ABSTRACT
Four cases of acute flaccid paralysis caused by slightly evolved (Sabin-like) vaccine polioviruses of serotype 2 were registered in July to August 2010 in an orphanage of Biysk (Altai Region, Russia). The Biysk cluster of vaccine-associated paralytic poliomyelitis (VAPP) had several uncommon, if not unique, features. (i) Until this outbreak, Sabin-like viruses (in distinction to more markedly evolved vaccine-derived polioviruses [VDPVs]) were reported to cause only sporadic cases of VAPP. Consequently, VAPP cases were not considered to require outbreak-type responses. However, the Biysk outbreak completely blurred the borderline between Sabin-like viruses and VDPVs in epidemiological terms. (ii) The outbreak demonstrated a very high disease/infection ratio, apparently exceeding even that reported for wild polioviruses. The viral genome structures did not provide any substantial hints as to the underlying reason(s) for such pathogenicity. (iii) The replacement of intestinal poliovirus lineages by other Sabin-like lineages during short intervals after the disease onsets was observed in two patients. Again, the sequences of the respective genomes provided no clues to explain these events. (iv) The polioviruses isolated from the patients and their contacts demonstrated a striking heterogeneity as well as rapid and uneven evolution of the whole genomes and their parts, apparently due to extensive interpersonal contacts in a relatively small closed community, multiple bottlenecking, and recombination. Altogether, the results demonstrate several new aspects of pathogenicity, epidemiology, and evolution of vaccine-related polioviruses and underscore several serious gaps in understanding these problems.
IMPORTANCE The oral poliovirus vaccine largely contributed to the nearly complete disappearance of poliovirus-caused poliomyelitis. Being generally safe, it can, in some cases, result in a paralytic disease. Two types of such outcomes are distinguished: those caused by slightly diverged (Sabin-like) viruses on the one hand and those caused by significantly diverged VDPVs on the other. This classification is based on the number of mutations in the viral genome region encoding a viral structural protein. Until now, only sporadic poliomyelitis cases due to Sabin-like polioviruses had been described, and in distinction from the VDPV-triggered outbreaks, they did not require broad-scale epidemiological responses. Here, an unusual outbreak of poliomyelitis caused by a Sabin-like virus is reported, which had an exceptionally high disease/infection ratio. This outbreak blurred the borderline between Sabin-like polioviruses and VDPVs both in pathogenicity and in the kind of responses required, as well as underscoring important gaps in understanding the pathogenicity, epidemiology, and evolution of vaccine-derived polioviruses.
INTRODUCTION
As a result of the implementation of the Global Polio Eradication Initiative launched by the World Health Organization (WHO) in 1988, the worldwide incidence of paralytic poliomyelitis decreased by approximately 2 orders of magnitude. The success has been due largely to the use of two outstanding vaccines: the Salk inactivated polio vaccine (IPV) and Sabin live oral polio vaccine (OPV). Notwithstanding their obvious merits, these vaccines have drawbacks: the former fails to trigger a robust intestinal immunity and is unable therefore to prevent transmission of wild polioviruses, whereas the latter can be associated, although quite rarely, with cases of paralytic poliomyelitis, a circumstance especially important now that the incidence of the disease caused by wild polioviruses has substantially decreased.
Two situations linked to the OPV-associated polio cases are usually distinguished. The disease may be triggered in vaccine recipients or their contacts by the original or slightly evolved (Sabin-like) vaccine viruses soon after the vaccination (1). Such cases are referred to as vaccine-associated paralytic poliomyelitis (VAPP). Other cases might be caused by markedly diverged vaccine viruses after their cryptic circulation or hidden evolution in a single organism before showing up. Such viruses are dubbed vaccine-derived polioviruses (VDPVs) (2). The current official criterion to classify an OPV-originated virus as a VDPV are the presence of 10 or more mutations in the region encoding the viral capsid protein VP1 for the vaccine serotypes 1 and 3 and at least 6 mutations there for serotype 2 (3).
The ability to cause the disease appears to be linked, at least to a large extent, to the intrinsic genetic instability of the Sabin strains, which had been deliberately selected to decrease viral fitness through the acquisition of fitness-suppressing, neurovirulence-attenuating mutations (4). After ingestion by a vaccinee, the virus undergoes evolution starting in its infected organism, and then, if conditions permit, during the subsequent transmission through chains of contacts. This evolution occurs in two steps (5). The first phase (a few weeks after the vaccination) consists of the rapid elimination of major attenuating mutations by reversions or compensating substitutions and consequent regaining of fitness and neurovirulence comparable to those of the wild polioviruses; intertypic recombination between vaccine serotypes could often take place after the use of trivalent OPV. The second phase involves a more or less linear accumulation of mutations (mostly synonymous), often accompanied by recombination with non-polioviruses of the enterovirus C species. This phase, eventually leading to the generation of VDPV, is not documented to be associated with a systematic parallel increase in fitness or virulence, although changes of this sort cannot be ruled out.
VAPP cases are known to be sporadic, each one considered to be an independent event (1, 2, 6), whereas VDPVs may cause not only sporadic illnesses in immunocompetent (7, 8) or immunocompromised (2, 9) persons but also outbreaks, sometimes quite large (2, 10). Because of the latter propensity, VDPVs are attracting special attention of public health authorities, requiring urgent large-scale responses to curb their transmission.
In spite of the generally accepted distinction between VAPP- and VDPV-caused cases of polio, the borderline between them is poorly demarcated. Here, we report a small cluster of cases of paralytic poliomyelitis, which was triggered by slightly diverged, Sabin-like, serotype 2 of OPV (i.e., VAPP cases) but was epidemiologically closely reminiscent of VDPV-caused outbreaks. Importantly, this cluster was characterized by an enormously high disease/infection ratio. This study provides new and unexpected information about the poliomyelitis epidemiology (in particular the epidemiology of VAPP), but on the other hand, it underscores significant gaps in our understanding of these topics as well as of the pathogenesis of the disease.
MATERIALS AND METHODS
Origin of samples.
Stool samples from the patients and their contacts were collected in the orphanage of the city of Biysk (Altai Region, Russia) in accordance with WHO guidelines (11).
Virological investigations.
Virus isolation from stools and serotyping were performed by standard methods (12). Intratypic differentiation (ITD) was carried out by enzyme-linked immunosorbent assay (ELISA) with cross-absorbed polyclonal antisera (kindly provided by H. G. van der Avoort, National Institute of Public Health and Environmental Protection [RIVM], The Netherlands) (12, 13) and real-time reverse transcription (RT)-PCR (14). For sequencing, poliovirus RNA was isolated from the infected cells with the RNeasy minikit (Qiagen) and was reverse transcribed using random hexamer primers (Syntol) and SuperScript II reverse transcriptase (Invitrogen). For VP3-to-VP1 region sequencing and full-genome sequencing, cDNAs of two and eight overlapping genomic fragments, respectively, were amplified by PCR. (The primer sequences are available upon request.) The products were purified with the QiaQuick DNA purification system (Qiagen). Sequencing was performed by using an ABI 3130 genetic analyzer.
Serological analysis.
Poliovirus-neutralizing antibodies in human sera were determined by the microneutralization assay (11). The analyses were performed by the virological laboratory of the Center for Hygiene and Epidemiology in the Altai Region, Barnaul, Russia.
Bioinformatics methods.
The genome sequences were assembled by using Lasergene software (version 7.0.0; DNAStar). Multiple alignments, the estimation of the degree of nucleotide divergence, and construction of phylogenetic trees were performed with the aid of the MEGA6 package (15). The similarity of the deduced nucleotide and amino acid sequences to the sequences available in the GenBank database was assayed by using BLAST (16).
Estimation of the age of viral isolates.
The estimation of the apparent duration of the evolution of viral isolates after their divergence from Sabin 2 was based on the following reported data: the rate of synonymous replacements per synonymous site within different regions of the viral genome of the consecutive Sabin 1-related viruses isolated from an immunodeficient patient (17), the rate of synonymous replacements per synonymous third-base codon positions in the genomes of Sabin 3-related viruses from another immunodeficient patient (18), and the rate of synonymous replacements per synonymous site within the P1 region of wild-type 1 polioviruses descended from a common ancestor (19). The values of the relevant parameters were either taken from references 18 and 19 or calculated with the aid of the MEGA6 program using the data reported in reference 17.
