Abstract
Thyroid carcinoma is the most common endocrine neoplasm, with the highest mortality rate of all the endocrine cancers. Among the endocrine malignancies, ~80% are papillary thyroid carcinomas (PTCs). In the initiation and progression of this tumor, genetic alterations in the mitogen-associated protein kinase pathway, including RAS point mutations, RET/PTC oncogene rearrangements and BRAF point mutations, play an important role, particularly in deciding targeted therapy. In the present study, a small population of thyroid tumor cells, known as tumor spheres, were isolated and characterized from PTC surgical samples. These spheres can be expanded indefinitely in vitro and give rise to differentiated adherent cells when cultivated in differentiative conditions. The present study showed by reverse transcription-polymerase chain reaction and flow cytometric analysis that the undifferentiated PTC cells exhibited a characteristic antigen expression profile of adult progenitor/stem cells. The cells were more resistant to chemotherapeutics, including bortezomib, taxol, cisplatin, etoposide, doxorubicin and vincristine, than differentiated PTC cells and the majority possessed a quiescent status, as revealed by the various cell cycle characteristics and anti-apoptotic protein expression. Such advances in cancer thyroid stem cell biology may provide relevant information for future targeted therapies.
Keywords: papillary thyroid carcinoma, cancer stem cells, tumor spheres, differentiation, chemoresistance
Introduction
Thyroid cancer is the most common carcinoma of the endocrine glands and represents ~1% of all malignancies (1). Papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and anaplastic and medullary thyroid carcinomas comprise >98% of all thyroid malignancies (2). While 80–85% of thyroid carcinomas are well-differentiated (PTC and FTC) and have a favorable prognosis, anaplastic thyroid cancer has an unfavorable prognosis and a fatal outcome.
According to reports from Western countries, PTC comprises 75–80% of all thyroid neoplasms. This carcinoma frequently demonstrates metastasis to the regional lymph nodes and exhibits multicentricity in the thyroid gland. FTC accounts for 10–20% of the thyroid carcinoma cases in the United States. In contrast to PTC, FTC is likely to metastasize to distant organs rather than the regional lymph nodes (1,2). These carcinomas generally have an indolent character, but following dedifferentiation of the lesion to become an undifferentiated carcinoma, it exhibits rapid growth with an adverse prognosis (1,2). Different cytogenetic events and oncogenic mechanisms occur in thyroid carcinoma (3–6); in particular, the ret proto-oncogene/PTC rearrangement is a specific genetic alteration observed in papillary carcinoma, but never in undifferentiated thyroid cancer (7). This oncogenic fusion protein fails to induce the carcinogenesis of mature thyrocytes (8), but introduction into the germ line is sufficient to induce PTC (9), indicating that the initiation of thyroid carcinoma may occur using transformed stem cells prior to or during terminal commitment.
According to the cancer stem cell hypothesis, only a rare subset of cells is able to initiate and sustain tumor growth. The existence of these cells, called cancer stem cells (CSCs) or cancer-initiating cells, was first demonstrated in acute myeloid leukemia (10), and was successively described in other hematological and solid tumors (11–18), including thyroid tumors (19). This small subpopulation of CSCs with unlimited proliferative potential possesses tumorigenic capacity and is consequently responsible for the development and maintenance of tumors (19). Thus, CSCs are a primary therapeutic target for complete tumor eradication.
Therefore, the present study investigated the cytotoxic effects of different chemotherapeutic agents on PTC spheres isolated and characterized at the Research Laboratories, Mediterranean Institute of Oncology (Viagrande, Italy). It was found that the PTC spheres were resistant to the chemotherapeutic drugs applied, which is consistent with the poor therapeutic effect observed when using conventional chemotherapy on relapsed or resistant PTC patients. Conversely, the drugs were effective on differentiated PTC (DPTC) cells, suggesting that undifferentiated cells become sensitive after differentiation.
