This trial evaluated the use of letrozole concomitantly or sequentially with radiotherapy in adjuvant breast cancer. No toxicity difference was found between the two arms with a long-term follow-up. This trial has changed the clinical practice and allowed physicians to start letrozole concomitantly with radiotherapy. Radiation-induced lymphocyte apoptosis was correlated with late breast fibrosis.
Keywords: radiation-induced late effects, breast cancer, radio-hormonotherapy
Abstract
Background
We present here final clinical results of the COHORT trial and both translational sub-studies aiming at identifying patients at risk of radiation-induced subcutaneous fibrosis (RISF): (i) radiation-induced lymphocyte apoptosis (RILA) and (ii) candidates of certain single-nucleotide polymorphisms (SNPs).
Patients and methods
Post-menopausal patients with stage I–II breast cancer (n = 150) were enrolled and assigned to either concurrent (arm A) or sequential radiotherapy (RT)-letrozole (arm B). Among them, 121 were eligible for RILA and SNP assays. Grade ≥2 RISF were the primary end point. Secondary end points were lung and heart events and carcinologic outcome. RILA was performed to predict differences in RISF between individuals. A genome-wide association study was performed to identify SNPs associated with RILA and RISF. Analyses were done by intention to treat.
Results
After a median follow-up of 74 months, 5 patients developed a grade ≥2 RISF. No significant difference was observed between arms A and B. Neither grade ≥2 lung nor symptomatic cardiac toxicity was observed. Median RILA value of the five patients who had grade ≥2 RISF was significantly lower compared with those who developed grade ≤1 RISF (6.9% versus 13%, P = 0.02). Two SNPs were identified as being significantly associated with RILA: rs1182531 (P = 4.2 × 10−9) and rs1182532 (P = 3.6 × 10−8); both located within the PHACTR3 gene on chromosome 20q13.33.
Conclusions
With long-term follow-up, letrozole can safely be delivered concomitantly with adjuvant breast RT. Translational sub-studies showed that high RILA values were correlated with patients who did not develop RISF.
Registered clinical trial
introduction
In 2005, a randomized trial (Concomitant HOrmono-RadioTherapy, CO-HO-RT, NCT00208273) was performed to evaluate whether letrozole given concomitantly or sequentially after breast-conserving surgery and adjuvant radiotherapy (RT) may alter radiation-induced side-effects and cosmetic outcomes. This trial was supported by the fact that letrozole showed radiosensitizing effects on breast cell lines in vitro [1]. Analyses of primary end point have been yet reported with a short follow-up and showed that letrozole in combination with RT did not increase the risk of radiation-induced subcutaneous fibrosis (RISF) [2].
In order to better predict the risk of RISF development, patients were proposed to be included in two translational sub-studies which were performed before adjuvant RT: (i) the radiation-induced CD8 T-lymphocyte apoptosis (RILA) assay (IRCM/Montpellier; David Azria) and (ii) the genome-wide association study (GWAS) (Mount Sinai School of Medicine/New York, Barry Rosenstein). RILA was one of the stratification factors to avoid imbalance between the two arms of randomization [3].
We present here final clinical results of the CO-HO-RT trial and results of ancillary translational sub-studies.
materials and methods
study design, patients' selection and treatment delivery
Post-menopausal patients with stage I–II breast cancer (n = 150) were enrolled and assigned to either concurrent (arm A; n = 75) or sequential letrozole-RT (arm B; n = 75). Randomization was open label with a minimization technique, stratified by investigational centres (Montpellier, Lille, Lausanne), chemotherapy (yes versus no), radiation boost (yes versus no), and value of RILA (≤16% versus >16%).
Details of adjuvant RT or chemotherapy were described elsewhere [2]. Briefly, patients underwent breast-conserving surgery and adjuvant letrozole was administered at a daily dose of 2.5 mg for 5 years (beginning 3 weeks pre-RT in arm A and 3 weeks post-RT in arm B).
toxicities assessments, quality-of-life questionnaires and cosmetic evaluation
Skin toxicity was assessed according to Common Toxicity Criteria Adverse Event version 3.0 (CTCAE v3.0) [4] at baseline, every week during RT, 3–6 weeks after the last RT fraction, every 3 months to month 24, every 6 months to month 60, and then every year for 5 more years. A different physician was asked to blindly score skin toxicities in cases of grade ≥2.
