ABSTRACT
In this study, we show that the HIV-1 Tat protein interacts with rapid kinetics to engage the Toll-like receptor 4 (TLR4) pathway, leading to the production of proinflammatory and anti-inflammatory cytokines. The pretreatment of human monocytes with Tat protein for 10 to 30 min suffices to irreversibly engage the activation of the TLR4 pathway, leading to the production of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10), two cytokines strongly implicated in the chronic activation and dysregulation of the immune system during HIV-1 infection. Therefore, this study analyzed whether the HIV-1 Tat protein is able to activate these two pathways separately or simultaneously. Using three complementary approaches, including mice deficient in the MyD88, TIRAP/MAL, or TRIF adaptor, biochemical analysis, and the use of specific small interfering RNAs (siRNAs), we demonstrated (i) that Tat was able to activate both the MyD88 and TRIF pathways, (ii) the capacity of Tat to induce TIRAP/MAL degradation, (iii) the crucial role of the MyD88 pathway in the production of Tat-induced TNF-α and IL-10, (iv) a reduction but not abrogation of IL-10 and TNF-α by Tat-stimulated macrophages from mice deficient in TIRAP/MAL, and (v) the crucial role of the TRIF pathway in Tat-induced IL-10 production. Further, we showed that downstream of the MyD88 and TRIF pathways, the Tat protein activated the protein kinase C (PKC) βII isoform, the mitogen-activated protein (MAP) kinases p38 and extracellular signal-regulated kinase 1/2 (ERK1/2), and NF-κB in a TLR4-dependent manner. Collectively, our data show that by recruiting the TLR4 pathway with rapid kinetics, the HIV-1 Tat protein leads to the engagement of both the MyD88 and TRIF pathways and to the activation of PKC, MAP kinase, and NF-κB signaling to induce the production of TNF-α and IL-10.
IMPORTANCE In this study, we demonstrate that by recruiting the TLR4 pathway with rapid kinetics, the HIV-1 Tat protein leads to the engagement of both the MyD88 and TRIF pathways and to the activation of PKC-βII, MAP kinase, and NF-κB signaling to induce the production of TNF-α and IL-10, two cytokines strongly implicated in the chronic activation and dysregulation of the immune system during HIV-1 infection. Thus, it may be interesting to target Tat as a pathogenic factor early after HIV-1 infection. This could be achieved either by vaccination approaches including Tat as an immunogen in potential candidate vaccines or by developing molecules capable of neutralizing the effect of the Tat protein.
INTRODUCTION
The immune system disorders observed in human immunodeficiency virus type 1 (HIV-1) infection emerge early in infected patients and contribute to the establishment of a chronic immune activation associated with loss of function of CD4+ T lymphocytes (T4 cells) and CD8+ T lymphocytes (T8 cells), impairment of dendritic cell functions (1), and progressive increases of proinflammatory and anti-inflammatory cytokines, including interleukin-10 (IL-10) (2, 3) and tumor necrosis factor alpha (TNF-α) (4). These physiological disorders occur in parallel with an increase in viral load and inevitably lead to AIDS disease progression (5–7). As in HIV-1-infected patients, a similar persistent proinflammatory reaction and AIDS disease development are also observed in the macaque, which is not a natural host for simian immunodeficiency virus (SIV), after experimental infection with the pathogenic SIVmac251 or SIVmac239 strain (8). Remarkably, SIV infection of sooty mangabeys or African green monkeys, the natural hosts of SIV, does not lead to chronic immune activation or an AIDS-like disease development, despite the presence of high viral loads (9, 10). The latter observation has led to the development of hypotheses considering immune system dysfunctions to be at the center of the pathogenesis of HIV-1 infection.
The induced hyperimmune activation following infection with pathogenic strains of HIV-1 or SIV is associated with a gradual depletion of circulating T4 cells in the blood and a rapid depletion, at 2 to 4 weeks postinfection, of those in the gut-associated lymphoid tissue (GALT). Interestingly, such chronic immune activation and GALT T4 cell depletion are more controlled and limited in the natural animal SIV hosts and also in human elite controllers, an HIV-1-infected patient population characterized by low viral loads, normal T4 cell levels, controlled immune activation, and slow evolution of AIDS development (11). However, in HIV-1-infected patients, as in nonnatural SIV host models, the T4 cell depletion in the GALT is accompanied by an alteration of the intestinal barrier, leading to microbial translocation to the blood, which generates an increase in bacterial products, including lipopolysaccharide (LPS), in the plasma (6). Thus, LPS, probably in combination with other bacterial pathogen-associated molecular patterns (PAMPs) once they are recognized by their specific pattern recognition receptors (PRRs), establishes continuous, persistent immune stimulation, inevitably leading to an abnormal activation and dysregulation of the immune system. However, in hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, these dysregulations can occur, even in the absence of the intestinal barrier alteration. So what are the mechanisms involved in immune activation during the acute phase, when the intestinal barrier is still intact? A likely hypothesis is the involvement of viral products, as has been shown for influenza A virus, Epstein-Barr virus (EBV), HBV, SIV, and lymphocytic choriomeningitis virus (LCMV) infections (11–13). To corroborate this hypothesis, many groups have characterized the presence of PAMPs associated with protein and nucleic fractions of HIV-1 and analyzed the corresponding recognized PRRs.
In agreement with the active role of HIV products in the establishment of early inflammation and the alteration of the intestinal barrier, Nazli et al. showed that exposure of the mucosal epithelial barrier to HIV-1 or to its envelope glycoprotein, gp120, stimulated the production of inflammatory cytokines and caused barrier dysfunctions (14). In addition to gp120, HIV-1 Vpr has been shown to activate the latent virus by inducing production of IL-6, an inflammatory cytokine, in a Toll-like receptor 4 (TLR4)- and MyD88 (myeloid differentiation primary response gene 88)-dependent manner (15). Furthermore, it has been shown that viral RNA contains multiple potential ligands of TLR7/8 (16) that may also be sensed by cytoplasmic RIG-I-like receptors (RLR), including RIG-I and MDA-5 (17, 18). In addition to RNA and viral proteins, HIV-1 DNA produced by reverse transcription can also be sensed, at least by cGAS (19) and IFI16 (20), which, like TLRs, can initiate NLRP inflammasome signaling, thus underlying the cross talk and the redundancy of innate immunity sensing. More recently, our group has shown that by interacting with high affinity with TLR4/MD2, the HIV-1 Tat protein stimulates the production of TNF-α and IL-10 (21) in addition to other inflammatory cytokines (22–24) and the positive modulation of immunosuppressive factors, including IDO (23) and PD-L1 (24), by dendritic cells.
Among the 10 TLRs identified in humans, TLR4 is distinguished by its ability to signal via two distinct pathways. The first is activated following LPS-TLR4/MD2 interaction at the cell surface membrane. Downstream of LPS-TLR4 formation, once phosphorylated by Btk (Bruton tyrosine kinase), TIRAP (Toll–interleukin-1 receptor domain-containing adaptor protein; also termed MAL [MyD88 adapter-like]) recruits MyD88 to the cell membrane (25). The complex formed between these two adaptors then induces signals leading to the recruitment of IRAK-TRAF6 family kinases and the activation of TAK1/TAB1/TAB2/TAB3 kinases. This last formed complex, in turn, activates the IKK signalosome, leading to NF-κB and AP1 activation and also to the activation of the mitogen-activated protein (MAP) kinases p38, extracellular signal-regulated kinase 1/2 (ERK1/2), and c-Jun N-terminal kinase (JNK) (26).
In parallel, due to the action of CD14 (27), a glycosylphosphatidylinositol (GPI)-linked protein, and the actions of Syk and PLCg2 (27), LPS-TLR4 is internalized into endosomes, where a second, MyD88-independent signaling pathway is activated. This pathway, which is dependent on two adaptors, TRIF (TIR domain-containing adapter-inducing beta interferon) and TRAM (TRIF-related adaptor molecule), activates the transcription factors interferon regulatory factor 3 (IRF3) and IRF7, leading to the production of type 1 interferon (IFN) (28). Generally, while the TRIF pathway is involved in type I interferon response, the MyD88-dependent pathway triggers essentially inflammatory cytokines (29). Downstream of the MyD88 and TRIF pathways, several studies have reported the implication of MAP kinases, NF-κB, and the serine/threonine kinases protein kinase C epsilon (PKC-ε), PKC-δ, PKC-ζ, and PKC-ι in TLR4 signaling (30–32).
In this study, we analyzed Tat-TLR4 interaction at the kinetic level, the implication of the MyD88 and TRIF pathways in Tat-induced TNF-α and IL-10, and the relationship between Tat-TLR4 interaction and activation of the MAP kinases p38 and ERK1/2, NF-κB, and PKC-δ and -βII.
MATERIALS AND METHODS
Ethics statement.