Since the majority of chemotherapeutic agents act through the cell cycle (20) by inducing cell death, the present study also investigated cell cycle features, including sub-G0, G0/G1, S and G2/M.
Materials and methods
Isolation and culture of PTC spheres
Papillary thyroid CSCs were obtained from 10 surgically-resected samples at the Mediterranean Institute of Oncology (Catania, Italy) between January 2008 and June 2012, as previously reported (19). The patient sample included 4 males and 6 females (age range, 36–78 years). All patients were informed of the study purpose and provided written informed consent. The study was approved by the Mediterranean Institute of Oncology Ethical Committee. Briefly, following mechanical and enzymatic dissociation of the tissue, the cells were cultured in serum-free Dulbecco' modified Eagle's medium/F12 medium containing 20 ng/ml epidermal growth factor (EGF) and 10 ng/ml basic fibroblast growth factor. These experimental conditions allowed the selection and growth of the tumor spheres. Factor deprivation and addition of 10% fetal bovine serum to the medium induced undifferentiated PTC (UPTC) cells to adhere to the flask and acquire the typical morphological features of differentiated cells.
Flow cytometry
The cells were checked for cluster of differentiation (CD)133 stem cell marker by cytofluorimetric analysis Freshly isolated cells were washed with cold phosphate-buffered saline (PBS) containing 1% bovine serum albumin and exposed to mouse monoclonal anti-CD133/1 primary antibody (clone AC133; dilution, 1:10; catalog no., 130-090-422; Miltenyi Biotec Inc., Cambridge, MA, USA) primary antibody or isotype control (mouse IgG1). Subsequent to being washed, the cells were labeled with phycoerthyrin-conjugated donkey anti-mouse secondary antibody (dilution, 1:100; catalog no., 715-116-150; Jackson ImmunoResearch Labs, West Grove, PA, USA) and fluorescence intensity was evaluated using a FACScan EPICS® XL™ flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
Musashi cDNA was amplified after total RNA was isolated from the UPTC and DPTC cells using the RNeasy Mini Spin Column kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocols. Total RNA was reverse transcribed with random examers. Briefly, 1.0 µg RNA was incubated for 5 min at 70°C, then the sample was placed on ice and reverse transcribed with M-MLV H- DNA polymerase (Promega, Madison, WI, USA) for 10 min at 25°C followed by 1 h incubation at 42°C. For conventional PCR, cDNA was diluted 1:5 with H2O and a 299 bp fragment was amplified using the GoTaq® Green Master Mix (Promega) and the following PCR primers (MWG-Biotech AG, Ebersberg, Germany) for Musashi DNA: GIOL393, sense 5′-CAAGATGTTCATCGGGGGACTCAGTT-3′ and GIOL394, antisense 5′-TATTGCTTCACGTCCTCCACCGTC-3′. The cycling conditions were as follows: Initial denaturation at 95°C for 30 sec, followed by 35 cycles of 62°C for 30 sec and extension at 72°C for 60 sec. Brain tumor stem cell (BTSC) cDNA was used as a positive control. The experiment was performed in triplicate using the Biometra TProfessional Gradient thermocycler (Biometra GmbH, Göttingen, Germany).
Chemotherapy resistance studies
Following dissociation, 3,000 cells obtained from PTC spheres and DPTC cells were seeded in 96-well plates. For the chemotherapy resistance studies, chemotherapeutic drugs were added to the cells at the following concentrations: 100 nM bortezomib (PS-341), 5 mM Taxol, 500 ng/ml cisplatin, 0.5 µM etoposide (VP-16), 5 mM doxorubicin and 1 µM vincristine. After 24, 48 and 72 h, cell viability was evaluated using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's protocol. This assay is based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-car- boxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt to a colored formazan product. The formazan is measured by absorbance at 490 nm, which is directly proportional to the number of living cells. The plates were read using a microplate reader (Synergy HT; BioTek, Winooski, VT, USA). Survival was expressed as the percentage of viable cells in the treated sample relative to the untreated control cells. Cell count was evaluated by Trypan blue exclusion test (catalog no., ECM0990D; EuroClone SpA, Pero MI, Italy). Data are presented as the mean of three independent experiments performed with the two experimental procedures.
