ABSTRACT
Although antibodies to the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein have been studied extensively for their ability to block viral infectivity, little data are currently available on nonneutralizing functions of these antibodies, such as their ability to eliminate virus-infected cells by antibody-dependent cell-mediated cytotoxicity (ADCC). HIV-1 Env-specific antibodies of diverse specificities, including potent broadly neutralizing and nonneutralizing antibodies, were therefore tested for ADCC against cells infected with a lab-adapted HIV-1 isolate (HIV-1NL4-3), a primary HIV-1 isolate (HIV-1JR-FL), and a simian-human immunodeficiency virus (SHIV) adapted for pathogenic infection of rhesus macaques (SHIVAD8-EO). In accordance with the sensitivity of these viruses to neutralization, HIV-1NL4-3-infected cells were considerably more sensitive to ADCC, both in terms of the number of antibodies and magnitude of responses, than cells infected with HIV-1JR-FL or SHIVAD8-EO. ADCC activity generally correlated with antibody binding to Env on the surfaces of virus-infected cells and with viral neutralization; however, neutralization was not always predictive of ADCC, as instances of ADCC in the absence of detectable neutralization, and vice versa, were observed. These results reveal incomplete overlap in the specificities of antibodies that mediate these antiviral activities and provide insights into the relationship between ADCC and neutralization important for the development of antibody-based vaccines and therapies for combating HIV-1 infection.
IMPORTANCE This study provides fundamental insights into the relationship between antibody-dependent cell-mediated cytotoxicity (ADCC) and virus neutralization that may help to guide the development of antibody-based vaccines and immunotherapies for the prevention and treatment of HIV-1 infection.
INTRODUCTION
The recent isolation of a new generation of monoclonal antibodies with remarkably potent and broad neutralizing activity against diverse human immunodeficiency virus type 1 (HIV-1) isolates has renewed interest in the use of antibodies to treat HIV-1 infection (1, 2). Passive transfer experiments in animal models have shown that many of these antibodies can protect against HIV-1 or simian-human immunodeficiency virus (SHIV) challenge (3, 4), and in some cases, they are able to suppress virus replication to undetectable levels when administered during chronic infection (5–7). While the ability to block viral infection is a defining property of neutralizing antibodies, nonneutralizing effector functions may also contribute to antiviral responses. The IgG constant (Fc) domain can recruit cellular mediators of antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis through interactions with Fcγ receptors (FcγRs) or initiate complement-mediated lysis by binding to soluble factors in plasma.
Studies of nonhuman primates and mice support a role for FcγR-dependent functions of antibodies in protection against immunodeficiency virus infection. Passive transfer experiments with Fc variants of an HIV-specific broadly neutralizing antibody (bNAb) revealed that protection of rhesus macaques against pathogenic SHIV challenge is dependent in part on FcγR interactions, but not on complement fixation (8, 9). The preferential engagement of activating, but not inhibitory, FcγRs was also shown to contribute to the clearance of cell-free virus by antibodies in murine models (10), and FcγR-mediated functions of bNAbs interfered with the establishment of persistent HIV-1 reservoirs in humanized mice (11). Thus, the therapeutic potential of HIV-1-specific antibodies may be significantly enhanced by optimizing FcγR-dependent antiviral activities.
Emerging evidence suggests that antibodies capable of engaging FcγRIIIa on NK cells to direct the lysis of virus-infected cells may be especially important for containing or preventing HIV-1 infection (12, 13). ADCC responses are detectable in plasma shortly after the resolution of acute viremia and correlate inversely with disease progression (14–20). Greater ADCC responses have also been observed in individuals who exhibit elite control of HIV-1 in the absence of antiretroviral therapy (21, 22). In the setting of mother-to-child transmission, higher ADCC activity in breast milk is associated with a lower risk of virus transmission by breastfeeding, and passively acquired ADCC correlates with reduced infant mortality (23, 24). ADCC may also have contributed to the modest protection observed in the RV144 trial as suggested by exploratory analyses revealing an association between ADCC and reduced risk of infection among vaccinated subjects with low IgA titers (25). Although passive transfer of a nonfucosylated bNAb with increased affinity for FcγRIIIa did not enhance the protection of macaques against pathogenic SHIV challenge relative to the fucosylated antibody (26), several studies of nonhuman primates have also revealed correlations between vaccine-induced ADCC and complete protection or reduced postchallenge viral loads (27–31).
While these studies suggest that ADCC, and possibly other FcγR-dependent functions, contribute to the antiviral activity of HIV-1-specific antibodies, the properties of antibodies that mediate ADCC are not well defined. We therefore tested monoclonal antibodies to diverse epitopes of the HIV-1 envelope glycoprotein, including potent bNAbs and nonneutralizing antibodies, for their ability to direct NK cell lysis of cells infected with primary versus lab-adapted HIV-1 and SHIV isolates. These antibodies were also tested for binding to Env on the surfaces of virus-infected cells and for neutralization of viral infectivity. Our results show that although ADCC generally correlates with Env binding and neutralization, there are cases where these functions do not correspond, revealing differences in epitopes exposed on virions versus infected cells that differentiate these antiviral activities.
MATERIALS AND METHODS
Virus production.
Virus stocks were produced by transfection of proviral DNA into HEK 293T cells using GenJet transfection reagent (SignaGen). Culture supernatants were collected 48 and 72 h posttransfection, cell debris was removed by centrifugation, and aliquots of virus-containing supernatant were stored at −80°C. Virus concentrations were determined by anti-p24 or anti-p27 enzyme-linked immunosorbent assay (ELISA). SHIVAD8-EO was provided by Malcolm Martin.
Antibodies.
2F5 and 4E10 were obtained from Polymun Scientific, and X5 and 17b were obtained from Strategic Biosolutions. b12, b6, and DEN3 were stably expressed in CHO-K1 cells. VRC01, PGV04, 2G12, PG9, PG16, PGT121, PGT126, and 10E8 were transiently expressed using the FreeStyle 293 Expression System (Invitrogen) as previously described (32, 33). A32, C11, 3BNC117, and 10-1074 were transiently expressed in HEK 293T or HEK 293-6E cells (5, 34, 35). 98-6, 126-7, 240D, and F240 were derived from Epstein-Barr virus (EBV)-transformed peripheral blood mononuclear cells (PBMCs) from HIV-1-positive (HIV-1+) donors fused with a human-mouse heteromyeloma cell line according to established methods (36–40). All antibodies were purified from cell-free supernatant using protein A or protein G affinity chromatography. Antibody concentrations were determined by absorbance at 280 nm and a mass extinction coefficient of 13.7 for a 1% (10-mg/ml) IgG solution (Nanodrop; Thermo Scientific) or by anti-human IgG ELISA using isotype-matched control immunoglobulins.
ADCC assay.
ADCC activity was measured as previously described (31, 41). CEM.NKR-CCR5-sLTR-Luc target cells, which express luciferase (Luc) under the control of a Tat-inducible promoter, were infected by spinoculation in the presence of 40 μg/ml Polybrene. At 4 days postinfection, infected cells were incubated with monoclonal antibodies and an NK cell line expressing human CD16 for 8 h. The dose-dependent loss of Luc activity was measured as an indication of antibody-mediated killing of virus-infected cells. Infected target cells incubated with NK cells in the absence of antibody were used to measure maximal Luc activity, and uninfected target cells cultured with NK cells were used to determine background Luc activity. Percent relative light units (RLU) were used to determine partial area under the ADCC curve (pAUC) values and antibody concentrations required for half-maximal killing (50% ADCC), as previously described (25, 41). Differences between log10-transformed percent RLU values and 100% RLU, indicating no activity, were calculated. pAUC values were determined by multiplying the sum of these differences at the four highest antibody concentrations tested by the log10-transformed dilution factor of 2, yielding an area. Standard deviations (SD) of individual measurements were propagated to yield the SD of the pAUC.
