Abstract
Background
Delayed cerebral ischemia (DCI) is the most common and disabling complication among patients hospitalized for subarachnoid hemorrhage (SAH). Clinical and radiographic methods often fail to detect DCI early enough to avert irreversible injury. We assessed the clinical feasibility of implementing a continuous electroencephalography (cEEG) ischemia monitoring service for early DCI detection as part of an institutional guideline.
Methods
An institutional neuromonitoring guideline was designed by an interdisciplinary team of neurocritical care, clinical neurophysiology and neurosurgery physicians as well as nursing staff and cEEG technologists. The interdisciplinary team focused on 1) selection criteria of high-risk patients, 2) minimization of safety concerns related to prolonged monitoring, 3) technical selection of quantitative and qualitative neurophysiologic parameters based on expert consensus and review of the literature, 4) a structured interpretation and reporting methodology, prompting direct patient evaluation and iterative neurocritical care, and 5) a two-layered quality assurance process including structured clinician interviews assessing for events of neurological worsening and an adjudicated consensus review of neuroimaging and medical records. The resulting guideline’s clinical feasibility was then prospectively evaluated.
Results
The institutional SAH monitoring guideline employed transcranial Doppler ultrasonography (TCD) and cEEG monitoring for vasospasm and ischemia monitoring in patients with either Fisher 3 or Hunt Hess 4–5 SAH. Safety criteria focused on prevention of skin breakdown and agitation. Technical components included monitoring of TCD velocities and cEEG features including quantitative alpha:delta ratio and percent alpha variability, qualitative evidence of new focal slowing, late-onset epileptiform activity, or overall worsening of background. Structured cEEG reports were introduced including verbal communication for findings concerning for neurological decline. The guideline was successfully implemented over 27 months, during which neurocritical care physicians referring 71 SAH patients for combined TCD and cEEG monitoring. The quality assurance process determined a DCI rate of 48% among the monitored population, over 90% of which occurred during the duration of cEEG monitoring (mean 6.9 days) beginning 2.7 days after symptom onset.
Conclusion
An institutional guideline implementing cEEG for SAH ischemia monitoring and reporting is feasible to implement and efficiently identify patients at high baseline risk of DCI during the period of monitoring.
Background
Delayed ischemic neurologic decline as a clinical phenomenon and delayed cerebral infarction as a radiologic finding, collectively called delayed cerebral ischemia (DCI), are the most common disabling events following hospitalization for aneurysmal subarachnoid hemorrhage (aSAH) (M. D. I. Vergouwen, Ilodigwe, & Macdonald, 2011). DCI occurrs in 30–40% of patients with aSAH (Hijdra et al., 1986) (Rabinstein et al.). Patients’ functional outcomes after aSAH have improved over recent decades (Komotar et al., 2009) (Rincon, Rossenwasser, & Dumont, 2013), but DCI remains a significant cause of disability and mortality (Molyneux et al., 2009) (Andaluz & Zuccarello, 2008). The population at risk is relatively young, placing heavy burdens on patients and their families, and the devastating consequences are a public health concern (Rinkel & Algra, 2011) (Qureshi et al., 2007).
Guidelines recommend that DCI symptoms be managed (Connolly et al., 2012) with avoidance of hypovolemia and induced hypertension with consideration of intra-arterial vasodilator therapy or balloon angioplasty for patients unresponsive to medical therapy. However, DCI detection is often challenging in the setting of agitation, coma, or baseline focal signs of aphasia, weakness, or neglect. Additionally, a significant portion of DCI involves subtle cognitive syndromes such as frontal executive dysfunction, which is challenging to reliably detect in the critical care setting.
Pathophysiologically, ischemia from cerebral vasospasm or electrophysiological cerebral dysfunction causing autoregulation and metabolic stress, are both mechanisms contributing to the development of DCI (Budohoski et al., 2013) (Macdonald, 2013). This dynamic process of mechanical, molecular and cellular responses can affect broad areas of brain tissue, at sites far from the aneurysmal rupture. For example, oxyhemoglobin leaked into the subarachnoid space can directly cause radiographic vasospasm, with a peaking incidence between 5–10 days. However, in vitro and in vivo evidence from animal models and single-center and multi-center randomized controlled trials in humans suggest that radiographic vasopasm does not uniformly overlap with DCI (M. D. I. Vergouwen et al., 2011) (Frontera et al., 2009). Up to 70% of patients with SAH have arterial narrowing, but only 20–30% develop focal deficits secondary to vasospasm. Microthrombosis (Mervyn D I Vergouwen, Vermeulen, Coert, Stroes, & Roos, 2008), microembolism (Romano et al., 2008), microvascular spasm (Uhl, Lehmberg, Steiger, & Messmer, 2003) and cortical spreading depolarizations (Dreier et al., 2009) may each mediate synergistic pathways that lead to parenchymal injury and poor clinical outcomes even in the absence of radiographic vasospasm. The pathophysiology of DCI is reviewed in detail in B. Foreman’s article in this issue of JCN.
Given the challenge of detecting DCI before deficits become irreversible, a number of diagnostic modalities have been examined for their potential to provide a dynamic biomarker of vasospasm or parenchymal ischemia. Electroencephalography (EEG) offers continuous monitoring that provides high temporal resolution sensitive to changes in cerebral blood flow (CBF) (see Gaspard in this issue, and (Foreman & Claassen, 2012) (Claassen, Mayer, & Hirsch, 2005), (Vespa et al., 1997)).
We sought to develop and implement a critical care ischemia monitoring guideline for the early detection of DCI after SAH. We developed an interdisciplinary guideline within an institutional clinical practice committee and subsequently prospectively assessed its feasibility in routine clinical practice.
