0.25 ml/mouse saline once per week. There was no difference in the V1 among the three groups (P>0.05). At 2 weeks after the initiation of treatment (V2), blood samples were collected from the orbit of the mice following removal of the eyeball. Subsequently, all animals were sacrificed with an overdose of pentobarbital (100 mg/kg), and the volume of EMS lesions following treatment was measured using the previously mentioned method.
ELISA
Blood samples were obtained from the orbit of the mice prior to sacrifice. Serum was collected by centrifugation for 15 min at 1,000 × g. Serum HIF-1α and VEGF protein expression levels were measured by ELISAs, according to the manufacturer's protocols. The serum concentrations of HIF-1α and VEGF were quantified using a standard curve constructed according to the absorbance and concentrations of standard samples.
Immunohistochemistry
The protein expression levels of HIF-1α, VEGF and CD34 in the EMS lesions of the mice were measured by immunohistochemical analysis. Briefly, the tissue samples of endometriotic foci obtained after sacrifice were fixed in 10% formalin and then embedded in paraffin. The tissues were cut into ~4-µm sections. Antigen repair was conducted by microwave heating in 0.1% citrate buffer (0.4 g citric acid dissolved in 3 g sodium citrate in 1 L deionized water). After blocking with 5% BSA at room temperature for 20 min, the slides were incubated overnight at 4°C with mouse anti-HIF-1α (1:100), anti-VEGF (1:200) or anti-CD34 (1:100) antibodies. Protein expression was detected according to the protocols of the SP and DAB kits. Brown staining in the cytoplasm, as observed under a light microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan), was indicative of HIF-1α, VEGF or CD34 protein expression.
The results of immunohistochemistry were reviewed independently by two senior pathologists blinded to the outcome of the tumors. Semi-quantitative analysis of HIF-1α, VEGF and CD34 expression levels was performed by consensus and comprised both the intensity of staining (negative as 0, light yellow as 1, brown as 2, tan as 3) for each cell and the extent of staining (ratio of positive cells/counted cells; 1 for 25%, 2 for 25–50%, 3 for 51–75% and 4 for 75%) for each random field. The scores for the intensity and extent of staining were multiplied to give a weighted score for each case (maximum possible score, 12). For statistical analysis, the weighted scores were grouped into four categories, wherein a score of 0 was considered negative, scores of 1–4 (+) were regarded as low positive expression levels, and scores of 5–8 (++) and 9–12 (+++) were regarded as high positive expression levels.
MVD
The MVD was assessed as previously described (18). Briefly, under a light microscope (Olympus Corporation), the immunostained section was scanned at low magnification (×40) and three areas with the highest density of microvessels were identified as ‘hotspots’. All the vessels in these ‘hotspot’ areas were then counted at high magnification (×200), and each slide was examined individually by two pathologists blinded to the research design.
Statistical analysis
The results are expressed as the mean ± standard deviation. Statistical analysis was conducted with SPSS 19.0 software (IBM SPSS, Armonk, NY, USA). Comparisons between groups were performed using one-way analysis of variance test. All the numerical data were tested for homogeneity and distribution. Ordinal data were tested by Wilcoxon signed-rank test. A P-value of <0.05 was considered to indicate a statistically significant difference.
Results
Effect of RAPA on the growth of endometriotic lesions in mice
In order to observe the effect of RAPA on the endometriotic lesions in mice, the volume of lesions was measured prior to treatment (V1) and after 2 weeks of treatment (V2). As shown in Table I, the V2 of the control and saline groups were comparable with V1 of all the groups. However, the V2 of the RAPA group was significantly decreased when compared with its corresponding V1, as well as compared with V2 of the control and saline groups (P<0.05). These findings indicated that RAPA significantly suppressed the growth of the endometriosis lesions.
Table I.
Endometriotic lesion volume in the mice (mean ± standard deviation; n=10 mice/group).
| Group | V1 (mm3) | V2 (mm3) |
|---|---|---|
| RAPA | 230.85±13.74 | 51.25±9.31a,b |
| Control | 219.56±14.79 | 229.44±10.01 |
| Saline | 231.34±13.41 | 210.71±10.96 |
P<0.05 vs. V1 in the same group
P<0.05 vs. V2 in the control and saline groups. RAPA, rapamycin; V1, lesion volume prior to treatment; V2, lesion volume following treatment.