Neutralization assay.
Neutralization of viral infectivity was measured using a TZM-bl reporter cell assay, according to standard methods (42, 43). In a flat-bottom 96-well plate, 5,000 cells per well were seeded the day before the neutralization assay. Either 4 ng p24 (HIV-1NL4-3), 10 ng p24 (HIV-1JR-FL), or 20 ng p27 (SHIVAD8-EO) of virus per well was incubated with serial dilutions of monoclonal antibody for 1 h at 37°C before being added to the reporter cells. After 3 days, luciferase activity in cell lysates was measured, and virus neutralization was calculated from reductions in RLU relative to cells incubated with virus but no antibody. Uninfected cells were measured to account for background luciferase activity. Partial area under the neutralization curve (pAUC) values and antibody concentrations for 50% neutralization (IC50) were calculated by using the same methods as for the ADCC assay.
Flow cytometry.
Surface envelope staining was performed 3 days postinfection as previously described (44, 45). Antibody binding to Env was detected using 5 μg/ml of monoclonal antibody followed by anti-human IgG F(ab′)2 (phycoerythrin [PE]; polyclonal). Cells were surface stained for CD45 (peridinin chlorophyll protein [PerCP]; clone 2D1) and CD4 (Alexa Fluor 700; clone RPA-T4), then permeabilized, and stained for intracellular Gag (fluorescein isothiocyanate [FITC]; clone FH190-1-1 for HIV-1; clone 55-2F12 for SHIV). Nonviable cells were excluded using LIVE/DEAD fixable dead cell aqua stain (Invitrogen), and data were collected using a SORP BD LSR-II flow cytometer (Becton Dickinson). After gating on viable CD45+ CD4low Gag+ cells, the geometric mean fluorescence intensity (gMFI) of Env staining was calculated using FlowJo, version 9.7.7 (Tree Star, Inc.).
Statistical analysis.
All statistical analysis was done using Prism version 6.0g (GraphPad Software, Inc.). Correlations were determined by calculating Pearson product-moment correlation coefficients. Significance levels of ADCC activity and neutralization were calculated by comparing pAUC values of samples to negative-control values by two-way analysis of variance (ANOVA) with a Dunnett correction for multiple comparisons. Negative controls for ADCC assays were pAUC values of the same antibody against SIVmac239-infected cells. For neutralization data, comparisons were drawn to the hypothetical pAUC of a negative sample with percent RLU of 100.
RESULTS
ADCC activity of HIV-1 Env-specific monoclonal antibodies.
Monoclonal antibodies targeting diverse epitopes of the HIV-1 envelope glycoprotein were tested for ADCC against target cells infected with HIV-1NL4-3 and HIV-1JR-FL, which represent lab-adapted and primary HIV-1 isolates, respectively, with tier 1 versus tier 2 sensitivity to neutralizing antibodies, SHIVAD8-EO, which is a chemokine (C-C motif) receptor 5 (CCR5)-tropic simian-human immunodeficiency virus isolate adapted for pathogenic infection of rhesus macaques (46–48), and SIVmac239 as a control for nonspecific killing. The antibodies (all IgG1) included bNAbs to the CD4 binding site (CD4bs) (49–54), glycan and proteoglycan epitopes in gp120 (33, 55–60), and the membrane-proximal external region (MPER) of gp41 (55, 61–63) and nonneutralizing antibodies to CD4-inducible (CD4i) epitopes in gp120 (53, 64–67) and cluster I and cluster II epitopes of gp41 (39, 40, 68–72). ADCC was assessed using an assay designed to measure the lysis of virus-infected cells expressing native conformations of Env, and antibody concentrations for half-maximal lysis (50% ADCC) and partial area under the curve (pAUC) values were calculated as previously described (25, 41).
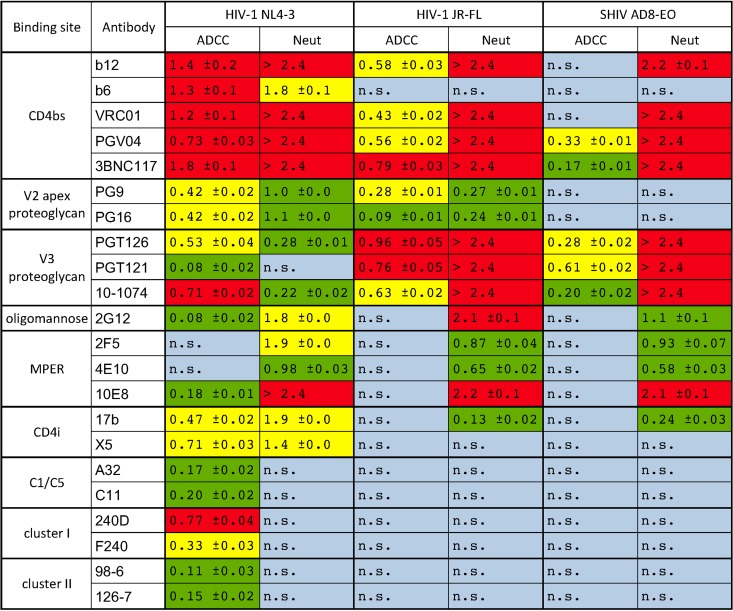
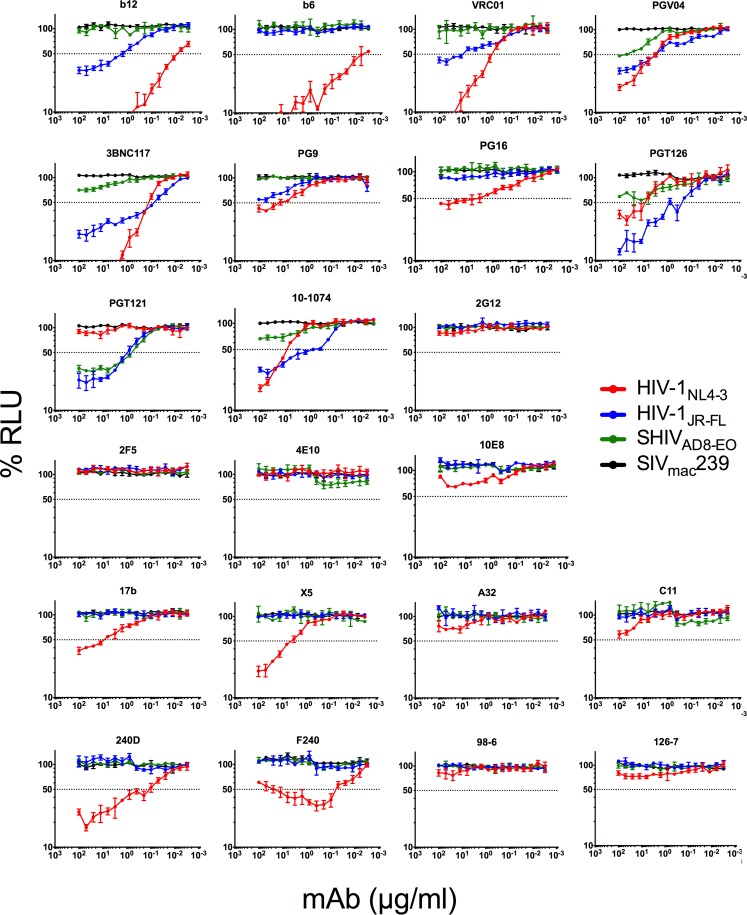
HIV-1NL4-3-infected cells exhibited the greatest susceptibility to ADCC. Of the 22 antibodies tested, 20 mediated significant lysis of cells infected with this virus (Table 1 and Fig. 1). These antibodies included most of the bNAbs (except 2F5 and 4E10), antibodies to CD4i epitopes in the coreceptor binding site (17b and X5), as well as nonneutralizing antibodies to the gp120 inner domain (A32 and C11) and nonneutralizing antibodies to gp41 (F240, 240D, 98-6, and 126-7). In most cases, HIV-1NL4-3-infected cells were also susceptible to ADCC at much lower antibody concentrations than cells infected with HIV-1JR-FL or SHIVAD8-EO (Fig. 1). Thus, consistent with the well-documented sensitivity of the lab-adapted isolate HIV-1NL4-3 to neutralizing antibodies (73–75), cells infected with HIV-1NL4-3 were highly susceptible to ADCC.