Nucleotide sequence accession numbers.
The nucleotide sequence accession numbers for the sequences submitted to GenBank are KU598883 to KU598891.
RESULTS
The outbreak.
In the summer of 2010, four children in an orphanage in Biysk, a city in the Altai Region of Russia, presented symptoms of acute flaccid paralysis (AFP). Vaccine-related polioviruses of serotype 2 were isolated from all of them. Paralysis persisted over 60 days in three patients. Upon the analysis of clinical and virological evidence, the National Commission on Diagnosis of Poliomyelitis and AFP had diagnosed all four of these patients as cases of vaccine-associated paralytic poliomyelitis (VAPP).
The orphanage hosted 115 children under 5 years, separated into isolated groups according to age. The first patient, PT1 (a 1-year-old boy) presented signs of paralysis on 22 July, followed by PT2 (a 16-month-old girl who first showed signs of the disease on 7 August), PT3 (an 11-month-old boy who first showed signs of the disease on August 7), and PT4 (a 10-month-old boy who first showed signs of the disease on 14 August). Before the outbreak, no vaccination with OPV had been carried out in the orphanage since 2007.
All four polio victims had prior histories of numerous diseases. Before presenting the signs of AFP, PT1 was admitted to the orphanage's isolation ward 4 times with respiratory tract infections and bilateral otitis. PT2 had a previous history of 3 admissions to this ward with respiratory infections and varicella. In addition, after the first of these admissions, she was treated for dysmetabolic nephropathy in the Biysk Children's Hospital for 6 weeks. Two other children were also admitted to the orphanage's isolation ward with respiratory infections.
Serological analyses performed twice (Table 1) confirmed that the patients had not been vaccinated against poliovirus before the outbreak. Indeed, all lacked antibodies against serotype 1, and three of them lacked antibodies against serotype 3. The presence of antibodies against serotype 2 was consistent with the infection with the relevant virus. An increase in the antibody titer against this serotype during the disease was demonstrated in two cases. It should be noted that PT2 had received a dose of trivalent IPV on 31 July, which, however, did not prevent the signs of AFP a week later and did not led to the appearance of antibodies to poliovirus serotypes 1 and 3.
TABLE 1.
Antipolio antibodies in the sera of the AFP cases
| Patient | Date (2010) | Antibody titer against poliovirus serotype: |
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| PT1 | 24 July | <1:8 | 1:128 | 1:16 |
| 3 August | <1:8 | 1:512 | 1:16 | |
| PT2 | 7 August | <1:8 | 1:64 | <1:8 |
| 23 August | <1:8 | 1:64 | <1:8 | |
| PT3 | 7 August | <1:8 | 1:256 | <1:8 |
| 23 August | <1:8 | 1:256 | <1:8 | |
| PT4 | 15 August | <1:8 | 1:32 | <1:8 |
| 26 August | <1:8 | 1:128 | <1:8 | |
Response to the outbreak.
In response to the outbreak, two rounds of IPV vaccination were carried out in the orphanage, on 26 July to 4 August and 6 to 10 September, involving 112 and 109 children, respectively. Fecal samples were collected from 91 people: 50 healthy children (1 of whom, PT4, later developed polio) and 41 orphanage staff on 9 to 13 August, and 31 of them gave positive PCR results for the presence of enterovirus RNA. Type 2 polioviruses were isolated from 20 (22%) of these samples.
After the vaccination (on 21 to 23 August and 4 to 6 September), the sera from 106 children were investigated for the presence of neutralizing antibodies. The overwhelming majority (89 children [84%]) possessed antibodies to all 3 poliovirus serotypes (it should be noted that according the official information, the majority of the orphanage children had been vaccinated with IPV also before the outbreak), whereas the rest lacked antibodies to one (1 child negative for serotype 2), two (13 children negative for serotypes 1 and 3), or three (3 children) poliovirus serotypes.
Characterization of the viral genomes.
Full sequencing of the genomes of polioviruses isolated early (0 to 6 days) after the onset of the symptoms was performed; a virus isolated from one patient (PT4) 2 days prior to the presentation of the first signs of the disease (genome PT4-0) was also analyzed in this way (Table 2). In addition to their unique mutations, all of these genomes shared 15 nucleotide (nt) differences from the Sabin 2 poliovirus: one substitution in the 5′-untranslated region (5′ UTR), 5 mutations resulting in amino acid changes, and 9 synonymous replacements in the protein-coding region. Noteworthy, all of the viruses possessed two known deattenuating mutations, A481→G in the 5′ UTR and U2909→C in the VP1-coding region (41). The former is expected to increase the efficiency of translation (42, 43), whereas the latter resulted in the VP1 amino acid replacement I143→T, the deattenuating effect of which could hypothetically consist of the stabilization of viral particle through changing the hydrophobic amino acid residue to hydrophilic in the region of the capsid 5-fold axes (44). Both mutations were very common among viruses excreted by the OPV vaccinees and their contacts (Table 2). One additional shared mutation, G1462→A, resulted in the A170→T amino acid substitution in another capsid protein, VP2, specifically within antigenic site 2 (45). The relevance of other common mutations is uncertain.
TABLE 2.
Mutations in the fully sequenced genomes of the Biysk isolates
| Genome region | Nucleotide (amino acid) mutation | Genome(s) of Biysk isolates harboring mutationa | Previous report(s) of same mutation in Sabin 2-derived virusesb |
|---|---|---|---|
| 5′ UTR | A145→U | PT1-1, PT1-2 | |
| U156→C | PT3, PT4 | 20, 21, 22, 23, 24 | |
| A248→G | C-1 | 10, 25, 26 | |