Lung toxicities were assessed through clinical examination using CTCAE v3.0 scale. Chest computed tomography (CT) scans were performed before randomization, and at 6, 12, 18, 24, 48 months, and every year up to 10 years after completion of RT. Functional pulmonary investigations as lung diffusion capacity for carbon monoxide (DLCO) and vital capacity (VC) were performed to identify effects of RT on breathing.
Heart toxicity was assessed according to CTCAE v3.0 scale, and heart hypertrophy according to the Hiatt et al. method [5]. The heart was considered hypertrophic when the measurement of cardiac surface was superior to 30% compared with the baseline measurement.
Cosmetic evaluation included clinical examination, photographs, and the two QLQ-C30/BR-23 EORTC quality-of-life questionnaires [6, 7].
ancillary translational sub-studies
All treated patients were included in the RILA assay (n = 121, 81% in Montpellier; n = 21, 14% in Lille; n = 7, 5% in Lausanne). Among them, only patients included in Montpellier (n = 121) were invited to participate in the GWAS sub-study. These blood samples were sent to NY for genomic screening. All ancillary translational sub-studies were performed before RT.
RILA [3]: Briefly, 200 µl of heparinized whole blood was cultured for 24 h and then were irradiated at 8 Gy (except control samples). After 24 h, CD8 lymphocytes were extracted from other blood cells and their apoptosis percentage was assessed through cytometry flow. RILA was defined as the population of cells with reduced DNA fluorescence and calculated as the percentage of total T-lymphocyte death induced by radiation dose (8 Gy) minus the spontaneous cell death (0 Gy).
GWAS: Genomic DNA was isolated from blood and genotyped using Affymetrix genome-wide SNP6.0 arrays (Affymetrix, San Diego, CA). After standard quality control filters (missingness >5% and minor allele frequency <5%), ∼650 000 single-nucleotide polymorphisms (SNPs) were included in the analysis. Overall call rate was >99%. Because genetic population structure is a common confounder in GWAS, principle components analysis (PCA) was carried out and samples from further SNP analysis were excluded if they showed non-Caucasian genetic ancestry. PCA was carried out using 100 000 randomly selected SNPs among the CO-HO-RT samples and 715 individuals from 11 populations of phase III of the International HapMap Project. A total of 7 individuals were identified as non-Caucasian and were excluded from further analysis, leaving 114 individuals of Caucasian ancestry included.
statistical analysis
The present study assessed the long-term outcomes of the treated patients included in Montpellier (n = 121; 81%) as only this centre accepted to participate to the extended follow-up more than 2 years and up to 10 years (protocol amendment #1; February 2007).
Occurrence of grade ≥2 RISF was the primary end point. Previous publication reported results about acute side-effects. Here, we only focused on late side-effects according to CTCAE v3.0 grading system. RISF was defined as fibrosis of the breast increased from grade 0 to 2 or 1 to 3, observed consecutively and never regressing over time.
Secondary end points were the incidence of lung- and heart-related side-effects, disease-free survival (DFS), and overall survival (OS).
Median follow-up was estimated by the inverse Kaplan–Meier method. All survival estimates were performed using the Kaplan–Meier method and comparisons were computed using the log-rank test. Complication-free survival (grade ≥2 RISF) was defined as the time from randomization to the occurrence of a grade ≥2 RISF. Otherwise, patients were censored at the last follow-up visit. DFS was defined as the time from randomization to the occurrence of the first carcinologic events among a local recurrence, a metastatic event, or death. OS was defined as the time from randomization to the occurrence of death whatever the cause.
Quality of life (QoL) and cosmetic results were analysed according to the EORTC criteria [6, 7] and Wilcoxon test was performed for arms comparison at each visit.
An exploratory analysis was performed to identify the possible risk factors of grade ≥2 RISF considering: (i) patient-related risk factors: age, body mass index, bra size, smoking status, diabetes mellitus, tumour volume, breast cancer phenotype), (ii) treatment-related risk factors: concomitant or sequential endocrine therapy, volume of tumour excision, mean lung dose, mean breast cutaneous dose, lung volume that encompassed 20 Gy (V20) and 30 Gy isodoses (V30), (iii) RILA using fisher's exact test for qualitative variables and Kruskal–Wallis test for quantitative variables. A linear mixed-regression model was used to determine the relationship between DLCO and VC over time. DLCO and VC parameters were log transformed to better fit the assumptions of linear mixed model.