Methods
We convened a multidisciplinary team of physician and nursing specialists in neurocritical care neurology, vascular and endovascular neurosurgery, and clinical neurophysiology. The team reviewed the literature as follows to formulate an evidence-based and feasible method of clinical monitoring including: 1) selection criteria of high-risk patients; 2) minimization of safety concerns related to prolonged monitoring; 3) technical selection of quantitative and qualitative parameters based on expert consensus and review of the literature; 4) a structured interpretation and reporting methodology, prompting direct patient evaluation and iterative neurocritical care in case of cEEG findings concerning for DCI; and 5) a two-layered quality assurance process for assessing DCI. We prospectively evaluated the resulting guideline’s clinical feasibility over a 27-month period.
Results
The SAH DCI neuromonitoring guideline developed by the hospital-based Neurosciences Intensive Care Unit (NeuroICU) Clinical Practice Committee was based on a review of primary literature and published guidelines, and included expert consensus regarding methods without published evidence such as the reporting and communication of neuromonitoring findings. A case review conference was designed for adjudication and consensus reporting of in-hospital outcomes, including DCI.
Selection criteria identifying high-risk patients
The monitoring selection criteria were selected to optimize the referral of high-risk patients. Upon admission to the NeuroICU, SAH patients are screened by the responsible clinician for inclusion into the cEEG and TCD monitoring protocol for detection of neurophysiologic ischemia, subclinical seizures, and sonographic vasospasm based on the following inclusion criteria: 1) non-traumatic SAH and either 2a) poor clinical grade, specifically Hunt-Hess (HH) Grade 4 or 5, or 2b) high radiologic grade due to thick cisternal blood (Fisher Group 3). Exclusion criteria were designated: 1) AVM-related or isolated perimesencephalic hemorrhage or 2) comfort measures only status (CMO).
Technical and safety protocol
In each patient meeting the clinical selection criteria, the guideline specified that the clinical team order cEEG monitoring and notify the clinical neurophysiologist and technologist of the request for cEEG monitoring. Monitoring begins during daytime hours, utilizing a standard 10–20 international arrangement of MRI-CT compatible conductive plastic scalp electrodes (Ives Electrodes, Newburyport, MA). If acute non-convulsive status epilepticus is suspected, an emergency setup can be requested, which during nighttime hours involves initial EEG placement by in-house medical staff.
Postoperative bandages may delay cEEG monitoring in patients treated by surgical clipping. Care is taken to avoid EEG electrode placement over the temporal windows to enable TCD monitoring, which is to be performed each morning prior to 10:00 AM. No clinical consent is sought for TCD or cEEG monitoring given their inclusion as standard medical care at our institution. TCD and cEEG monitoring was stipulated to be discontinued when the patient is considered by the clinical team no longer to be at clinical risk from vasospasm and DCI.
The default cEEG monitoring duration is 10 days, which covers the peak risk period for DCI after the occurrence of SAH. Indications were stipulated for early discontinuation: 1) discharge from the NeuroICU; 2) transition to comfort care; 3) significant skin breakdown not amenable to lead adjustment or replacement; or 4) a request by the neurocritical attending or neurosurgeon related to patient safety concerns, such as agitation. Monitoring can continue beyond 10 days in certain situations, such as in case of nonconvulsive status epilepticus or DCI under active clinical management.
The guideline recommends that nursing staff mark the cEEG recording at the time of each exam and while the patient is off sedation with a single button press.
The clinical neurophysiology team monitoring cEEG was directed to generate reports twice daily, before 11:00 AM and before 8:00 PM. These reports should be communicated to the NeuroICU staff by written entries that are immediately available in the electronic medical record system. Parameters chosen for monitoring and reporting included quantitative trends such as alpha:delta ratio (ADR) or percent alpha variability (PAV) as well as emergence of new background slowing or interictal discharges on raw EEG.
Minimization of safety concerns related to prolonged monitoring is an important constituent of the monitoring guideline. A detailed protocol for prevention of skin breakdown related to EEG electrodes was created, including avoidance of tight head wrapping of electrodes, daily evaluation beneath frontal electrodes for early erythema, contact allergy, or signs of pressure, and periodic movement of each electrode. Additionally, frequent mobilization to a chair and ambulation when possible were recognized as standards requiring tolerance of intermittent cEEG electrode disconnection. Of the 71 patients evaluated, 21% (15/71) experienced some degree of skin irritation. No patients experienced EEG-related skin pressure ulcers that were beyond stage II.
EEG and TCD Reporting Measures
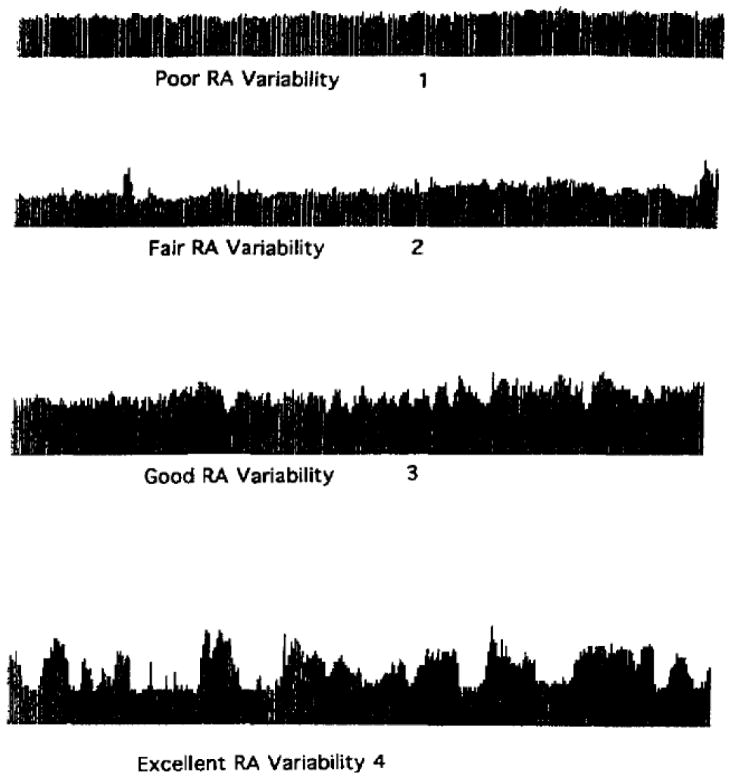
Technical selection of quantitative and qualitative parameters were guided by review of the literature, in which studies to date have involved the retrospective analysis of which cEEG patterns and trends were present in patients found to have DCI. The method of Vespa et al., (Vespa et al., 1997) is based on the semi-quantitative scoring of the variability of the relative alpha power over an 8–12 hour period (Figure 1). The technique relies on qualitative visual inspection of monitoring data. Percent alpha variability (PAV) in our protocol was calculated by a moving average of 2-minute samples and visualized with a sliding 4-hour trend panel (Persyst, Inc.; Prescott, AZ).