| U344→C | PT1-1, PT1-2 | 21, 22 | |
| U398→C | PT2, PT3, PT4-0, PT4 | 8, 10, 20, 21, 22, 23, 24, 26, 27, 28, 29 | |
| A481→G | All | 8, 10, 20, 21, 22, 23, 24, 25, 26, 27, 28 | |
| U690→C | C-2 | 20 | |
| A718→G | C-1 | ||
| U722→C | C-1 | 8, 27, 30 | |
| U744→C | C-2 | 21, 23, 30 | |
| VP4 | A888→G | C-1 | |
| C909→U | C-1 | 10, 27 | |
| VP2 | U984→C | PT1-2 | 26 |
| A1042→G (N30→D) | C-2 | ||
| C1059→U | Main group | 31 | |
| C1080→U | PT3-1 | ||
| U1125→C | C-1 | 22 | |
| C1161→U | PT3-1 | ||
| C1281→U | PT3, PT4-0, PT4 | 10 | |
| U1317→C | C-2 | ||
| U1392→C | C-2 | ||
| A1407→G | Main group | 10 | |
| A1429→G (S159→G) | C-2 | 32 | |
| G1462→A (A170→T) | Main group | 33 | |
| A1468→G (N172→D) | PT3-1 | ||
| A1512→G | C-1 | 8 | |
| C1623→U | PT3-1 | ||
| A1758→G | PT1-1 | ||
| VP3 | G1855→A (V30→I) | Main group | |
| C1935→U | PT1-2 | 10 | |
| A1997→G (H77→R) | C-1 | 10 | |
| C1998→A (H77→Q) | PT3-1 | ||
| A2005→G (T80→A) | PT3-1 | 10, 22, 29 | |
| C2020→U (L85→F) | Main group and C-2 | ||
| G2188→A (A141→T) | C-1 | ||
| C2193→U | PT1-2 | 8 | |
| A2223→U | PT3, PT4-0, PT4 | 10 | |
| G2250→A | PT3, PT4-0, PT4 | 10, 28 | |
| C2310→U | PT1-2 | 8 | |
| VP1 | A2492→C (D4→A) | Main group | |
| A2494→U (M5→L) | PT1-2 | 34 | |
| Deletion of nt 2497–2499 (I6) | C-1 | ||
| C2550→U | PT1-2 | 29, 35 | |
| A2628→G | C-2 | 10 | |
| U2909→C (I143→T) | All | 10, 24, 25, 27, 28, 29, 33, 34, 36 | |
| C3279→U | Main group | 36 | |
| G3343→A (D288→N) | PT1-1, PT1-2 | 10 | |
| 2A | A3459→G | Main group | 7 |
| A3491→G (N36→S) | PT1-2 | ||
| A3559→G (T59→A) | PT3, PT4-0, PT4 | ||
| U3597→C | PT1-1, PT1-2 | 21 | |
| C3639→G | C-1 | ||
| A3726→G | PT1-2 | ||
| 2B | C3966→U | PT3-1 | 10, 37 |
| U4044→A | PT1-2 | 38 | |
| 2C | U4216→C | C-2 | |
| A4335→G | C-2 | 39 | |
| C4653→U | Main groupc | ||
| G4909→A (A263→T) | PT1-1 | ||
| G5063→A (R314→K) | PT1-2 | ||
| 3A | G5121→U (Q4→H) | C-2 | |
| G5168→A (C20→Y) | C-1 | ||
| A5223→G | PT1-2 | 21, 24 | |
| U5238→C | PT1-2 | ||
| C5365→U (H86→Y) | PT2, PT3-1, C-1 | ||
| 3B | C5418→U | C-2 | |
| 3C | A5457→G | C-2 | 7 |
| U5532→C | All | ||
| C5559→U | PT3, PT4-0, PT4 | ||
| A5592→G | PT3-1 | ||
| A5625→G | C-1 | ||
| U5640→C | PT1-1 | ||
| A5823→G | PT1-1, PT1-2 | ||
| A5852→G (N139→S) | PT3-1 | ||
| G5949→A | PT3, PT4-0, PT4, PT1-2 | 25, 40 | |
| C5970→U | PT3, PT4-0, PT4 | ||
| A5985→G | PT1-2 | ||
| 3D | U6075→C | PT2, PT3-1, C-1 | 24, 28, 39 |
| G6084→A | PT3-1 | ||
| G6142→A (D53→N) | PT1-2 | ||
| A6156→G | C-2 | ||
| C6438→U | All | ||
| U6618→C | PT3, PT4-0, PT4 | ||
| C6789→U | All | ||
| C7071→U | Main group | ||
| U7119→C | PT1-1, PT1-2, C-2 | AJ544513d | |
| C7326→U | PT1-2 | ||
| 3′ UTR | G7405→A | PT3, PT4-0, PT4 | 10, 20, 22, 26, 28, 29, 37, 38, 39 |
The “main group” represents all fully sequenced genomes, except PT1-1, PT1-2, and C-2.
Reference numbers in boldface reported on the relevant mutation in more than one isolate.
C/U heterogeneity was detected at this position in the PT1 genome.
GenBank accession number.
Importantly, all these early virus isolates (genomes PT1, PT2, PT3, PT4-0, and PT4) harbored only 3 mutations in the VP1-coding sequence and thus should be classified, according to the currently accepted definition (3), as Sabin-like rather than VDPV.
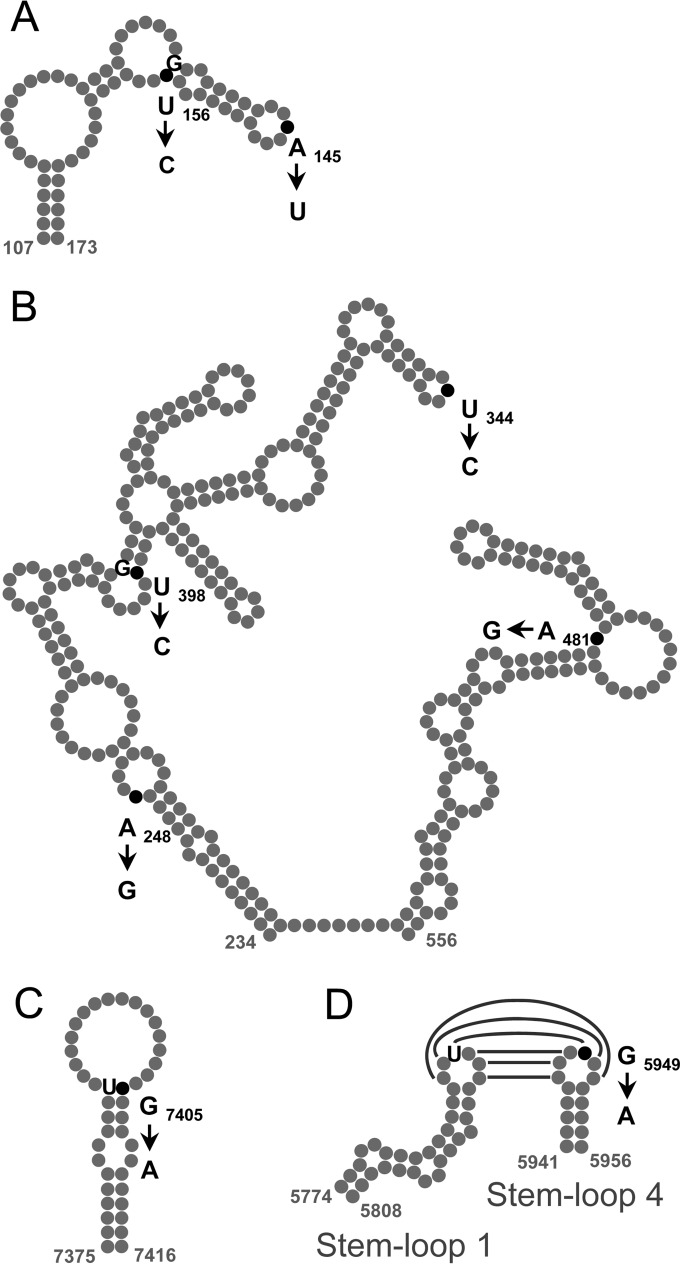
The distribution of mutations (in addition to the 15 commonly shared ones) in these early isolates may be summarized as follows. The PT1 genome had no additional mutations. The PT2 genome exhibited a close similarity to PT1, having only 3 additional nucleotide substitutions, implying their close relationship. The isolates from the two other patients were less related to the PT1 virus, differing from it at 11 positions. The phenotypic effects, if any, of these mutations are uncertain, but it may be noted that the replacements U156→C, U398→C, G5949→A, and G7405→A are expected to stabilize the secondary structure of domains II and IV of the 5′ UTR (46), the RNase L competitive inhibitor RNA (ciRNA) cis-acting element (47), and the 3′ UTR (48), respectively (Fig. 1).
FIG 1.
Nucleotide substitutions in the phylogenetically conserved structural elements of the genomes of the poliovirus isolates in comparison with the Sabin 2 strain. The positions of changed nucleotides are represented by black dots, and the corresponding mutations are indicated; the nucleotides involved in the resulting base pairing (if formed) are also shown. (A) Domain II of the 5′ UTR. (B) Domains IV and V of the 5′ UTR. (C) Domain Y of the 3′ UTR. The structures are given as proposed previously (46, 48). (D) Possible tertiary (“kissing”) interaction between stem-loops 1 and 4 of the RNase L competitive inhibitor element of viral RNA (ciRNA) (47).
The genomes of viruses excreted by PT1 (two isolates) and PT3 (one isolate) during the subsequent course of infection were also sequenced. The virus excreted by PT1 a month after the onset of the symptoms (genome PT1-1) exhibited a strikingly great divergence from the first one: it lacked 10 PT1-specific mutations (including 2 in the VP1-coding region) but possessed 9 new nucleotide substitutions (including one in the VP1-coding region) (Table 2). A similarly marked difference distinguished the next isolate from the same patient (PT1-2) obtained still another month later than PT1-1: 3 mutations were lost, and 15 were acquired (Table 2). Such a significant difference suggests that the viruses with the PT1, PT1-1, and PT1-2 genomes did not comprise a lineage of successive descendants but rather were sister strains, sharing a common predecessor (see below). It may be added that a mutation shared by the PT1-1 and PT1-2 strains, G3343→A, resulted in an amino acid replacement at capsid protein VP3 position 288, known to be located within antigenic site 3 (45).