The use of human cells in this study was approved by the Research Ethical Committee, Haute-Garonne, France. Human peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coats of samples from healthy human donors. Buffy coats were provided anonymously by the Établissement Français du Sang (EFS; Toulouse, France). Written informed consent was obtained from each donor under EFS contract 21/PVNT/TOU/INSERM01/2011-0059, according to decree 2007-1220 (articles L1243-4 and R1243-61). The use of mice as animal models in this study was conducted in accordance with European Union regulations and with the French national charter for ethics of animal experiments (articles R214-87 to -90 of the rural code). The protocol was approved by the committee on the ethics of animal experiments of the Region Midi-Pyrénées and by IFR 150 (permit numbers 04-U563-DG-06 and MP/18/26/04/04). This study included both human studies and animal studies, which conformed to the guides for IRB and IACUC published by the U.S. National Institutes of Health.
Isolation of human monocytes.
PBMCs were isolated from the buffy coats of samples from healthy donors by standard Ficoll-Paque density centrifugation (GE Healthcare). Briefly, the blood was diluted 1:1 in phosphate-buffered saline (PBS) prewarmed to 37°C and carefully layered over the Ficoll-Paque gradient. The tubes were centrifuged for 25 min at 2,000 rpm and 20°C. The cell interface layer was harvested carefully, and the cells were washed twice in PBS (for 10 min at 1,200 rpm followed by 10 min at 800 rpm) and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 1% penicillin (100 IU/ml) and streptomycin (100 μg/ml). Monocytes were separated from lymphocytes by adherence to tissue culture plastic (Becton Dickinson). PBMCs were seeded in 24-well plates (107 PBMCs/well). After incubation for 1 h at 37°C, nonadherent cells were removed by 3 washes with PBS, and adherent cells (>94% CD14+ by flow cytometry analysis) were cultured in complete RPMI 1640 medium.
Human promonocytic cell line.
THP-1 cells were obtained from the American Type Culture Collection (ATCC) and grown in RPMI 1640 medium supplemented with 10% FCS, penicillin (100 IU/ml), and streptomycin (100 μg/ml) in a 37°C humidified atmosphere with 5% CO2.
HEK cell line expressing TLR4/MD2-CD14.
A human embryonic kidney (HEK) cell line stably expressing TLR4 and its cofactors (MD2 and CD14) was purchased from Invivogen and maintained in culture in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS, Normocin (100 μg/ml), blasticidin (10 μg/ml), and Hygrogold (50 μg/ml) at 37°C, according to the manufacturer's instructions (Invivogen).
Primary mouse peritoneal macrophages.
C57BL/6 mice (wild type [WT]) were purchased from Charles Rivers. TLR4 knockout (KO), MyD88 KO, TRIF KO, and TIRAP/MAL KO mice with the C57BL/6 strain background (TLR4−/−, MyD88−/−, TRIF−/−, and TIRAP−/− mice) were a generous gift from B. Ryffel (Laboratory of Molecular Immunology and Embryology, CNRS, Orléans, France). Primary macrophages were isolated as previously described (22). Briefly, mice were injected intraperitoneally with 1 ml of 3% thioglycolate medium (bioMérieux). Three days later, the mice were sacrificed and macrophages were collected by peritoneal washes with PBS and then enriched by adherence selection for 1 h in complete medium (DMEM supplemented with 2% FCS, penicillin [100 IU/ml], and streptomycin [100 μg/ml]). Isolated macrophages were >95% CD11b+ by flow cytometry analysis.
Tat protein and TLR ligands.
Recombinant glutathione S-transferase (GST), a full-length GST-Tat protein (amino acids [aa] 1 to 101) from HIV-1 strain SF2, or Tat-deleted mutant (GST-Tat 1-45 and GST-Tat 30-72) proteins were produced and purified in our laboratory as previously described (33). A chemically synthesized Tat (aa 1 to 86) protein from the HIV-1 Lai strain and the analogue K50 form of Tat were produced as described previously (24). The level of endotoxin contamination was assessed using the Limulus amebocyte lysate (LAL) assay (Bio-Sepra, Villeneuve la Garenne, France). All these recombinant proteins contained <0.3 endotoxin unit (EU)/μg LPS, the limit of detection of this test. The TLR (TLR4) agonist LPS from E. coli serotype R515 was purchased from Alexis Biochemicals. Pam3CSK4 (TLR2/1 ligand) was purchased from Invitrogen.
Antibodies and chemical products. (i) Antibodies for TLR4 inhibition assays.
Monoclonal antibodies (MAbs) against human TLR4 (anti-TLR4; clone HTA125) and MAbs of the mouse IgG2a isotype, used for neutralization assays, were purchased from eBioscience. Monoclonal anti-Tat antibodies were obtained from the Agence National de Recherche sur le SIDA (ANRS). These three monoclonal antibodies recognize epitopes localized in domains 1-15, 46-60, and 78-86 of the Tat sequence. The anti-gp120 monoclonal antibody (Mab110-4), obtained from Genetic Systems Corporation, recognizes an epitope within the V3 region.
(ii) Plasmids.
The following plasmids were purchased from Invivogen: psiRNA-hTLR4, psiRNA-hTLR2, psiRNA-hMyD88, psiRNA-hTIRAP/MAL, psiRNA-Control, pORF-LacZ, and pNiFty2-SEAP.
(iii) Antibodies for Western blot analysis.
Anti-PKC-βII (C-18), anti-PKC-δ (C-17), anti-human/mouse p65, anti-human/mouse TFIIB (clone II B8), and anti-IκBα were purchased from Santa Cruz Biotechnology. Anti-TIRAP/MAL antibodies were obtained from eBioscience. Anti-P-ERK1/2, anti-ERK1/2, anti-P-p38, and anti-p38 were purchased from Cell Signaling. For a loading control, monoclonal anti-human/mouse β-actin (clone AC-15) was obtained from Sigma-Aldrich. Monoclonal rabbit anti-Na+/K+ ATPase (clone EP184Y) was obtained from Abcam. Mouse monoclonal anti-tubulin (clone DM1A) was obtained from Sigma-Aldrich. Rabbit polyclonal anti-goat–horseradish peroxidase (HRP), anti-mouse–HRP, and polyclonal swine anti-rabbit–HRP were purchased from Dako.
(iv) Chemicals.
Phorbol myristate acetate (PMA), a PKC activator, was purchased from Calbiochem. MG132, an inhibitor of proteasome activity (Z-Leu-Leu-Leu-al), was purchased from Sigma-Aldrich.
Isolation of cytoplasmic and membrane proteins.
Following treatment with PMA or Tat in the presence or absence of anti-TLR4 blocking antibodies, human monocytes were harvested and rapidly lysed at 4°C in 100 μl of hypotonic buffer A (200 mM Tris-HCl, 2 mM EDTA, 1 mM dithiothreitol [DTT], 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF]; pH 7.5) by repeated aspiration through a syringe fitted with a 21-gauge needle. After addition of 300 μl of ice-cold sample buffer B (20 mM Tris-HCl, 2 mM EDTA, 1 mM DTT, 10 μg/ml leupeptin, 1 mM PMSF, 0.33 M sucrose; pH 7.5), the lysate was centrifuged (100,000 × g, 4°C, 40 min). The supernatant, corresponding to the cytoplasmic fraction, was then collected, and proteins were quantified using the Bradford assay and stored at −20°C. The membrane pellets were solubilized in 50 μl of cold sample buffer B containing 1% Triton X-100, sonicated (1 min, power 2.5), and stored at −20°C.
Isolation of cytoplasmic and nuclear protein extracts.
After treatment with LPS or Tat in the presence or absence of anti-TLR4 blocking antibodies, human monocytes or mouse macrophages were harvested and rapidly lysed at 4°C in 200 μl of hypotonic buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 0.2 mM Na3VO4, 0.05 mM NaF) for 15 min. Next, 12.5 μl of 10% Nonidet P-40 was added, and the lysate was vortexed (20 s) before centrifugation (1 min, 14,000 rpm, 4°C). The supernatant, corresponding to the cytoplasmic fraction, was collected, and proteins were quantified using the Bradford assay and stored at −20°C. The nuclear pellets were solubilized in 100 μl of cold sample buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 0.2 mM Na3VO4, 0.05 mM NaF) and shaken strongly for 15 min at 4°C. After centrifugation, nuclear proteins were collected in the supernatant, quantified by use of the Bradford assay, and stored at −20°C.
Western blot analysis.
Equal amounts of protein (10 to 40 μg) were subjected to 10% SDS-PAGE, and the separated proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% low-fat milk in Tris-buffered saline with 0.05% Tween 20 (TTBS) for 1 h, washed with TTBS, and then incubated with the primary antibody overnight at 4°C. Na3VO4 (0.2 mM) was added to the wash and saturation buffer to prevent phosphatase activity. Immunoreactive bands were detected by incubation for 2 h with the appropriate secondary antibodies conjugated with horseradish peroxidase (Dako A/S, Roskilde, Denmark). Proteins of interest were visualized using ECL substrate (Pierce, Rockford, IL).
Cytokine detection by ELISA.
Adherent human monocytes (106/well) or murine macrophages (5 × 105/well) were washed 3 times with PBS. Monocytes were then cultured in RPMI 1640 medium completed with 1% FCS, and murine macrophages were cultured in DMEM plus 2% FCS before treatment. After 24 h of cell treatment, the cell supernatants were collected, and the amounts of human and mouse TNF-α and IL-10 were quantified using enzyme-linked immunosorbent assay (ELISA) kits from eBiosciences according to the manufacturer's instructions.
