Abstract
Purpose
Vitamins A, C and E and folate have anticarcinogenic properties and thus might protect against cancer. Few known modifiable risk factors for ovarian cancer exist. We examined the associations between dietary and total (food and supplemental) vitamin intake and the risk of invasive epithelial ovarian cancer.
Methods
The primary data from 10 prospective cohort studies in North America and Europe were analyzed. Vitamin intakes were estimated from validated food frequency questionnaires in each study. Study-specific relative risks (RR) were estimated using the Cox proportional hazards model and then combined using a random-effects model.
Results
Among 501,857 women, 1,973 cases of ovarian cancer occurred during a maximum follow-up of 7-22 years across studies. Dietary and total intake of each vitamin was not significantly associated with ovarian cancer risk. The pooled multivariate RRs (95% confidence intervals (CIs)) for incremental increases in total intake of each vitamin were 1.02 (0.97-1.07) for vitamin A (increment: 1300 mcg/day), 1.01 (0.99-1.04) for vitamin C (400 mg/day), 1.02 (0.97-1.06) for vitamin E (130 mg/day) and 1.01 (0.96-1.07) for folate (250 mcg/day). Multivitamin use (vs. non-use) was not associated with ovarian cancer risk (pooled multivariate RR=1.00, 95% CI: 0.89-1.12). Associations did not vary substantially by study, or by subgroups of the population. Greater vitamin intakes were associated with modestly higher risks of endometrioid tumors (n=156 cases), but not other histological types.
Conclusion
These results suggest that consumption of vitamins A, C, and E and folate during adulthood does not play a major role in ovarian cancer risk.
Keywords: ovarian cancer, vitamin A, vitamin C, vitamin E, folate, pooled analysis, cohort studies
Ovarian cancer is the most deadly of the gynecological cancers [1], primarily reflecting that the disease is identified at late stages when treatment success is limited. Given that effective measures to identify the disease early, either through symptom identification or screening, are currently unavailable at the population level, prevention is important for reducing the burden of this deadly malignancy.
The consumption of foods containing potentially cancer-preventive vitamins may reduce the risk of ovarian cancer. Vitamin A activity is important for the normal control of cellular differentiation and proliferation [2], vitamins C and E have strong antioxidant activity [3], and folate serves as a methyl group donor for DNA synthesis and repair [4]; thus, inadequate levels of these vitamins could enhance carcinogenesis. In a 2007 international systematic review of the literature published through 2006, the available data on the associations between intake of vitamins A, C and E and folate and ovarian cancer risk were judged to be limited and inconclusive [5]. Relatively few studies had been published on each of these vitamins and collectively the reported results were not consistent. Statistical power may have been limited in the studies as most had sample sizes of less than 500 cases. Among subsequent studies [6-9], sample sizes have been large in some (>1000 cases) [7, 8] though results have remained inconsistent.
The Pooling Project of Prospective Studies of Diet and Cancer (Pooling Project) is an international consortium of prospective cohort studies. In the Pooling Project, the primary data from each included study is analyzed, thus definitions of dietary and covariate variables are standardized, minimizing heterogeneity due to variable definitions. Because the included studies are all prospective, the potential for selection and information biases, which may influence results from case-control studies, is minimized. Moreover, by pooling data from several studies, we can maximize statistical power to detect small but potentially important associations. Thus, using the data from 10 cohort studies in the Pooling Project, among which only 4 studies had previously published on vitamin intake and ovarian cancer risk [10-16], we analyzed dietary and supplemental intake of vitamins A, C, and E and folate in relation to ovarian cancer risk overall, by histological type and among subgroups of the population defined by other ovarian cancer risk factors.