Similarly, the genome of the virus excreted by PT3 a month after the onset of the disease (PT3-1) displayed significant differences from the genome of the original isolate. The PT3 and PT3-1 genomes differed from one another at as many as 23 positions. The sets of mutations distinguishing these two RNAs from PT1 were unique without any overlapping (Table 2). A mutation in VP2 (N172→D) and two mutations in VP3 (H77→Q and T80→A) targeting antigenic sites 2 and 3b, respectively (45), were among the differences between the consecutive isolates from this patient.
In addition, full genomes of the viruses isolated on 12 to 13 August from two healthy orphanage children were sequenced. The C-1 and C-2 genomes shared with PT1 15 and 6 nucleotide substitutions, respectively, and harbored numerous nonoverlapping mutations in addition (Table 2). Among the C-1 mutations, there was a 3-nt deletion at positions 2497 to 2499 in the VP1-coding region, resulting in the loss of an amino acid (I6).
Finally, a portion of the poliovirus genome encompassing the VP3- and VP1-coding regions and a 5′-adjacent part of the 2A gene (nt 3385 to 3460) of an additional 17 viruses isolated between 8 and 13 of August from healthy contacts were sequenced (Table 3). This was done to ascertain to what extent isolates from contacts were related to the isolates from the polio victims. All of the partially sequenced contact isolates contained the whole set of mutations found in this region of PT1, suggesting that they were derived from the latter or had with it a common predecessor. Six of the isolates possessed no other substitutions, whereas the rest had 1 to 4 additional mutations. Nearly all of these mutations were unique, but one genome possessed a substitution present also in C-1, whereas another RNA shared two mutations with PT3, PT4-0, and PT4 (Table 3). Of note, additional single mutations in the VP1-coding region were detected in 4 genomes, and two mutations were present there in one RNA. Even the latter exceptional genome (C-13), possessing a maximally diverged VP1-coding region (5 substitutions), should still be classified as Sabin-like.
TABLE 3.
Mutations in the partially sequenced derivatives of Sabin 2 polioviruses isolated from healthy contacts during the Biysk outbreaka
| Genome region | Additional nucleotide (amino acid) mutation compared with PT1 sequence | Strain genome(s) | Genome(s) of fully- sequenced strain(s) with same mutation |
|---|---|---|---|
| VP3 | G1827→A | C-4 | |
| A1997→G (H77→R) | C-10, C-13, C-14 | C-1 | |
| A2157→G | C-7 | ||
| A2223→U | C-19 | ||
| G2250→A | C-19 | PT3, PT4-0, PT4 | |
| U2364→C | C-13 | PT3, PT4-0, PT4 | |
| VP1 | G2608→A (A43→T) | C-16 | |
| A2707→G (T76→A) | C-9 | ||
| A2901→U | C-18 | ||
| U3178→A (S233→T) | C-13 | ||
| U3204→C | C-7 | ||
| G3237→C | C-13 | ||
| 5′ part of 2Ab | C3445→U | C-6, C-11 |
All of these isolates harbored the whole set of mutations present in this region of the PT1 RNA.
Nucleotides 3385 to 3460.
Possible relationships among the isolates.
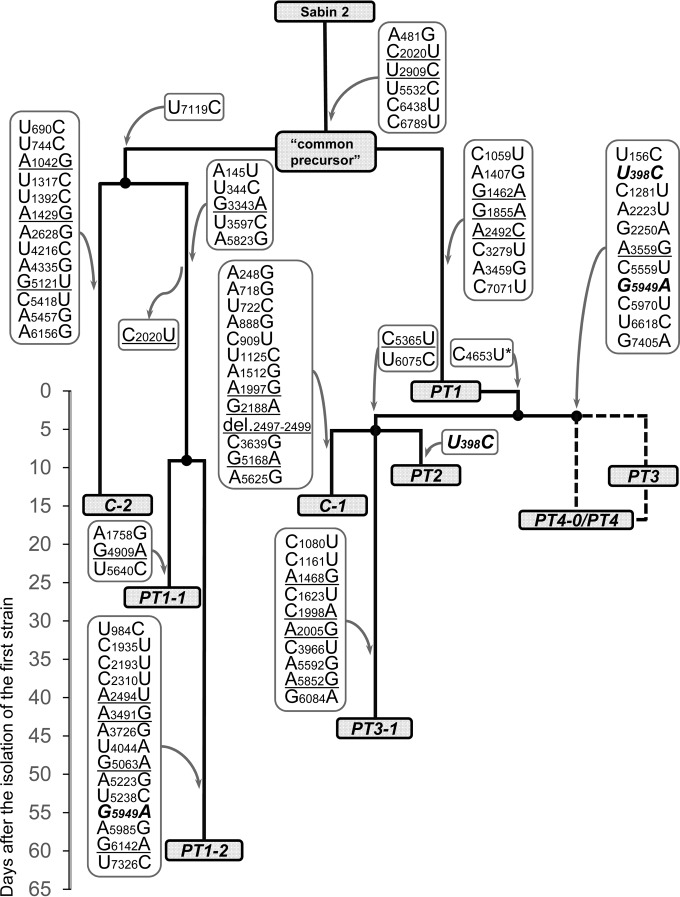
The set of the genomes isolated in the orphanage exhibited a quite unusual combination of features. All of them were Sabin-like, implying that they were close descendants of Sabin 2. Judging by the possession of a large number of shared mutations, all of them had a common precursor (genome CP). On the other hand, this set was highly heterogeneous, containing a collection of exemplars differing from one another by numerous mutations. A model of their interrelationships compatible with the pattern of harbored mutation is displayed in Fig. 2. The most significant features of this model are as follows.
FIG 2.
A hypothetical evolutionary pathway of the polioviruses isolated in Biysk in 2010. The names of the isolates' genomes are given in gray rectangles. The nucleotide changes of their genomes are shown in frames, with the arrows directed from and to these frames indicating acquisition and loss of the relevant mutations, respectively. (The exact timing of these changes is unknown.) The mutations resulting in amino acid substitutions are underlined, and those that apparently appeared independently in different strains are highlighted in boldface and italics. The substitution C4653U (marked with *) was present in a portion of PT1 molecules. Dashed lines connecting isolated PT3 virus with PT4-0/PT4 virus reflect the uncertainty of their real interrelationship. The timeline on the left of the figure shows the number of days after the isolation of the first strain (genome PT1).
We postulate that the common precursor (genome CP) of all investigated viruses, containing (among other common mutations) both major deattenuating replacements, gave rise to at least two descending lineages. One of them resulted in the generation, through an unknown number of intermediates, of the PT1 genome, which is considered to be a common precursor of the initial isolates from the three other patients. However, the path to these isolates was not straightforward. A short branch led to the PT2 genome, suggesting that the latter received the virus from PT1 either directly or via a small number of intermediate carriers. This notion was in line with the fact that both of these children, although attending different age groups, spent some time (20 to 31 May and 17 to 21 June) together in the isolation ward before the onset of the PT1 disease. On the other hand, the branches leading to the PT3 as well as PT4-0/PT4 genomes appeared to be more extended. The existing data do not permit to define their exact topology, which appeared to be compatible with two different scenarios, both involving the existence of unknown number of intermediates: the PT4-0/PT4 viruses could be descendants of the PT3 virus (or vice versa), or the isolates from the two patients were independent descendants of a common intermediate (Fig. 2).
As noted above, the virus isolated from PT1 a month after the onset of the disease, genome PT1-1, appeared to be not a direct descendant of the initial isolate, genome PT1, but rather was a representative of a distinct lineage originated from the common precursor (genome CP). The isolate obtained from the same patient after an additional month, genome PT1-2, likely belonged to the same branch as the PT1-1 virus but did not appear to be its direct descendant either. Similarly, the genome (PT3-1) of a virus isolated from patient PT3 a month after the first isolate (PT3) did not appear to descend from the latter but rather was a representative of another PT1-derived branch, which also hosted PT2 and a genome (C-1) of a healthy contact.
The above model was also supported by the phylogenetic relationships between strains calculated by the neighbor-joining (NJ) method implemented in the MEGA6 package, although it should be admitted that some branches of the tree are not supported enough statistically (not shown).