Cell transfection and reporter gene expression assays.
HEK TLR4/MD2-CD14 cells were seeded into 24-well plates at 4 × 105 cells/well the day before transfection. After 24 h, the cells (60 to 70% confluence) were cotransfected with pORF-LacZ (Invivogen) and an NF-κB reporter plasmid (p-Nifty2-Seap; Invivogen) together with the indicated amounts of plasmid encoding small interfering RNA (siRNA) targeting a specific gene of interest (control, TLR4, TLR2, MyD88, or TIRAP) by use of a calcium phosphate transfection system as described previously (22). After 24 h of transfection, cells were washed with PBS and stimulated with Tat or LPS or left untreated. After 24 h of stimulation, NF-κB-driven secreted embryonic alkaline phosphatase (SEAP) reporter gene expression was assayed in the supernatant according to the manufacturer's instructions (Invivogen). For normalization, cells were lysed, and expression of the β-galactosidase gene was analyzed.
Statistical analyses.
Statistical analysis was performed using GraphPad Prism software. All results are expressed as means ± standard deviations (SD). All experiments were performed a minimum of three times. Differences in the means for the different groups were tested using one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test or two-way ANOVA followed by the Bonferroni post hoc test (as indicated in the figure legends). P values of <0.05 were considered to be statistically significant.
RESULTS
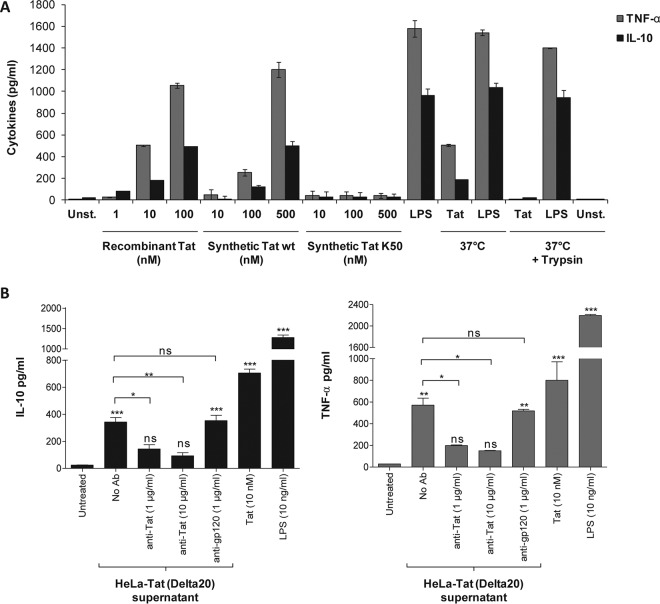
As previously demonstrated by our group and others (4, 21, 22, 33–37), we showed that the HIV-1 Tat protein activates the production of TNF-α and IL-10 by human monocytes (Fig. 1A). This action is specific to the Tat protein and is not due to possible contaminants in HIV-1 Tat protein preparations. The capacity of Tat to induce cytokine production was totally inhibited if it was pretreated with trypsin (Fig. 1A), while the same treatment applied to LPS had no effect on the capacity of Tat to induce TNF-α and IL-10 production (Fig. 1A). Using the LAL assay, we showed that the level of endotoxins in the purified recombinant Tat protein was below the detection threshold of the kit (<0.3 EU/μg). In addition, treatment with a chemically synthesized Tat protein which had never been in contact with bacterial proteins, but not treatment with its acetylated lysine 50 analogue (K50), stimulated the production of TNF-α and IL-10 in human monocytes (Fig. 1A). Moreover, in more physiological settings, the Tat protein secreted from a HeLa cell line stably transfected with the HIV-1 Lai tat gene was also able to stimulate TNF-α and IL-10 production in human monocytes (Fig. 1B). The specificity of Tat-induced cytokines was further demonstrated by the capacity of anti-Tat antibodies to strongly inhibit TNF-α and IL-10 production (Fig. 1B), while the same amount of anti-gp120 antibodies did not interfere with cytokine induction. Previously, we showed that the full-length Tat protein (Tat 1-101) or its N-terminal fragment (Tat 1-45), but not the central region (Tat 30-72), was able to stimulate TNF-α and IL-10 production in a TLR4-dependent manner (21). However, the characteristics of Tat-TLR4 interaction and the activated signaling pathways leading to TNF-α and IL-10 production remained to be investigated. Thus, in the present study, we further analyzed the kinetic characteristics of Tat-TLR4 interaction and studied the signaling pathways activated and their roles in the production of TNF-α and IL-10, two cytokines strongly implicated in the hyperactivation of the immune system and its dysregulation during HIV-1 infection.
FIG 1.
The Tat protein induces TNF-α and IL-10 production in human monocytes in a specific manner. (A) Human monocytes were incubated with increasing amounts of full-length HIV-1 Tat protein, either recombinant (Tat), chemically synthesized (synthetic Tat), or acetylated at lysine 50 (K50). As a control, the recombinant Tat protein (10 nM) or LPS (100 ng/ml) was pretreated with trypsin for 1 h at 37°C or kept at 37°C for 1 h without trypsin. After 24 h of treatment, levels of TNF-α (gray bars) and IL-10 (black bars) in the supernatants of the cells were quantified by ELISA. Unst., unstimulated cells. (B) Human monocytes were incubated with HeLa-Tat cells (Delta20 line) stably transfected with tat-rev-vpu gene-conditioned medium (100 μl) in the absence or presence of a mixture of three monoclonal antibodies (1 μg or 10 μg/ml), recognizing epitopes localized in domains 1-15, 46-60, and 78-86 of the Tat sequence, or 10 μg/ml of a monoclonal antibody against HIV-1 gp120 (Mab110-4), which recognizes an epitope within the V3 region. As positive controls, human monocytes were treated with either recombinant Tat (10 nM) or LPS (10 ng/ml). After 24 h, the cell supernatants were collected, and TNF-α (gray bars) and IL-10 (black bars) levels were quantified by ELISA. The data represent means and SD for three independent experiments. Statistical significance was analyzed by one-way ANOVA follow by Bonferroni posttests and is marked as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, nonsignificant. All bars were compared to those for untreated cells unless otherwise specified (indicated with a black line above the bars).
Tat activates the TLR4 pathway with rapid kinetics.
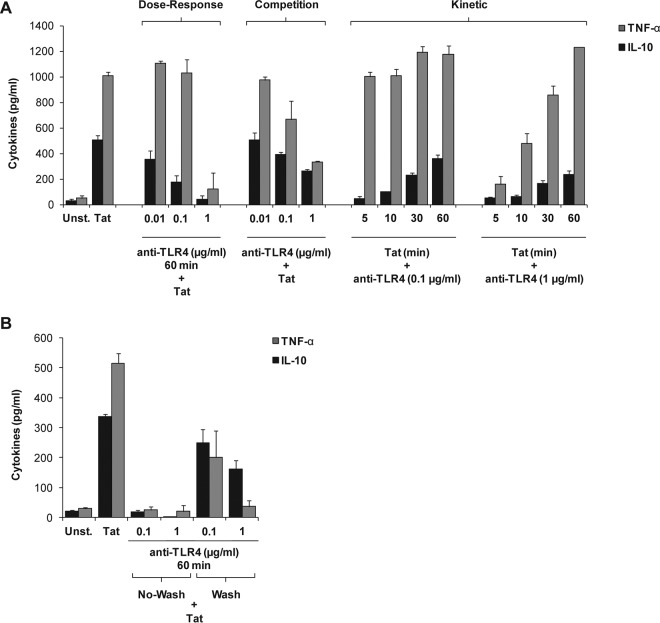
To further analyze the physicochemical characteristics of Tat-TLR4 interaction, we explored this interaction at the kinetic level by using a set of 4 complementary conditions, including dose-response, competition, kinetics, and chase studies.
Under the first condition, monocytes were previously treated with increasing amounts of anti-TLR4 MAbs (0.01 to 1 μg/ml) for 60 min before treatment with Tat protein. Under these conditions, a dose-dependent inhibition of TNF-α and IL-10 production was observed. The most inhibition was obtained with anti-TLR4 antibodies at 1 μg/ml (Fig. 2A, dose-response data).
FIG 2.
Tat activates the TLR4 pathway with rapid kinetics. Human monocytes were incubated with 10 nM full-length HIV-1 Tat protein (Tat 1-86) in the presence or absence of various concentrations of anti-TLR4 antibodies as described below. After 24 h of treatment, levels of TNF-α (gray bars) and IL-10 (black bars) in the supernatants of the cells were quantified by ELISA. (A) (Dose-response) Cells were first preincubated with escalating amounts of anti-TLR4 (0.01 to 1 μg/ml) for 60 min before treatment with Tat. (Competition) Anti-TLR4 antibodies (0.01 to 1 μg/ml) and Tat protein (10 nM) were added to the cells at the same time. (Kinetic) Cells were first stimulated with Tat (10 nM) for 5 to 60 min before anti-TLR4 MAbs were added at 0.1 μg/ml or 1 μg/ml. (B) Cells (106 human monocytes) were first preincubated with 0.1 or 1 μg/ml of anti-TLR4 blocking antibodies for 60 min, washed twice or left unwashed, and then stimulated with Tat (10 nM). Cytokine production levels in cell supernatants were quantified after 24 h of treatment. The data represent means and SD for three independent experiments.