Figure 1.
Relative alpha variability scoring chart. Adapted from Vespa et al. 1997 (with permission from Elsevier Limited).
Based on findings from Claassen et al. (Claassen et al., 2004), our guideline stipulated two conditions for reporting an ADR decrement intended to prospectively predict DCI: a) an ADR decrease of 10% below baseline lasting 6 consecutive hours (100% sensitive and 76% specific) or b) an ADR decrement of at least 50% below baseline lasting 2 or more hours (89% sensitive and 84% specific). We also required qualitative visual inspection of cEEG data to exclude trends contaminated by artifact. Unlike the method of Claassen et al, we did not mandate that only post-stimulation epochs be evaluated.
The guideline also recommended that emergence of new discrete or periodic epileptiform discharges be reported as a potential indicator of ischemia. This decision was based on the association of epileptiform discharges with cortical spreading depolarization (Leng, Fink, & Iadecola, 2011) (Woitzik et al., 2012) and cortical spreading ischemia (Dreier et al., 2009) (Strong & Macdonald, 2012). The practice committee felt that it was not possible to distinguish the late development of new epileptiform findings from those findings of periodic discharges first documented more than 50 years ago as a feature of ischemic stroke (Chatrian, Shaw, & Leffman, 1964). Additionally, studies of periodic discharges after SAH reveal an association with prolonged neurologic decline; in one cohort of 116 poor-grade SAH patients, periodic epileptiform discharges and electrographic status epilepticus were also found to be significant predictors of poor subsequent clinically outcome (Claassen et al., 2006).
To facilitate the implementation of these literature-based cEEG monitoring criteria, we developed a structured reporting method for interpreting and reporting cEEG data. For each monitoring epoch, clinical electroencephalographers reported on: 1) cEEG background activity and reactivity including the development of any new focal slowing; 2) changes in ADR trends; 3) grading of relative alpha variability (RAV); 4) development of new epileptiform waveforms, periodic or rhythmic patterns, or seizures; or 5) an overall impression of whether the combined findings were concerning for ischemia. The guideline encouraged phone communication for worsening in any of these 5 parameters. The elements included in reports for cEEG monitoring in SAH are summarized in Table 1 and a sample report is shown in Figure 2.
Table 1.
Elements of an EEG report tailored to ischemia monitoring in the setting of aSAH.
| Summary of clinical monitoring |
|
| Detailed description of each epoch |
|
| Supplemental report |
|
Figure 2.
Sample epoch from an EEG report tailored for SAH ischemia monitoring. The portion of the report shown corresponds to the “Detailed description of each epoch” from Table 1. This template is used to report each 8–12-hour epoch, while the “Summary” and “Supplement” parts of the report are provided once at the beginning and end of the report.
The guideline also established operational definitions for sonographic TCD vasospasm, based on our historical institutional practice of trending TCD peak systolic velocity (PSV), as follows. Mild sonographic vasospasm: PSV > 200 cm/sec; moderate sonographic vasospasm, PSV >250 cm/sec, and severe sonographic vasospasm, PSV > 300 cm/sec.
Quality assurance, outcomes assessment and adjudication processes
As part of the quality improvement process the committee periodically adjudicated clinical outcomes using a clinical definition of worsening due to DCI proposed by Vergouwen et al: 1) the occurrence of focal neurological impairment or 2) a decrease of at least 2 points on the Glasgow Coma Scale for at least 1 hour, that cannot be attributed to other causes including rebleeding, hydrocephalus, procedure-related complications, seizures and systemic or metabolic abnormalities (M. D. I. Vergouwen et al., 2011). By this definition, DCI is a diagnosis of exclusion and relies heavily on both clinical judgment combined with data from imaging and laboratory tests. The most utilized imaging studies include cerebral angiography, non-contrast CT scans, perfusion CT and MRI as well as TCDs. The increasing availability of long-term continuous EEG and the high temporal resolution it provides, has made this modality a preferred option among many experts.
To ensure quality during the process of prospective evaluation of our ischemia monitoring protocol, a process was established for structured interviews with the clinical team (neurocritical care physician and nurse, separately) determining the daily incidence of either a focal neurological deficit (e.g. new abulia, aphasia, focal weakness, neglect, field cut, etc) or a global decrease in arousal (2-point decrement of the Glasgow Coma Scale) persisting for 2 hours or more. We performed a secondary review of each patient’s medical record to ensure no clinical events were missed using the structured interview process. The presence of a DCI event was determined after a case review and adjudication of these events in order to ensure they met the minimal severity and duration thresholds. Physicians were asked if they noted any new focal neurological deficits while conducting the daily neurological exam or if the patient developed any new persistent change in arousal. If so, they were asked to attribute these findings to specific causative factors such as rebleeding, hydrocephalus, procedure-related complications, seizures, and infectious or metabolic systemic abnormalities. Deficits that could not be reasonably attributed to any such alternative causes were judged as DCI. Treatment options were also discussed with the care team. Similar questions were also asked of the nurse.