A P-value of <0.05 was regarded as significant. All statistical tests were two-sided. Analyses were carried out using the Stata software, v13.
GWAS was performed to identify single-nucleotide polymorphisms (SNPs) associated with RILA and RISF. As other skin toxicity outcomes were rare, there were insufficient numbers of cases to investigate these in the GWAS. Association with each SNP was analysed by a χ2 test. Four different genetic models were considered: allelic, genotypic (2 degrees of freedom), dominant, and recessive. The percentage of CD8 lymphocytes undergoing radiation-induced apoptosis was analysed as a continuous outcome and the association with each SNP was analysed by linear regression among the four different genetic models (allelic, genotypic, dominant, and recessive). The genomic inflation factor for was 1.0168 for RISF and 1.0002 for RILA. The genetic analysis software PLINK was used for all association tests (http://pngu.mgh.harvard.edu/purcell/plink/). The standard P value threshold of 5 × 10−8 was used to determine genome-wide significance, accounting for multiple hypothesis testing.
results
patients' baseline characteristics, treatment delivered and Consort diagram
Updated consort diagram is depicted in Figure 1. One hundred and twenty-one patients from Montpellier entered follow-up as mandated in the protocol, and 105 patients were assessable at year 2 for 8 years more (10 years in total). Seventy of them (58%) were followed up for at least 72 months according to the first amendment of the protocol. The other patients (19 in the concurrent group and 16 in the sequential group) interrupted the planned follow-up before 72 months. The main reasons are detailed in Figure 1.
Figure 1.
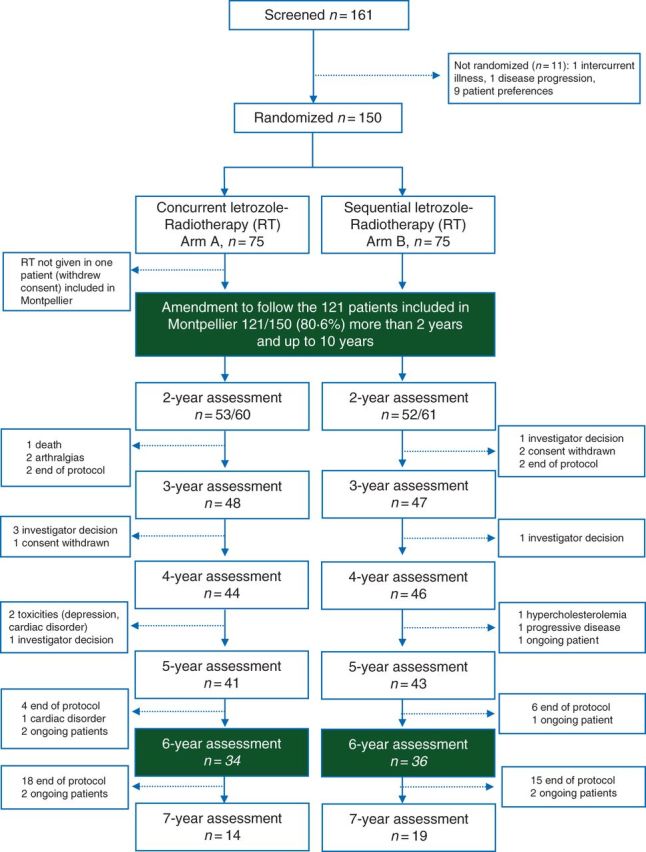
CONSORT diagram.
Baseline patient, disease characteristics, and treatment delivered in this population were similar to the entire cohort described in the first report [2]. Significantly more women smoked or were ex-smokers in the sequential group than in the concurrent group (P = 0.03) (supplementary Table S1, available at Annals of Oncology online). The majority of this cohort did not receive a boost and were not delivered adjuvant chemotherapy (supplementary Table S1, available at Annals of Oncology online). Nodes areas were irradiated in 18 patients (arm A) and 17 patients (arm B).
late radiation-induced toxicities, QoL, and cosmetic outcomes
grade ≥2 RISF
After a median follow-up of 74 months (range 48−85 months), a total of 5 patients developed a grade ≥2 RISF, one and four patients in arms A and B, respectively (P = 0.17). Three events appeared within the first 2 years and two patients presented a late progressing breast fibrosis 6 and 7 years after adjuvant RT. None of the selected risk factors related to patients' characteristics were correlated with grade ≥2 RISF occurrence (Table 1). Among parameters related to treatment planning, grade ≥2 RISF was associated with a high median lung dose (P < 0.01) and a large ipsilateral lung exposure (V20, P < 0.01 and V30, P = 0.01).