MATERIALS AND METHODS
Study population
The Pooling Project has been described previously [17]. Each of the studies included in these analyses met the following predefined criteria: at least 50 incident invasive epithelial ovarian cancer cases; an assessment of usual diet; and a validation study of the diet assessment method or a closely related instrument. Although the Adventist Health Study has been included in previous Pooling Project investigations of ovarian cancer risk [18, 19], data on individual vitamin intake were available only for vitamin E in this study. Therefore, to keep the study population consistent for the analyses of different vitamins, this study was excluded. Because the Women's Health Study was a randomized trial of vitamin E and β-carotene, we restricted the study population to the placebo arm; however, only 23 cases remained, thus this study was excluded from these analyses based on not meeting the inclusion criterion of having at least 50 incident cases. The cases occurring and person-time experienced during follow-up in the Nurses’ Health Study was considered as two different cohorts (1980-1986, Nurses’ Health Study (a); 1986-2000, Nurses’ Health Study (b)) so that data from the more detailed dietary assessment conducted in 1986 could be utilized. According to the underlying theory of survival analysis, blocks of person-time in different time periods are asymptotically uncorrelated, regardless of the extent to which they are derived from the same people [20]. Thus, pooling the estimates from these two time periods is a statistically valid alternative to using a single time period.
The exclusion criteria used by each study were first applied, after which we excluded participants who reported a history of any cancer (except non-melanoma skin cancer) at baseline, had a bilateral oophorectomy prior to baseline, or reported energy intakes greater than three standard deviations above or below the study-specific loge-transformed mean energy intake of the baseline population. The exclusion based on bilateral oophorectomy was not made in the New York State Cohort because this information was not collected.
Ovarian cancer ascertainment
Incident invasive epithelial ovarian cancer cases were identified in each study using follow-up questionnaires with subsequent medical record review [11, 21, 22], linkage with a cancer registry [10, 23-26], or both [27-29]. Mortality registries served as an additional source of incident cases in some studies [10, 11, 21, 22, 25, 27, 28]. Non-epithelial and borderline ovarian cancers were not identified in all studies and thus were not included in these analyses. Invasive epithelial ovarian cancers were further classified by histology according to the International Classification of Diseases for Oncology morphology codes [30] or the histological classification provided by the original study investigators.
Dietary assessment
Usual consumption of specific food items was assessed at baseline in each study with a self-administered food frequency questionnaire (FFQ). Each study determined daily consumption of each vitamin from food sources only (dietary vitamin intake) using food composition databases specific to that cohort. In addition, the use of vitamin supplements was also assessed, allowing for the analysis of vitamin intake from food and supplemental sources together (total vitamin intake). Supplemental intakes of vitamins A, C and E and folate were ascertained from study-specific questionnaire items on the use of individual vitamin supplements and multivitamins. The measure of supplemental folate intake was primarily based on the use of multivitamins, as only four studies assessed the use of individual folic acid supplements [10, 11, 21, 25]. In these four studies, the prevalence of folic acid supplement use was very low (<3%).
In the New York State Cohort and the Netherlands Cohort Study, vitamin supplement intake was assessed only as use versus nonuse, without information on frequency and dose. To include these studies in the analyses of total vitamin intake, we assumed an intake frequency of one per day for users and a usual dose, which, for the New York State Cohort was the dose for generic multivitamins and vitamins A, C and E supplements used in the Nurses’ Health Study, and for the Netherlands Cohort Study was the most common dose of vitamin A, C and E in individual supplements and multivitamins reported by participants in their FFQ validation study. Folate was not included in multivitamin preparations in the Netherlands at the time that the study was initiated thus folate intake in this study is from food sources only.
Vitamin intakes were adjusted for total energy intake according to the residual method [31] using the predicted nutrient intake for a daily total energy intake of 1,600 kilocalories. Among the validation studies of the FFQs [32-39], the median value across studies of the correlation coefficients comparing dietary vitamin intakes estimated using the FFQ versus the reference method (multiple dietary records or 24-hour recalls) were 0.42 for vitamin A, 0.63 for vitamin C, 0.28 for vitamin E and 0.46 for folate [17]. The study-specific correlation coefficients were generally above 0.30 [17]. Correlations for total intakes of these vitamins were not reported. In a biomarker-based validation study conducted in the Nurses’ Health Study, the Pearson correlation coefficient for dietary vitamin E intake from the FFQ versus the plasma concentration was 0.41 [40].