Under the second experimental condition, i.e., the competition assay, when similar amounts of anti-TLR4 antibodies (i.e., 0.01 to 1 μg/ml) were added at the same time as Tat (10 nM), less inhibition of TNF-α and IL-10 production was observed with anti-TLR4 MAbs used at 0.1 and 1 μg/ml, thus demonstrating the efficiency and rapidity of the Tat-TLR4 interaction to activate the TLR4 pathway (Fig. 2A, competition data). In contrast, no significant inhibition of IL-10 was obtained with the lowest concentration of anti-TLR4 antibodies (0.01 μg/ml).
In the third kinetic assay protocol, monocytes were first stimulated with Tat (10 nM) for 5 to 60 min before anti-TLR4 MAbs were added at 0.1 μg/ml or 1 μg/ml. Under these conditions, the results obtained showed that the sooner the anti-TLR4 antibodies were added, the greater was the intensity of inhibition of Tat-induced TNF-α and IL-10 production (Fig. 2A, kinetic data). These results indicate that Tat and anti-TLR4 antibodies compete for the same cell membrane receptor and that Tat pretreatment for 5 to 30 min is sufficient to trigger and prime the activation of the signaling pathways leading to significant production of TNF-α and IL-10 by human monocytes (Fig. 2A, kinetic data). In agreement with this interpretation, the fourth set of experimental conditions showed that when monocytes were first preincubated for 60 min with 0.1 or 1 μg/ml of anti-TLR4 blocking antibodies, washed twice or left unwashed, and then stimulated with Tat (10 nM), a decrease of the inhibitory effect of anti-TLR4 antibodies on Tat-induced TNF-α and IL-10 production was observed (Fig. 2B), in agreement with the results found under the third set of conditions. These data suggest a partial TLR4–anti-TLR4 dissociation following the two washes, at least with the smallest amount (0.1 μg/ml) of anti-TLR4 antibodies (Fig. 2B). However, this phenomenon was less visible in the presence of saturating concentrations of anti-TLR4 antibodies (1 μg/ml) (Fig. 2B).
Altogether, these results indicate that the Tat protein efficiently induces the production of TNF-α and IL-10 in a TLR4-dependent manner in human monocytes.
HIV-1 Tat protein induces TNF-α and IL-10 production by activating NF-κB in an MyD88- and TRIF-dependent pathway.
Activation of the TLR4 pathway by its prototypic ligand LPS leads to the activation of both MyD88-dependent and TRIF-dependent pathways. Thus, the present study investigated whether the Tat protein was able to activate one or both pathways. To this end, three complementary approaches were used.
(i) Effects of Tat protein on TNF-α and IL-10 production in peritoneal macrophages from mice deficient in TLR4, MyD88, TIRAP, or TRIF.
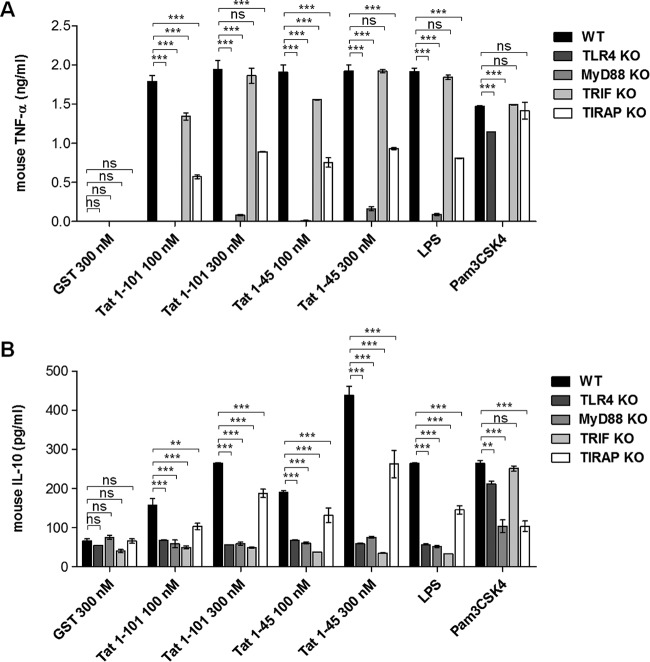
To validate the mouse as an animal model, we first showed that as in human monocytes, the Tat protein was also able to induce TNF-α and IL-10 in a TLR4-dependent manner, as previously reported (21) (see Fig. S1 in the supplemental material). We then used cells from mice deficient in MAL/TIRAP and MyD88, two adaptors of the MyD88 pathway, or TRIF, the essential adaptor of the MyD88-independent pathway. Peritoneal macrophages from each of these mutant KO mice were isolated and treated with increasing concentrations of Tat protein. After 20 h, the amounts of TNF-α and IL-10 present in cell supernatants were quantified by ELISA. As expected, we showed that the Tat protein, like the positive controls, i.e., LPS (TLR4 ligand) and Pam3CSK4 (TLR2 ligand), stimulated the production of TNF-α and IL-10 in peritoneal macrophages isolated from WT mice (Fig. 3A and B, black bars). However, the deletion of MyD88 totally abrogated the capacity of Tat to stimulate the production of both TNF-α and IL-10 (Fig. 3A and B, gray bars), while the deletion of MAL/TIRAP, an adaptor involved in the recruitment of MyD88 to the intracytoplasmic region of TLR4, resulted in a partial inhibition of TNF-α and IL-10 production, suggesting the possibility of partial MyD88 recruitment in the absence of MAL/TIRAP expression (Fig. 3A and B, white bars).
FIG 3.
HIV-1 Tat protein induces TNF-α and IL-10 production via TLR4-, MyD88-, and TRIF-dependent pathway. Primary mouse peritoneal macrophages isolated from WT (black bars), TLR4−/− (dark gray bars), MyD88−/− (gray bars), TRIF−/− (light gray bars), and TIRAP/MAL−/− (white bars) mice were incubated with increasing amounts of recombinant GST-Tat (full length; Tat 1-101) or the GST-tagged N-terminal fragment (Tat 1-45; carrying the first 45 amino acids) or with an equal amount of GST alone. LPS (100 ng/ml; TLR4 ligand) and Pam3CSK4 (100 ng/ml; TLR1/2 ligand) were used as positive controls. After 20 h of incubation, mouse TNF-α (A) and mouse IL-10 (B) levels in the cell supernatants were quantified by ELISA. The data represent means and SD for three independent experiments. The statistical significance of differences between different groups (indicated with black lines above the bars) was analyzed by two-way ANOVA followed by Bonferroni posttests and is marked as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, nonsignificant.
After TLR4-MyD88 activation, the TLR4-ligand complex is internalized and localized in the early endosome, where activation of the TRIF pathway can be initiated. Using macrophages from mice deficient in TRIF, we showed that TRIF deletion abolished IL-10 production without significantly affecting Tat-induced TNF-α production (Fig. 3A and B, light gray bars). This result is in line with the major implication of the MyD88 pathway in the induction of proinflammatory cytokines by Tat.
Altogether, our results demonstrated that, in mouse macrophages, the MyD88 pathway seems to be essential for TNF-α and IL-10 production, while the TRIF pathway seems to be essential for the production of IL-10 and only partially necessary for TNF-α production.
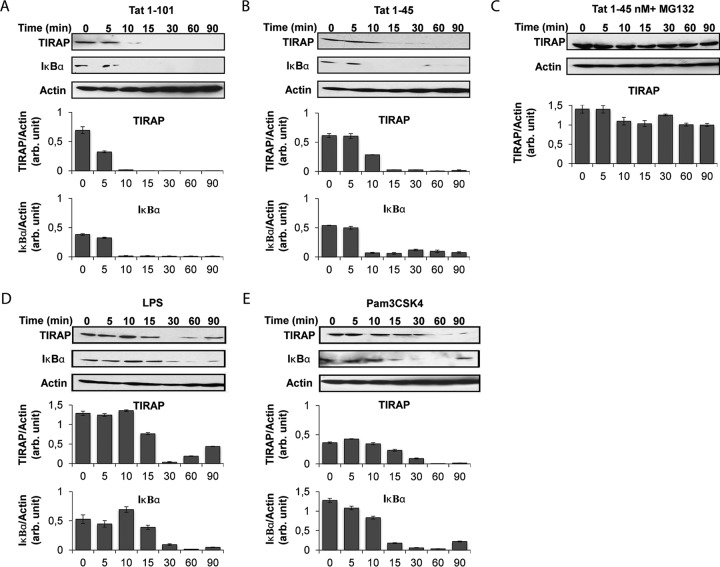
(ii) Stimulation of monocytes by Tat leads to MAL degradation.