TABLE 1.
Comparison of pAUC values for ADCC and virus neutralizationa
Percent RLU values at the four highest antibody concentrations tested were used to calculate partial area under the curve (pAUC) values as previously described (25, 41). Standard deviations were calculated from triplicate measurements. Red indicates potent ADCC or neutralization (Neut) (top tertile), yellow indicates intermediate activity, green indicates weak activity (bottom tertile), and blue indicates a lack of significant antiviral activity (P > 0.01). The tertiles for ADCC activity and neutralization were calculated separately from the respective values against HIV-1NL4-3. n.s., not significant.
FIG 1.
ADCC activity of HIV-1 Env-specific monoclonal antibodies. CEM.NKR-CCR5-sLTR-Luc cells infected with HIV-1NL4-3, HIV-1JR-FL, SHIVAD8-EO, or SIVmac239 were incubated with an NK cell line expressing human CD16 at a 10:1 effector-to-target ratio in the presence of the indicated concentrations of monoclonal antibodies (mAbs). ADCC responses were measured as the dose-dependent loss of luciferase activity in relative light units (RLU) after an 8-h incubation in comparison to control wells containing NK cells and either infected (maximal) or uninfected (background) CEM.NKR-CCR5-sLTR-Luc cells in the absence of antibody. Values are the means ± standard deviations (error bars) for triplicate wells, and the dotted line indicates half-maximal lysis of infected cells.
In contrast, cells infected with HIV-1JR-FL and SHIVAD8-EO were susceptible to lysis only by bNAbs. With the exception of the oligomannose-specific antibody 2G12 and the MPER-specific antibodies 2F5, 4E10, and 10E8, HIV-1JR-FL-infected cells were sensitive to all of the bNAbs (Table 1 and Fig. 1); however, SHIVAD8-EO-infected cells were resistant to all but a handful of antibodies. ADCC was detected against SHIVAD8-EO-infected cells for PGV04, 3BNC117, PGT126, PGT121, and 10-1074, but only PGV04 and PGT121 mediated potent killing at 50% ADCC concentrations of less than 100 μg/ml (58 μg/ml and 0.67 μg/ml, respectively) (Table 2). ADCC activity was also measured at lower antibody concentrations for HIV-1JR-FL-infected cells than SHIVAD8-EO-infected cells (Fig. 1). These observations indicate that SHIVAD8-EO-infected cells are less sensitive to recognition by most HIV-1-specific antibodies, perhaps as a consequence of extensive adaptation of this virus for replication in rhesus macaques, as reflected by changes in the neutralization profile of SHIVAD8-EO relative to the parental HIV-1AD8 strain (76). In comparison to HIV-1NL4-3, the greater resistance of HIV-1JR-FL- and SHIVAD8-EO-infected cells to ADCC is also consistent with the resistance of these primary isolates to neutralizing antibodies.
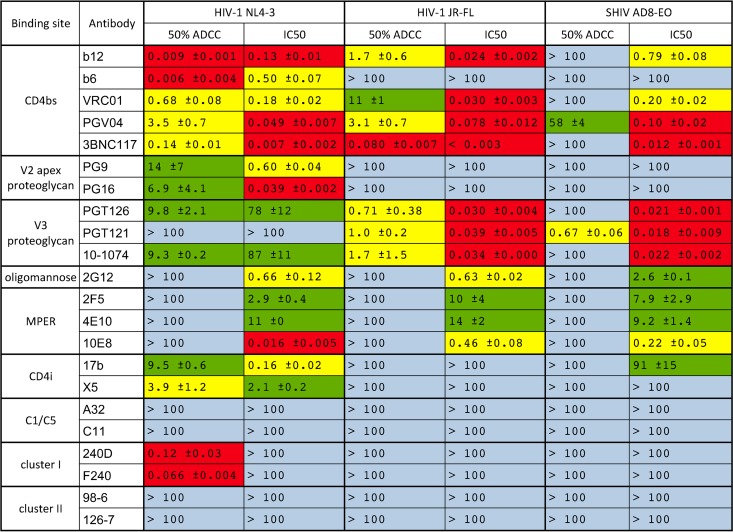
TABLE 2.
Antibody concentrations for 50% ADCC and 50% neutralizationa
Antibody concentrations (μg/ml) for half-maximal ADCC (50% ADCC) and virus neutralization (IC50) were calculated as previously described (41). Standard deviations were calculated from triplicate neutralization curves. Red indicates potent ADCC or neutralization (top tertile), yellow indicates intermediate activity, green indicates weak activity (bottom tertile), and blue indicates less than 50% activity at 100 μg/ml. The tertiles for ADCC activity and neutralization were calculated separately from the respective values against HIV-1NL4-3.
ADCC activity correlates with binding to Env on the surfaces of virus-infected cells.
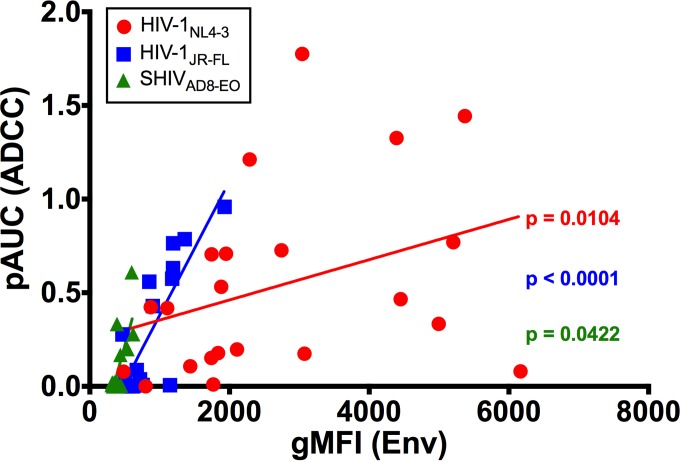
Antibody binding to virus-infected cells is a prerequisite for ADCC. Cells infected with HIV-1NL4-3, HIV-1JR-FL, and SHIVAD8-EO were therefore stained with each of the HIV-1 Env-specific monoclonal antibodies and analyzed by flow cytometry to determine the extent to which binding correlates with susceptibility to ADCC (Fig. 2). The geometric mean fluorescence intensities of Env staining (Table 3) were compared by nonparametric Spearman correlation to partial area under the curve values for ADCC (Fig. 3), which capture responses for antibodies that did not achieve 50% ADCC at concentrations less than 100 μg/ml.