Cell cycle analysis
Following dissociation, 5,000 cells obtained from PTC spheres and DPTC cells were washed and resuspended in cold 80% ethanol to a final concentration of 0.5×106 cells/ml for 1 h at 4°C. The ethanol-fixed cells were centrifuged at 300 × g for 10 min to remove ethanol and the pellet was resuspended in propidium iodide staining reagent (0.1% triton X-100, 0.1 mM ethylenediaminetetraacetic acid, 0.05 mg/ml RNase A and 50 µg/ml propidium iodide). The cells were stored in the dark at room temperature for ~3 h. The cells were then analyzed with a flow cytometer (FC500; Beckman Coulter Inc.) for cell cycle analysis.
Western blot analysis
Proteins (30 µg) extracted from the UPTC and DPTC cells were processed for western blot analysis. Nitrocellulose membranes were incubated with the following primary anti-human antibodies: Rabbit polyclonal anti-p27 (clone, C-19; dilution, 1:200; catalog no., sc-528), rabbit polyclonal anti-cyclin E (clone, M-20; dilution, 1:500; catalog no., sc-481), mouse monoclonal anti-BCL2-like 1 isoform 1 (Bcl-xL; clone, H-5; dilution, 1:500; catalog no., sc-8392), and goat polyclonal anti-actin (clone, I-19; dilution, 1:500; catalog no., sc-1616) (all obtained from Santa Cruz Biotechnology Inc., Dallas, TX, USA). After washing with PBS, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (dilution, 1:2,000; catalog no., sc-2005), goat anti-rabbit (dilution, 1:5,000; catalog no., sc-2313) and donkey anti-goat IgG (dilution, 1:5,000; catalog no., sc-2020) secondary antibodies (all obtained from Santa Cruz Biotechnology Inc.). For detection, the enhanced chemiluminescence kit (ECL plus; GE Healthcare Life Sciences, Chalfont, UK) was used.
Statistical analysis
All statistical analysis was performed using Microsoft Excel 14.5.3 software (Microsoft Corporation, Redmond, WA, USA). Data are presented as the mean ± standard deviation. A paired t-test was used to analyze the statistical significance of the results. P<0.05 was considered to indicate a statistically significant difference.
Results
RNA-binding protein Musashi is expressed by UPTC spheres but not by DPTCs
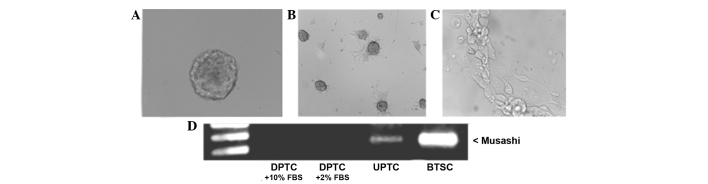
As previously reported, experimental conditions based on culturing freshly dissociated tumor cells in serum-free medium containing EGF and bFGF, allowed the selective growth of cellular clusters resembling tumor spheres (12,17,18,19,21). In the present study, such spheres maintained an undifferentiated state (UPTC), as confirmed by morphological analysis (Fig. 1A) and the expression of stem cell markers such as CD133 (data not shown), as found in previous studies (17,22). Morphological analysis revealed that these cell cultures were exclusively formed by cellular clusters resembling so called ‘tumor spheres’. In the presence of 10% serum, the PTC spheres became adherent and acquired the typical morphological features of differentiated cells (DPTC), which is a fibroblast-like phenotype (Fig. 1B and C); furthermore, CD133 antigen was lost, confirming its specific expression in the undifferentiated cells (data not shown).
Figure 1.
