Abstract
Human noroviruses interact with both human histo-blood group antigens (HBGAs) and human milk oligosaccharides (HMOs). The former are believed to be important for a virus infection, while the latter might act as natural decoys in the host during an infection. However, certain noroviruses are known to bind poorly to HBGAs and yet still cause infections; some interact with numerous HBGA types but are nonprevalent; and yet others bind HBGAs and seem to be increasing in prevalence. HBGAs and HMOs can be found as soluble antigens in humans, can be structurally alike, and can interact with equivalent residues at identical binding pockets on the capsid. In this Gem, we discuss HBGA and HMO binding studies for human noroviruses, concentrating on the clinically important genogroup II noroviruses. In short, the roles of HBGA and HMO interactions in norovirus infections are still unclear.
INTRODUCTION
Human noroviruses are the dominant cause of outbreaks of acute gastroenteritis. These viruses are also responsible for significant numbers of sporadic infections, are often found in asymptomatic cases, can cause chronic infections, and infect all age groups. Noroviruses are transmitted via the fecal-oral route, and infections often result from consumption of contaminated foods such as bivalves (clams and oysters). Noroviruses have a single-stranded, positive-sense RNA genome of ∼7.5 kb. The genome contains three open reading frames (ORFs): ORF1 encodes nonstructural proteins, ORF2 encodes a capsid protein (VP1), and ORF3 encodes a minor capsid protein. Human norovirus is difficult to grow in cell cultures, and the exact locations of the cells and the cell type(s) that norovirus infect remain unknown. Expression of the capsid protein in insect cells can generate self-assembled virus-like particles (VLPs) that are morphologically and antigenically similar to the native virion (1). The norovirus particle is composed of 180 copies of VP1, which forms 90 VP1 homodimers. The X-ray crystal structure of norovirus VLPs identified two main domains, the shell (S) domain and the protruding (P) domain (2). The S domain surrounds the viral RNA, whereas the P domain is further subdivided into P1 and P2 subdomains and contains determinants for cell attachment and antigenicity.
HUMAN NOROVIRUS AND HISTO-BLOOD GROUP ANTIGENS
Human noroviruses are known to interact with histo-blood group antigens (HBGAs), and this interaction could be important for infection (3–7). HBGAs can be found as soluble antigens in saliva and are expressed on the mucosal epithelium of the digestive tract. Therefore, noroviruses could interact several times with HBGAs during the course of an infection. Interestingly, human noroviruses can naturally bioaccumulate in bivalves, where they are thought to bind HBGA-like carbohydrates in the gastrointestinal epithelial cells (8). However, this accumulation does not appear to cause harm to the bivalves.
Multiple gene families control human HBGA biosynthesis through the action of glycosyltransferases (9). As HBGAs can be either lipid or protein linked, distinct core sugars are found in glycosphingolipids (glucose [Glc]) and mucin-type O-glycoproteins or mucins (N-acetylgalactosamine [GalNAc]). The former are largely restricted to the plasma membrane, whereas mucin-type O-glycoproteins can be membrane tethered or secreted and hence play quite antagonistic roles in virus infections. While membrane-exposed HBGAs serve as decoys for virus entry into the cells, soluble mucins form viscoelastic gel layers on epithelial surfaces and are claimed to play essential roles in defense by physical and chemical entrapment of the pathogens. In this context, the same HBGAs as those mediating infection might be involved in innate immunity mediated by secretory mucins.
HBGAs on mucin-type O-glycoproteins are formed by sequential addition of sugars in the Golgi compartments. Up to eight different core-type structures are expressed in an organ-characteristic manner and elongated to linear or branched polylactosamine-type chains, which can terminate in blood group-active oligosaccharides. The latter are derived from type 1 or type 2 lactosamine chains by sequential addition of either H-type fucose (α1-2 linked to Gal) or Lewis-type fucose (α1-3/4 linked to GlcNAc). The H-type structures and Lewisb/y glycans serve as the substrates for glycosyltransferases, adding α3-GalNAc (blood group A) or α3-Gal (blood group B). So, HBGAs as mostly peripheral variations of O-linked glycans actually represent a subfraction of the complex family with numerous structural isomers in several hundred variants with lengths of up to 20 monosaccharide units. The blood group O/H and Lewisa/x types are distinguished by the different connections of a fucose moiety.