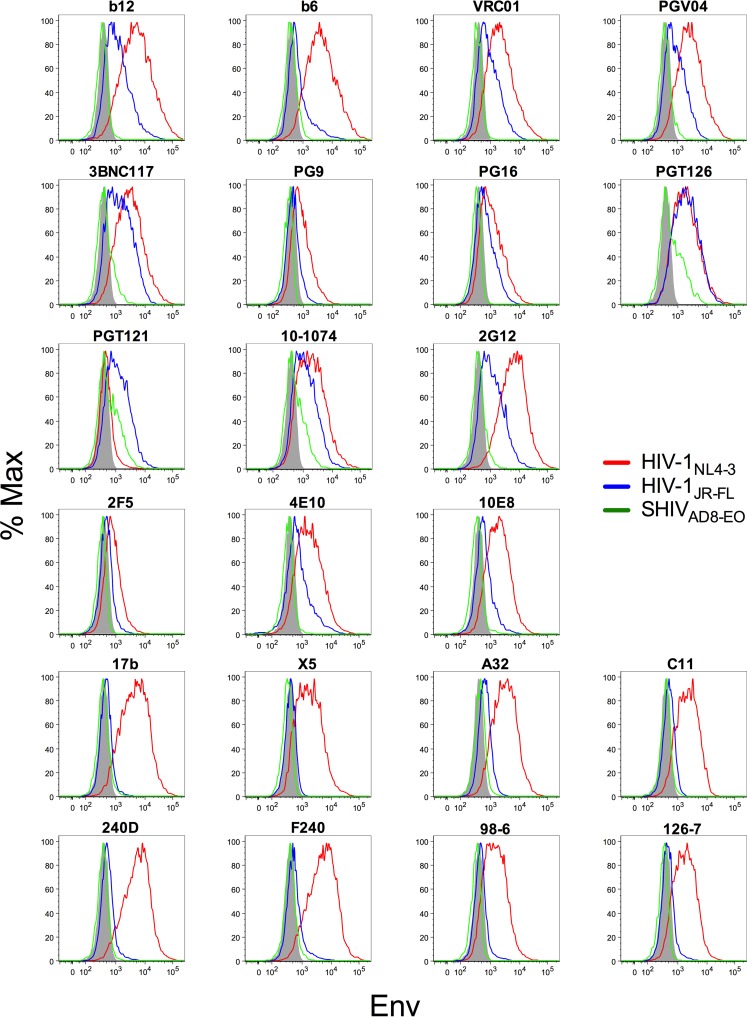
FIG 2.
Antibody binding to Env on the surfaces of virus-infected cells. CEM.NKR-CCR5-sLTR-Luc cells infected with HIV-1NL4-3, HIV-1JR-FL, or SHIVAD8-EO were stained with HIV-1 Env-specific antibodies followed by a PE-conjugated anti-human IgG F(ab′)2. The cells were also stained for surface expression of CD45 and CD4, intracellular expression of the viral Gag protein, and with a viability dye. The histograms show Env staining on virus-infected (Gag+ CD4low) cells of the viable CD45+ population. The shaded area indicates nonspecific staining with the DEN3 control antibody. Max, maximum.
TABLE 3.
Env staining on the surface of virus-infected cellsa
| Antibody | gMFI of Env staining |
||
|---|---|---|---|
| HIV-1NL4-3 | HIV-1JR-FL | SHIVAD8-EO | |
| DEN3 | 391 | 361 | 311 |
| b12 | 5,370 | 1,179 | 355 |
| b6 | 4,391 | 719 | 396 |
| VRC01 | 2,289 | 901 | 351 |
| PGV04 | 2,744 | 851 | 389 |
| 3BNC117 | 3,041 | 1,358 | 437 |
| PG9 | 872 | 466 | 313 |
| PG16 | 1,109 | 672 | 335 |
| PGT126 | 1,882 | 1,930 | 620 |
| PGT121 | 483 | 1,193 | 599 |
| 10-1074 | 1,951 | 1,191 | 540 |
| 2G12 | 6,167 | 1,148 | 411 |
| 2F5 | 800 | 482 | 333 |
| 4E10 | 1,768 | 756 | 341 |
| 10E8 | 1,836 | 619 | 349 |
| 17b | 4,451 | 473 | 382 |
| X5 | 1,743 | 412 | 322 |
| A32 | 3,073 | 642 | 446 |
| C11 | 2,108 | 504 | 396 |
| 240D | 5,205 | 533 | 373 |
| F240 | 4,996 | 560 | 402 |
| 98-6 | 1,438 | 473 | 327 |
| 126-7 | 1,740 | 471 | 331 |
CEM.NKR-CCR5-sLTR-Luc cells infected with HIV-1NL4-3, HIV-1JR-FL, or SHIVAD8-EO were stained with HIV-1 Env-specific antibodies followed by a PE-conjugated anti-human IgG F(ab′)2. The cells were also stained for surface expression of CD45 and CD4, intracellular expression of the viral Gag protein, and with a viability dye. Values indicate the geometric mean fluorescence intensity (gMFI) of Env staining on the surfaces of virus-infected (Gag+ CD4low) cells of the viable CD45+ population. DEN3 is a dengue virus-specific monoclonal antibody included as a control for nonspecific staining.
FIG 3.
Comparison of ADCC activity and Env binding by HIV-1 Env-specific antibodies. Partial area under the curve values (pAUC) for ADCC activity were calculated from percent RLU measurements at the four highest antibody concentrations tested, as previously described (25, 41). pAUC values for HIV-1NL4-3, HIV-1JR-FL, and SHIVAD8-EO were compared to the geometric mean fluorescence intensities (gMFIs) of Env staining on the surfaces of virus-infected cells by Spearman correlation.
Antibody binding correlated with ADCC for each of the three viruses tested. Whereas binding was highly predictive of ADCC for HIV-1JR-FL (P < 0.0001), less robust, but nevertheless significant, associations were also observed for HIV-1NL4-3 (P = 0.0104) and SHIVAD8-EO (P = 0.0422) (Fig. 3). The relationship between binding and ADCC for HIV-1NL4-3-infected cells reflects greater variability in these measurements and instances of antibody binding in the absence of detectable ADCC. For instance, 2F5 and 4E10 stained cells infected with HIV-1NL4-3 (Fig. 2), but they did not mediate cell lysis (Fig. 1). The reason for this discrepancy is unclear, but it may be related to the limited accessibility of these antibodies for engagement by FcγRs on NK cells when bound to virus-infected cells due to their specificity for an epitope consisting of phospholipids and sequences exposed at the base of gp41 (77–80). For SHIVAD8-EO-infected cells, there was almost complete correspondence between antibody binding and susceptibility to ADCC (Fig. 1 and 2); however, the significance of this relationship was limited in comparison to HIV-1JR-FL by lower responses that were detectable for a smaller subset of antibodies.
Correlation of ADCC activity with virus neutralization.
The antiviral activity of HIV-1-specific antibodies is typically defined by their ability to neutralize viral infectivity. Each of the HIV-1 Env-specific monoclonal antibodies was therefore tested for neutralization of HIV-1NL4-3, HIV-1JR-FL, and SHIVAD8-EO to investigate the relationship between their ability to block viral infectivity and to mediate NK cell lysis of virus-infected cells. Antibody concentrations for 50% neutralization (IC50) (Table 2) were calculated from neutralization curves (Fig. 4), and corresponded well to previously published data (33, 50, 51, 59, 63, 81–84). pAUC values for neutralization were also determined (Table 1) and compared to pAUC values for ADCC by nonparametric Spearman correlation (Fig. 5).