UPTC spheres and DPTC cells: Differential expression of the RNA-binding protein Musashi. (A) A typical PTC sphere obtained in serum-free medium containing epidermal growth factor and basic fibroblast growth factor (magnification, ×40). Tumor spheres cultivated in differentiative conditions (10% serum) for (B) 3 and (C) 7 days (magnification, ×10). (D) Musashi reverse transcription-polymerase chain reaction in UPTC and DPTC cells. BTSC cDNA was used as a positive control. PTC, papillary thyroid carcinoma; BTSC, brain tumor stem cell; UPTC, undifferentiated PTC; DPTC, differentiated PTC; FBS, fetal bovine serum.
Notably, it was found that the RNA-binding protein Musashi, associated with stem cell identity and recently considered as a master regulator in a number of stem cell populations (23,24), was expressed by the UPTC spheres, while its expression was lost in the DPTCs (Fig. 1D).
UPTC cells are highly resistant to different chemotherapeutic agents
The establishment of PTC stem-like cell cultures may allow the direct evaluation of the cytotoxic activity of antineoplastic agents on the putative cells responsible for tumor growth and spreading, which represent the optimal cellular targets for successful therapies. Therefore, the present study investigated the cytotoxic effect of different chemotherapeutic agents on PTC spheres. Taxol, cisplatin, VP-16, doxorubicin, vincristine and PS-341 were used at doses comparable with the higher plasma levels reached in patients. Since in preliminary experiments these cells proved to be rather resistant to chemotherapeutic drugs after 24 and 48 h of treatment, the viability of the UPTC cells was evaluated after 3 days of treatment. These drugs displayed modest cytotoxic activity in all UPTC cells examined at any time point (P>0.05). Conversely, the drugs were effective against the DPTC cells after 24 h, suggesting that undifferentiated cells become sensitive after differentiation (P<0.05; Fig. 2). Thus, similar to glioblastoma and lung cancer stem cells (18,25), PTC spheres are resistant to chemotherapeutic drugs, which is in agreement with the poor therapeutic effect observed when using conventional chemotherapy on relapsed or resistant PTC patients.
Figure 2.
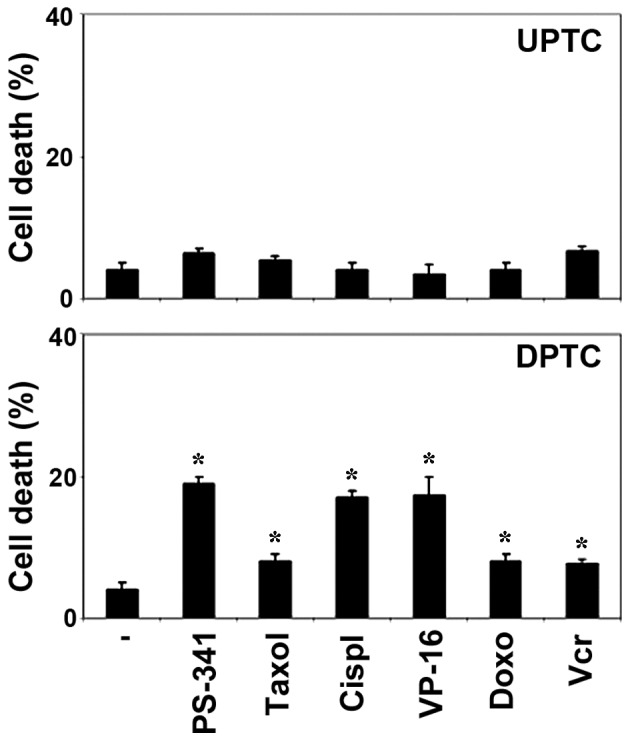
Chemotherapeutic resistance as determined by cell death. The UPTC cells were highly resistant to almost all the tested chemotherapeutic drugs compared with the DPTC cells. The figure shows the percentage of cell death in the UPTC and DPTC cells treated for 24 h with PS-341 (100 nM), Taxol (5 mM), cisplatin (Cispl; 500 ng/ml), VP-16 (0.5 µM), doxorubicin (Doxo; 5 mM) and vincristine (Vcr; 1 µM). *P<0.05 vs. control. Results represent the mean ± standard deviation of three independent experiments for each UPTC and DPTC cell sample. PTC, papillary thyroid carcinoma; UPTC, undifferentiated PTC; DPTC, differentiated PTC.