The engagement of the MyD88 pathway in human monocytes was explored by following the degradation of the adaptor MAL/TIRAP. The THP-1 human monocyte cell line was treated for various times (between 0 and 90 min) with the Tat protein or its N-terminal active fragment (Tat 1-45). After cell lysis, cellular proteins were separated by SDS-PAGE and analyzed by Western blotting. Labeling was performed with specific anti-MAL/TIRAP antibodies. Under these conditions, the results showed a time-dependent decrease of the level of MAL/TIRAP in Tat-treated cells (Fig. 4A and B). After 15 min of Tat treatment, MAL/TIRAP became undetectable, indicating its total degradation. Moreover, MAL/TIRAP degradation could also be observed when stimulation was performed with the N-terminal fragment of the Tat protein (Tat 1-45) (Fig. 4B). The latter result is in agreement with our previous data showing that the Tat 1-45 mutant was also able to stimulate the TLR4 pathway (21, 22). The degradation of MAL/TIRAP following Tat stimulation seems to be related to its proteasomal degradation. In the presence of MG132, an inhibitor of proteasome activity, MAL/TIRAP degradation was totally inhibited (Fig. 4C). As positive controls, we showed that monocyte stimulation with LPS (Fig. 4D) or Pam3CSK4 (Fig. 4E), both known for their capacity to stimulate TLR4 and TLR2 pathways in an MyD88-dependent manner, also led to MAL/TIRAP degradation. These results suggest that the Tat protein activates the MyD88 pathway in human THP-1 cells via its N-terminal domain (aa 1 to 45), in agreement with the mouse data presented in Fig. 3. These results are also in line with the rapid kinetics of TLR4 pathway activation following Tat treatment, within 10 to 15 min, as presented in Fig. 2A (kinetic data). All these results indicate that the Tat protein activates the TLR4 pathway by recruiting the MyD88 pathway, as demonstrated by its capacity to lead to the degradation of MAL/TIRAP.
FIG 4.
HIV-1 Tat protein leads to time-dependent and proteasome-dependent TIRAP/MAL degradation via its N-terminal domain. THP-1 cells (106 cells/condition) were treated with recombinant GST-Tat (full-length; Tat 1-101) (A) or the GST-tagged N-terminal fragment (Tat 1-45) (B) at 180 nM. (C) Cells were preincubated with MG132 (20 μM) for 60 min before treatment with Tat 1-45. As positive controls, cells were treated with LPS (1 μg/ml) (D) and Pam3CSK4 (1 μg/ml) (E). Cells were lysed after different times (0 to 90 min). Cellular proteins were separated by SDS-PAGE and analyzed by Western blotting for TIRAP/MAL and IκBα expression. Actin was also quantified as a loading control. Quantification of the bands obtained from 3 independent experiments was performed using ImageJ software. Data represent TIRAP/MAL expression or IκB expression normalized to actin expression.
(iii) The MyD88, TIRAP/MAL, and TRIF pathways are essential for NF-κB activation.
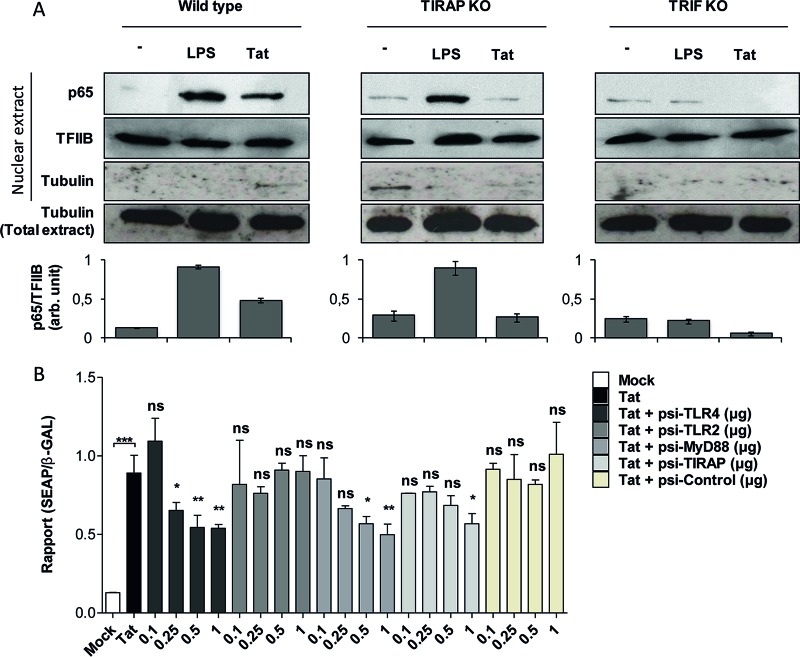
In previous work, we showed that Tat activates NF-κB in a TLR4-dependent manner (22). Downstream of TLR4 activation, MyD88 and MAL/TIRAP work together to mediate the early activation of NF-κB, while the TRIF pathway is involved in the late phase of NF-κB activation. Therefore, the effects of these adaptors on Tat-induced NF-κB activation were analyzed by testing nuclear p65–NF-κB translocation in Tat-treated macrophages from mice deficient in MAL/TIRAP or TRIF. While the p65 level in the nuclear fraction from unstimulated cells was undetectable, the treatment of WT peritoneal macrophages with Tat protein (100 nM) or with LPS (10 ng/ml), as a positive control, led to NF-κB activation as demonstrated by the nuclear translocation of p65 (Fig. 5A). When the same assay was performed using macrophages from mice deficient in MAL/TIRAP or TRIF, Tat-induced NF-κB activation was strongly inhibited (Fig. 5A). These results show that downstream of TLR4, NF-κB activation by Tat seems to be dependent on both the MyD88 and TRIF pathways.
FIG 5.
HIV-1 Tat protein activates NF-κB downstream of TLR4-, MyD88-, TIRAP/MAL-, and TRIF-dependent pathways. (A) Primary mouse peritoneal macrophages isolated from WT, TIRAP/MAL−/−, or TRIF−/− mice were incubated with LPS (10 ng/ml) or the full-length Tat protein (100 nM) or left untreated (−) for 30 min. Cells were lysed, and the p65 subunit of NF-κB was detected in the nuclear fraction of the cells by Western blotting. TFIIB and tubulin were used as loading controls. Quantification of the bands obtained from 3 independent experiments was performed using ImageJ software. Data represent NF-κB (p65) expression in the nucleus normalized to TFIIB expression. (B) HEK cells expressing TLR4/MD2-CD14 were cotransfected with an NF-κB reporter plasmid (SEAP) together with the same amount of pORF-LacZ. Cells were also cotransfected with the indicated amounts (in micrograms) of plasmids encoding siRNAs targeting TLR4, TLR2, MyD88, and TIRAP/MAL or control siRNA or were left without transfection with siRNA (white bar, mock transfection; black bar, Tat stimulation). After 24 h of transfection, cells were left untreated (mock) or treated with Tat 1-45 (100 nM). Twenty-four hours after stimulation, NF-κB-driven SEAP reporter gene expression in the culture supernatants was measured. For normalization, cells were lysed and expression of β-galactosidase was quantified. The data represent means and SD for three independent experiments. Statistical significance was analyzed by one-way ANOVA followed by Bonferroni posttests and is marked as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, nonsignificant. All bars were compared to Tat-treated cells in the absence of cotransfection with any siRNA (black bars) unless otherwise specified.
In line with NF-κB activation by Tat in human monocytes, the results presented in Fig. 3 show that MAL/TIRAP degradation also paralleled the degradation of IκB, an inhibitor of NF-κB, suggesting an IκB–NF-κB dissociation, p65–NF-κB nuclear translocation, and its proteasomal degradation. To further explore the implication of the MyD88 pathway in Tat-induced NF-κB activation, we used a cellular model comprising HEK293 cells stably transfected with TLR4 in association with its cofactors, CD14 and MD2 (HEK TLR4/MD2-CD14 cells). Before stimulation with Tat, HEK TLR4/MD2-CD14 cells were transiently cotransfected with two plasmids, the first carrying the gene for SEAP under the control of NF-κB and the second carrying the gene for β-galactosidase under the control of a constitutively active promoter, used for normalization. To evaluate the relationship of MyD88 and its adaptor MAL/TIRAP to NF-κB, SEAP/β-galactosidase-cotransfected HEK TLR4/MD2-CD14 cells were also transfected with escalating amounts of siRNA specific to MyD88 or MAL before their treatment with the HIV-1 Tat protein, and SEAP and β-galactosidase activities were determined 48 h later. In the presence of MyD88 siRNA (si-MyD88), a dose-response inhibition was observed (Fig. 5B). With 1 μg of si-MyD88, an inhibition of about 40% of SEAP activity was obtained (Fig. 5B). In a positive-control experiment in the presence of 1 μg of siRNA specific to TLR4, a similar inhibition of Tat-induced TLR4 signaling (about 40%) was observed (Fig. 5B). In line with the data obtained with macrophages from MAL/TIRAP KO mice (Fig. 3), only a relatively weak inhibition was observed with MAL siRNA (Fig. 5B). HEK TLR4/MD2-CD14 cell transfection with siRNA specific to TLR2, used as a negative control, had no effect on Tat-induced SEAP activity (Fig. 5B). These results show that MyD88 is an essential adaptor that is recruited following TLR4 engagement by the HIV-1 Tat protein to activate NF-κB and to trigger the production of IL-10 and TNF-α, while TIRAP/MAL is partially required and TRIF seems to be essential only for IL-10 production. As a control, the same experiment was performed with the natural TLR4 ligand LPS (see Fig. S2 in the supplemental material). The results show that in the HEK TLR4/MD2-CD14 cell line, NF-κB activation by LPS is tightly dependent on TLR4, MyD88, and TIRAP/MAL expression but independent of TLR2 expression (see Fig. S2).