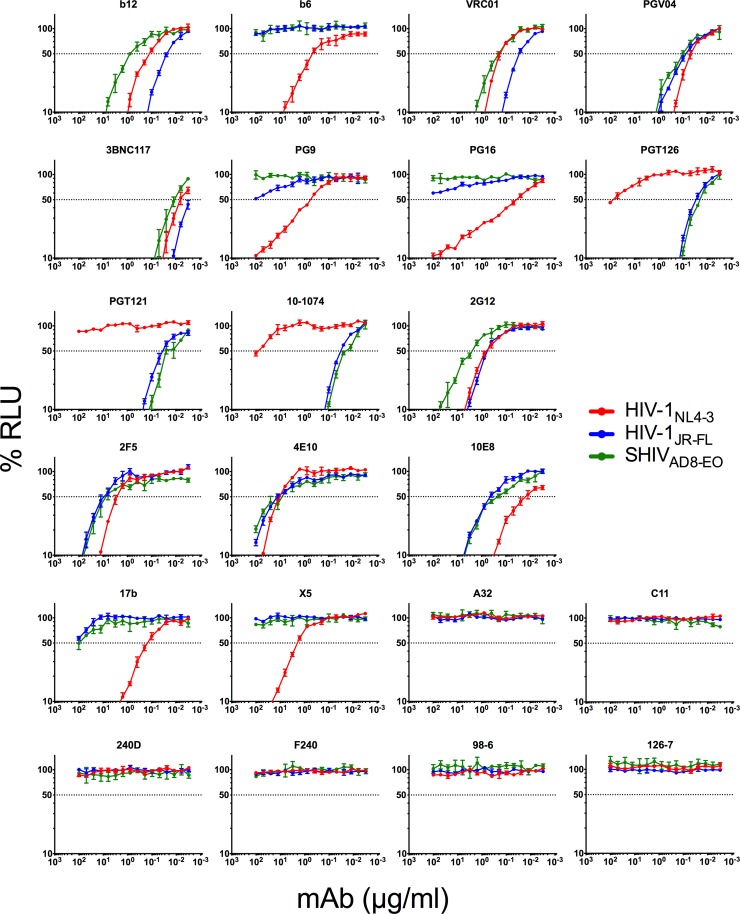
FIG 4.
Neutralization of HIV-1NL4-3, HIV-1JR-FL, and SHIVAD8-EO by Env-specific antibodies. HIV-1 Env-specific monoclonal antibodies were tested for the ability to block viral infectivity. HIV-1NL4-3, HIV-1JR-FL, and SHIVAD8-EO were incubated with serial dilutions of each antibody for 1 h before addition to TZM-bl reporter cells. Three days postinfection, neutralization was calculated from the luciferase activity (RLU) in TZM-bl cell lysates for cells inoculated with virus plus antibody relative to cells inoculated with virus in the absence of antibody. The error bars indicate standard deviations of the means for triplicate wells, and the dotted line indicates half-maximal infection or 50% neutralization.
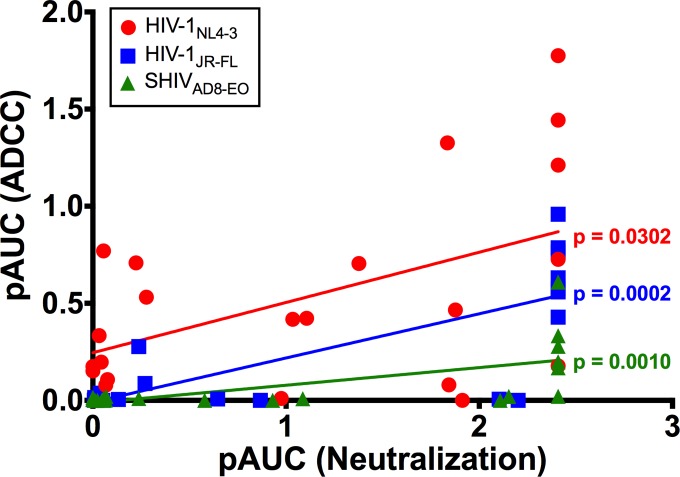
FIG 5.
Comparison of ADCC and neutralizing activity of HIV-1 Env-specific antibodies. Partial area under the curve values (pAUC) for ADCC and neutralization were calculated from percent RLU measurements at the four highest concentrations of each antibody, as previously described (25, 41). pAUC values for ADCC and neutralization against HIV-1NL4-3, HIV-1JR-FL, and SHIVAD8-EO were compared by Spearman correlation.
Neutralization correlated with ADCC for all three viruses. This relationship was strongest for HIV-1JR-FL (P = 0.0002), followed by SHIVAD8-EO (P = 0.0010) and HIV-1NL4-3 (P = 0.0302) (Fig. 5). Several instances of neutralization in the absence of detectable ADCC were observed, and in most cases, antibodies that directed ADCC against cells infected with a particular isolate also neutralized that virus (Tables 1 and 2). Indeed, all of the antibodies with ADCC activity against HIV-1JR-FL- or SHIVAD8-EO-infected cells also neutralized these viruses (Table 1). Furthermore, antibody concentrations for 50% neutralization were generally lower than for 50% ADCC (Table 2). However, in the case of HIV-1NL4-3, a number of instances of ADCC in the absence of detectable neutralization were observed (Table 1), and antibody concentrations for 50% ADCC were sometimes lower than for 50% neutralization (Table 2). Notably, 240D and F240, which recognize epitopes exposed in gp41 that are nonneutralizing, mediated efficient NK cell lysis of HIV-1NL4-3-infected cells (50% ADCC of 0.12 and 0.066 μg/ml, respectively) (Table 2). Overall, these results indicate that most antibodies that are able to bind to Env expressed on the surfaces of virus-infected cells to mediate ADCC are also able to bind to Env trimers on the surfaces of virions to neutralize infectivity. However, neutralization is not always predictive of ADCC, and for lab-adapted viruses such as HIV-1NL4-3, conformations of Env may be exposed on the surfaces of virus-infected cells that render them susceptible to antibody-dependent killing but are not relevant to blocking viral infectivity.
DISCUSSION
Increasing evidence suggests that Fcγ receptor-dependent functions of antibodies are important for the optimal antiviral activity of HIV-1-specific antibodies (8–11, 85). Thus, a better understanding of the relationship between neutralizing and nonneutralizing functions of antibodies is needed to guide the development of immunotherapies and antibody-based vaccines for the treatment and prevention of HIV-1 infection. In the present study, antibodies targeting diverse epitopes of the HIV-1 Env protein were tested for ADCC against cells infected with HIV-1 or SHIV isolates, and their ADCC activity was compared to their ability to bind to Env expressed on the surfaces of virus-infected cells and to neutralize viral infectivity. Consistent with recent findings (86, 87), ADCC activity correlated with Env binding and with neutralization for each of the viruses tested, indicating that these functions are largely overlapping; however, instances of ADCC in the absence of detectable neutralization and neutralization in the absence of detectable ADCC were also observed, revealing differences in Env epitopes exposed on the surfaces of HIV-1-infected cells and virions that confer susceptibility to these antiviral activities.