UPTC and DPTC cells show different cell cycle features and anti-apoptotic protein expression
Since the majority of chemotherapeutic agents acts through the cell cycle and/or by activation of apoptotic mechanism to induce cell death (20,26,27), it is likely that defects or dysregulation of different steps of these pathways may be important determinants of resistance to anticancer drugs. To this aim, the present study investigated cell cycle features and found that the UPTC cells had an higher percentage of cells (82.6%) in a quiescent status (G0/G1) than the DPTC cells (66.9%) (Fig. 3A). In addition, the UPTC and DPTC cells exhibited differing cell cycle protein expression; in particular, the p27 and cyclin E proteins, involved in G0-G1 progression, were expressed to a lesser degree in the UPTC cells compared with the DPTC cells (Fig. 3B). Furthermore, the expression level of the anti-apoptotic protein Bcl-xL was also evaluated and Bcl-xL was found to be strongly expressed by the UPTC cells, but not expressed by the DPTC cells (Fig. 3B).
Figure 3.
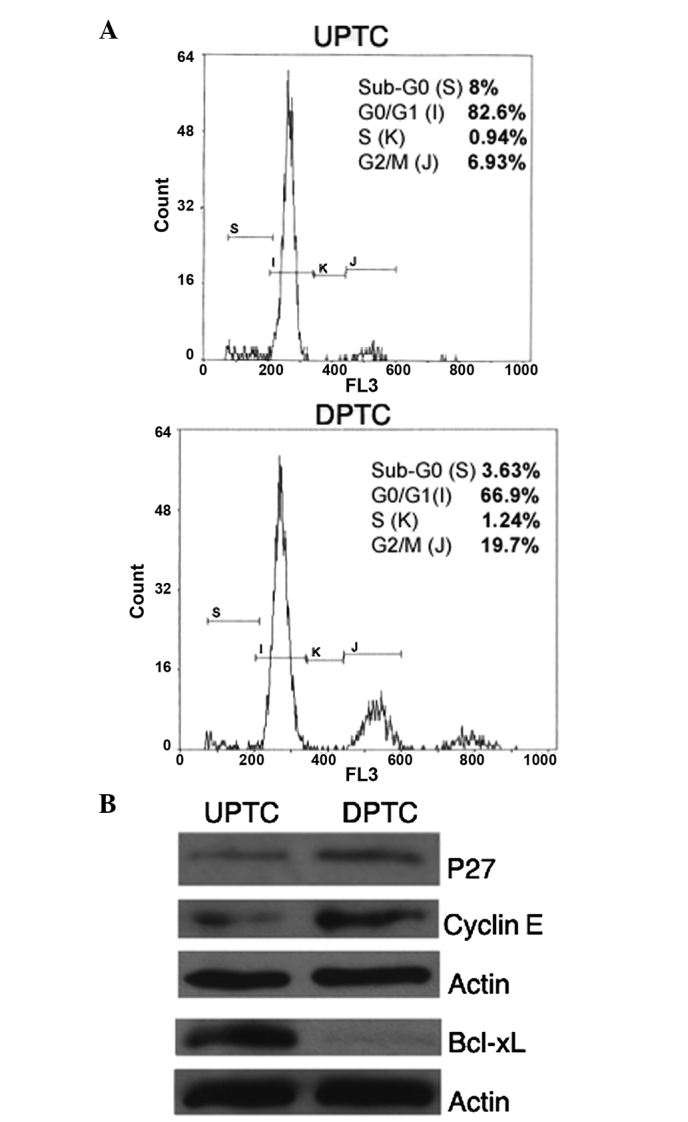
UPTC and DPTC cells show different cell cycle features and anti-apoptotic protein expression. (A) Cytofluorimetric analysis of cell cycle status (Sub-G0, G0/G1, S, G2/M) of UPTC and DPTC cells. (B) Immunoblot analysis of the cell cycle proteins: p27 and cyclin E, and the anti-apoptotic protein Bcl-xL, in UPTC and DPTC cells. PTC, papillary thyroid carcinoma; UPTC, undifferentiated PTC; DPTC, differentiated PTC; Bcl-xL, BCL2-like 1 isoform.