Adjudication of outcomes was designed to manage the challenge of diagnosis outlined by Vergouwen. As reported by Zafar et al in this issue of the journal, when DCI is identified by a CT hypodensity or MRI restricted diffusion, only fair-to-moderate agreement was found between 5 independent raters (κ = 39.2%). Detecting DCI by clinical means (through either a decrease in the GCS > 2, a new neurological focal deficit or the combination of both findings, has a better inter-rater agreement (IRA) (κ = 48.7%, 56.6% and 54.7% respectively). However, as per proposed guidelines, clinical diagnosis of DCI is reached by exclusion of alternative explanations for the deterioration, which include rebleeding, hydrocephalus, metabolic abnormalities, seizures or an interventional procedure, among others. When any clinical or radiologic deterioration was considered DCI without prejudice to the diagnostic modality, the overall percentage agreement was high (96%) in adjudication. However, independent raters of these outcomes often did not initially agree on the categorization of the event that had occurred (e.g., new abulia was determined by one rater as a focal neurologic deficit and by another as a new decline in arousal). As a result, the quality assurance process further specified such that DCI events be determined by consensus agreement on a neurologic or radiologic finding occurring on the same day but without regard to the focality of deficits or the radiologic modality.
Invasive Multimodality monitoring
In our cohort, a portion of the patients underwent invasive multimodality monitoring at the discretion of the care team as part of clinical care when deemed to be clinically indicated at by the neurosurgical and neurocritical care teams. Multimodality monitoring was subsequently implemented in revisions of the clinical guideline for management of SAH patients with high clinical grade (HH 4–5) at our center. The clinical guideline above has since been amended to detail the use of multimodality monitoring for continuous measurements of cerebral perfusion pressure (CPP) (QFlow 500™ Perfusion Probe; Hemedex Inc., Cambridge, MA), cerebral blood flow (Licox Brain Tissue Oxygen Monitoring; Integra, Plainsboro, NJ), brain activity (Spencer Probe Depth Electrode; Ad-Tech Medical, Racine, WI) and intracranial pressure (ICP) (OLM Intracranial Pressure Monitoring; Camino, Integra Neurosciences, San Diego, CA), in addition to TCD and scalp EEG displayed using an integrative bedside monitor (CNS-210; Moberg ICU Solutions, Ambler, PA). This decision was based on the observation that seizures are detected at a nearly five-fold rate (38%) when cEEG is supplemented with depth electrode monitoring (Claassen et al., 2013). Additionally, the inclusion of the electrocorticography (ECoG) electrodes in comatose patients affords an opportunity to assess for perfusion-dependent ischemia and for cortical spreading depolarizations (CSDs) (Jeffcote et al., 2014). The guideline recommends that multimodality monitoring be performed in comatose patients and discontinued upon emergence from coma, stability of trends, or upon the need to perform MRI.
Analysis of the feasibility of cEEG ischemia monitoring
Between July 2012 and May 2014, 71 patients underwent combined cEEG and TCD monitoring. Prospective medical record review was done in 100% of patients, and clinical interviews for outcomes assessment were performed in 52 (73%) through daily interviews with physicians and nurses as per the quality assurance process that was developed. Outcome assessment in the remaining patients relied on secondary retrospective chart review only. The structured interviews with clinicians required approximately <5 minutes per patient per day inclusive of both nurse and physician interviews. Through the process of adjudication, 34 patients were determined to have had at least one DCI event during their stay in the NeuroICU, and, 22 (65%) underwent angiography as treatment.
During the study period, the mean duration of monitoring was 6.9 days (n=71). Patients with high clinical grade (HH4–5) underwent an average of 8.1 days of monitoring compared to 6.2 days among patients with good or moderate clinical grade. The mean time from symptom onset that cEEG was initiated was 2.7 days. Patients treated endovascular alone versus with craniotomy had EEG monitoring started with a 2.1 vs. a 3.2-day delay from symptom onset (n=68).
Of 34 events of DCI, 91.2% (31) occurred during the period of EEG monitoring and 97.1% (33) occurred during the period of TCD monitoring. A total of 705 epochs of EEG monitoring were reported, of which 104 (14.8%) were difficult to interpretable due to artifact.
A detailed analysis of the statistical performance characteristics of the overall cEEG monitoring process, accounting for the duration of monitoring, timing of the guideline-specified EEG and TCD warnings, and timing of DCI, is currently in process and will be reported separately from this clinical feasibility data.
Below we review two clinical vignettes and the associated cEEG data from our case records. These cases illustrate how the established aSAH cEEG monitoring protocol works in practice.
Case study 1
A 61-year-old female with hyperlipidemia presented with initially severe left hemicranial headache. In the emergency department she complained of neck stiffness without photophobia, meningismus, nausea, focal weakness, numbness, parasthesias or visual disturbances (HH 1). A head CT showed Fisher 3 SAH in basal cisterns and left Sylvian fissure. CT angiogram demonstrated a 2-mm posterior communicating artery aneurysm projecting posteriorly and inferiorly. Nimodipine was started. Prophylactic levetiracetam was administered while the aneurysm was unsecured. She immediately underwent cerebral angiogram, confirming a neck-dome configuration unfavorable for endovascular management. The following day, she underwent a left craniotomy and aneurysm clipping with right ventriculostomy placement.
Transcranial Doppler ultrasound (TCD) and continuous EEG monitoring were started approximately 32 hours after symptom onset and within 24 hours after admission to the hospital. Figures 3 and 4 show a snapshot of the raw EEG and quantitative trends at key points during the admission (day 2 vs. day 6).