The rate of accumulation of mutations.
To further characterize the evolutionary time course of the isolates, we attempted to evaluate their apparent “ages” by comparing the rates of mutation accumulation in their genomes with those reported in the literature (17–19). It goes without saying that molecular clocks do not work rhythmically enough during relatively short time intervals (especially, if negative/positive selection is involved), and the results of such calculations should therefore be regarded rather cautiously. Nevertheless, the data presented in Table 4 confirmed some of the conclusions described in the preceding section and also suggested the existence of some additional events.
TABLE 4.
Calculated ages of fully sequenced strains and their hypothetical common precursor
| Strain genome | Age (mo) of genomic regiona |
||||||
|---|---|---|---|---|---|---|---|
| P1 |
P2/P3 |
Entire genome |
|||||
| a | b | c | a | b | a | b | |
| CPb | 0.0 | 0.0 | 0.0 | 1.1 | 1.0 | 0.6 | 0.4 |
| PT1 | 1.4 | 1.0 | 2.0 | 1.7 | 2.0 | 1.7 | 1.7 |
| PT1-1 | 0.6 | 0.4 | 0.8 | 2.3 | 2.5 | 1.7 | 1.7 |
| PT1-2 | 2.5 | 2.1 | 3.6 | 4.6 | 5.1 | 3.7 | 3.5 |
| PT2 | 1.4 | 1.0 | 2.0 | 2.3 | 2.5 | 2.0 | 2.2 |
| PT3/PT4 | 2.8 | 2.5 | 4.0 | 3.4 | 4.0 | 3.1 | 3.0 |
| PT3-1 | 2.8 | 2.8 | 4.0 | 3.4 | 4.0 | 3.1 | 3.5 |
| C-1 | 3.3 | 2.8 | 4.8 | 3.1 | 3.5 | 3.1 | 3.0 |
| C-2 | 1.4 | 1.0 | 2.0 | 3.1 | 3.5 | 2.2 | 2.2 |
The results indicated that the postulated common predecessor of all of the isolates was indeed a very close descendant of Sabin 2, hardly being even 1 month old. It cannot be excluded that it was a recombinant, since the capsid-coding region of its RNA was calculated to be “younger” than the rest of the genome. (It should be taken into account that the two deattenuating mutations existing in the genome were ignored during the calculations.) In any case, this asymmetry could to some extent contribute to a similar noncongruency of the ages of different genome parts in the most of the CP descendants.
As expected, some discrepancies between the calculated ages and the real timing of virus isolation were also evident. Thus, the apparent age of the PT3-1 virus was equal to that of the PT3 virus despite the 1-month interval between the dates of their isolation, whereas the capsid-coding region of genome PT1-1 seemed to be even “younger” than this region of the PT1 virus isolated a month earlier. Although such discrepancies could well be due to the above-mentioned inaccuracy of the molecular clocks, they could possibly also be explained by the involvement of recombination, which was especially likely in the origin of genome PT1-1.
DISCUSSION
Genetic structure of the isolates.
The genomes of the viruses isolated from the patients early after the onset of the disease displayed a typical pattern characteristic of slightly evolved (Sabin-like) vaccine viruses excreted by the vaccinees or their more or less close contacts. A distinctive feature of this pattern is the loss of attenuating mutations, primarily in the 5′ UTR (nucleotide change A481→G) and the capsid protein VP1 (amino acid replacement I143→T) (41, 49). In addition, these viruses acquired an amino acid mutation, A170→T, in VP2 known to be located within an antigenic site. Some of these genomes also contained additional individual mutations in antigenic sites (Table 5) as well as substitutions stabilizing the structure of the 5′ UTR (46) and 3′ UTR (48) as well as a cis-acting element in the coding region reported to exhibit anti-RNase L activity (47) (Fig. 1). Although some fitness-enhancing effects of at least some of these mutations cannot be ruled out, we are aware of no reports that any of them (except the two mentioned in the beginning of this paragraph) could markedly increase pathogenicity or transmissibility of the virus. It would be quite interesting if further studies show that certain of these mutations are nevertheless linked to high pathogenicity.
TABLE 5.
Possible biological relevance of some mutations detected in the Biysk isolates
| Genome region | Nucleotide (amino acid) mutation | Known or assumed biological relevance (figure or reference) |
|---|---|---|
| 5′ UTR | U156→C | Stabilization of domain II structure (Fig. 1A) |
| U398→C | Stabilization of domain IV structure (Fig. 1B) | |
| A481→G | Deattenuation (41) | |
| VP2 | A1429→G (S159→G) | Region of antigenic site 2 (32) |
| G1462→A (A170→T) | Antigenic site 2 (45) | |
| A1468→G (N172→D) | Antigenic site 2 (45) | |
| VP3 | A1997→G (H77→R) | Antigenic site 3b (45) |
| C1998→A (H77→Q) | Antigenic site 3b (45) | |
| A2005→G (T80→A) | Antigenic site 3b (45) | |
| VP1 | U2909→C (I143→T) | Deattenuation (41) |
| G3343→A (D288→N) | Antigenic site 3 (45) | |
| 3C | G5949→A | Stabilization of ciRNA structure (Fig. 1D) |
| 3′ UTR | G7405→A | Stabilization of oriR structure (Fig. 1C) |
It should perhaps be specified that the investigated viruses were all derived from stools and might not represent what was in the central nervous system (CNS). However, the simultaneous independent appearance of an unusually pathogenic mutation in the CNS of 4 children in a close community seems rather unlikely.
Heterogeneity of the viral isolates and peculiarities of the transmission path.
Genome sequencing of the viruses isolated from the patients and their contacts allowed us to suggest a likely chain of virus transmission (Fig. 2). This chain reveals a peculiar pattern that includes some quite uncommon aspects. Given the recent common origin, the very small population of hosts (∼100 persons), their living/working in a single orphanage, and the short time interval of isolation, the population of polioviruses circulating there exhibited a striking genetic heterogeneity, as revealed by the full genome sequencing (Table 2). Indeed, there were at least two independently evolving branched lineages, with the most diverged isolates differing from one another in as many as 44 nt positions. Partial sequencing of the capsid-coding region (Table 3) provided additional evidence for unusual heterogeneity of the local viral population.
Such a pattern was likely due to the involvement of numerous bottlenecking situations during the virus transmission within a close community lacking sufficient intestinal immunity to the virus, resulting in haphazard picking up of small minority populations from inherently heterogeneous (quasispecies) viral communities. Interestingly, there were several discrepancies between the timing of isolation of the viruses and calculated “ages” (the time intervals after their divergence from the parental Sabin 2) of their genomes or parts thereof (Table 4). Partly, such incongruences could be due to the inaccuracy of the molecular clocks within short time periods. However, multiple recombination events between viruses belonging to different lineages/branches might also be involved. Such a possibility is supported by the acquisition of identical mutations (having no known adaptive properties) at different stages of evolution of viruses belonging to separate lineages (Table 2). Which (if any) of these recombination events resulted in an increase of viral fitness remains unknown. However, their contribution to the diversification of the viral populations, generating a rich soil for further evolution, seems quite significant.
Another unusual feature of the investigated outbreak was the replacement of an intestinal population by another independent population, both of which are OPV derived. Indeed, the PT1-1 genome belonged to a distinct lineage compared to the PT1 genome, while PT1-2 did not appear to be a direct descendant of PT1-1, as evidenced by its lacking 3 and acquiring 15 nt substitutions (Fig. 2). These facts demonstrated again cocirculation of different viral populations in a closed community and suggested that these populations exhibited different levels of fitness. However, the only mutation distinguishing PT1-1 from PT1 virus having a known phenotypic significance was amino acid change D288→N in antigenic site 3b of VP1. This mutation, however, could hardly be solely responsible for the apparent PT1-1 advantage. Among mutations distinguishing PT1-2 from PT1-1, there was also a single one with a known effect, namely, that affecting the secondary structure of the cis-acting element capable of suppressing the activity of the host defensive enzyme RNase L. To what extent this mutation could confer a significant fitness gain is uncertain. A similar pattern was displayed by another pair of isolates, namely, the viruses excreted by PT3 with a monthly interval. The genomes PT3 and PT3-1 clearly belonged to different branches of a lineage, and among numerous genetic distinctions, there were some with known or likely phenotypic effects. Thus, PT3-1 virus had one and two amino acid replacements in antigenic sites 2 and 3b, respectively (Table 5), which potentially might facilitate its reproduction in the PT3 intestine with already partially raised mucosal immunity. On the other hand, PT3-1 virus was devoid of the mutations present in PT3 virus that could stabilize the secondary structures of the RNase L-suppressing cis-acting element and a domain in the 3′ UTR.