Role of the TLR4 pathway in the activation of PKC and MAP kinases p38 and ERK1/2.
The interrelation between the engagement of the TLR4 pathway by the Tat protein and the downstream activation of PKC and MAP kinases p38 and ERK1/2 was then investigated.
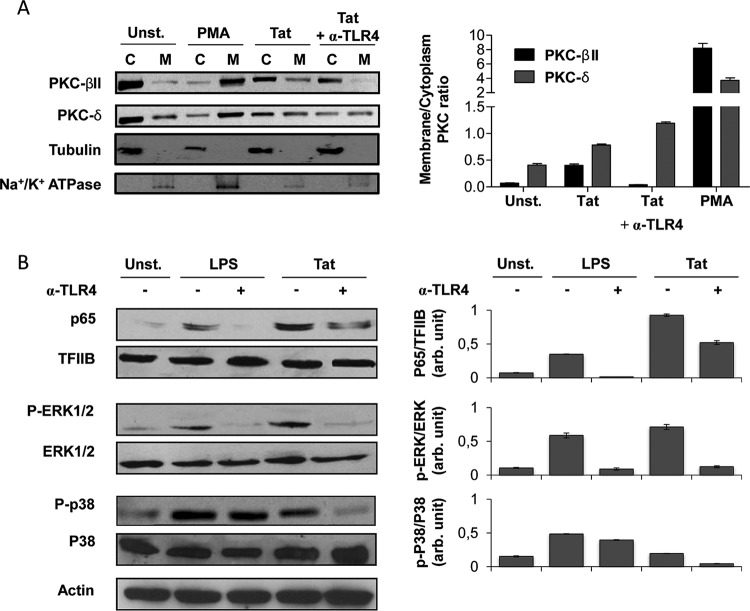
First, the role of the TLR4 pathway in the activation of the PKC pathway by Tat was evaluated. Previously, our group showed that the HIV-1 Tat protein was able to activate PKC isoforms α, βII, δ, and ε (38). We also demonstrated that among these Tat-activated PKC isoforms, only PKC-βII and -δ were essential for Tat-induced TNF-α and IL-10 production in human monocytes (38, 39). To understand whether the activation of PKC-βII and PKC-δ by Tat is TLR4 dependent, the present work tested the effect of anti-TLR4 blocking antibodies on Tat-induced PKC activation in human monocytes. The results presented in Fig. 6A show that the treatment of human monocytes with the Tat protein or PMA (positive control) induced the activation of PKC-βII and PKC-δ as demonstrated by their translocation from the cytoplasmic to the membrane compartment. In fact, in their inactive forms, the PKC isoforms are localized in the cytoplasm, but upon activation, they adopt conformational changes and translocate to the membrane. This translocation to the membrane compartment is used as a signature of PKC activation. However, when the activation was performed in the presence of anti-TLR4 blocking antibodies, an almost complete inhibition of PKC-βII was observed (Fig. 6A), while under the same conditions, Tat-induced PKC-δ did not seem to be impaired significantly (Fig. 6A).
FIG 6.
HIV-1 Tat protein activates NF-κB, MAP kinases ERK1/2 and p38, and PKC-βII in a TLR4-dependent manner. (A and B) Primary human monocytes were incubated with Tat protein (100 nM) in the presence or absence of anti-TLR4 antibodies (1 μg/ml). LPS (100 ng/ml) treatment was used as a positive control for TLR4 pathway activation, and PMA (100 ng/ml) was used as a positive control for PKC activation. After 30 min, cells were lysed as described in Materials and Methods to prepare cytoplasm/membrane (A) or cytoplasm/nucleus (B) protein extracts. (A) Membrane (M) and cytoplasmic (C) protein extracts were separated by SDS-PAGE and analyzed by Western blotting using specific antibodies for PKC isoforms (PKC-βII and PKC-δ). Quantification of the membrane/cytoplasm signal ratios for 3 independent experiments was performed using ImageJ software. The histogram shows the ratios of membrane/cytoplasm PKC-βII (black bars) and PKC-δ (gray bars). Detection of tubulin and Na+/K+ ATPase were performed to control for the quality of protein extracts from the cytoplasmic and membrane compartments, respectively. (B) Cytoplasmic and nuclear extracts were separated by SDS-PAGE and analyzed by Western blotting using specific antibodies for NF-κB/p65, ERK1/2, phospho-ERK1/2, p38, and phospho-p38. Cytoplasmic and nuclear fractions were normalized by use of anti-actin and anti-TFIIB, respectively. Quantification of the bands obtained from 3 independent experiments was performed using ImageJ software.
Overall, these results suggest that PKC-βII but not PKC-δ isoform activation seems to be at least partially dependent on the TLR4 signaling pathway.
Because MAP kinases are key actors in Tat-induced cytokine production, and given that the PKC pathway can also activate MAP kinases downstream (39, 40), we tested the effect of TLR4 blockade on the capacity of Tat to trigger p38 and ERK1/2 MAP kinase activation in human monocytes. The results presented in Fig. 6B show that the p38 and ERK1/2 MAP kinases were activated in human monocytes following stimulation by Tat (100 nM). However, when the stimulation was performed in the presence of anti-TLR4 antibodies (1 μg/ml), a strong inhibition of the activation of these MAP kinases, by about 80% for ERK1/2 and 70% for p38, was observed (Fig. 6B), thus indicating a direct link between TLR4 recruitment by Tat and activation of the p38 and ERK1/2 MAP kinases.
DISCUSSION
This study has shown that the recruitment of the TLR4 pathway by Tat occurs in a dose-dependent manner, with rapid kinetics. The pretreatment of human monocytes with Tat for 5 to 30 min before the addition of saturating concentrations of anti-TLR4 blocking antibodies seems to be sufficient to irreversibly engage the TLR4 pathway leading to the production of TNF-α and IL-10.
This capacity of HIV-1 to activate the TLR4 pathway, an innate immune response normally involved in the antiviral response, could be considered a paradoxical, suicidal response when induced by a pathogen. However, in order to escape control by the immune system, many viruses, including HIV-1, HBV, HCV, influenza A virus, and herpesviruses, have evolved various strategies to manipulate the immune system (41). In general, two essential mechanisms have been developed by viruses: either they initiate inappropriate and continuous PRR stimulations, which may establish chronic immune activation, or they interfere with several key steps in PRR signaling cascades. Both of these strategies are associated with immune dysregulation and immunosuppression, which are deleterious to the control of viral infection. For example, respiratory syncytial virus, by its F protein (42), and mouse mammary tumor virus (MMTV), by its envelope glycoprotein (43), hijack the TLR4 pathway leading to the production of IL-10, a highly immunosuppressive cytokine which is involved in T-cell exhaustion and the weakening of dendritic cell function, thus contributing to the establishment of persistent viral infections, while HCV inhibits the PRR signaling pathway by blocking MyD88 and catalyzing IRF3 degradation by its proteins NS5A (44) and NS3-4A, respectively (45).
However, the most important conclusions on the mechanisms that control viral persistence were drawn from the prototypic model of LCMV Cl-13 infection of mice (46–48). In this model, chronic infection was accompanied by high viral loads, a strong type 1 IFN response, chronic immune activation, disruption of lymphoid architecture, and induction of immunosuppressive factors, including IL-10 and PD-1/PD-L1 (46–48).
Despite the known antiviral function of type 1 IFN, several convergent studies have also shown that it has a proviral action by inducing immunoregulatory factors, including IDO, IL-10, and PD-1/PD-L1, leading to an immunosuppressive state beneficial for the persistence of the virus. More recently, Ng et al. (46) showed that the proviral action induced by LCMV Cl-13 seems to be mediated by type 1 IFN-β but not type 1 IFN-α. Blockade of type-1 IFN-β, but not IFN-α, signaling by specific antibodies allowed clearance of the virus and the restoration of immune system functions (46–48). This kind of strategy also seems to be exploited in the mechanisms of persistence of HIV-1, SIV, and HCV (49–51).