Table 1.
Risk factors of radiation-induced subcutaneous fibrosis
| Grade <2 RISF (N = 116) | Grade ≥2 RISF (N = 5) | P value | |
|---|---|---|---|
| Patients' characteristics | |||
| Median age, years (range) | 62 (45−82) | 60 (51−71) | 0.355 |
| BMI (overweight and obese; number of patients) | 59 | 3 | 0.689 |
| Bra size (large, number of patients) | 81 | 3 | 0.572 |
| Smokers (number of patients) | 11 | 1 | 0.441 |
| Diabetes mellitus/hypertension (number of patients) | 39 | 2 | 0.768 |
| Volume of surgical specimen, cm3 (median) | 53.2 | 65.5 | 0.333 |
| Tumour volume, cm3 (median) | 3.03 | 0.43 | 0.078 |
| ER−/PR+ | 24 | 1 | 0.188 |
| ER+/PR− | 4 | 1 | |
| ER+/PR+ | 88 | 3 | |
| RILA, % (median) | 13 | 6.9 | 0.019 |
| Treatment planning | |||
| Mean lung dose, Gy (median) | 5.2 | 9.7 | 0.007 |
| Lung V20, Gy (median) | 8.4 | 19.5 | 0.008 |
| Lung V30, Gy (median) | 7.2 | 15.1 | 0.013 |
| Mean breast cutaneous dose, Gy (median) | 41.9 | 35.9 | 0.126 |
| Dosimetric breast volume, cm3 (median) | 728.7 | 701.1 | 0.641 |
BMI, body mass index; ER, oestrogen receptor; PR, progesterone receptor; RISF: radiation-induced subcutaneous fibrosis.
lung toxicities
Patients' population was well balanced between arms A and B according to dosimetric parameters, DLCO values and VC at baseline. No grade ≥2 lung toxicity was observed by CT scan. Mean lung dose was 5.3 Gy (range 2−18.5), V20 at 8.6% (range 1.4−37.3), and V30 at 7.4% (range 0.9−32.1). Median baseline DLCO was 82% (range 57−135). Median VC was 2.83 l (range 1.6−4.2 l) and significantly decreased over time (P = 0.02).
cardiac events
Among the 121 patients, 98 chest CT scans were prospectively performed and analysed. Measurement of the cardiac contact distance (CCD) showed that 81 patients exhibited a 30% increase in their CCD and in their heart surface measurement as early as 12 months after completion of RT that worsened in time. No statistical difference was observed between the two arms. Nonetheless, no symptomatic late cardiac event was reported.
Mean heart dose was 2.45 Gy (range 0.78−4.97) and V30 at 1.26% (range 0.00−4.90).
QoL evaluation
Compliance for filling up questionnaires was 81% until 24 months and 77% until 72 months. Patients' QoL remained more than 70% from 24 to 72 months, which could be considered as very good. No significant difference was observed regarding breast symptoms or body image. At 6 months, arm symptoms' mean scores were significantly higher for patients treated with concomitant letrozole (23.3 versus 16.2, P = 0.02). Twelve months after the end of RT, sexual functioning' mean score was significantly lower in the sequential endocrine therapy arm (14.5 versus 23.2, P = 0.03) but improved over time without border significance at month 72 (14 versus 25.7, P = 0.06).
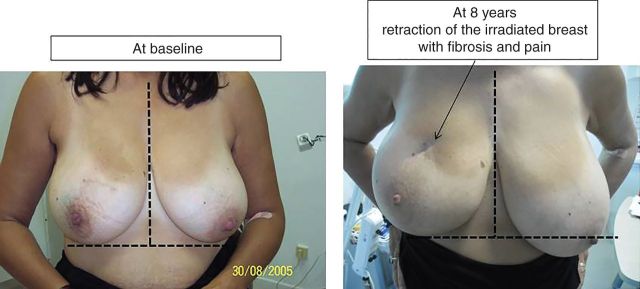
Figure 2 shows one patient at baseline and after 8 years of follow-up. This patient presented a grade 3 fibrosis with retraction of the breast that increased over time with pain impairing her QoL.
Figure 2.