Thus, it remains enigmatic why the colonization of the vaccinees' gut by other OPV-derivative of the same serotype could occur and why the newcomers could outcompete the direct descendants of the originally received viruses. It is also unknown whether such changes resulted from reinfections or from a kind of “struggle for the survival of the fittest” among the members of inherently heterogeneous original populations.
VAPP- versus VDPV-triggered paralytic disease.
As indicated in the introduction, the two conditions described above, while both being caused by OPV-derived viruses, are considered to differ from one another in at least two respects: the level of divergence of their causative agents from the Sabin strains as well as their epidemiological characteristics. The cluster of diseases described here was caused by closely related variants of the OPV-2 virus (three mutations in the genome region encoding the capsid VP1 protein). Hence, this cluster should be regarded as a bona fide VAPP outbreak. This seems to be quite an unusual situation since, as far as we are aware, only sporadic, independent cases of VAPP have been described previously (1, 6). The ability of Sabin-like viruses to trigger outbreaks of poliomyelitis demonstrated here blurred the borderline between the VAPP- and VDPV-caused paralytic disease. In fact, the former may be considered a subset of the latter, the more so as in order to curb outbreaks of VAPP, epidemiological measures identical to those aimed to stop VDPV outbreaks can be required.
It seems obvious that one of the conditions required for the emergence of the outbreak caused by OPV derivatives is the existence of a large enough cohort of people nonimmune against poliovirus. VAPP cases are usually classified into 3 categories: those in the recipients of OPV and those in the known contacts of the vaccinees, as well as so-called “community-acquired” cases, when the primary virus-shedding person is not known (6). The cluster described here belonged to the latter category. It would be important to know how large the relevant community of people susceptible to polio was and how broadly the OPV-derived virus was spread. We have only partial answers to these questions.
The lower limit of the size of the community susceptible to polio is less than 100 of the orphanage inhabitants (since a significant part of this community had been immunized with IPV before the outbreak). No information is available on whether the susceptible community was really significantly larger. We do not know the primary vaccinee, who excreted the progenitor of the viruses circulating in the orphanage. Nor is the route of transmission between this initial excretor and the victims of the disease known, although the involvement of either orphanage personnel or contacts in the hospital seems quite plausible.
Although the availability of a susceptible cohort is a necessary condition for the emergence of an outbreak, it is not a sufficient one, as is discussed below.
Disease/infection ratio.
It is known that less than 1% of immunocompetent but nonimmune persons infected with wild polioviruses develop the paralytic disease, whereas this value may be as low as 0.05% for serotype 2 (50). The incidence of VAPP is several orders of magnitude more rare, and being different in different countries may be as low as less than 1 case per million doses of OPV (1). In the example described here, this ratio was unprecedentedly high. Notably, as mentioned above, the majority of the orphanage children were reported to have been previously vaccinated with IPV. The fact that judging by the presence of the virus or its RNA, at least 23 of them (out of 53 investigated) were infected with the Sabin-like virus was in line with the known inability of IPV to curb poliovirus circulation, while efficiently preventing the disease (51). However, four unvaccinated children of this mostly vaccinated small community developed VAPP, demonstrating a uniquely high disease/infection ratio, markedly exceeding that for the wild poliovirus. Admittedly, given that some unknown number of people outside the orphanage also were likely to be nonsymptomatically infected, this ratio could be somewhat overestimated. However, it seems to remain exceptionally high.
Although our understanding of the nature of the viral genomic features responsible for the neurovirulence is obviously incomplete, there is no clear evidence or even substantiated speculation that the viruses circulating in the orphanage were peculiar in this respect. The virus did find its way to the orphanage from somewhere outside—e.g., from personnel of the hospital to which some children had been admitted before the outbreak. Nevertheless, no polio cases outside the orphanage had been reported, suggesting that the situation in the orphanage was in some unknown aspects quite special.
Several potentially contributing factors may be considered. Undoubtedly, an important circumstance was the fact that the polio victims (and possibly some of other orphanage children) had not been vaccinated before the outbreak. However, when any antipolio vaccination was cancelled for 3 years (1963 to 1966) in a district of Belorussia with a population of around 160,000, this resulted in a widespread circulation of vaccine-related viruses of three serotypes. Nevertheless, no paralytic diseases have been reported (27). Similarly, introduction of a cVDPV-1 in an unvaccinated community in the United States resulted in a single case of polio (in an immunocompromised child) (9).
Other possible risk-enhancing factors may also be mentioned, such as the close interpersonal contacts and poor health of many children. These factors are, however, surely not exceptional for this orphanage. An analysis of incidence of VAPP in Romania suggested that intramuscular injections soon after the receipt of the vaccine or contact with vaccinees might be a risk factor increasing the likelihood of the disease (52). No information is available on whether this procedure was implemented in the Biysk orphanage during the VAPP outbreak. Although a contribution of this factor cannot be completely ruled out, its decisive role is hardly likely because the reported increase in the probability of triggering the disease in Romania was not so great and also even such an increase is not a common phenomenon, not being documented in the United States (53). Also, the possibility that the reaction of the patients to the infection with a Sabin-like virus was exaggerated by coinfection with another virus/microbe cannot be excluded. In this regard, it may be noted that coinfection with an OPV-derived poliovirus increased the severity of the enterovirus 71-triggered disease (54). However, it should be admitted that we are unable to provide any evidence-based explanation for the unusual disease/infection ratio during the Biysk outbreak.
Concluding remarks.
This article describes a small outbreak of paralytic poliomyelitis caused by slightly diverged derivatives of OPV (Sabin-like rather than VDPV, according to the current nomenclature). It strengthens the view that there is no clear borderline between the VAPP and VDPV-triggered diseases. This is not a merely semantic issue, since the lack of substantial differences means that both situations may demand similar epidemiological responses. The unusually high, if not unique, disease/infection ratio observed in this orphanage remains unexplained and underscores the existence of important gaps in our understanding of pathogenesis and epidemiology of poliomyelitis. The outbreak represents additional evidence that orphanages and similar institutions may represent zones of enhanced risk (see also references 7 and 33) requiring special attention from the health authorities. Finally, the fact that the VAPP outbreak was caused by a derivative of Sabin 2 virus has direct relevance to the planned removal of this virus from the OPV and replacement of the trivalent OPV by a bivalent (types 1 and 3) one (55, 56). On the one hand, the Biysk outbreak may be considered an additional argument in favor of such plans. On the other hand, this outbreak demonstrates a significant hazard associated with the existence of even relatively small cohorts nonimmune against this poliovirus serotype. In our view, the optimal solution of this uncertainty would be not simply stopping the use of Sabin 2 virus but rather replacing it with an alternative, a safer live vaccine virus of the same serotype or non-live vaccine able to mount intestinal immunity, rendering its recipients unsusceptible to the infection and unable to be cryptic carriers in transmission chains.