In the case of HIV-1, our group has shown that the Tat protein, in addition to gp120 (52) and Vpr (15), by activating the TLR4 pathway, leads to the production of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-8, and type 1 IFN-α1, and to stimulation of immunosuppressive factors, including IL-10, PD-L1, and IDO, by dendritic cells (22–24), thus supporting the implication of the HIV-1 Tat protein as a potential pathogenic factor. The product of the tat gene is a protein of 14 to 16 kDa, composed of 86 to 101 amino acids, which is expressed during the early phase of the HIV-1 cycle. Despite the lack of a signal peptide, the Tat protein can be secreted by infected cells and taken up by neighboring cells, whether they are infected or not. Then, by its intracellular and cell surface actions, Tat may lead to HIV-1 transactivation in latently infected cells and to the activation or inhibition of a variety of cellular host factors, including proinflammatory cytokines and immunosuppressive factors known for their involvement in immune dysregulation (23, 24, 35), neurocognitive disorders (4), and intestinal glucose absorption dysfunctions (53). In association with the inflammatory response, the latter dysfunctions may contribute to the early alteration of the intestinal barrier observed in HIV-1-infected patients. The Tat protein is found at nanomolar levels in the sera of HIV-1-infected patients (54–56), but this Tat concentration is probably underestimated due to the fact that a large proportion of the secreted Tat protein is probably found complexed with heparan sulfate at the cell surface, essentially in the neighborhood of HIV-1-infected cells in lymphoid organs (37). In line with this interpretation, Santosuosso et al. showed that while the concentration of the external envelope glycoprotein gp120 remains undetectable in the plasma of HIV-1-infected patients, its concentration in secondary lymphoid tissues can be estimated to be about 2.5 × 10−9 M (57). We observed that the Tat protein produced under more physiological conditions, released by HeLa cells stably transfected with the HIV-1 Tat protein or by HIV-1-infected Jurkat cells, was able to induce the production of TNF-α and IL-10 in human monocytes (not shown). However, the production of these cytokines cannot be attributed solely to the effect of Tat because of the presence in the culture supernatant of HIV-1-infected cells of several HIV-1 proteins, including Nef, gp120, gp41, Vpu, and Vpr, and many HIV-1-induced cytokines, which are also known for their capacity to induce the production of TNF-α and IL-10. Finally, by using a chemically synthesized Tat protein, we showed that the synthetic Tat protein, but not its acetylated (at K50) derivative, stimulates TNF-α and IL-10 production in human monocytes. The unexpected loss of activity of Tat with acetylated K50 in comparison with that of the active Tat 1-45 construct may be related to structural modifications in the N-terminal active domain of Tat following its acetylation at lysine residue 50.
Starting from the observations that in human monocytes, macrophages, and dendritic cells, the HIV-1 Tat protein can induce the production of TNF-α and IL-10 in a PKC-, MAP kinase-, and NF-κB-dependent manner and that the same signaling pathways are also activated downstream of the TLR4 pathway, we advanced the hypothesis of the recruitment of the TLR4 pathway by the Tat protein. This hypothesis is supported by our previous data showing that (i) Tat-induced cytokines are totally inhibited in the presence of anti-TLR4 blocking antibodies, (ii) Tat interacts physically and with high affinity with the TLR4-MD2 complex, and (iii) Tat loses its capacity to induce TNF-α and IL-10 production in macrophages from KO mice deficient in TLR4, MD2, or CD14 (21, 22).
The activation of the TLR4 pathway by LPS, the prototypic ligand of TLR4, leads to the activation of both MyD88 and TRIF (26). Thus, the present study first investigated whether the HIV-1 Tat protein was able to activate these two pathways separately or simultaneously and then analyzed the role of each pathway in the control of TNF-α and IL-10 production in human monocytes or macrophages from WT mice or mice deficient in MyD88 or TRIF. Three complementary approaches were used, including mice deficient for each adaptor, biochemical analysis targeting MAL/TIRAP degradation, and use of siRNA specific for TLR4, MAL/TIRAP, or MyD88.
Overall, our findings showed (i) the ability of Tat to activate both the MyD88 and TRIF pathways; (ii) the capacity of Tat to induce MAL/TIRAP degradation, which is considered a signature of MyD88 engagement; (iii) the crucial role of the MyD88 pathway for Tat-induced TNF-α and IL-10 production; (iv) a reduction, but not abrogation, of IL-10 and TNF-α production by Tat-stimulated macrophages from mice deficient in MAL/TIRAP; (v) the crucial role of the TRIF pathway in Tat-induced IL-10, while the absence of this adaptor seems to have a limited effect on Tat-induced TNF-α; and (vi) a direct relationship between MyD88 inhibition with a specific siRNA and the capacity of Tat to activate NF-κB.
The results showing that Tat-induced IL-10 production is MyD88 dependent are in agreement with the high efficiency of IL-10 production via TLR2, a pathway that is MyD88 dependent only in macrophages and dendritic cells (58). However, IL-10 production can also occur via TLR3 in macrophages where only the TRIF pathway can be engaged (59). These conclusions are in agreement with the fact that optimal LPS induction of IL-10 in macrophages necessitates both the MyD88 and TRIF pathways (59, 60). It is important to note that both the MyD88 and TRIF pathways are essential for Tat-induced IL-10 production in mouse macrophages, while only the MyD88 pathway is essential for Tat-induced TNF-α production in the same model.
The analysis of the subsequently activated signaling pathways demonstrated that the Tat protein activated the MAP kinases ERK1/2 and p38, NF-κB, and at least the PKC-βII isoform, in a TLR4-dependent manner. When the stimulation by Tat protein was performed in the presence of anti-TLR4 blocking antibodies, the activation of these pathways was abrogated.
Overall, the novelty of our finding can be summarized in the following three points: (i) Tat interacts with and activates the TLR4 pathway with a rapid kinetics; (ii) Tat activates both the MyD88 and TRIF pathways, leading to the production of TNF-α and IL-10; and (iii) Tat activates the PKC-βII isoform, the p38 and ERK1/2 MAP kinases, and NF-κB in a TLR4-dependent manner.
We previously showed that Tat-induced IL-10 production in human monocytes is essentially dependent on PKC-βII and -δ isoform activation (38). In the present study, we showed that only PKC-βII activation seems to be related to TLR4 pathway signaling by the Tat protein. However, the possibility cannot be excluded that PKC-δ activation is mediated following the signaling pathways activated after Tat interaction with its other potential receptors, including the CD26 receptor, L-type calcium channels, integrins αvβ3 and α5β1, membrane lipids, and the Flk-1/KDR receptor (37).
Considering that the Tat protein is (i) expressed in the early phase of the viral cycle, (ii) secreted by infected cells, (iii) present in the plasma of infected patients, (iv) capable of inducing an inflammatory response following the engagement of the TLR4 pathway, and (v) able to disturb several physiological functions, including the immune, nervous, and gastrointestinal systems, it seems very important to target Tat as a pathogenic factor early after HIV-1 infection. This could be achieved either by vaccination approaches including Tat as an immunogen in potential candidate vaccines or by developing molecules capable of neutralizing the effect of the Tat protein.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Ryffel from the Université d'Orléans for providing the TLR4 KO, MyD88 KO, TRIF KO, and TIRAP/MAL KO mice used in this study, the staff of the INSERM U1043 flow cytometry imaging facility, J. Auriol and S. Ethuin from UMR 5547 CNRS for animal housing, N. Jabrane, J. Gouilly, and H. Elcosta for their support, and S. Becker for English editing.
R.P. was supported by an MRT fellowship. N.B.H. was supported by MRT and SIDACTION fellowships.
All the authors concurred with the manuscript submission and have no financial/commercial conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00262-16.