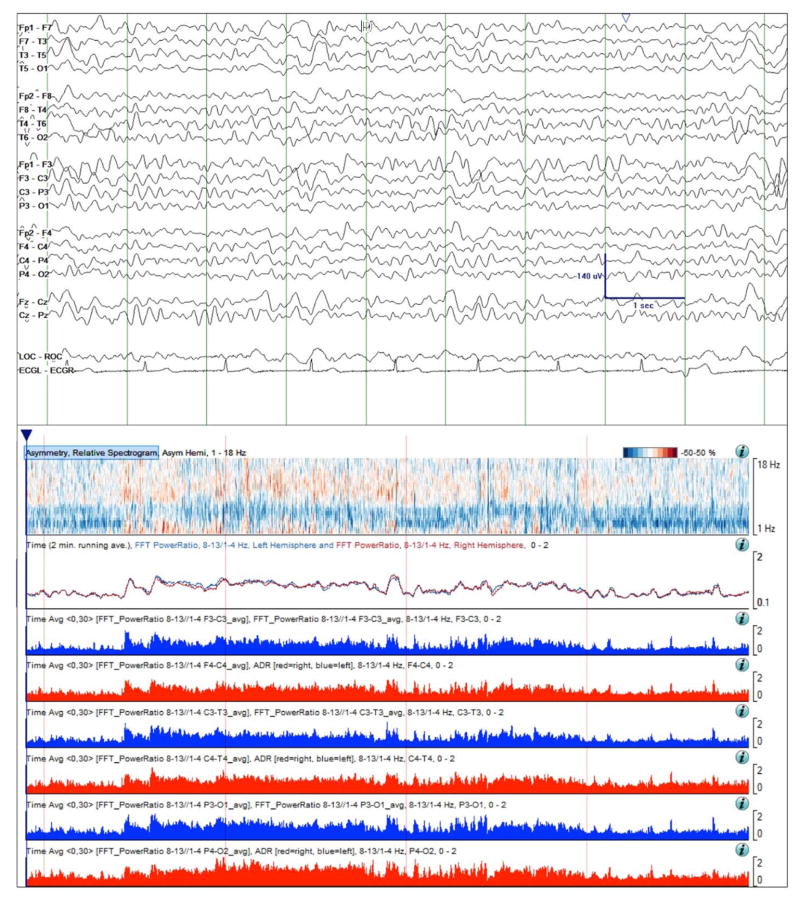
Figure 3.
Case study 1. Raw EEG Data (top) and quantitative trends on day 2 of monitoring. The RAV was scored as 4 and the ADR trend was similar between the left and right hemispheres, indicating minimal asymmetry. While not evident in the sample of raw EEG data shown, during this baseline period intermittent mild slowing was seen over the left hemisphere on careful visual inspection of the prolonged cEEG data. This finding is evident in the asymmetry spectrogram as light blue shading delta band. Time windows: raw EEG (top panel), 10 seconds; asymmetry spectrogram, ADR, and RAV panels: 4 hours.
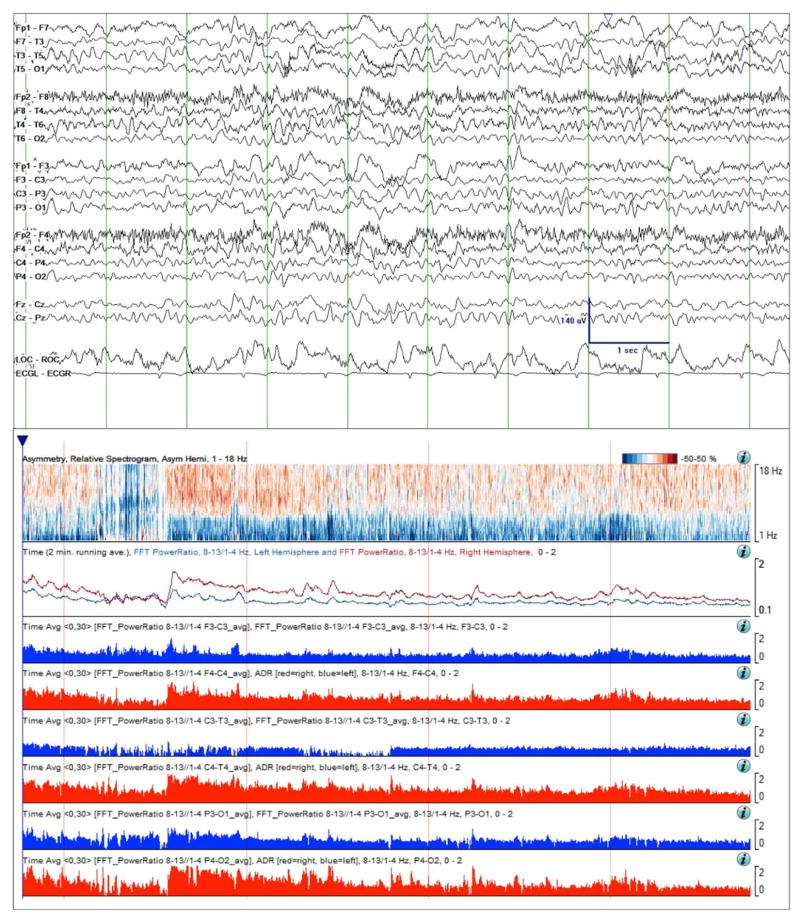
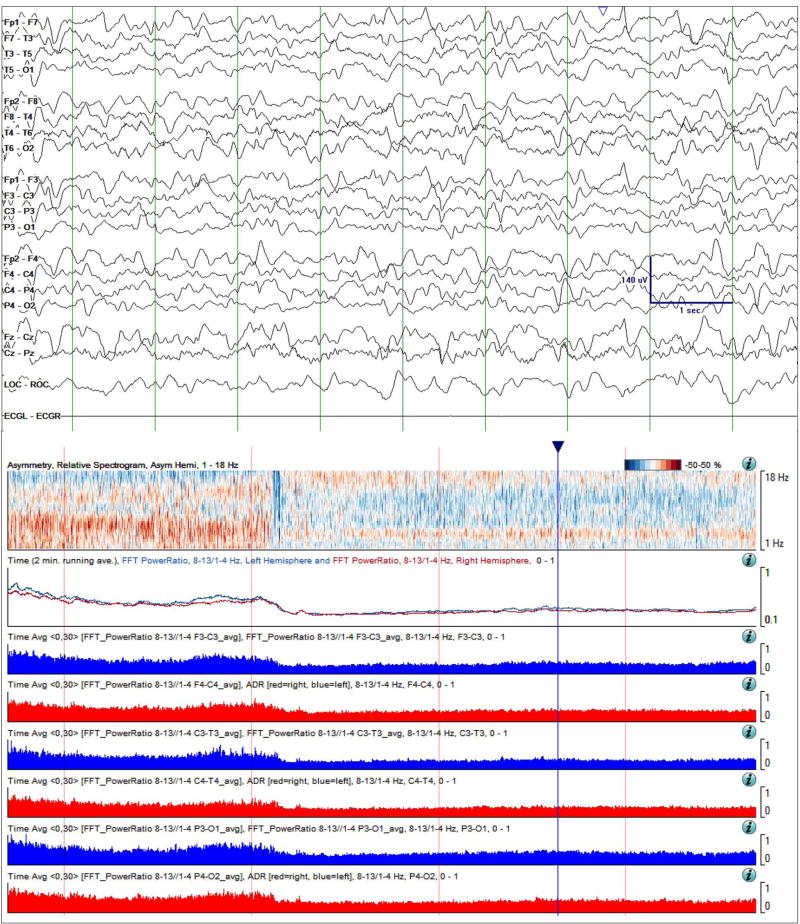
Figure 4.
Continuation of Case study 1. Raw EEG Data (top) and quantitative trends on day 6 of monitoring. Focal slowing over the left hemisphere is still intermittent, but is now more pronounced and is evident in the shown EEG sample. This change is also evident over the 4-hour asymmetry spectrogram as a deepening of the blue shading in the delta frequency band. The ADR trends have now separated, the left hemisphere ADR (blue line) consistently below that on the right (red line). Finally, the RAV grade has decreased in all regions, and particularly in the left temporal region, from grade 4 (“excellent”) to grade 3 (“good”). Taken together, these changes are concerning for ischemia. Time windows: same as in Figure 3.
On monitoring day 2, relative alpha variability was given a score of 4 (“excellent”) in all regions and the ADR was symmetrical (Figure 3). On monitoring day 4, the patient showed a decrease in the relative alpha variability to 3 in the bilateral frontocentral head regions. On day 4 there were new epileptiform discharges on the raw EEG. By day 6 there was an increase in left frontotemporal delta and theta slowing that became near continuous and correspondingly affected the ADR (Figure 