Photographies of one patient with grade ≥3 breast fibrosis at baseline (left) and at long-term follow-up (right): 8 years. She presented retraction and whole breast fibrosis with pain and impaired quality of life. The radiation-induced lymphocyte apoptosis value for this patient was 8.5%.
translational sub-studies
radiation-induced lymphocyte apoptosis
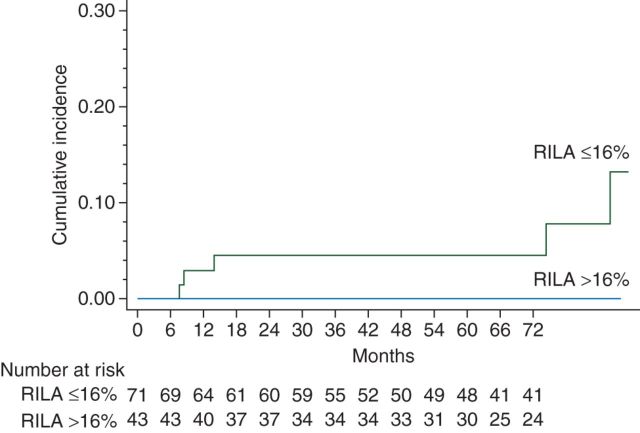
Median RILA value of the entire cohort was 12.9% (range 1.5−42). Median RILA value of the five patients who had grade ≥2 breast fibrosis was 6.9% (range 4.6−9.3) regardless of treatment arm and was significantly lower compared with those who developed grade ≤1 breast fibrosis (6.9% versus 13%, P = 0.02) (Figure 3). No grade 3 side-effects were observed for patients with RILA >24%. Among all risk factors studied, RILA was significantly predictive of grade ≥2 breast fibrosis (Table 1).
Figure 3.
Cumulative incidence of grade ≥2 fibrosis curves according to radiation-induced lymphocyte apoptosis (RILA) in the whole population.
genomic assay
Upon genome-wide SNP analysis of RILA (available for 99 patients), two SNPs were identified as being significantly associated with RILA: rs1182531 (P = 4.2 × 10−9) and rs1182532 (P = 3.6 × 10−8). To account for any population structure remaining after exclusion of individuals with non-European ancestry, these SNPs were tested in multivariable models adjusting for the first five principal components from PCA. The association remained after adjustment, though the strength of association decreased slightly (P = 1.2 × 10−7 for rs1182531 and P = 4.9 × 10−7 for rs1182532). The mean percent apoptosis was 35%, 15.4%, and 11.5%, respectively, for individuals with two, one or no copies of the rare allele of rs1182531, and 35%, 15.2%, and 12%, respectively, for two, one, or no copies of the rare allele of rs1182532. These SNPs are in moderately strong linkage disequilibrium (r2 = 0.74), tagging a single locus, and are both located within the PHACTR3 gene on chromosome 20q13.33 (supplementary Table S2, available at Annals of Oncology online). Other SNPs with marginally significant P values were clustered on chromosome 5p14.3, near the CHD18 gene but none reached genome-wide significance.
From patients with a severe grade ≥3 RISF, a number of SNPs were identified (either homozygous for the minor allele or heterozygous), and for which none of the controls (grade 0) carried the minor allele. SNP analysis was carried out with cases defined as patients who presented at least one time grade ≥2 RISF in the follow-up (n = 25 patients versus n = 85 controls, i.e. grade 0 or 1 toxicities). The top SNP was rs9421747 on chromosome 10q11.22 (P = 2.2 × 10−6, odds ratio 5.8 [95% confidence interval (CI) 2.7−12.6] for an allelic inheritance model; P = 2.5 × 10−5 after adjustment for the first five principal components; supplementary Table S2, available at Annals of Oncology online), though there were a relatively small number of cases for fibrosis in this analysis. This SNP lies downstream of growth differentiation factor 10 (GDF-10).
survival
The median follow-up was 74 months (IQ range 47.7−85.5). All patients are still alive. Three recurrences or new cancers were observed (one lymphoma, one local recurrence, and one contralateral breast cancer with no difference between the two arms (i.e. sequential versus concomitant letrozole). At 6 years, DFS was 97.4% (95% CI 92−99).
discussion
The long-term follow-up of the CO-HO-RT study confirmed that letrozole can be safely delivered shortly after surgery and concomitantly with RT. No increase of RISF was observed, only five patients developed severe breast fibrosis regardless letrozole administration timing. Apart from breast fibrosis, no grade ≥2 lung fibrosis was observed confirming lung safety with modern breast RT techniques. Functional tests were reassuring and showed that two tangential fields decreased significantly VC over time but without clinical impact. These findings were similarly published in a cohort of breast cancer patients after a median of 11 years following RT [8, 9]. No cardiac toxicity was observed in the regardless letrozole delivering. Finally, sexual functioning was lower in the sequential arm, which may correspond to a longer overall treatment time.