Sensitivity to ADCC corresponded closely with sensitivity to neutralization for antibody-resistant primary isolates. All of the antibodies that directed ADCC against HIV-1JR-FL- and SHIVAD8-EO-infected cells also neutralized these viruses. Thus, most antibodies capable of binding to Env on the cell surface and directing the lysis of virus-infected cells are also able to bind functional Env trimers on virions to block viral infectivity; however, this was not always the case for HIV-1NL4-3. In accordance with the greater exposure of the Env proteins of lab-adapted viruses to antibodies (73–75), HIV-1NL4-3-infected cells were generally more sensitive to ADCC, both in terms of the number of antibodies and the magnitude of responses. Although ADCC corresponded with neutralization for many of the bNAbs, cells infected with HIV-1NL4-3 were also susceptible to killing by nonneutralizing antibodies. This was particularly evident for F240 and 240D, which mediated potent ADCC against HIV-1NL4-3-infected cells despite their inability to block viral infection (Fig. 4) (88). ADCC responses were also detected for 98-6 and 126-7, which recognize epitopes exposed in the postfusion conformation of gp41 (39, 70, 71), and for A32 and C11, which target CD4i epitopes of the gp120 inner domain (66, 67, 89–91). The ADCC activity of nonneutralizing antibodies against HIV-1NL4-3-infected cells suggests that Env epitopes that are not relevant to blocking viral infectivity are exposed on the surfaces of cells infected with lab-adapted viruses that render them susceptible to ADCC.
The striking difference in the susceptibility of HIV-1NL4-3- versus HIV-1JR-FL-infected cells to ADCC illustrates the importance of using primary virus isolates for studying antiviral functions of antibodies. To facilitate virus replication in the face of ongoing immune responses, the HIV-1 envelope glycoprotein has evolved structural features that make it inherently resistant to antibodies (92–95). These features can become attenuated as virus is passaged in T cell lines, accounting for the well-documented increase in the susceptibility of lab-adapted HIV-1 to neutralizing antibodies (73–75, 96, 97). The much greater sensitivity of HIV-1NL4-3-infected cells to opsonization by Env-specific monoclonal antibodies, including antibodies that do not neutralize this virus, suggests that this is also true for ADCC. These observations therefore advocate for the use of primary HIV-1 isolates expressing physiologically relevant conformations of Env on the surfaces of infected cells for studies investigating ADCC or other FcγR-dependent functions of antibodies.
Contrary to earlier reports identifying A32 as a potent mediator of ADCC (98, 99), we found that HIV-1- and SHIV-infected cells are highly resistant to lysis by this antibody. Indeed, ADCC was detected against HIV-1NL4-3-infected cells only at high concentrations of A32, and not at all against HIV-1JR-FL- or SHIVAD8-EO-infected cells. Recent evidence suggests that this disparity probably reflects differences in the methods used to measure ADCC. A32 is specific for an epitope on the inner domain of gp120 that is normally occluded in the unliganded Env trimer but can be exposed upon CD4 engagement (53, 94). The accumulation of Env-CD4 complexes on the surfaces of cells infected with viruses deficient for Nef- and/or Vpu-mediated CD4 downregulation was accordingly found to increase exposure of this epitope (100, 101). Shed gp120 released from productively infected cells was also found to sensitize uninfected bystander cells to A32-mediated ADCC (102). These studies help to explain the robust ADCC activity for A32 initially observed using target cells pulsed with soluble gp120 or chronically infected with a Nef-deficient HIV-1 (98). The measurement of NK cell degranulation as a surrogate for the direct lysis of virus-infected cells, which cannot differentiate ADCC responses to virus-infected cells from responses to uninfected cells coated with gp120, may also explain the detection of ADCC activity for A32 against cells infected with HIV-1 isolates that retain CD4 downmodulation (98, 99). In accordance with this interpretation, other recent studies using ADCC assays that directly measure the elimination of virus-infected cells have found that viruses that downmodulate CD4 are resistant to A32-mediated lysis (100, 101, 103). These observations therefore further argue for the use of ADCC assays that directly measure the killing of cells infected with HIV-1 isolates expressing functional accessory proteins and native conformations of Env.
Instances of neutralization in the absence of ADCC include the MPER-specific antibodies 2F5, 4E10, and 10E8, and the glycan-specific antibody 2G12. The lack of ADCC activity for the MPER bNAbs is probably due in part to the lower affinity of these antibodies for Env on virus-infected cells, which is consistent with their specificity for an epitope consisting of gp41 sequences that are transiently exposed during fusion and phospholipids that are preferentially enriched in viral membranes (77–80). Yet these antibodies still bound to HIV-1NL4-3- and HIV-1JR-FL-infected cells, as indicated by levels of Env staining similar to other antibodies with ADCC against these viruses, such as the V2 apex bNAbs PG9 and PG16. The reason for this discrepancy in binding versus ADCC is unclear at this time but potentially reflects the orientation of MPER-specific antibodies bound to gp41, which may hinder their accessibility for engagement by NK cells. The lack of detected ADCC activity for 2G12 was also surprising considering the ability of this antibody to stain Env on the surfaces of HIV-1NL4-3- and HIV-1JR-FL-infected cells. 2G12 is specific for a cluster of high-mannose glycans on the outer domain of gp120 that should not limit its accessibility (56, 104); however, 2G12 has an unusual domain-swapped configuration and propensity for dimerization that may impair FcγR interactions (105, 106). Although monomeric and dimeric forms of 2G12 were shown to mediate ADCC against a cell line expressing HIV-1 HXB2 gp160 (107), our data are consistent with recent reports that have found negligible ADCC activity for 2G12 against HIV-1-infected cells (86, 98, 103).
Overall, our results reveal a general correlation between ADCC and neutralization by HIV-1 Env-specific antibodies, which implies, perhaps not surprisingly, that most antibodies that are able to bind to functional Env trimers on virions to block infectivity are also able to bind to Env expressed on the surfaces of virus-infected cells to direct their elimination by ADCC. This correlation was imperfect, however, as several instances where these antiviral activities did not correspond were observed. These exceptions point to underlying differences in Env epitopes on the surfaces of virions and infected cells that differentiate susceptibility to neutralization versus ADCC. Hence, this study provides new insights into the relationship between neutralization and ADCC that may help to guide the development of antibody-based vaccines and immunotherapies for the prevention and treatment of HIV-1 infection.
ACKNOWLEDGMENTS
We thank Michel Nussenzweig (The Rockefeller University) for contributing antibodies 3BNC117 and 10-1074.