4). The patient was diagnosed with DCI on day 7 manifesting as acute onset aphasia. TCD peak systolic velocities (PSV) were elevated (PSV maximum 132 cm/s one prior to vasospasm report) but below the threshold for mild vasospasm until monitoring day 11, when mild vasospasm of the right middle cerebral artery (MCA) was reported with a PSV of 248.
Case study 2
A 50-year-old right-handed female who was found down unresponsive at home was diagnosed with a HH4, Fisher 4 SAH with a 3x3cm right frontal intra-parenchymal hemorrhage and intra-ventricular extension. A computed tomography angiogram (CTA) of the head showed a ruptured 7 mm distal right A2 aneurysm with a positive “spot sign” within the right frontal intraparenchymal hemorrhage, consistent with active hemorrhage. She was treated with an extra-ventricular drain and coiling of the aneurysm on day 1.
Continuous EEG monitoring was begun on day 2 (Figure 5). EEG findings remained stable until day 4 when she the RAV decreased from a grade 4 to a grade 2 (Figure 6). TCDs also showed evidence of vasospasm on day 4, reported as moderate vasospasm in the right middle cerebral artery and in the left middle cerebral artery. A repeat CTA of the head performed on day 4 showed vasospasm of the azygous A1 segment of the anterior cerebral artery (ACA), the anterior-most A3 segment, two posterior A3 segments, and the M1 segment of the right MCA.
Figure 5.
Case study 2. Raw EEG Data (top) and quantitative trends on day 2 of monitoring. The EEG shows diffuse irregular delta-theta slowing, with a mild asymmetry, and likewise the asymmetry spectrogram shifting shows differences in the “pastel” range (only mild asymmetry). The RAV was scored as grade 4 (“excellent”) in all regions. ADR trend lines for the right (red line) and left (blue line) hemisphere fluctuated, but stayed largely superimposed and did not show any systematic long term trends. Time windows: same as in Figure 3.