Effect of RAPA on the serum levels of HIF-1α and VEGF
In order to investigate the effect of RAPA on the expression of HIF-1α and VEGF, the serum levels of these factors were determined by ELISA. As shown in Table II, the serum levels of HIF-1α and VEGF in the saline groups were comparable with those in the corresponding control group levels. Notably, there was no statistically significant difference in the serum HIF-1α levels among the RAPA, control and saline groups. However, treatment with RAPA markedly reduced the serum levels of VEGF, when compared with those in the control and saline groups (P<0.05).
Table II.
Serum levels of HIF-1α and VEGF in the mice (mean ± standard deviation; n=10 mice/group).
| Group | HIF-1α (pg/ml) | VEGF (pg/ml) |
|---|---|---|
| RAPA | 1.575±0.290 | 0.128±0.030 |
| Control | 1.668±0.223 | 0.169±0.029 |
| Saline | 1.660±0.205 | 0.162±0.030 |
RAPA, rapamycin; HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor.
Effect of RAPA on the expression of HIF-1α and VEGF in endometriotic lesions
In order to confirm the effect of RAPA on the location and expression of HIF-1α and VEGF, their expression in endometriotic lesions was determined by immunohistochemical staining. The results showed that HIF-1α and VEGF were mainly present in the cytoplasm of glandular epithelium. In addition, weak immunoreactivity for HIF-1α and VEGF was observed in the ectopic endometrial stromal cells in the control group (Figs. 1 and 2). The results of the staining intensity are summarized in Tables III and IV. There was no significant difference in the staining for HIF-1α among the RAPA, control and saline groups. However, the VEGF expression in the RAPA group was significantly lower compared with that of the control and saline groups.
Figure 1.
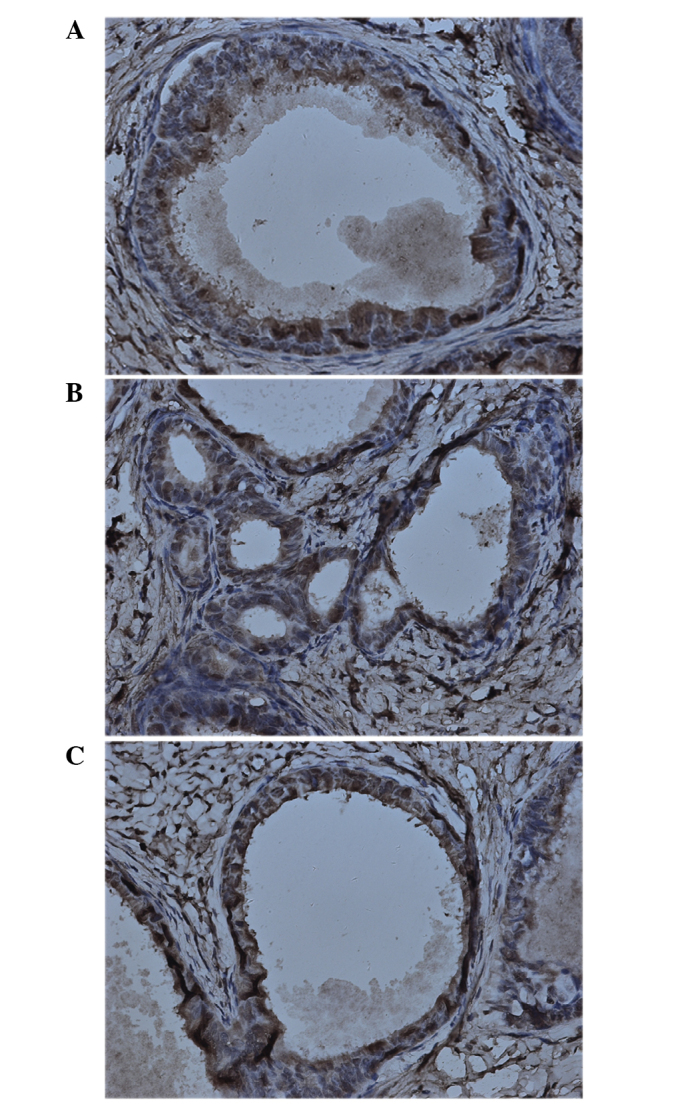
Representative image of immunohistochemical analysis of hypoxia-inducible factor-1α in endometriotic lesions of (A) control, (B) saline and (C) rapamycin-treated groups. Staining was performed with the streptomycin avidin-peroxidase method. Magnification, ×400.