Discussion
Growing evidence suggests that CSCs are responsible for tumor initiation, growth, metastasis, therapy resistance, relapse and poor prognosis. It has recently been demonstrated that CSCs isolated from different types of cancer tissues as tumor spheres are able to reproduce the original human tumor in immunocompromised mice (10–19). These cells should be primary therapeutic target in order to achieve complete eradication of the malignancy. Therefore, the establishment of PTC stem-like cell cultures may enable the direct evaluation of the cytotoxic activity of antineoplastic agents on the putative cells responsible for the growth and spread of thyroid tumors, which represent optimal cellular targets for successful therapies. For this purpose, the present study isolated PTC spheres from surgical pathological tissues and PTC stem-like cell cultures were set up and characterized. Notably, the present study found that the undifferentiated cells expressed Musashi, an RNA-binding protein associated with stem cell identity, and that once differentiated, the expression of the protein was lost. Subsequently, the present study investigated the cytotoxic effect of several chemotherapeutic agents on the PTC spheres. It was found that the PTC spheres were resistant to all the chemotherapeutic drugs tested, which is in line with the poor therapeutic effect observed for the use of conventional chemotherapy on relapsed or resistant PTC patients. This is also in agreement with various studies that revealed that CSCs are crucial in chemoresistance against different anti-tumoral drugs, including cisplatin, paclitaxel, VP-16 and doxorubicin (28–31), in a variety of tumors, including glioblastoma (28), breast (29), ovarian (30) and prostate (31) cancers. However, in the present study, the drugs became effective on the cells once they were induced to differentiate into DPTC cells, suggesting that the undifferentiated cells become sensitive to the treatment only after differentiation.
Since the majority of chemotherapeutic agents act through the cell cycle and the activation of apoptosis to induce cell death in susceptible cells, the altered expression of proteins involved in such mechanisms could explain the chemoresistance phenomenon found in UPTC cells. Indeed, chemotherapy preferentially targets fast proliferating cells. In addition, it is now generally theorized that normal and cancer stem cells remain in a quiescent status for the majority of time (32,33), thus preventing the attack by most chemotherapeutic drugs. The present study investigated cell cycle features and found that UPTC cells exhibited a higher percentage of cells (82.6%) in a quiescent status (G0/G1) compared to DPTC cells (66.9%). Similarly, the present study demonstrated, by western blot analysis, a reduced expression of p27 and cyclin E, cell cycle proteins involved in G0-G1 progression, in UPTC cells compared with DPTC cells. In addition, several studies report that BCL-2 family proteins are critical role for cancer cell survival and chemoresistance (34,35). For this reason, the present study evaluated the expression of the anti-apoptotic protein, Bcl-xL by western blot analysis, and revealed that it was strongly expressed by UPTC cells, whereas it was almost totally absent in DPTC cells.
The data obtained by the present study may explain the resistance of UPTC cells to chemotherapeutic drugs. However, further studies are required to better understand the chemoresistance mechanisms in papillary thyroid CSCs, allowing the development of novel successful therapies for thyroid cancer treatment.