Human noroviruses have a preference to certain types of HBGAs (10, 11). One study showed that individuals expressing the O type (H antigen) had higher infection rates than individuals expressing other blood types, while those with the B type were resistant to infections (5). Human noroviruses are genetically and antigenetically distinct (1). There are two main genogroups (GI and GII), and those can be further subdivided into numerous genotypes. Over the past decade, GII genotype 4 (GII.4) has been the dominant cause of outbreaks worldwide. However, recent molecular epidemiological studies have speculated that GII.17 noroviruses might replace the GII.4 noroviruses, since their prevalence appears to be increasing worldwide (12). One possible reason for the recent increase in the prevalence of GII.17 noroviruses might be related to a change in their HBGA binding profiles (13, 14). The previous nonprevalent GII.17 noroviruses were thought to bind poorly to HBGAs, while the more recent GII.17 noroviruses that appeared in 2014 and 2015 might bind a greater panel of HBGA types (13, 14). On the other hand, a nonprevalent GII.10 norovirus isolated in 2004 bound numerous HBGA types, and yet this genotype remains relatively rare (15, 16).
A number of studies have also found that some norovirus genotypes do not bind or poorly bind HBGAs (4, 17). Whether or not a norovirus interaction with HBGAs is important for the infection process remains unclear. One study showed that some of the earliest known GII.4 strains (isolated around 1974) were able to bind many HBGA types (18). Variants of these GII.4 noroviruses have retained this HBGA binding ability (19). However, it was not until the middle 1990s that the GII.4 noroviruses began to dominate. The reason for a particular norovirus to bind or not to bind HBGAs is not so evident. Moreover, the corresponding consequence of the HBGA interaction or noninteraction is still unclear.
STRUCTURAL BASIS FOR NOROVIRUS BINDING TO HBGAs
The GI and GII noroviruses bind HBGAs at different locations on the capsid and typically involve monomeric and dimeric interactions, respectively. The GII.4 and GII.10 noroviruses interact with the largest panel of HBGAs by far (Fig. 1). A common set of residues (i.e., Asn355, Arg356, Asp385, Tyr452, and Gly451 [GII.10 strain numbering]) generally holds the ABH-type and Lewis-type fucose through direct hydrogen bonds with side chains and main chains (15, 19). The other saccharide units are usually held less firmly, with variable residues and water-mediated interactions. X-ray crystal structures of three P domains from epidemic GII.4 variants in 2004, 2006, and 2012 showed that the region immediately beneath the HBGA binding pocket had few amino acid changes (19). The regions that contributed to binding terminal saccharides of Lewis HBGAs had more noticeable substitutions, but how these changes related to HBGA binding remains unclear.
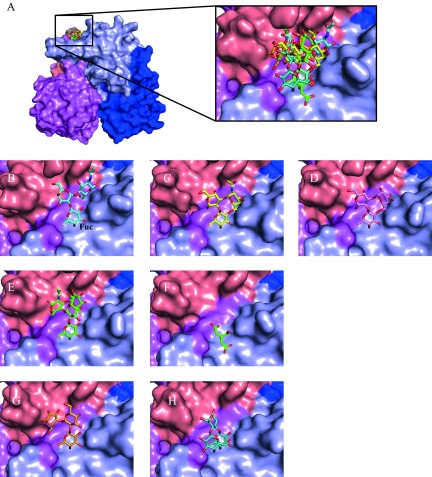
FIG 1.
Surface representation of GII.10 norovirus P domain in complex with HBGAs, HMOs, and citrate. (A) The GII.10 P dimer was colored according to chain A and B and P1 and P2 subdomains as follows: for chain A, P1 is dark blue and P2 is light blue; for chain B, P1 is violet and P2 is salmon. Data represent superposition and a closeup of the various orientations of the HBGAs, HMOs, and citrate bound to the P dimer. (B) The H type 2 trisaccharide (cyan sticks) is α-l-fucose-(1-2)-β-d-galactose-(1-4)-2-N-acetyl-β-d-glucosamine; however, the α-fucose was shown as an β-fucose in this structure, due to impurities in the commercially available stock. (C) The type A trisaccharide (yellow sticks) is α-l-fucose-(1-2)-β-d-galactose-(3-1)-2-N-acetyl-α-d-galactosamine. (D) The type B trisaccharide HBGA (pink sticks) is α-l-fucose-(1-2)-β-d-galactose-(3-1)-α-d-galactose. (E) The Lewisy tetrasaccharide (green sticks) is α-l-fucose-(1-2)-β-d-galactose-(1-4)-2-N-acetyl-β-d-glucosamine-(3-1)-α-l-fucose. (F) The citrate molecule (green sticks) bound at the HBGA pocket and showed a high degree of mimicry to fucose moiety. Seven P domain residues, many of which are conserved, were involved in hydrogen bonding interactions with citrate. (G) The 2′FL (orange sticks) is an α-l-fucose-(1-2)-β-d-galactose-(1-4)-α-d-glucose. (H) The 3FL (teal sticks) is an α-l-fucose-(1-3)-[β-d-galactose-(1-4)]-β-d-glucose.