Figure 2.
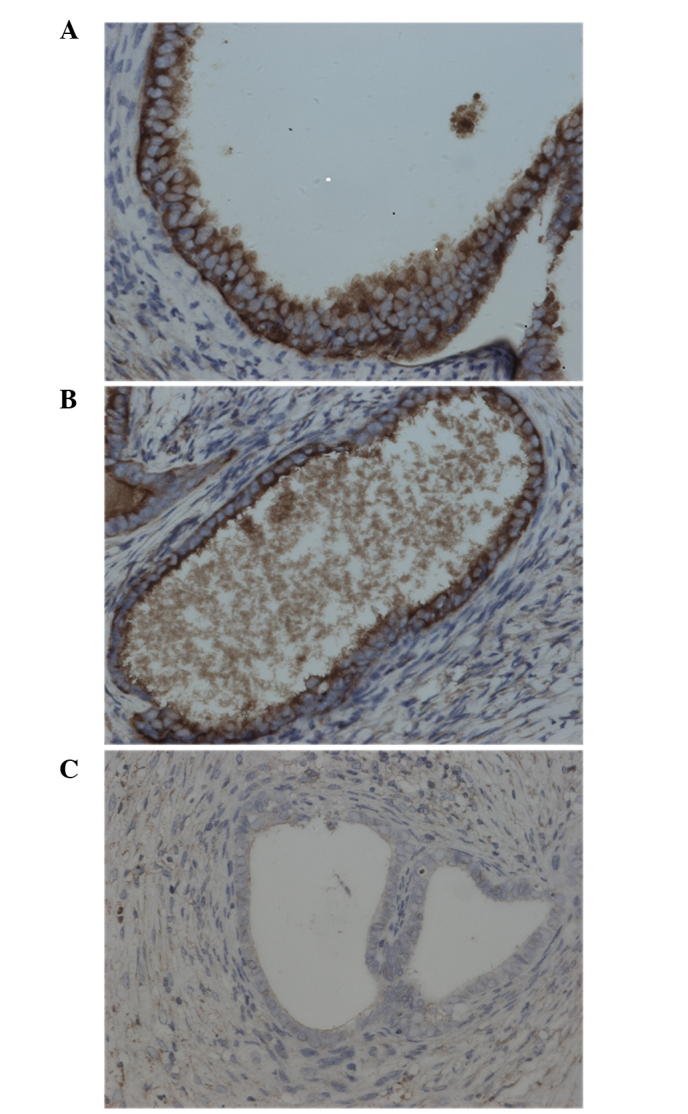
Representative image of immunohistochemical analysis of vascular endothelial growth factor in endometriotic lesions of (A) control, (B) saline and (C) rapamycin-treated groups. Staining was performed with the streptomycin avidin-peroxidase method. Magnification, ×400.
Table III.
Intensity of immunohistochemical staining for HIF-1α in endometriotic lesions (n=10 mice/group).
| HIF-1α | ||||
|---|---|---|---|---|
| Group | − | + | ++ | +++ |
| RAPA | 0 | 4 | 2 | 4 |
| Control | 0 | 4 | 3 | 3 |
| Saline | 0 | 3 | 4 | 3 |
RAPA, rapamycin; HIF-1α, hypoxia-inducible factor-1α.
Table IV.
Intensity of immunohistochemical staining for VEGF in endometriotic lesions (n=10 mice/group).
| VEGF | ||||
|---|---|---|---|---|
| Group | − | + | ++ | +++ |
| RAPA | 7 | 3 | 0 | 0 |
| Control | 0 | 5 | 3 | 2 |
| Saline | 0 | 4 | 5 | 1 |
RAPA, rapamycin; VEGF, vascular endothelial growth factor.