While analysis of clinical parameters and treament's modalities failed at identifying risk factor of RISF, the analysis of translational research showed promising predictive biological tools. RILA seems particularly capable of predicting among all patients treated by RT those whom will not develop severe subcutaneous fibrosis occurrence. Besides RILA, a GWAS showed promising results with new SNPs strongly associated with the apoptosis pathway and moderately with severe subcutaneous fibrosis.
Identifying patients at high risk of late radiation-induced effects is important at an individual scale as these late toxicities could impair QoL of long-term cancer survivors. As we and others published for the last 10 years [2, 3, 10–12], the present results showed that high RILA score is associated with the absence of late toxicity occurrence, while patients with severe late radiation-induced toxicities exhibit a low RILA score. Some limitations of the present trial could be listed as (i) this study is exploratory; (ii) the events of interest are rare, hence statistical analysis is underpowered to definitively demonstrate that RILA is a validated biomarker. Nevertheless, the clinical results of the prospective multicentric French trial (NCT00893035) evaluating the predictive role of RILA as a predictor of late effect after RT in 502 breast cancer patients have confirmed our current results in a greater extent and have been presented in abstract form at the 2015 ASTRO Annual Meeting (Azria et al., Proc ASTRO 2015).
A GWAS was conducted to identify SNPs that could eventually improve identification of patients at high risk of severe late radiation-induced toxicities. As the sample size of this cohort was very limited, this GWAS was viewed as a discovery stage, and validation studies will be needed to be certain if these SNPs were truly associated with severe fibrosis, given the high probability of false positives. Though fine-mapping and functional studies will be needed, it lies downstream of a gene whose function suggests a plausible role in development of fibrosis: growth differentiation factor 10 (GDF-10), also called bone morphogenic protein-3b (BMP-3b). The protein encoded by this gene belongs to the TGF-β superfamily and shares similar amino acid sequence with BMP-3. GDF-10 is not known to be involved in fibrosis but BMPs family-members usually act through Smad 1 and 5 [13]. Indeed, GDF-10 may act as a fibrotic cofactor but this role warrants confirmation in a large dataset of patients [14]. The GWAS also tested SNPs for association with RILA and therefore to individual radiosensitivity [15]. The locus identified that reached genome-wide significance lies within the phosphatase and actin regulator 3 (PHATCR3) belongs to the family of protein phosphatase 1 and actin regulatory proteins [16] that inhibit the catalytic subunit of protein phosphatase-1 (PP1) [17]. PP1 is a ubiquitously expressed, abundant phosphatase. After irradiation, PP1 is dephosphorylated in an ATM-dependent manner and then activated [18]. PP1 interacts with various proteins [PP1-interacting proteins (PIPs)] as targeting subunits, substrates and/or inhibitors of PP1 [19]. Recently, SNP in TXNRD2 was shown to be associated with radiation-induced fibrosis [20]. TXNRD2 is a mitochondrial enzyme which plays a key role in reactive oxygen species scavenging. These new findings suggested that other proteins or enzymes such as PP1 could be involved in fibrosis development.
Results from these translational sub-studies need to be validated in large prospective cohort. These assays are currently assessed within a FP7 grant (REQUITE trial).
In conclusion, letrozole can safely be delivered concomitantly with adjuvant breast RT. Clinical lung and cardiac fibrosis did not appear during long-term follow-up. Late subcutaneous fibrosis is a rare event but strongly influenced by intrinsic radioreactivity evaluated by RILA. Two SNPs were identified as being significantly associated with radiation-induced CD8 lymphocytes apoptosis necessitating a large validation set as proposed in the radiogenomic consortium [14].
acknowledgements
The authors thank Carole Jouet for data monitoring and Sarah Kerns for editorial assistance.
funding
This study was supported by Novartis S.A.S., France, with an unrestricted educational grant. Novartis Oncology provided funding support to the Montpellier Cancer Institute. This latter independently promoted the trial. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. No grant numbers applied. The corresponding author had full access to all of the data and the final responsibility for the decision to submit for publication.