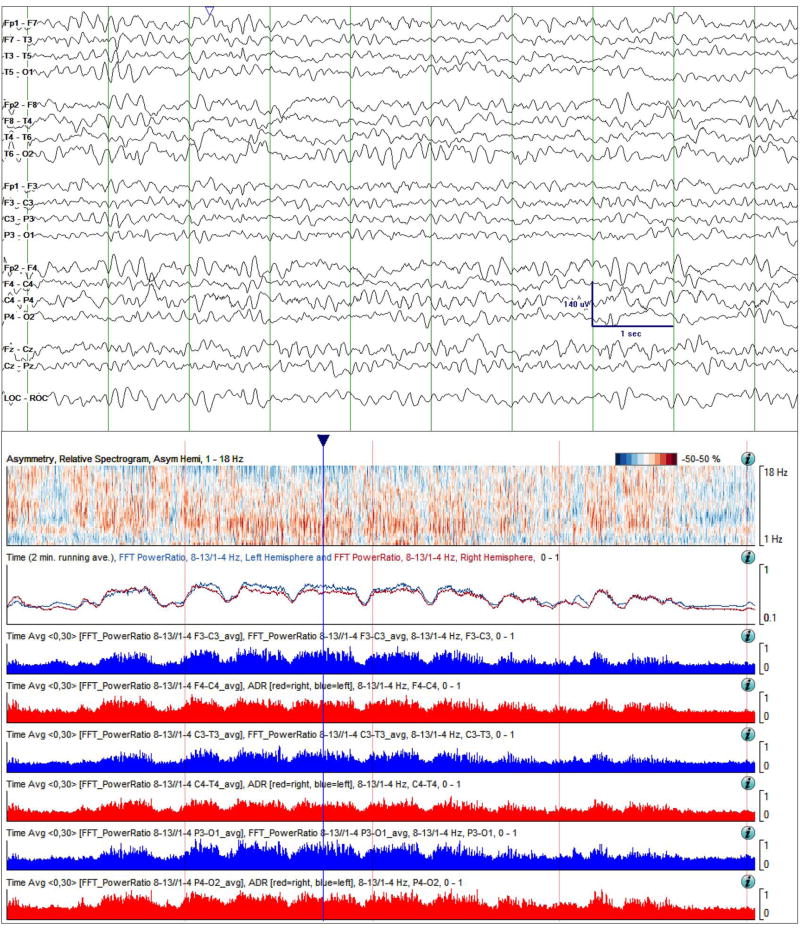
Figure 6.
Case study 2. Raw EEG Data (top) and quantitative trends on day 4 of monitoring. Slowing in the raw EEG is more prominent compared with day 2 (Figure 5) in the 10-second sample shown. Overall asymmetry is reduced in the asymmetry spectrogram. Of concern, is a persistent global decrease in ADR, and a decrease in RAV from grade 4 (“excellent”) to grade 2 (“fair”). These changes are concerning for ischemia. Time windows: same as in Figure 3.
Subsequently on day 5 she had a clinical decline of the GCS and a diagnosis of DCI was made. She underwent a decrompressive bifrontal craniectomy on day 5 of admission.
Future Directions
Cerebral ischemia monitoring in the setting of aSAH is feasible and can be efficiently deployed such that a high-risk population can be identified and that cEEG can be targeted to a time period in which over 90% of DCI events occur during a 6–7-day period. A prospective process for consensus determination of clinical outcomes was also found to be also feasible and efficient, and therefore is recommended to assure quality.
This work serves to describe the constituent clinical, technical and safety parameters that can enable implementation of cEEG ischemia monitoring following SAH as well as the feasibility of the method. Further work will focus on reporting the predictive accuracy of these prospectively implemented methods for early DCI detection.
Because of the length of the study and the large amount of data generated, cEEG utilizes substantially more technical and professional resources to record and interpret each tracing. Thus, advances in the field of ischemia monitoring will be dependent on breakthroughs in both the technical and clinical domains.
Given the frequent occurrence of artifacts in the ICU environment, artifact reduction at the hardware and software ends as well as safer MRI-compatible electrodes will be important constituent components of system refinement. Similarly, the development of reliable automated software ischemia detection software would ensure timely identification of abnormalities. Unfortunately, methods which appeared promising in careful retrospective studies do not appear effective when implemented as part of completely automated ischemia detection schemes, as reported in the manuscript by Wickering et al in this issue. Thus further advances in statistical signal processing in this area are required before ischemia monitoring in SAH can be performed without a heavy reliance on visual EEG interpretation by experts.
Clinical validation in a large population will be important to justify the widespread adoption of cEEG for SAH ischemia monitoring. Epidemiological studies of cost-effectiveness and patient outcomes as well as clinical trials of early therapeutic interventions could provide valuable information to move the field forward.
Contributor Information
CF Muniz, Email: cmuniz@mgh.harvard.edu.
AV Shenoy, Email: ashenoy2@mgh.harvard.edu.
MB Westover, Email: mwestover@mgh.harvard.edu.
ES Rosenthal, Email: erosenthal@mgh.harvard.edu.