Effect of RAPA on MVD in entometriotic lesions
As a marker of endothelial cells, CD34 was assayed by immunohistochemistry in order to evaluate the effect of RAPA on MVD in endometriotic lesions. As shown in Table V, the MVD in the saline group was comparable with that in the control group (Table V). As expected, treatment with RAPA significantly reduced the MVD when compared with that the in control and saline groups. In addition, as shown in Fig. 3, positive staining of CD34 was observed in the endothelial cells, which were comma, stripe or tube-shaped with clear lumens. The incidence of angiogenesis was also significantly suppressed in the RAPA-treated group compared with that in the control group (Fig. 3). Therefore, these findings indicated that RAPA inhibited endothelial cell proliferation and the resulting angiogenesis.
Table V.
MVD in endometriotic lesions in mice (mean ± standard deviation; n=10 mice/group).
| Group | MVD |
|---|---|
| RAPA | 5.38±0.18a |
| Control | 35.54±1.19 |
| Saline | 36.26±1.28 |
P<0.05 vs. control and saline groups. RAPA, rapamycin; MVD, microvessel density.
Figure 3.
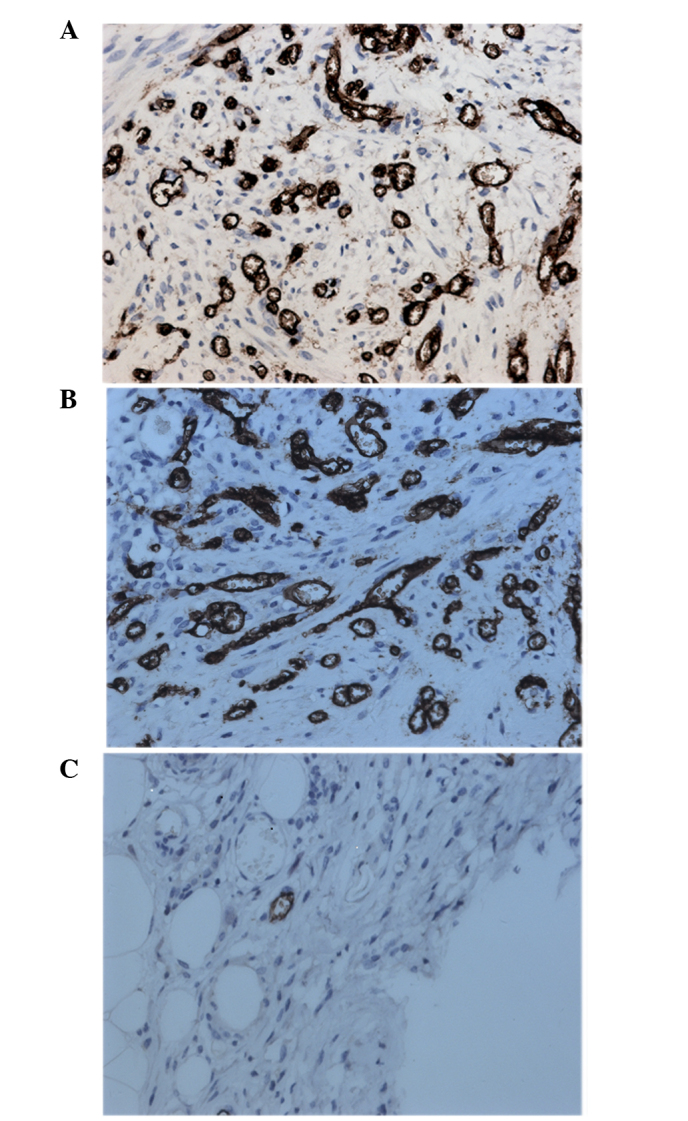
Representative images of immunohistochemical analysis of CD34 in endometriotic lesions of the (A) control, (B) saline and (C) rapamycin-treated groups. Staining was performed with the streptomycin avidin-peroxidase method. Magnification, ×400.