disclosure
Montpellier Cancer Institute (Montpellier) received an unrestricted educational grant to cover the expenses of the investigators for undertaking this trial. DA served as consultant for advisory board and received support for travel to meetings for the study. All remaining authors have declared no conflicts of interest.
references
- 1.Azria D, Larbouret C, Cunat S et al. Letrozole sensitizes breast cancer cells to ionizing radiation. Breast Cancer Res 2005; 7: R156–R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azria D, Belkacemi Y, Romieu G et al. Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): a phase 2 randomised trial. Lancet Oncol 2010; 11: 258–265. [DOI] [PubMed] [Google Scholar]
- 3.Ozsahin M, Crompton NE, Gourgou S et al. CD4 and CD8 T-lymphocyte apoptosis can predict radiation-induced late toxicity: a prospective study in 399 patients. Clin Cancer Res 2005; 11: 7426–7433. [DOI] [PubMed] [Google Scholar]
- 4.Trotti A, Colevas AD, Setser A et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176–181. [DOI] [PubMed] [Google Scholar]
- 5.Hiatt JR, Evans SB, Price LL et al. Dose-modeling study to compare external beam techniques from protocol NSABP B-39/RTOG 0413 for patients with highly unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys 2006; 65: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 6.Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 7.Sprangers MA, Groenvold M, Arraras JI et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996; 14: 2756–2768. [DOI] [PubMed] [Google Scholar]
- 8.Blom Goldman U, Svane G, Anderson M et al. Long-term functional and radiological pulmonary changes after radiation therapy for breast cancer. Acta Oncol 2014; 53: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 9.Erven K, Weltens C, Nackaerts K et al. Changes in pulmonary function up to 10 years after locoregional breast irradiation. Int J Radiat Oncol Biol Phys 2012; 82: 701–707. [DOI] [PubMed] [Google Scholar]
- 10.Bordon E, Henriquez-Hernandez LA, Lara PC et al. Prediction of clinical toxicity in locally advanced head and neck cancer patients by radio-induced apoptosis in peripheral blood lymphocytes (PBLs). Radiat Oncol 2010; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foro P, Algara M, Lozano J et al. Relationship between radiation-induced apoptosis of T lymphocytes and chronic toxicity in patients with prostate cancer treated by radiation therapy: a prospective study. Int J Radiat Oncol Biol Phys 2014; 88: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 12.Schnarr K, Boreham D, Sathya J et al. Radiation-induced lymphocyte apoptosis to predict radiation therapy late toxicity in prostate cancer patients. Int J Radiat Oncol Biol Phys 2009; 74: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 13.Upadhyay G, Yin Y, Yuan H et al. Stem cell antigen-1 enhances tumorigenicity by disruption of growth differentiation factor-10 (GDF10)-dependent TGF-beta signaling. Proc Natl Acad Sci USA 2011; 108: 7820–7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstein BS, West CM, Bentzen SM et al. Radiogenomics: radiobiology enters the era of big data and team science. Int J Radiat Oncol Biol Phys 2014; 89: 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azria D, Ozsahin M, Kramar A et al. Single nucleotide polymorphisms, apoptosis, and the development of severe late adverse effects after radiotherapy. Clin Cancer Res 2008; 14: 6284–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen PB, Greenfield AT, Svenningsson P et al. Phactrs 1–4: a family of protein phosphatase 1 and actin regulatory proteins. Proc Natl Acad Sci USA 2004; 101: 7187–7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagara J, Higuchi T, Hattori Y et al. Scapinin, a putative protein phosphatase-1 regulatory subunit associated with the nuclear nonchromatin structure. J Biol Chem 2003; 278: 45611–45619. [DOI] [PubMed] [Google Scholar]
- 18.Guo CY, Brautigan DL, Larner JM. Ionizing radiation activates nuclear protein phosphatase-1 by ATM-dependent dephosphorylation. J Biol Chem 2002; 277: 41756–41761. [DOI] [PubMed] [Google Scholar]
- 19.Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 2010; 35: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edvardsen H, Landmark-Hoyvik H, Reinertsen KV et al. SNP in TXNRD2 associated with radiation-induced fibrosis: a study of genetic variation in reactive oxygen species metabolism and signaling. Int J Radiat Oncol Biol Phys 2013; 86: 791–799. [DOI] [PubMed] [Google Scholar]