Bibliography
- Andaluz N, Zuccarello M. Recent trends in the treatment of cerebral aneurysms: analysis of a nationwide inpatient database. Journal of Neurosurgery. 2008;108(6):1163–9. doi: 10.3171/JNS/2008/108/6/1163. http://doi.org/10.3171/JNS/2008/108/6/1163. [DOI] [PubMed] [Google Scholar]
- Budohoski KP, Czosnyka M, Kirkpatrick PJ, Smielewski P, Steiner LA, Pickard JD. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nature Reviews Neurology. 2013;9(3):152–163. doi: 10.1038/nrneurol.2013.11. http://doi.org/10.1038/nrneurol.2013.11. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Shaw CM, Leffman H. The significance of periodic lateralized epileptiform discharges in EEG: an electrographic, clinical and pathological study. Electroencephalogr Clin Neurophysiol. 1964;17(2):177–193. doi: 10.1016/0013-4694(64)90149-x. Available at: http://doi.org/10.1016/0013-4694(64)90149-X. [DOI] [PubMed] [Google Scholar]
- Claassen J, Hirsch LJ, Frontera JA, Fernandez A, Schmidt M, Kapinos G, … Mayer SA. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocritical Care. 2006;4(2):103–12. doi: 10.1385/NCC:4:2:103. http://doi.org/10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- Claassen J, Hirsch LJ, Kreiter KT, Du EY, Connolly ES, Emerson RG, Mayer SA. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2004;115(12):2699–2710. doi: 10.1016/j.clinph.2004.06.017. http://doi.org/10.1016/j.clinph.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society. 2005;22(2):92–8. doi: 10.1097/01.wnp.0000145006.02048.3a. [DOI] [PubMed] [Google Scholar]
- Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, … Hripcsak G. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Annals of Neurology. 2013;74(1):53–64. doi: 10.1002/ana.23859. http://doi.org/10.1002/ana.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, … Vespa P. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737. doi: 10.1161/STR.0b013e3182587839. http://doi.org/10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, … Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain: A Journal of Neurology. 2009;132(Pt 7):1866–81. doi: 10.1093/brain/awp102. http://doi.org/10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman B, Claassen J. Quantitative EEG for the detection of brain ischemia. Critical Care (London, England) 2012;16(2):216. doi: 10.1186/cc11230. http://doi.org/10.1186/cc11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, … Mayer SA. Defining Vasospasm After Subarachnoid Hemorrhage: What Is the Most Clinically Relevant Definition? Stroke. 2009;40(6):1963–1968. doi: 10.1161/STROKEAHA.108.544700. http://doi.org/10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- Hijdra A, Van Gijn J, Stefanko S, Van Dongen KJ, Vermeulen M, Van Crevel H. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology. 1986;36(3):329–33. doi: 10.1212/wnl.36.3.329. [DOI] [PubMed] [Google Scholar]
- Jeffcote T, Hinzman JM, Jewell SL, Learney RM, Pahl C, Tolias C, … Boutelle MG. Detection of spreading depolarization with intraparenchymal electrodes in the injured human brain. Neurocritical Care. 2014;20(1):21–31. doi: 10.1007/s12028-013-9938-7. http://doi.org/10.1007/s12028-013-9938-7. [DOI] [PubMed] [Google Scholar]
- Komotar RJ, Schmidt JM, Starke RM, Claassen J, Wartenberg KE, Lee K, … Mayer SA. Resuscitation and Critical Care of Poor-Grade Subarachnoid Hemorrhage. Neurosurgery. 2009;64(3):397–411. doi: 10.1227/01.NEU.0000338946.42939.C7. http://doi.org/10.1227/01.NEU.0000338946.42939.C7. [DOI] [PubMed] [Google Scholar]
- Leng LZ, Fink ME, Iadecola C. Spreading Depolarization. Archives of Neurology. 2011;68(1):31–36. doi: 10.1001/archneurol.2010.226. http://doi.org/10.1001/archneurol.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nature Reviews Neurology. 2013;10(1):44–58. doi: 10.1038/nrneurol.2013.246. http://doi.org/10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. The Lancet Neurology. 2009;8(5):427–433. doi: 10.1016/S1474-4422(09)70080-8. http://doi.org/10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Suri MFK, Nasar A, Kirmani JF, Ezzeddine MA, Divani Aa, Giles WH. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38(7):2180–2184. doi: 10.1161/STROKEAHA.106.467506. http://doi.org/10.1161/STROKEAHA.106.467506. [DOI] [PubMed] [Google Scholar]
- Rabinstein AA, Friedman JA, Nichols DA, et al. Predictors of outcome after endovascular treatment of cerebral vasospasm. AJNR Am J Neuroradiol. 2004 Nov-Dec;25(10):1778–82. [PMC free article] [PubMed] [Google Scholar]
- Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2013;73(2):217–222. doi: 10.1227/01.neu.0000430290.93304.33. http://doi.org/10.1227/01.neu.0000430290.93304.33. [DOI] [PubMed] [Google Scholar]
- Rinkel GJE, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. The Lancet Neurology. 2011;10(4):349–356. doi: 10.1016/S1474-4422(11)70017-5. http://doi.org/10.1016/S1474-4422(11)70017-5. [DOI] [PubMed] [Google Scholar]
- Romano JG, Rabinstein Aa, Arheart KL, Nathan S, Campo-Bustillo I, Koch S, Forteza AM. Microemboli in aneurysmal subarachnoid hemorrhage. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 2008;18:396–401. doi: 10.1111/j.1552-6569.2007.00215.x. http://doi.org/10.1111/j.1552-6569.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Macdonald RL. Cortical spreading ischemia in the absence of proximal vasospasm after aneurysmal subarachnoid hemorrhage: evidence for a dual mechanism of delayed cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2012;32(2):201–202. doi: 10.1038/jcbfm.2011.170. http://doi.org/10.1038/jcbfm.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl E, Lehmberg J, Steiger HJ, Messmer K. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery. 2003;52(6):1307–15. doi: 10.1227/01.neu.0000065154.04824.9e. disacussion 1315–7. [DOI] [PubMed] [Google Scholar]
- Vergouwen MDI, Ilodigwe D, Macdonald RL. Cerebral Infarction After Subarachnoid Hemorrhage Contributes to Poor Outcome by Vasospasm-Dependent and -Independent Effects. Stroke. 2011;42(4):924–929. doi: 10.1161/STROKEAHA.110.597914. http://doi.org/10.1161/STROKEAHA.110.597914. [DOI] [PubMed] [Google Scholar]
- Vergouwen MDI, Vermeulen M, Coert BA, Stroes ESG, Roos YBWEM. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2008;28(11):1761–1770. doi: 10.1038/jcbfm.2008.74. http://doi.org/10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- Vespa PM, Nuwer MR, Juhász C, Alexander M, Nenov V, Martin N, Becker DP. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalography and Clinical Neurophysiology. 1997;103(6):607–15. doi: 10.1016/s0013-4694(97)00071-0. [DOI] [PubMed] [Google Scholar]
- Woitzik J, Dreier JP, Hecht N, Fiss I, Sandow N, Major S, … Vajkoczy P. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2012;32(2):203–212. doi: 10.1038/jcbfm.2011.169. http://doi.org/10.1038/jcbfm.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]