Discussion
As a common gynecologic disease, the incidence of EMS has been increasing in recent years (2). The implantation of ectopic endometrium, the resultant infertility and repeated abdominal pain greatly affect EMS patients (2,19). A previous study demonstrated that angiogenesis serves an important role not only in the development and repair of normal endometrium, but also in the occurrence and development of EMS (20). Excessive angiogenesis may be critical for the ectopic implantation and growth of the endometrium, which results in EMS (21,22). In the present study, we hypothesized that the effective inhibition of angiogenesis may block the occurrence and development of EMS.
HIF-1, a heterodimer composed of the HIF-1α and HIF-1β subunits, is a transcription factor that is widely found in the tissues of mammals under hypoxic or anoxic stress (23). HIF-1α is the unique oxygen-regulated subunit and determines the activity of HIF-1. Furthermore, it is a hypoxia inducible global regulator and contributes to the transcriptional regulation of the expression of VEGF and certain enzymes associated with glycolysis (24–26). Additionally, HIF-1α is the regulator directly involved in the entire process of angiogenesis (10). VEGF, also known as vascular permeability factor, is an important proangiogenic factor, which selectively binds VEGF receptor in endothelial cells, serving a proangiogenic role (27). Angiogenesis during EMS has been found to be closely associated with hypoxia in the endometrium (28). As a regulator of proangiogenic factors, particularly of VEGF, HIF-1α serves a critical role in the angiogenesis process in EMS (28). Our previous study demonstrated that HIF-1α functionally contributes to angiogenesis during EMS (15).
mTOR, an atypical serine/threonine kinase, is important for cell growth and is involved in a wide variety of physiological functions. This kinase also regulates the expression of proangiogenic factors (such as VEGF) at the transcriptional and translational levels. An abnormal mTOR signaling pathway has been identified in various types of malignant neoplasm, while the aberrant activation of mTOR has been confirmed to mediate angiogenesis and contribute to neoplasm development (29). Based on these functions, the mTOR signaling pathway has been a novel target in the treatment of neoplasm and mTOR inhibitors have been identified as the third generation of antiangiogenic pharmaceuticals (30). RAPA is the first identified specific inhibitor of mTOR. A previous study has demonstrated that RAPA is able to significantly inhibit the growth of cell lines derived from various tumors, including breast, lung, colorectal and renal carcinoma (31). In addition, RAPA has an anti-angiogenesis activity, and this effect is most probably due to at least the following two mechanisms: i) Direct inhibition of the proliferation of endothelial cells mediated by VEGF; and ii) inhibition of the proliferative effect of HIF-1 on endothelial cells. Hudson et al (32) has shown that RAPA inhibits the expression levels of VEGF and platelet-derived growth factor through the downregulation of HIF-1α expression, leading to suppression of new vascular occurrence in prostate cancer. Another study found that, in multiple myeloma, RAPA reduced VEGF receptor expression and therefore inhibited the endothelial cell differentiation and vascular sprouting, reducing angiogenesis in multiple myeloma (33). Laschke et al (34) also demonstrated that RAPA attenuated ectopic endometrium in vivo through inhibition of angiogenesis and cell proliferation.
In the present study, RAPA was shown to significantly attenuate the development of endometriotic lesions, as well as to inhibit MVD and the expression of VEGF in endometriotic lesions. However, RAPA had no significant effect on HIF-1α expression in the lesions, which may be due to the direct inhibitory role of RAPA in the VEGF expression, supported by presence of various mTOR-independent pathways to regulate HIF-1α expression in EMS. Although the role of mTOR in the expression of HIF-1α remains controversial, it is widely agreed that the ultimate effect of the complicated signaling pathways regulated by hypoxia determines the expression of HIF-1α.
In conclusion, the present study observed the effect of RAPA on the development of endometriotic lesions and investigated the mechanism of EMS to provide new evidence for the treatment of the disease with RAPA. mTOR inhibitors, represented by RAPA in the current study, may be potentially used in targeted therapy for EMS. However, the clinical efficacy, side effects and the use of mTOR inhibitors in combination with other targeted pharmaceuticals require further evaluation. Biomarkers for the clinical efficacy of mTOR inhibitors also require further investigation.