As mentioned, not all noroviruses interact with HBGAs (4, 17). Structural studies indicated that neighboring loops on the capsid could influence the orientation and stabilization of the aspartic acid side chain that primarily holds the fucose of the HBGAs (20). Interestingly, these genotypes contain the common set of fucose binding residues observed in GII.4 and GII.10 noroviruses. On the other hand, the recent GII.17 noroviruses may have acquired the ability to bind HBGAs (13, 14). The earlier nonprevalent GII.17 noroviruses had a valine residue at the HBGA pocket (Val444 [GII.17 numbering]) which was typically a tyrosine residue (Tyr444 [GII.4 numbering]) and which provided a critical hydrophobic interaction to the fucose methyl group (19). The recent prevalent GII.17 strains have obtained a tyrosine (Tyr444 [GII.17 numbering]), and this substitution might have permitted binding to HBGAs. Overall, these data suggest that some nonprevalent noroviruses might alter their HBGA pocket and acquire the ability to bind HBGAs.
It should be noted that most studies have examined only those HBGAs containing only three or four saccharide units. Therefore, it remains uncertain if longer HBGA saccharides can directly interact with the capsid. In most cases, the terminal HBGA units project outward from the capsid surface, suggesting that three or four saccharide units of the HBGAs were the main components of the binding interactions. We have shown that HBGA binding interactions are more complex than previously thought (19). We found that a capsid loop can be repositioned in order to allow HBGA binding. We showed that the binding mechanisms of Lewis HBGAs involved more-complex interactions than A-trisaccharide and B-trisaccharide binding interactions. Moreover, we recently showed that the GII.4 and GII.10 noroviruses actually have four fucose binding pockets per dimer (21, 22). The affinity between norovirus and HBGAs is weak and in the high micromolar range (23), and, based on the number of direct hydrogen bonds and water-mediated interactions, small changes in P domain affinities to HBGAs may exist.
HUMAN NOROVIRUS AND HMOs
Human milk oligosaccharides (HMOs), the third-most-abundant components of human milk, are known to provide health benefits for breast-fed infants (24). HMOs are thought to act as a “receptor decoy” for certain pathogens (25). HMOs consist of monosaccharide building blocks similar to those of HBGAs and are unconjugated complex glycans with more than 200 known isomers. All HMOs are build up from the precursor disaccharide lactose (Galβ1-4Glc), which is the most abundant milk oligosaccharide, with a concentration of about 60 g/liter. HMOs structurally resemble mucin-type O-glycans with their terminal HBGA variants, refrained from the different core structures. The more complex HMOs are characterized by polylactosamine-type extensions with 3/6 branching at galactoses and multiple fucosylation sites giving rise to the formation of Lewis-type sequences, such as Lewisa-Lewisx.
The HMO structures, compositions, and sizes differ over longer terms of lactation. The content of HMOs is higher in colostrum and early milk than in later phases of lactation, which is in accordance with the protective functions of breast milk feeding of the immunologically immature neonate. The composition of breast milk over periods of 4 months shows an increase in lactose content but a decrease in the level of 2′-fucosyllactose (2′FL) (suggesting reduced activity of the FUT2 secretor enzyme), whereas the level of 3-fucosyllactose (3FL) structural isomer increases over the same period, pointing to increased activities of secretor- and Lewis-gene-independent fucosyltransferases (FUT3, -4, -5, -6, -7, and -9). The structural variation of fucose substitution patterns is dependent on maternal genes encoding the secretor- and Lewis blood group-type fucosyltransferases.
One study found that human milk might provide some protection to infants with a norovirus infection (26). Several studies showed that human milk or HMOs could compete with the HBGA binding sites on the norovirus capsid (27, 28). We recently showed that two HMOs, 2′FL and 3FL, blocked norovirus from binding to HBGAs in a mostly dose-dependent manner (29). HMOs can resist degradation in the gut, and the majority is excreted intact in the feces (∼1% is absorbed by the infant). This implies that HMOs can essentially travel the same path as norovirus in the host.