ACKNOWLEDGMENTS
We thank M. A. Vaitovich and A. P. Toptygina for the help in the epidemiological investigation and analysis of immunological data, respectively, and M. S. Trofimchik for technical assistance.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Platt LR, Estívariz CF, Sutter RW. 2014. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 210:S380–S389. doi: 10.1093/infdis/jiu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns CC, Diop OM, Sutter RW, Kew OM. 2014. Vaccine-derived polioviruses. J Infect Dis 210:S283–S293. doi: 10.1093/infdis/jiu295. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2015. Reporting and classification of vaccine-derived polioviruses. GPEI guidelines. World Health Organization, Geneva, Switzerland: http://www.polioeradication.org/Portals/0/Document/Resources/VDPV_ReportingClassification.pdf. [Google Scholar]
- 4.Sabin AB, Boulger LR. 1973. History of Sabin attenuated oral live vaccines. J Biol Stand 1:115–118. doi: 10.1016/0092-1157(73)90048-6. [DOI] [Google Scholar]
- 5.Agol VI. 2006. Vaccine-derived polioviruses. Biologicals 34:103–108. doi: 10.1016/j.biologicals.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. 2005. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol 59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 7.Cherkasova EA, Korotkova EA, Yakovenko ML, Ivanova OE, Eremeeva TP, Chumakov KM, Agol VI. 2002. Long-term circulation of vaccine-derived poliovirus that causes paralytic disease. J Virol 76:6791–6799. doi: 10.1128/JVI.76.13.6791-6799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakovenko ML, Korotkova EA, Ivanova OE, Eremeeva TP, Samoilovich E, Uhova I, Gavrilin GV, Agol VI. 2009. Evolution of the Sabin vaccine into pathogenic derivatives without appreciable changes in antigenic properties: need for improvement of current poliovirus surveillance. J Virol 83:3402–3406. doi: 10.1128/JVI.02122-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander JP, Ehresmann K, Seward J, Wax G, Harriman K, Fuller S, Cebelinski EA, Chen Q, Jorba J, Kew OM, Pallansch MA, Oberste MS, Schleiss M, Davis JP, Warshawsky B, Squires S, Hull HF, Vaccine-Derived Poliovirus Investigations Group . 2009. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis 199:391–397. doi: 10.1086/596052. [DOI] [PubMed] [Google Scholar]
- 10.Burns CC, Shaw J, Jorba J, Bukbuk D, Adu F, Gumede N, Ali Pate M, Abanida EA, Gasasira A, Iber J, Chen Q, Vincent A, Chenoweth P, Henderson E, Wannemuehler K, Naeem A, Umami RN, Nishimura Y, Shimizu H, Baba M, Adeniji A, Williams AJ, Kilpatrick DR, Oberste MS, Wassilak SG, Tomori O, Pallansch MA, Kew O. 2013. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J Virol 87:4907–4922. doi: 10.1128/JVI.02954-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 1997. Manual for the virological investigation of poliomyelitis. WHO/EPI/GEN/97.1. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.World Health Organization. 2004. Manual for the virological investigation of poliomyelitis. WHO/IVB/04.10. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 13.van der Avoort HG, Hull BP, Hovi T, Pallansch MA, Kew OM, Crainic R, Wood DJ, Mulders MN, van Loon AM. 1995. A comparative study of five methods of intratypic differentiation of polioviruses. J Clin Microbiol 33:2562–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpatrick DR, Yang CF, Ching K, Vincent A, Iber J, Campagnoli R, Mandelbaum M, De L, Yang SJ, Nix A, Kew OM. 2009. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J Clin Microbiol 47:1939–1941. doi: 10.1128/JCM.00702-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschul S, Gish W, Miller W, Myers E, Lipman D. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Bellmunt A, May G, Zell R, Pring-Akerblom P, Verhagen W, Heim A. 1999. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265:178–184. doi: 10.1006/viro.1999.0003. [DOI] [PubMed] [Google Scholar]
- 18.Martín J, Dunn G, Hull R, Patel V, Minor PD. 2000. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J Virol 74:3001–3010. doi: 10.1128/JVI.74.7.3001-3010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorba J, Campagnoli R, De L, Kew O. 2008. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol 82:4429–4440. doi: 10.1128/JVI.02354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakoto-Andrianarivelo M, Guillot S, Iber J, Balanant J, Blondel B, Riquet F, Martin J, Kew O, Randriamanalina B, Razafinimpiasa L, Rousset D, Delpeyroux F. 2007. Co-circulation and evolution of polioviruses and species C enteroviruses in a district of Madagascar. PLoS Pathog 3:e191. doi: 10.1371/journal.ppat.0030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avellón A, Cabrerizo M, de Miguel T, Pérez-Breña P, Tenorio A, Pérez JL, de Aragón MV, Trallero G. 2008. Paralysis case and contact spread of recombinant vaccine-derived poliovirus, Spain. Emerg Infect Dis 14:1807–1809. doi: 10.3201/eid1411.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Yan D, Zhu S, Wen N, Li L, Wang H, Liu J, Ye X, Ding Z, Wang D, Zhu H, Chen L, Hou X, An H, Liang X, Luo H, Kew O, Xu W. 2010. Type 2 vaccine-derived poliovirus from patients with acute flaccid paralysis in China: current immunization strategy effectively prevented its sustained transmission. J Infect Dis 202:1780–1788. doi: 10.1086/657410. [DOI] [PubMed] [Google Scholar]
- 23.Sharif S, Abbasi BH, Khurshid A, Alam MM, Shaukat S, Angez M, Rana MS, Zaidi SS. 2014. Evolution and circulation of type-2 vaccine-derived polioviruses in Nad Ali District of southern Afghanistan during June 2009-February 2011. PLoS One 9:e88442. doi: 10.1371/journal.pone.0088442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Famulare M, Chang S, Iber J, Zhao K, Adeniji JA, Bukbuk D, Baba M, Behrend M, Burns CC, Oberste MS. 2016. Sabin vaccine reversion in the field: a comprehensive analysis of Sabin-like poliovirus isolates in Nigeria. J Virol 90:317–331. doi: 10.1128/JVI.01532-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adu F, Iber J, Bukbuk D, Gumede N, Yang SJ, Jorba J, Campagnoli R, Sule WF, Yang CF, Burns C, Pallansch M, Harry T, Kew O. 2007. Isolation of recombinant type 2 vaccine-derived poliovirus (VDPV) from a Nigerian child. Virus Res 127:17–25. doi: 10.1016/j.virusres.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Yan D, Zhang Y, Zhu S, Chen N, Li X, Wang D, Ma X, Zhu H, Tong W, Xu W. 2014. Limited and localized outbreak of newly emergent type 2 vaccine-derived poliovirus in Sichuan, China. Clin Vaccine Immunol 21:1012–1018. doi: 10.1128/CVI.00196-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korotkova EA, Park R, Cherkasova EA, Lipskaya GY, Chumakov KM, Feldman EV, Kew OM, Agol VI. 2003. Retrospective analysis of a local cessation of vaccination against poliomyelitis: a possible scenario for the future. J Virol 77:12460–12465. doi: 10.1128/JVI.77.23.12460-12465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savolainen-Kopra C, Samoilovich E, Kahelin H, Hiekka AK, Hovi T, Roivainen M. 2009. Comparison of poliovirus recombinants: accumulation of point mutations provides further advantages. J Gen Virol 90:1859–1868. doi: 10.1099/vir.0.010942-0. [DOI] [PubMed] [Google Scholar]