Acknowledgements
The present study was funded by Guangzhou Medical Science and Technology Project 2013 (grant no. 2013A011030016) and the Guangdong Province Science and Technology Plan Projects 2015 (grant no. 20140212).
References
- 1.Zhan L, Wang W, Zhang Y, Song E, Fan Y, Wei B. Hypoxia-inducible factor-1alpha: A promising therapeutic target in endometriosis. Biochimie. 2016;123:130–137. doi: 10.1016/j.biochi.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Khan KN, Kitajima M, Fujishita A, Hiraki K, Matsumoto A, Nakashima M, Masuzaki H. Pelvic pain in women with ovarian endometrioma is mostly associated with coexisting peritoneal lesions. Hum Reprod. 2013;28:109–118. doi: 10.1093/humrep/des364. [DOI] [PubMed] [Google Scholar]
- 3.Huynh H. Molecularly targeted therapy in hepatocellular carcinoma. Biochem Pharmacol. 2010;80:550–560. doi: 10.1016/j.bcp.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: Implications for diabetes. Mol Cell Endocrinol. 2009;302:128–139. doi: 10.1016/j.mce.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Makker A, Goel MM, Das V, Aqarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: An update. Gnnecol Endocrinol. 2012;28:175–81. doi: 10.3109/09513590.2011.583955. [DOI] [PubMed] [Google Scholar]
- 6.McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD. Kinase signalling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Hum Reprod Update. 2016 Jan 5; doi: 10.1093/humupd/dmv060. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Ashworth RE, Wu J. Mammalian target of rapamycin inhibition in hepatocellular carcinoma. World J Hepatol. 2014;6:776–782. doi: 10.4254/wjh.v6.i11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck JT, Ismail A, Tolomeo C. Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: An emerging treatment strategy for squamous cell lung carcinoma. Cancer Treat Rev. 2014;40:980–989. doi: 10.1016/j.ctrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Leconte M, Nicco C, Ngô C, Chéreau C, Chouzenoux S, Marut W, Guibourdenche J, Arkwright S, Weill B, Chapron C, et al. The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am J Pathol. 2011;179:880–889. doi: 10.1016/j.ajpath.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 11.Lu Z, Zhang W, Jiang S, Zou J, Li Y. Effect of oxygen tensions on the proliferation and angiogenesis of endometriosis heterograft in severe combined immunodeficiency mice. Fertil Steril. 2014;101:568–576. doi: 10.1016/j.fertnstert.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 12.Lu Z, Zhang W, Jiang S, Zou J, Li Y. Effect of lesion location on endometriotic adhesion and angiogenesis in SCID mice. Arch Gynecol Obstet. 2014;289:823–830. doi: 10.1007/s00404-013-3048-9. [DOI] [PubMed] [Google Scholar]
- 13.Sharkey AM, Day K, McPherson A, Malik S, Licence D, Smith SK, Charnock-Jones DS. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metab. 2000;85:402–409. doi: 10.1210/jc.85.1.402. [DOI] [PubMed] [Google Scholar]
- 14.Ren X, He YL, Pan SL, Peng DX. Expression of hypoxia-inducible factor-1alpha in endometriosis. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:538–540. (In Chinese) [PubMed] [Google Scholar]
- 15.Ren X, He Y, Peng D. The expression of hypoxia-inducible factor-1α and the relationship with microvessel density in endometriosis. Guangdong yi xue. 2007;28:229–231. (In Chinese) [Google Scholar]
- 16.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv Cancer Res. 2002;86:1–39. doi: 10.1016/S0065-230X(02)86001-8. [DOI] [PubMed] [Google Scholar]
- 17.Katsuki Y, Takano Y, Futamura Y, Shibutani Y, Aoki D, Udagawa Y, Nozawa S. Effects of dienogest, a synthetic steroid, on experimental endometriosis in rats. Eur J Endocrinol. 1998;138:216–226. doi: 10.1530/eje.0.1380216. [DOI] [PubMed] [Google Scholar]