Variable definitions
Dietary and total intake of each of the vitamins were analyzed as both continuous variables and by categories based on study-specific quintiles. Quintiles were assigned based on the distributions in each original cohort or subcohort for the Canadian National Breast Screening Study and the Netherlands Cohort Study, which were analyzed as case-cohort studies [41]. The prevalence of multivitamin use in the Netherlands Cohort Study was much lower (6%) than in the other studies (33-49%). Furthermore, folate intake was not included in their multivitamin preparations. Because multivitamins are a major source of vitamins A, C and E and folate, total vitamin intakes in the Netherlands Cohort Study was much lower than in the other studies. Thus, when categorizing total vitamin intake according to study-specific quintiles, the intake levels in the highest quintile in the Netherlands Cohort Study was not comparable to intake in the other studies, and mainly reflected dietary vitamin intake. Therefore, the Netherlands Cohort Study was excluded from the quintile analyses of total intake of each vitamin. For total intake of each of the individual vitamins, in addition to analyzing associations using study-specific quintiles we also created categories based on cut points of absolute intake that were identical across studies. The category cut points were determined so as to differentiate multivitamin nonusers, users of multivitamins only and users of individual vitamin supplements [42]. Since categories were based on absolute intake and were identical across studies, the lower total vitamin intake in the Netherlands Cohort Study would be appropriately classified, thus, this study was included in these analyses.
Total energy intake was modeled as a continuous variable. Analyses also included variables for smoking habits, physical activity, body mass index, parity, age at menarche, oral contraceptive use, menopausal status and postmenopausal hormone use, which were assessed by self-administered questionnaires at baseline in each study, and were categorized in a consistent manner across the studies as described previously [18, 19]. An indicator variable for missing responses was created for these covariates, if needed. The proportion of missing values generally was less than 8% in each study that measured the covariate.
Statistical analysis
We first estimated the study-specific relative risks (RR) and 95% confidence intervals (CI) using the Cox proportional hazards model [43, 44]. Person-years of follow-up were calculated from the date of the baseline questionnaire until the date of ovarian cancer diagnosis, death, loss to follow-up or end of follow-up, whichever came first. Age and calendar time were accounted for by stratifying on age at baseline (in years) and the year the baseline questionnaire was returned, which allows for baseline incidence rates to vary jointly and arbitrarily by age at enrollment and calendar year and is equivalent to a left-truncated survival analysis using age as the time scale. After estimating the study-specific RRs, pooled RRs were calculated by combining study-specific loge RRs, weighted by the inverse of their variance, using a random effects model [45]. The presence of heterogeneity between studies was tested for by using the Q statistic [45, 46]. To calculate the P-value for the test for trend across categories of intake, participants were assigned the median value of their category and this variable was entered as a continuous term in the regression model, the coefficient for which was evaluated by the Wald test.
Before analyzing vitamin intakes as continuous variables, we assessed whether associations were consistent with linearity by examining nonparametric regression curves using restricted cubic splines [47, 48]. The model fit including the linear and cubic spline terms selected by a stepwise regression procedure was compared with the model fit including only the linear term, using the likelihood ratio test. For these analyses, all studies were combined into a single data set and stratified by study, age at baseline and the year the questionnaire was returned. The results indicated that the associations were consistent with linearity (P, tests for linearity > 0.05). For the analyses of vitamin intake as continuous variables, the RRs were calculated for increments of intake roughly based on the mean of the standard deviation of the intake across studies. In the analyses of dietary vitamin intake, for which we had validation study data, we corrected the RRs for measurement error using regression coefficients between dietary vitamin intake estimated by the FFQs and reference methods [49].