Acknowledgements
The authors would like to thank Mr. G. Anastasi (Mediterranean Institute of Oncology-Research) for providing skillful technical assistance and Dr. Giovanna Calabrese (Mediterranean Institute of Oncology-Research) for providing helpful advice and for support with organizing the figures of this study.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.de Groot JW, Links TP, Plukker JT, Lips CJ, Hofstra RM. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev. 2006;27:535–560. doi: 10.1210/er.2006-0017. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 5.Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: Molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. doi: 10.1677/ERC-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivan M, Bond JA, Prat M, Comoglio PM, Wynford-Thomas D. Activated ras and ret oncogenes induce over-expression of c-met (hepatocyte growth factor receptor) in human thyroid epithelial cells. Oncogene. 1997;14:2417–2423. doi: 10.1038/sj.onc.1201083. [DOI] [PubMed] [Google Scholar]
- 7.Salvatore D, Barone MV, Salvatore G, Melillo RM, Chiappetta G, Mineo A, Fenzi G, Vecchio G, Fusco A, Santoro M. Tyrosines 1015 and 1062 are in vivo autophosphorylation sites in ret and ret-derived oncoproteins. J Clin Endocrinol Metab. 2000;85:3898–3907. doi: 10.1210/jc.85.10.3898. [DOI] [PubMed] [Google Scholar]
- 8.Portella G, Vitagliano D, Borselli C, Melillo RM, Salvatore D, Rothstein JL, Vecchio G, Fusco A, Santoro M. Human N-ras, TRK-T1, and RET/PTC3 oncogenes, driven by a thyroglobulin promoter, differently affect the expression of differentiation markers and the proliferation of thyroid epithelial cells. Oncol Res. 1999;11:421–427. [PubMed] [Google Scholar]
- 9.Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, Xing S, Ledent C. Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology. 1996;137:375–378. doi: 10.1210/en.137.1.375. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 13.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 14.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: First steps into uncharted territory. Cell Stem Cell. 2007;1:241–242. doi: 10.1016/j.stem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 18.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 19.Todaro M, Iovino F, Eterno V, Cammareri P, Gambara G, Espina V, Gulotta G, Dieli F, Giordano S, De Maria R, Stassi G. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. doi: 10.1158/0008-5472.CAN-10-1994. [DOI] [PubMed] [Google Scholar]
- 20.Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci. 2009;122:2579–2585. doi: 10.1242/jcs.039719. [DOI] [PubMed] [Google Scholar]
- 21.Forte S, Pagliuca A, Maniscalchi ET, Gulino R, Calabrese G, Ricci-Vitiani L, Pallini R, Signore M, Parenti R, De Maria R, Gulisano M. Gene expression analysis of PTEN positive glioblastoma stem cells identifies DUB3 and Wee1 modulation in a cell differentiation model. PLoS One. 2013;8:e81432. doi: 10.1371/journal.pone.0081432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke CC, Liu RS, Yang AH, Liu CS, Chi CW, Tseng LM, Tsai YF, Ho JH, Lee CH, Lee OK. CD-133-expressing thyroid cancer cells are undifferentiated, radioresistant and survive radioiodide therapy. Eur J Nucl Mol Imaging. 2013;40:61–71. doi: 10.1007/s00259-012-2242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306:349–356. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland JM, McLaughlin EA, Hime GR, Siddall NA. The Musashi family of RNA binding proteins: Master regulators of multiple stem cell populations. Adv Exp Med Biol. 2013;786:233–245. doi: 10.1007/978-94-007-6621-1_13. [DOI] [PubMed] [Google Scholar]
- 25.Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/S0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 27.Portugal J, Bataller M, Mansilla S. Cell death pathways in response to antitumor therapy. Tumori. 2009;95:409–421. doi: 10.1177/030089160909500401. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, Stanbridge EJ, Lee EY. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102:1276–1283. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W, Liu Z. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010;340:265–273. doi: 10.1007/s11010-010-0426-5. [DOI] [PubMed] [Google Scholar]
- 32.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Chonghaile Ni T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettaieb A, Dubrez-Daloz L, Launay S, Plenchette S, Rébé C, Cathelin S, Solary E. Bcl-2 proteins: Targets and tools for chemosensitisation of tumor cells. Curr Med Chem Anticancer Agents. 2003;3:307–318. doi: 10.2174/1568011033482396. [DOI] [PubMed] [Google Scholar]