STRUCTURAL BASIS FOR NOROVIRUS BINDING TO HMOs
The HMO binding site on the GII.10 capsid was recently discovered (29). The 2′FL and 3FL bound at the equivalent HBGA pocket on the norovirus capsid (Fig. 1). These HMOs were held in place by a network of hydrophilic and hydrophobic interactions at the dimeric interface. The fucose of the HMOs was held by the common set of conserved residues described above for the HBGAs. The central saccharides of HMOs were both poorly held by the protein, while the terminal saccharides interacted with various residues. Interestingly, these HMO binding interactions were typical of HBGA interactions (15, 19). Moreover, the GII.4 and GII.17 noroviruses also bound 2′FL and 3FL at their equivalent HBGA pockets (G. S. Hansman, unpublished data), indicating broad reactivity to genetically distinct GII noroviruses.
STRUCTURAL MIMICRY OF HBGAs AND HMOs
The GII.4 and GII.10 noroviruses bind both HBGAs and HMOs at the same pocket. Structural data show that HBGAs and HMOs bind in copious orientations (Fig. 1). The fucose moieties of HBGAs and HMOs are always held in identical positions, whereas the other saccharide units are orientated slightly differently. The terminal saccharide units are usually held with only a few if any direct hydrogen bonds, suggesting a weaker interaction. Interestingly, we found that citrate can also occupy the HBGA pocket (Fig. 1) and might perform as a weak natural inhibitor against HBGAs (23, 30), comparably to HMOs.
In summary, the HBGA/HMO site on the GII capsid is a multipurpose binding pocket for norovirus. It appears that some noroviruses might require HBGAs for an infection, while others may have reduced HBGA interactions. The HMOs (2′FL and 3FL) may act as natural decoys in an infection by mimicking HBGAs and binding at the HBGA pocket.
ACKNOWLEDGMENTS
Funding for this study was provided by the CHS Foundation, the Helmholtz-Chinese Academy of Sciences (HCJRG-202), the Deutsche Forschungsgemeinschaft (DFG; FOR2327), and the Federal Ministry of Education and Research (BMBJ; PTJ-BIO2 and BIO-428-066).
REFERENCES
- 1.Hansman GS, Natori K, Shirato-Horikoshi H, Ogawa S, Oka T, Katayama K, Tanaka T, Miyoshi T, Sakae K, Kobayashi S, Shinohara M, Uchida K, Sakurai N, Shinozaki K, Okada M, Seto Y, Kamata K, Nagata N, Tanaka K, Miyamura T, Takeda N. 2006. Genetic and antigenic diversity among noroviruses. J Gen Virol 87:909–919. doi: 10.1099/vir.0.81532-0. [DOI] [PubMed] [Google Scholar]
- 2.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 3.Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, Morrow AL, Altaye M, Pickering LK, Newburg DS, LePendu J, Jiang X. 2003. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis 188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 4.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. 2002. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol 76:12335–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockx BH, Vennema H, Hoebe CJ, Duizer E, Koopmans MP. 2005. Association of histo-blood group antigens and susceptibility to norovirus infections. J Infect Dis 191:749–754. doi: 10.1086/427779. [DOI] [PubMed] [Google Scholar]
- 6.Hutson AM, Atmar RL, Graham DY, Estes MK. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis 185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 7.Marionneau S, Ruvoen N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacois G, Huang P, Jiang X, Le Pendu J. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Guyader F, Loisy F, Atmar RL, Hutson AM, Estes MK, Ruvoen-Clouet N, Pommepuy M, Le Pendu J. 2006. Norwalk virus-specific binding to oyster digestive tissues. Emerg Infect Dis 12:931–936. doi: 10.3201/eid1206.051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Ruvoen N, Clement M, Le Pendu J. 2001. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83:565–573. doi: 10.1016/S0300-9084(01)01321-9. [DOI] [PubMed] [Google Scholar]