- 18.Weidner N. Current pathologic methods for measuring intratumoral microvessel density with in breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 19.Berkes E, Bokor A, Rigó J., Jr. Current treatment of endometriosis with laparoscopic surgery. Orv Hetil. 2010;151:1137–1144. doi: 10.1556/OH.2010.28904. (In Hungarian) [DOI] [PubMed] [Google Scholar]
- 20.Young VJ, Ahmad SF, Brown JK, Duncan WC, Horne AW. Peritoneal VEGF-A expression is regulated by TGF-β1 through an ID1 pathway in women with endometriosis. Sci Rep. 2015;5:16859. doi: 10.1038/srep16859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimoto J, Sakaguchi H, Hirose R, Wen H, Tamaya T. Angiogenesis in endometriosis and angiogenic factors. Gynecol Obstet Invest. 1999;48(Suppl 1):14–20. doi: 10.1159/000052864. [DOI] [PubMed] [Google Scholar]
- 22.Soysal D, Kizildag S, Saatli B, Posaci C, Soysal S, Koyuncuoglu M, Dogan O. A novel angiogenesis inhibitor bevacizumab induces apoptosis in the rat endometriosis model. Balkan J Med Genet. 2015;17:73–80. doi: 10.2478/bjmg-2014-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: Implication of natural antisense HIF-1alpha. J Biol Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 24.Jian-Lin Z, Hong-Song F, Hao P, Shuang D, Shen C, Jian-Ping L, Bo Q, Jin-Qing W, Feng L. The relationship between HIF-2α and VEGF with radiographic severity in the primary osteoarthritic knee. Yonsei Med J. 2016;57:735–740. doi: 10.3349/ymj.2016.57.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae WY, Choi JS, Kim JE, Jeong JW. Cinnamic aldehyde suppresses hypoxia-induced angiogenesis via inhibition of hypoxia-inducible factor-1α expression during tumor progression. Biochem Pharmacol. 2015;98:41–50. doi: 10.1016/j.bcp.2015.08.095. [DOI] [PubMed] [Google Scholar]
- 26.Remels AH, Gosker HR, Verhees KJ, Langen RC, Schols AM. TNF-α-induced NF-κB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1α. Endocrinology. 2015;156:1770–1781. doi: 10.1210/en.2014-1591. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machado-Linde F, Pelegrin P, Sanchez-Ferrer ML, Leon J, Cascales P, Parrilla JJ. 2-methoxyestradiol in the pathophysiology of endometriosis: Focus on angiogenesis and therapeutic potential. Reprod Sci. 2012;19:1018–1029. doi: 10.1177/1933719112446080. [DOI] [PubMed] [Google Scholar]
- 29.Dufour M, Dormond-Meuwly A, Demartines N, Dormond O. Tarageting the mammalian target of rapamycin (mTOR) in cancer therapy: Lessions from past and future perspectives. Cancers (Basel) 2011;3:2478–2500. doi: 10.3390/cancers3022478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganjoo K, Jacobs C. Antiangiogenesis agents in the treatment of soft tissue sarcomas. Cancer. 2010;116:1177–1183. doi: 10.1002/cncr.24859. [DOI] [PubMed] [Google Scholar]
- 31.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of AKT/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 32.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor1 alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirsgahi P, Toprak SK, Faussat AM, Dubrulle S, Marie JP, Soria C, Soria J, Mirshahi M. Malignant hematopoietic cells induce an increased expression of VEGFR-1 and VEGFR-3 on bone marrow endothelial cells via AKT and mTOR signalling pathways. Biochem Biophys Res Commun. 2006;349:1003–1010. doi: 10.1016/j.bbrc.2006.08.132. [DOI] [PubMed] [Google Scholar]
- 34.Laschke MW, Elitzsch A, Scheuer C, Holstein JH, Vollmar B, Menger MD. Rapamycin induces regression of endometriotic lesions by inhibiting neovascularization and cell proliferation. Br J Pharmacol. 2006;149:137–144. doi: 10.1038/sj.bjp.0706857. [DOI] [PMC free article] [PubMed] [Google Scholar]