We evaluated whether associations for vitamin intake and ovarian cancer risk varied by levels of other potential risk factors for ovarian cancer. For oral contraceptive use, parity, and alcohol consumption, we first calculated the pooled RRs for vitamin intake as continuous variables stratified by levels of these risk factors and then assessed the statistical significance of the cross-product term between the vitamin variable and potential effect modifier, using a Wald test [17]. For smoking status and postmenopausal hormone use, which are nominal variables and were analyzed in three categories (never, past and current), we used a mixed-effects meta-regression model, and evaluated the statistical significance of the parameter estimate using a Wald test [17, 50]. We also examined associations separately for the main histological types of epithelial ovarian cancer (serous, endometrioid and mucinous). Differences in the pooled RRs by histological type were evaluated using a contrast test [51]. Analyses were conducted using SAS. All statistical tests were two-sided and a p-value of 0.05 was considered as statistically significant.
RESULTS
A total of 1,973 women were diagnosed with epithelial ovarian cancer among 501,857 women over a median follow-up period ranging from 7 to 16 years across the 10 studies, (table 1). The mean of the mean age at diagnosis for each study was 62 years; 746 women were diagnosed before the age of 62 years and 1,227 women were diagnosed at age 62 years or later. Among the 1,973 cases, 165 were premenopausal at both baseline and diagnosis, where menopausal status at diagnosis was determined using a previously described algorithm [52].
Table 1.
Characteristics of the cohort studies included in the pooled analyses of vitamin intake and ovarian cancer risk
| Study | Follow-up period | Baseline age range (years) | Baseline cohort size1 | No. of cases2 |
|---|---|---|---|---|
| Breast Cancer Detection Demonstration Project Follow-up Cohort (BCDDP) | 1987-1999 | 40-93 | 32,885 | 142 |
| Canadian National Breast Screening Study (CNBSS) | 1980-2000 | 40-59 | 49,613 | 223 |
| Cancer Prevention Study II Nutrition Cohort (CPS II) | 1992-2001 | 50-74 | 61,201 | 278 |
| Iowa Women's Health Study (IWHS) | 1986-2001 | 55-69 | 28,486 | 208 |
| Netherlands Cohort Study (NLCS) | 1986-1995 | 55-69 | 62,412 | 208 |
| New York State Cohort (NYSC) | 1980-1987 | 50-93 | 22,550 | 77 |
| New York University Women's Health Study (NYUWHS) | 1985-1998 | 34-65 | 12,401 | 65 |
| Nurses’ Health Study (a) (NHSa) | 1980-1986 | 34-59 | 80,195 | 120 |
| Nurses’ Health Study (b) (NHSb) | 1986-2002 | 40-65 | 59,5383 | 315 |
| Nurses’ Health Study II (NHS II) | 1991-2000 | 27-44 | 91,514 | 52 |
| Swedish Mammography Cohort (SMC) | 1987-2004 | 40-74 | 60,600 | 285 |
Cohort sizes after applying study-specific exclusion criteria and then excluding women with loge-transformed energy intake values greater than three standard deviations from the study-specific mean, with previous cancer diagnoses (other than nonmelanoma skin cancer) and who had previously had a bilateral oophorectomy (except in the New York State Cohort where this information was not collected); the Canadian National Breast Screening Study and the Netherlands Cohort Study are analyzed as case-cohort studies so their baseline cohort size does not reflect the above exclusions; total cohort size is 501,857
Total number of cases is 1,973
Nurses’ Health Study (b) is not included as part of total cohort size since they are a subset of the women in Nurses’ Health Study (a)
The prevalence of individual supplement use across the studies ranged from 2-9% for vitamin A, 7-37% for vitamin C, 2-26% for vitamin E and 1 to 3% for folate (table 2). The prevalence of multivitamin use was higher, ranging from 33-49% across all studies except the Netherlands Cohort Study, where the prevalence of multivitamin use was 6%. Median Pearson correlation coefficients (r) across studies between intake of each of the vitamin variables ranged from 0.06 for dietary vitamin A with total vitamin E (range across studies: 0.04-0.08) to 0.60 for total vitamin A with total folate (range across studies: 0.57-0.75).
Table 2.