D.T.E. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Corti D, Lanzavecchia A. 2013. Broadly neutralizing antiviral antibodies. Annu Rev Immunol 31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 2.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. 2013. Antibodies in HIV-1 vaccine development and therapy. Science 341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moldt B, Rakasz EG, Schultz N, Chan-Hui P-Y, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. 2014. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med 211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu R-B, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang H-W, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diskin R, Klein F, Horwitz JA, Halper-Stromberg A, Sather DN, Marcovecchio PM, Lee T, West AP, Gao H, Seaman MS, Stamatatos L, Nussenzweig MC, Bjorkman PJ. 2013. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J Exp Med 210:1235–1249. doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, Lanigan CMS, Landucci G, Forthal DN, Parren PWHI, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 9.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PWHI, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halper-Stromberg A, Lu C-L, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Ravetch JV. 2008. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman ME, Dugast A-S, Alter G. 2012. Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annu Rev Med 63:113–130. doi: 10.1146/annurev-med-050310-085221. [DOI] [PubMed] [Google Scholar]
- 14.Sawyer LA, Katzenstein DA, Hendry RM, Boone EJ, Vujcic LK, Williams CC, Zeger SL, Saah AJ, Rinaldo CR, Phair JP. 1990. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res Hum Retroviruses 6:341–356. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- 15.Tyler DS, Lyerly HK, Weinhold KJ. 1989. Anti-HIV-1 ADCC. AIDS Res Hum Retroviruses 5:557–563. doi: 10.1089/aid.1989.5.557. [DOI] [PubMed] [Google Scholar]
- 16.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol 157:2168–2173. [PubMed] [Google Scholar]
- 17.Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol 21:227–233. doi: 10.1023/A:1011087132180. [DOI] [PubMed] [Google Scholar]
- 18.Banks ND, Kinsey N, Clements J, Hildreth JEK. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses 18:1197–1205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 19.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, Kaplan J. 1999. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis 180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 20.Dugast A-S, Stamatatos L, Tonelli A, Suscovich TJ, Licht AF, Mikell I, Ackerman ME, Streeck H, Klasse PJ, Moore JP, Alter G. 2014. Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection. Eur J Immunol 44:2925–2937. doi: 10.1002/eji.201344305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambotte O, Ferrari G, Moog C, Yates NL, Liao H-X, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy J-F. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, Delfraissy J-F, Saez-Cirion A, Ferrari G. 2013. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS One 8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. 2015. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe 17:500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes BF, Gilbert PB, McElrath JM, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H- X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast A-S, Parren PWHI, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ, Burton DR. 2012. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol 86:6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gómez-Román VR, Wang L, Kalyanaraman VS, Markham PD, Robey FA, Robert-Guroff M. 2004. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol 78:2212–2221. doi: 10.1128/JVI.78.5.2212-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-Román VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol 174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 29.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, Montefiori DC, Robert-Guroff M. 2009. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol 83:791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M, Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT. 2012. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIVmac251 challenge. PLoS Pathog 8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch RM, Rong R, Boliar S, Sethi A, Li B, Mulenga J, Allen S, Robinson JE, Gnanakaran S, Derdeyn CA. 2011. The B cell response is redundant and highly focused on V1V2 during early subtype C infection in a Zambian seroconverter. J Virol 85:905–915. doi: 10.1128/JVI.02006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong R, Li H, Bibollet-Ruche F, Decker JM, Zheng NN, Gottlieb GS, Kiviat NB, Sow PS, Georgiev I, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2012. Broad and potent neutralizing antibody responses elicited in natural HIV-2 infection. J Virol 86:947–960. doi: 10.1128/JVI.06155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng NN, Lam KS, Calvo Riera F, Kaplan HS. 1983. Construction and testing of mouse–human heteromyelomas for human monoclonal antibody production. Proc Natl Acad Sci U S A 80:7308–7312. doi: 10.1073/pnas.80.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorny MK, Xu JY, Gianakakos V, Karwowska S, Williams C, Sheppard HW, Hanson CV, Zolla-Pazner S. 1991. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A 88:3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorny MK, Moore JP, Conley AJ, Karwowska S, Sodroski J, Williams C, Burda S, Boots LJ, Zolla-Pazner S. 1994. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol 68:8312–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorny MK, Gianakakos V, Sharpe S, Zolla-Pazner S. 1989. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc Natl Acad Sci U S A 86:1624–1628. doi: 10.1073/pnas.86.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu JY, Gorny MK, Palker T, Karwowska S, Zolla-Pazner S. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol 65:4832–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alpert MD, Heyer LN, Williams DEJ, Harvey JD, Greenough T, Allhorn M, Evans DT. 2012. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol 86:12039–12052. doi: 10.1128/JVI.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 44.von Bredow B, Arias JF, Heyer LN, Gardner MR, Farzan M, Rakasz EG, Evans DT. 2015. Envelope glycoprotein internalization protects human and simian immunodeficiency virus-infected cells from antibody-dependent cell-mediated cytotoxicity. J Virol 89:10648–10655. doi: 10.1128/JVI.01911-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. 2014. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura Y, Shingai M, Willey R, Sadjadpour R, Lee WR, Brown CR, Brenchley JM, Buckler-White A, Petros R, Eckhaus M, Hoffman V, Igarashi T, Martin MA. 2010. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol 84:4769–4781. doi: 10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gautam R, Nishimura Y, Lee WR, Donau O, Buckler-White A, Shingai M, Sadjadpour R, Schmidt SD, LaBranche CC, Keele BF, Montefiori D, Mascola JR, Martin MA. 2012. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIVAD8 in rhesus macaques: implications for use in vaccine studies. J Virol 86:8516–8526. doi: 10.1128/JVI.00644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shingai M, Donau OK, Schmidt SD, Gautam R, Plishka RJ, Buckler-White A, Sadjadpour R, Lee WR, LaBranche CC, Montefiori DC, Mascola JR, Nishimura Y, Martin MA. 2012. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc Natl Acad Sci U S A 109:19769–19774. doi: 10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton DR, Barbas CF, Persson MA, Koenig S, Chanock RM, Lerner RA. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A 88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O'Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang Z-Y, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TYK, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore JP, Sodroski J. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol 70:1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pantophlet R, Ollmann Saphire E, Poignard P, Parren PW, Wilson IA, Burton DR. 2003. Fine mapping of the interaction of neutralizing and nonneutralizing monoclonal antibodies with the CD4 binding site of human immunodeficiency virus type 1 gp120. J Virol 77:642–658. doi: 10.1128/JVI.77.1.642-658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses 10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 56.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 70:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunert R, Rüker F, Katinger H. 1998. Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res Hum Retroviruses 14:1115–1128. doi: 10.1089/aid.1998.14.1115. [DOI] [PubMed] [Google Scholar]
- 58.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PNP, Spencer DIR, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang G-Y, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang Z-Y, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang L-X, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol 67:6642–6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, Sodroski J. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol 67:3978–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moulard M, Phogat SK, Shu Y, Labrijn AF, Xiao X, Binley JM, Zhang M-Y, Sidorov IA, Broder CC, Robinson J, Parren PWHI, Burton DR, Dimitrov DS. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc Natl Acad Sci U S A 99:6913–6918. doi: 10.1073/pnas.102562599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robinson J, Yoshiyama H, Holton D, Elliott S, Ho D. 1992. Distinct antigenic sites on HIV gp120 identified by a panel of human monoclonal antibodies. J Cell Biochem 50(Suppl 16E):71. [Google Scholar]