REFERENCES
- 1.Miller E, Bhardwaj N. 2013. Dendritic cell dysregulation during HIV-1 infection. Immunol Rev 254:170–189. doi: 10.1111/imr.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orsilles MA, Pieri E, Cooke P, Caula C. 2006. IL-2 and IL-10 serum levels in HIV-1-infected patients with or without active antiretroviral therapy. APMIS 114:55–60. doi: 10.1111/j.1600-0463.2006.apm_108.x. [DOI] [PubMed] [Google Scholar]
- 3.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Mayne M, Power C, Nath A. 1997. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem 272:22385–22388. [DOI] [PubMed] [Google Scholar]
- 5.Boasso A, Shearer GM. 2008. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol 126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 7.d'Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V. 2011. HIV-associated immune activation: from bench to bedside. AIDS Res Hum Retroviruses 27:355–364. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- 8.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 9.Rey-Cuille MA, Berthier JL, Bomsel-Demontoy MC, Chaduc Y, Montagnier L, Hovanessian AG, Chakrabarti LA. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol 72:3872–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvestri G. 2005. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J Med Primatol 34:243–252. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 11.Centlivre M, Sala M, Wain-Hobson S, Berkhout B. 2007. In HIV-1 pathogenesis the die is cast during primary infection. AIDS 21:1–11. doi: 10.1097/QAD.0b013e3280117f7f. [DOI] [PubMed] [Google Scholar]
- 12.Skoner DP, Gentile DA, Patel A, Doyle WJ. 1999. Evidence for cytokine mediation of disease expression in adults experimentally infected with influenza A virus. J Infect Dis 180:10–14. doi: 10.1086/314823. [DOI] [PubMed] [Google Scholar]
- 13.Zaunders JJ, Kaufmann GR, Cunningham PH, Smith D, Grey P, Suzuki K, Carr A, Goh LE, Cooper DA. 2001. Increased turnover of CCR5+ and redistribution of CCR5− CD4 T lymphocytes during primary human immunodeficiency virus type 1 infection. J Infect Dis 183:736–743. doi: 10.1086/318827. [DOI] [PubMed] [Google Scholar]
- 14.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. 2010. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino S, Konishi M, Mori M, Shimura M, Nishitani C, Kuroki Y, Koyanagi Y, Kano S, Itabe H, Ishizaka Y. 2010. HIV-1 Vpr induces TLR4/MyD88-mediated IL-6 production and reactivates viral production from latency. J Leukoc Biol 87:1133–1143. doi: 10.1189/jlb.0809547. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 17.Berg RK, Melchjorsen J, Rintahaka J, Diget E, Soby S, Horan KA, Gorelick RJ, Matikainen S, Larsen CS, Ostergaard L, Paludan SR, Mogensen TH. 2012. Genomic HIV RNA induces innate immune responses through RIG-I-dependent sensing of secondary-structured RNA. PLoS One 7:e29291. doi: 10.1371/journal.pone.0029291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wang X, Li J, Zhou Y, Ho W. 2013. RIG-I activation inhibits HIV replication in macrophages. J Leukoc Biol 94:337–341. doi: 10.1189/jlb.0313158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. 2013. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakobsen MR, Bak RO, Andersen A, Berg RK, Jensen SB, Tengchuan J, Laustsen A, Hansen K, Ostergaard L, Fitzgerald KA, Xiao TS, Mikkelsen JG, Mogensen TH, Paludan SR. 2013. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci U S A 110:E4571–E4580. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben Haij N, Leghmari K, Planes R, Thieblemont N, Bahraoui E. 2013. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-alpha and IL-10. Retrovirology 10:123. doi: 10.1186/1742-4690-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben Haij N, Planes R, Leghmari K, Serrero M, Delobel P, Izopet J, BenMohamed L, Bahraoui E. 2015. HIV-1 Tat protein induces production of proinflammatory cytokines by human dendritic cells and monocytes/macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-kappaB pathway. PLoS One 10:e0129425. doi: 10.1371/journal.pone.0129425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Planes R, Bahraoui E. 2013. HIV-1 Tat protein induces the production of IDO in human monocyte derived-dendritic cells through a direct mechanism: effect on T cells proliferation. PLoS One 8:e74551. doi: 10.1371/journal.pone.0074551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Planes R, BenMohamed L, Leghmari K, Delobel P, Izopet J, Bahraoui E. 2014. HIV-1 Tat protein induces PD-L1 (B7-H1) expression on dendritic cells through tumor necrosis factor alpha- and Toll-like receptor 4-mediated mechanisms. J Virol 88:6672–6689. doi: 10.1128/JVI.00825-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piao W, Song C, Chen H, Wahl LM, Fitzgerald KA, O'Neill LA, Medvedev AE. 2008. Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. J Biol Chem 283:3109–3119. doi: 10.1074/jbc.M707400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat Rev Immunol 4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 27.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. 2011. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the Toll adapters TRAM and TRIF. J Exp Med 198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT. 2012. Selective TRIF-dependent signaling by a synthetic Toll-like receptor 4 agonist. Sci Signal 5:ra13. doi: 10.1126/scisignal.2001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGettrick AF, Brint EK, Palsson-McDermott EM, Rowe DC, Golenbock DT, Gay NJ, Fitzgerald KA, O'Neill LA. 2006. Trif-related adapter molecule is phosphorylated by PKC{epsilon} during Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A 103:9196–9201. doi: 10.1073/pnas.0600462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dallot E, Mehats C, Oger S, Leroy MJ, Breuiller-Fouche M. 2005. A role for PKCzeta in the LPS-induced translocation NF-kappaB p65 subunit in cultured myometrial cells. Biochimie 87:513–521. doi: 10.1016/j.biochi.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Kubo-Murai M, Hazeki K, Sukenobu N, Yoshikawa K, Nigorikawa K, Inoue K, Yamamoto T, Matsumoto M, Seya T, Inoue N, Hazeki O. 2007. Protein kinase Cdelta binds TIRAP/Mal to participate in TLR signaling. Mol Immunol 44:2257–2264. doi: 10.1016/j.molimm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Badou A, Bennasser Y, Moreau M, Leclerc C, Benkirane M, Bahraoui E. 2000. Tat protein of human immunodeficiency virus type 1 induces interleukin-10 in human peripheral blood monocytes: implication of protein kinase C-dependent pathway. J Virol 74:10551–10562. doi: 10.1128/JVI.74.22.10551-10562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennasser Y, Badou A, Tkaczuk J, Bahraoui E. 2002. Signaling pathways triggered by HIV-1 Tat in human monocytes to induce TNF-alpha. Virology 303:174–180. doi: 10.1006/viro.2002.1676. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Boppana R, Mishra GC, Saha B, Mitra D. 2008. HIV-1 Tat suppresses gp120-specific T cell response in IL-10-dependent manner. J Immunol 180:79–88. doi: 10.4049/jimmunol.180.1.79. [DOI] [PubMed] [Google Scholar]
- 36.Leghmari K, Bennasser Y, Bahraoui E. 2008. HIV-1 Tat protein induces IL-10 production in monocytes by classical and alternative NF-kappaB pathways. Eur J Cell Biol 87:947–962. doi: 10.1016/j.ejcb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Noonan D, Albini A. 2000. From the outside in: extracellular activities of HIV Tat. Adv Pharmacol 48:229–250. doi: 10.1016/S1054-3589(00)48008-7. [DOI] [PubMed] [Google Scholar]
- 38.Bennasser Y, Bahraoui E. 2002. HIV-1 Tat protein induces interleukin-10 in human peripheral blood monocytes: involvement of protein kinase C-betaII and -delta. FASEB J 16:546–554. doi: 10.1096/fj.01-0775com. [DOI] [PubMed] [Google Scholar]
- 39.Leghmari K, Contreras X, Moureau C, Bahraoui E. 2008. HIV-1 Tat protein induces TNF-alpha and IL-10 production by human macrophages: differential implication of PKC-betaII and -delta isozymes and MAP kinases ERK1/2 and p38. Cell Immunol 254:46–55. doi: 10.1016/j.cellimm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Leghmari K, Bennasser Y, Tkaczuk J, Bahraoui E. 2008. HIV-1 Tat protein induces IL-10 production by an alternative TNF-alpha-independent pathway in monocytes: role of PKC-delta and p38 MAP kinase. Cell Immunol 253:45–53. doi: 10.1016/j.cellimm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Bowie AG, Unterholzner L. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 43.Jude BA, Pobezinskaya Y, Bishop J, Parke S, Medzhitov RM, Chervonsky AV, Golovkina TV. 2003. Subversion of the innate immune system by a retrovirus. Nat Immunol 4:573–578. doi: 10.1038/ni926. [DOI] [PubMed] [Google Scholar]
- 44.Abe T, Kaname Y, Hamamoto I, Tsuda Y, Wen X, Taguwa S, Moriishi K, Takeuchi O, Kawai T, Kanto T, Hayashi N, Akira S, Matsuura Y. 2007. Hepatitis C virus nonstructural protein 5A modulates the Toll-like receptor–MyD88-dependent signaling pathway in macrophage cell lines. J Virol 81:8953–8966. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M Jr, Lemon SM. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A 102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng CT, Sullivan BM, Teijaro JR, Lee AM, Welch M, Rice S, Sheehan KC, Schreiber RD, Oldstone MB. 2015. Blockade of interferon beta, but not interferon alpha, signaling controls persistent viral infection. Cell Host Microbe 17:653–661. doi: 10.1016/j.chom.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. 2013. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolen CR, Robek MD, Brodsky L, Schulz V, Lim JK, Taylor MW, Kleinstein SH. 2013. The blood transcriptional signature of chronic hepatitis C virus is consistent with an ongoing interferon-mediated antiviral response. J Interferon Cytokine Res 33:15–23. doi: 10.1089/jir.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, Silvestri G, Muller-Trutwin M, Vasile-Pandrea I, Apetrei C, Hirsch V, Lifson J, Brenchley JM, Estes JD. 2010. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol 84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sedaghat AR, German J, Teslovich TM, Cofrancesco J Jr, Jie CC, Talbot CC Jr, Siliciano RF. 2008. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol 82:1870–1883. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, Dizzell S, Chauvin S, Mian MF, Ouellet M, Tremblay MJ, Mossman KL, Ashkar AA, Kovacs C, Bowdish DM, Snider DP, Kaul R, Kaushic C. 2013. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol 191:4246–4258. doi: 10.4049/jimmunol.1301482. [DOI] [PubMed] [Google Scholar]
- 53.Canani RB, De Marco G, Passariello A, Buccigrossi V, Ruotolo S, Bracale I, Porcaro F, Bifulco G, Guarino A. 2006. Inhibitory effect of HIV-1 Tat protein on the sodium-d-glucose symporter of human intestinal epithelial cells. AIDS 20:5–10. doi: 10.1097/01.aids.0000198088.85572.68. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein G. 1996. HIV-1 Tat protein as a potential AIDS vaccine. Nat Med 2:960–964. doi: 10.1038/nm0996-960. [DOI] [PubMed] [Google Scholar]
- 55.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 56.Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. 2000. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A 97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santosuosso M, Righi E, Lindstrom V, Leblanc PR, Poznansky MC. 2009. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J Infect Dis 200:1050–1053. doi: 10.1086/605695. [DOI] [PubMed] [Google Scholar]
- 58.Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol 172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 59.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, Bates EE, Akira S, Vieira P, Liu YJ, Trinchieri G, O'Garra A. 2006. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol 177:7551–7558. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 60.Chang EY, Guo B, Doyle SE, Cheng G. 2007. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol 178:6705–6709. doi: 10.4049/jimmunol.178.11.6705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.