- 29.Tao Z, Zhang Y, Liu Y, Xu A, Lin X, Yoshida H, Xiong P, Zhu S, Wang S, Yan D, Song L, Wang H, Cui N, Xu W. 2013. Isolation and characterization of a type 2 vaccine-derived poliovirus from environmental surveillance in China, 2012. PLoS One 8:e83975. doi: 10.1371/journal.pone.0083975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razafindratsimandresy R, Joffret ML, Rabemanantsoa S, Andriamamonjy S, Heraud JM, Delpeyroux F. 2013. Reemergence of recombinant vaccine-derived polioviruses in healthy children, Madagascar. Emerg Infect Dis 19:1008–1010. doi: 10.3201/eid1906.130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma DK, Nalavade UP, Varose SY, Deshpande JM. 2015. Vaccine-derived polioviruses not detected by global surveillance screening assay. Emerg Infect Dis 21:1880–1881. doi: 10.3201/eid2110.150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yakovenko ML, Cherkasova EA, Rezapkin GV, Ivanova OE, Ivanov AP, Eremeeva TP, Baykova OY, Chumakov KM, Agol VI. 2006. Antigenic evolution of vaccine-derived polioviruses: changes in individual epitopes and relative stability of the overall immunological properties. J Virol 80:2641–2653. doi: 10.1128/JVI.80.6.2641-2653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherkasova EA, Yakovenko ML, Rezapkin GV, Korotkova EA, Ivanova OE, Eremeeva TP, Krasnoproshina LI, Romanenkova NI, Rozaeva NR, Sirota L, Agol VI, Chumakov KM. 2005. Spread of vaccine-derived poliovirus from a paralytic case in an immunodeficient child: an insight into the natural evolution of oral polio vaccine. J Virol 79:1062–1070. doi: 10.1128/JVI.79.2.1062-1070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Sanden S, Pallansch MA, van de Kassteele J, El-Sayed N, Sutter RW, Koopmans M, van der Avoort H. 2009. Shedding of vaccine viruses with increased antigenic and genetic divergence after vaccination of newborns with monovalent type 1 oral poliovirus vaccine. J Virol 83:8693–8704. doi: 10.1128/JVI.02388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuura K, Ishikura M, Yoshida H, Nakayama T, Hasegawa S, Ando S, Horie H, Miyamura T, Kitamura T. 2000. Assessment of poliovirus eradication in Japan: genomic analysis of polioviruses isolated from river water and sewage in Toyama Prefecture. Appl Environ Microbiol 66:5087–5091. doi: 10.1128/AEM.66.11.5087-5091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esteves-Jaramillo A, Estivariz CF, Penaranda S, Richardson VL, Reyna J, Coronel DL, Carrion V, Landaverde JM, Wassilak SG, Perez-Sanchez EE, Lopez-Martinez I, Burns CC, Pallansch MA. 2014. Detection of vaccine-derived polioviruses in Mexico using environmental surveillance. J Infect Dis 210:S315–S323. doi: 10.1093/infdis/jiu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buttinelli G, Donati V, Fiore S, Marturano J, Plebani A, Balestri P, Soresina AR, Vivarelli R, Delpeyroux F, Martin J, Fiore L. 2003. Nucleotide variation in Sabin type 2 poliovirus from an immunodeficient patient with poliomyelitis. J Gen Virol 84:1215–1221. doi: 10.1099/vir.0.18974-0. [DOI] [PubMed] [Google Scholar]
- 38.Blomqvist S, Bruu AL, Stenvik M, Hovi T. 2003. Characterization of a recombinant type 3/type 2 poliovirus isolated from a healthy vaccinee and containing a chimeric capsid protein VP1. J Gen Virol 84:573–580. doi: 10.1099/vir.0.18708-0. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Zhu S, Yan D, Liu G, Bai R, Wang D, Chen L, Zhu H, An H, Kew O, Xu W. 2010. Natural type 3/type 2 intertypic vaccine-related poliovirus recombinants with the first crossover sites within the VP1 capsid coding region. PLoS One 5:e15300. doi: 10.1371/journal.pone.0015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dedepsidis E, Pliaka V, Kyriakopoulou Z, Brakoulias C, Levidiotou-Stefanou S, Pratti A, Mamuris Z, Markoulatos P. 2008. Complete genomic characterization of an intertypic Sabin 3/Sabin 2 capsid recombinant. FEMS Immunol Med Microbiol 52:343–351. doi: 10.1111/j.1574-695X.2008.00381.x. [DOI] [PubMed] [Google Scholar]
- 41.Macadam AJ, Pollard SR, Ferguson G, Skuce R, Wood D, Almond JW, Minor PD. 1993. Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology 192:18–26. doi: 10.1006/viro.1993.1003. [DOI] [PubMed] [Google Scholar]
- 42.Muzychenko AR, Lipskaya GY, Maslova SV, Svitkin YV, Pilipenko EV, Nottay BK, Kew OM, Agol VI. 1991. Coupled mutations in the 5′-untranslated region of the Sabin poliovirus strains during in vivo passages: structural and functional implications. Virus Res 21:111–122. doi: 10.1016/0168-1702(91)90002-D. [DOI] [PubMed] [Google Scholar]
- 43.Svitkin YV, Alpatova GA, Lipskaya GY, Maslova SV, Agol VI, Kew O, Meerovitch K, Sonenberg N. 1993. Towards development of an in vitro translation test for poliovirus neurovirulence. Dev Biol Stand 78:27–32. [PubMed] [Google Scholar]
- 44.Lentz KN, Smith AD, Geisler SC, Cox S, Buontempo P, Skelton A, DeMartino J, Rozhon E, Schwartz J, Girijavallabhan V, O'Connell J, Arnold E. 1997. Structure of poliovirus type 2 Lansing complexed with antiviral agent SCH48973: comparison of the structural and biological properties of three poliovirus serotypes. Structure 5:961–978. doi: 10.1016/S0969-2126(97)00249-9. [DOI] [PubMed] [Google Scholar]
- 45.Shulman LM, Manor Y, Handsher R, Delpeyroux F, McDonough MJ, Halmut T, Silberstein I, Alfandari J, Quay J, Fisher T, Robinov J, Kew OM, Crainic R, Mendelson E. 2000. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J Clin Microbiol 38:3729–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilipenko EV, Blinov VM, Romanova LI, Sinyakov AN, Maslova SV, Agol VI. 1989. Conserved structural domains in the 5′-untranslated region of picornaviral genomes: an analysis of the segment controlling translation and neurovirulence. Virology 168:201–209. doi: 10.1016/0042-6822(89)90259-6. [DOI] [PubMed] [Google Scholar]
- 47.Han JQ, Townsend HL, Jha BK, Paranjape JM, Silverman RH, Barton DJ. 2007. A phylogenetically conserved RNA structure in the poliovirus open reading frame inhibits the antiviral endoribonuclease RNase L. J Virol 81:5561–5572. doi: 10.1128/JVI.01857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilipenko EV, Poperechny KV, Maslova SV, Melchers WJ, Slot HJ, Agol VI. 1996. Cis-element, oriR, involved in the initiation of (−) strand poliovirus RNA: a quasi-globular multi-domain RNA structure maintained by tertiary (‘kissing') interactions. EMBO J 15:5428–5436. [PMC free article] [PubMed] [Google Scholar]
- 49.Georgescu MM, Delpeyroux F, Tardy-Panit M, Balanant J, Combiescu M, Combiescu AA, Guillot S, Crainic R. 1994. High diversity of poliovirus strains isolated from the central nervous system from patients with vaccine-associated paralytic poliomyelitis. J Virol 68:8089–8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nathanson N, Kew OM. 2010. From emergence to eradication: The epidemiology of poliomyelitis deconstructed. Am J Epidemiol 172:1213–1229. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anis E, Kopel E, Singer SR, Kaliner E, Moerman L, Moran-Gilad J, Sofer D, Manor Y, Shulman LM, Mendelson E, Gdalevich M, Lev B, Gamzu R, Grotto I. 2013. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill 18:20586. doi: 10.2807/1560-7917.ES2013.18.38.20586. [DOI] [PubMed] [Google Scholar]
- 52.Strebel PM, Ion-Nedelcu N, Baughman AL, Sutter RW, Cochi SL. 1995. Intramuscular injections within 30 days of immunization with oral poliovirus vaccine—a risk factor for vaccine-associated paralytic poliomyelitis. N Engl J Med 332:500–506. doi: 10.1056/NEJM199502233320804. [DOI] [PubMed] [Google Scholar]
- 53.Izurieta HS, Sutter RW, Baughman AL, Strebel PM, Stevenson JM, Wharton M. 1995. Vaccine-associated paralytic poliomyelitis in the United States: no evidence of elevated risk after simultaneous intramuscular injections of vaccine. Pediatr Infect Dis J 14:840–846. doi: 10.1097/00006454-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Ma S, Du Z, Feng M, Che Y, Li Q. 2015. A severe case of co-infection with enterovirus 71 and vaccine-derived poliovirus type II. J Clin Virol 72:25–29. doi: 10.1016/j.jcv.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization. 2013. Global Polio Eradication Initiative. Polio eradication and endgame strategic plan (2013-2018). WHO/POLIO/13.02. World Health Organization, Geneva, Switzerland: http://www.polioeradication.org/Default.aspx?tabid=116&Tag=strategy. [Google Scholar]
- 56.World Health Organization. 2015. Global Polio Eradication Initiative. World Health Assembly resolution WHA68.3. http://www.polioeradication.org/Default.aspx?tabid=123&Tag=resolution.