- 67.Moore JP, Thali M, Jameson BA, Vignaux F, Lewis GK, Poon SW, Charles M, Fung MS, Sun B, Durda PJ. 1993. Immunochemical analysis of the gp120 surface glycoprotein of human immunodeficiency virus type 1: probing the structure of the C4 and V4 domains and the interaction of the C4 domain with the V3 loop. J Virol 67:4785–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson WE Jr, Gorny MK, Xu JY, Mitchell WM, Zolla-Pazner S. 1991. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol 65:4169–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cavacini LA, Emes CL, Wisnewski AV, Power J, Lewis G, Montefiori D, Posner MR. 1998. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res Hum Retroviruses 14:1271–1280. doi: 10.1089/aid.1998.14.1271. [DOI] [PubMed] [Google Scholar]
- 70.Yuan W, Li X, Kasterka M, Gorny MK, Zolla-Pazner S, Sodroski J. 2009. Oligomer-specific conformations of the human immunodeficiency virus (HIV-1) gp41 envelope glycoprotein ectodomain recognized by human monoclonal antibodies. AIDS Res Hum Retroviruses 25:319–328. doi: 10.1089/aid.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vincent N, Kone A, Chanut B, Lucht F, Genin C, Malvoisin E. 2008. Antibodies purified from sera of HIV-1-infected patients by affinity on the heptad repeat region 1/heptad repeat region 2 complex of gp41 neutralize HIV-1 primary isolates. AIDS 22:2075–2085. doi: 10.1097/QAD.0b013e3283101260. [DOI] [PubMed] [Google Scholar]
- 72.Gorny MK, Zolla-Pazner S. 2000. Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41. J Virol 74:6186–6192. doi: 10.1128/JVI.74.13.6186-6192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawyer LS, Wrin MT, Crawford-Miksza L, Potts B, Wu Y, Weber PA, Alfonso RD, Hanson CV. 1994. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol 68:1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wrin T, Loh TP, Vennari JC, Schuitemaker H, Nunberg JH. 1995. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. J Virol 69:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammonds J, Chen X, Ding L, Fouts T, De Vico A, zur Megede J, Barnett S, Spearman P. 2003. gp120 stability on HIV-1 virions and Gag-Env pseudovirions is enhanced by an uncleaved Gag core. Virology 314:636–649. doi: 10.1016/S0042-6822(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 76.Boyd DF, Peterson D, Haggarty BS, Jordan APO, Hogan MJ, Goo L, Hoxie JA, Overbaugh J. 2015. Mutations in HIV-1 envelope that enhance entry with the macaque CD4 receptor alter antibody recognition by disrupting quaternary interactions within the trimer. J Virol 89:894–907. doi: 10.1128/JVI.02680-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci U S A 105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Frey G, Peng H, Rits-Volloch S, Garrity J, Seaman MS, Chen B. 2014. Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. J Virol 88:1249–1258. doi: 10.1128/JVI.02664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cardoso RMF, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 80.Irimia A, Sarkar A, Stanfield RL, Wilson IA. 2016. Crystallographic identification of lipid as an integral component of the epitope of HIV broadly neutralizing antibody 4E10. Immunity 44:21–31. doi: 10.1016/j.immuni.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chuang G-Y, Acharya P, Schmidt SD, Yang Y, Louder MK, Zhou T, Kwon YD, Pancera M, Bailer RT, Doria-Rose NA, Nussenzweig MC, Mascola JR, Kwong PD, Georgiev IS. 2013. Residue-level prediction of HIV-1 antibody epitopes based on neutralization of diverse viral strains. J Virol 87:10047–10058. doi: 10.1128/JVI.00984-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Georgiev IS, Doria-Rose NA, Zhou T, Kwon YD, Staupe RP, Moquin S, Chuang G-Y, Louder MK, Schmidt SD, Altae-Tran HR, Bailer RT, McKee K, Nason M, O'Dell S, Ofek G, Pancera M, Srivatsan S, Shapiro L, Connors M, Migueles SA, Morris L, Nishimura Y, Martin MA, Mascola JR, Kwong PD. 2013. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science 340:751–756. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 83.Liao H-X, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, Becker J, Benjamin B, Blakesley R, Bouffard G, Brooks S, Coleman H, Dekhtyar M, Gregory M, Guan X, Gupta J, Han J, Hargrove A, Ho S, Johnson T, Legaspi R, Lovett S, Maduro Q, Masiello C, Maskeri B, McDowell J, Montemayor C, Mullikin J, Park M, Riebow N, Schandler K, Schmidt B, Sison C, Stantripop M, Thomas J, Thomas P, Vemulapalli M, Young A, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia S-M, et al. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Imamichi H, Georgiev IS, Chuang G-Y, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Peña AT, Derking R, Klasse P-J, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, Bailey-Kellogg C, Ackerman ME, Scanlan C, Zolla-Pazner S, Alter G. 2014. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS 28:2523–2530. doi: 10.1097/QAD.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko S-Y, Hallahan CW, Wong H, Liu B, You L, Scheid J, Kappes JC, Ochsenbauer C, Nabel GJ, Mascola JR, Connors M. 2012. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol 86:8672–8680. doi: 10.1128/JVI.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noël N, Lambotte O, Mouquet H, Schwartz O. 2016. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nyambi PN, Mbah HA, Burda S, Williams C, Gorny MK, Nádas A, Zolla-Pazner S. 2000. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J Virol 74:7096–7107. doi: 10.1128/JVI.74.15.7096-7107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore JP, Willey RL, Lewis GK, Robinson J, Sodroski J. 1994. Immunological evidence for interactions between the first, second, and fifth conserved domains of the gp120 surface glycoprotein of human immunodeficiency virus type 1. J Virol 68:6836–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol 69:5723–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Finzi A, Xiang SH, Pacheco B, Wang L, Haight J, Kassa A, Danek B, Pancera M, Kwong PD, Sodroski J. 2010. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol Cell 37:656–667. doi: 10.1016/j.molcel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 94.Julien J-P, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse P-J, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee JH, Ozorowski G, Ward AB. 2016. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moore JP, Burkly LC, Connor RI, Cao Y, Tizard R, Ho DD, Fisher RA. 1993. Adaptation of two primary human immunodeficiency virus type 1 isolates to growth in transformed T cell lines correlates with alterations in the responses of their envelope glycoproteins to soluble CD4. AIDS Res Hum Retroviruses 9:529–539. doi: 10.1089/aid.1993.9.529. [DOI] [PubMed] [Google Scholar]
- 97.Pugach P, Kuhmann SE, Taylor J, Marozsan AJ, Snyder A, Ketas T, Wolinsky SM, Korber BT, Moore JP. 2004. The prolonged culture of human immunodeficiency virus type 1 in primary lymphocytes increases its sensitivity to neutralization by soluble CD4. Virology 321:8–22. doi: 10.1016/j.virol.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 98.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang K-K, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao C-Y, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao H-X, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Veillette M, Désormeaux A, Medjahed H, Gharsallah N-E, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alsahafi N, Ding S, Richard J, Markle T, Brassard N, Walker B, Lewis GK, Kaufmann DE, Brockman MA, Finzi A. 2015. Nef proteins from HIV-1 elite controllers are inefficient at preventing antibody-dependent cellular cytotoxicity. J Virol 90:2993–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richard J, Veillette M, Ding S, Zoubchenok D, Alsahafi N, Coutu M, Brassard N, Park J, Courter JR, Melillo B, Smith AB, Shaw GM, Hahn BH, Sodroski J, Kaufmann DE, Finzi A. 2016. Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine 3:122–134. doi: 10.1016/j.ebiom.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding S, Veillette M, Coutu M, Prévost J, Scharf L, Bjorkman PJ, Ferrari G, Robinson JE, Stürzel C, Hahn BH, Sauter D, Kirchhoff F, Lewis GK, Pazgier M, Finzi A. 2015. A highly conserved residue of the HIV-1 gp120 inner domain is important for antibody-dependent cellular cytotoxicity responses mediated by anti-cluster A antibodies. J Virol 90:2127–2134. doi: 10.1128/JVI.02779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrabi R, Voss JE, Liang C-H, Briney B, McCoy LE, Wu C-Y, Wong C-H, Poignard P, Burton DR. 2015. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope V2 apex to facilitate vaccine design. Immunity 43:959–973. doi: 10.1016/j.immuni.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 106.West AP, Galimidi RP, Foglesong CP, Gnanapragasam PNP, Huey-Tubman KE, Klein JS, Suzuki MD, Tiangco NE, Vielmetter J, Bjorkman PJ. 2009. Design and expression of a dimeric form of human immunodeficiency virus type 1 antibody 2G12 with increased neutralization potency. J Virol 83:98–104. doi: 10.1128/JVI.01564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klein JS, Webster A, Gnanapragasam PNP, Rachel P, Bjorkman PJ. 2010. A dimeric form of the HIV-1 antibody 2G12 elicits potent antibody-dependent cellular cytotoxicity. AIDS 24:1633–1640. doi: 10.1097/QAD.0b013e32833ad8c8. [DOI] [PMC free article] [PubMed] [Google Scholar]