- 10.Tan M, Jiang X. 2005. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol 13:285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Cao S, Lou Z, Tan M, Chen Y, Liu Y, Zhang Z, Zhang XC, Jiang X, Li X, Rao Z. 2007. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol 81:5949–5957. doi: 10.1128/JVI.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Graaf M, van Beek J, Vennema H, Podkolzin AT, Hewitt J, Bucardo F, Templeton K, Mans J, Nordgren J, Reuter G, Lynch M, Rasmussen LD, Iritani N, Chan MC, Martella V, Ambert-Balay K, Vinje J, White PA, Koopmans MP. 2015. Emergence of a novel GII.17 norovirus—end of the GII.4 era? Euro Surveill 20:pii=21178. doi: 10.2807/1560-7917.ES2015.20.26.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan MC, Lee N, Hung TN, Kwok K, Cheung K, Tin EK, Lai RW, Nelson EA, Leung TF, Chan PK. 2015. Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat Commun 6:10061. doi: 10.1038/ncomms10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang XF, Huang Q, Long Y, Jiang X, Zhang T, Tan M, Zhang QL, Huang ZY, Li YH, Ding YQ, Hu GF, Tang S, Dai YC. 2015. An outbreak caused by GII.17 norovirus with a wide spectrum of HBGA-associated susceptibility. Sci Rep 5:17687. doi: 10.1038/srep17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansman GS, Biertumpfel C, Georgiev I, McLellan JS, Chen L, Zhou T, Katayama K, Kwong PD. 2011. Crystal structures of GII.10 and GII.12 norovirus protruding domains in complex with histo-blood group antigens reveal details for a potential site of vulnerability. J Virol 85:6687–6701. doi: 10.1128/JVI.00246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansman GS, Doan LT, Kguyen TA, Okitsu S, Katayama K, Ogawa S, Natori K, Takeda N, Kato Y, Nishio O, Noda M, Ushijima H. 2004. Detection of norovirus and sapovirus infection among children with gastroenteritis in Ho Chi Minh City, Vietnam. Arch Virol 149:1673–1688. [DOI] [PubMed] [Google Scholar]
- 17.Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, Jiang X. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol 79:6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bok K, Abente EJ, Realpe-Quintero M, Mitra T, Sosnovtsev SV, Kapikian AZ, Green KY. 2009. Evolutionary dynamics of GII.4 noroviruses over a 34-year period. J Virol 83:11890–11901. doi: 10.1128/JVI.00864-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh BK, Leuthold MM, Hansman GS. 2015. Human noroviruses' fondness for histo-blood group antigens. J Virol 89:2024–2040. doi: 10.1128/JVI.02968-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh BK, Leuthold MM, Hansman GS. 2016. Structural constraints on human norovirus binding to histo-blood group antigens. mSphere 1:e00049-16. doi: 10.1128/mSphere.00049-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koromyslova AD, Leuthold MM, Bowler MW, Hansman GS. 2015. The sweet quartet: binding of fucose to the norovirus capsid. Virology 483:203–208. doi: 10.1016/j.virol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Mallagaray A, Lockhauserbaumer J, Hansman G, Uetrecht C, Peters T. 2015. Attachment of norovirus to histo blood group antigens: a cooperative multistep process. Angew Chem Int Ed Engl 54:12014–12019. doi: 10.1002/anie.201505672. [DOI] [PubMed] [Google Scholar]
- 23.Hansman GS, Shahzad-Ul-Hussan S, McLellan JS, Chuang GY, Georgiev I, Shimoike T, Katayama K, Bewley CA, Kwong PD. 2012. Structural basis for norovirus inhibition and fucose mimicry by citrate. J Virol 86:284–292. doi: 10.1128/JVI.05909-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 25.Etzold S, Bode L. 2014. Glycan-dependent viral infection in infants and the role of human milk oligosaccharides. Curr Opin Virol 7:101–107. doi: 10.1016/j.coviro.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. 2004. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr 145:297–303. doi: 10.1016/j.jpeds.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 27.Shang J, Piskarev VE, Xia M, Huang P, Jiang X, Likhosherstov LM, Novikova OS, Newburg DS, Ratner DM. 2013. Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance. Glycobiology 23:1491–1498. doi: 10.1093/glycob/cwt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X, Huang P, Zhong W, Tan M, Farkas T, Morrow AL, Newburg DS, Ruiz-Palacios GM, Pickering LK. 2004. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J Infect Dis 190:1850–1859. doi: 10.1086/425159. [DOI] [PubMed] [Google Scholar]
- 29.Weichert S, Koromyslova A, Singh BK, Hansman S, Jennewein S, Schroten H, Hansman GS. 2016. Structural basis for norovirus inhibition by human milk oligosaccharides. J Virol 90:4843–4848. doi: 10.1128/JVI.03223-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koromyslova AD, White PA, Hansman GS. 2015. Treatment of norovirus particles with citrate. Virology 485:199–204. doi: 10.1016/j.virol.2015.07.009. [DOI] [PubMed] [Google Scholar]