Median vitamin intakes and prevalence of supplement use in the cohort studies included in the pooled analyses of vitamin intake and ovarian cancer risk
| Study1 | Energy-adjusted median intake (10th-90th percentile)2 |
Prevalence of supplement use | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary intake3 | Total intake4 | ||||||||||||
| Vitamin A (μg/day) |
Vitamin C (mg/day) |
Vitamin E (mg/day) |
Folate (μg/day) |
Vitamin A (μg/day) |
Vitamin C (mg/day) |
Vitamin E (mg/day) |
Folate (μg/day) |
Multivitamins (%) |
Vitamin A (%) |
Vitamin C (%) |
Vitamin E (%) |
Folate7 (%) |
|
| BCDDP | 1269 (735-2203) | 148 (65-273) | 9 (6-16) | 300 (183-502) | 1611 (801-3417) | 193 (78-847) | 13 (7-278) | 380 (200-832) | 33 | 3 | 20 | 15 | -- |
| CNBSS5 | 1006 (605-1716) | 130 (66-213) | 16 (11-23) | 242 (168-341) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| CPS II | 1096 (680-1704) | 128 (57-220) | 9 (6-16) | 271 (164-435) | 1452 (758-3130) | 183 (72-757) | 18 (7-286) | 370 (181-776) | 41 | 6 | 28 | 22 | -- |
| IWHS | 1474 (737-2669) | 132 (71-217) | 8 (6-11) | 248 (169-363) | 1871 (816-4048) | 176 (83-670) | 10 (7-232) | 280 (178-678) | 33 | 7 | 28 | 14 | 1 |
| NLCS | 814 (561-1281) | 101 (59-162) | 11 (7-18) | 183 (137-261) | 834 (569-1328) | 107 (62-168) | 11 (7-18) | --6 | 6 | 4 | 7 | 2 | -- |
| NYSC | 1586 (833-3500) | 182 (99-295) | 7 (5-10) | 378 (263-552) | 2275 (987-5423) | 237 (119-769) | 11 (5-312) | 501 (289-861) | 44 | 9 | 30 | 22 | 3 |
| NYUWHS | 1119 (641-2022) | 163 (81-277) | 8 (6-11) | 269 (154-451) | 2027 (775-3501) | 247 (106-1192) | 17 (7-287) | 445 (181-767) | 49 | 7 | 37 | 26 | -- |
| NHSa | 1366 (730-2693) | 120 (61-208) | 4 (3-6) | 239 (150-377) | 1751 (802-4058) | 153 (70-668) | 5 (3-204) | 276 (158-665) | 34 | 4 | 19 | 13 | -- |
| NHSb | 1323 (778-2313) | 140 (76-233) | 6 (4-9) | 273 (189-395) | 1721 (865-3959) | 196 (90-786) | 8 (5-406) | 320 (201-707) | 42 | 5 | 29 | 16 | 1 |
| NHS II | 1168 (648-2142) | 106 (59-180) | 6 (4-8) | 272 (188-393) | 1535 (732-3186) | 142 (68-489) | 7 (5-38) | 336 (203-770) | 44 | 2 | 20 | 7 | 1 |
| SMC5 | 1303 (721-2314) | 66 (30-124) | 5 (4-7) | 218 (170-277) | -- | -- | -- | -- | -- | -- | -- | -- | -- |
BCDDP=Breast Cancer Detection Demonstration Project Follow-up Cohort, CNBSS=Canadian National Breast Screening Study, CPS II=Cancer Prevention Study II Nutrition Cohort, IWHS=Iow a Women's Health Study, NLCS =Netherlands Cohort Study, NYSC = New York State Cohort, NYUWHS=New York University Women's Health Study, NHSa=Nurses' Health Study (a), NHSb=Nurses' Health Study (b), NHS II=Nurses' Health Study II, SMC=Swedish Mammography Cohort
Units are retinol equivalents μg/day for vitamin A and alpha-tocopherol equivalents mg/day for vitamin E
Intake from food only
Intake from food and supplements
Total vitamin intake is not available for the Canadian National Breast Screening Study and Swedish Mammography Cohort because supplement use data at baseline were not available in these studies
Total folate intake is equivalent to dietary folate intake in the Netherlands Cohort Study because folate was not included in multivitamin preparations in the Netherlands when the study was initiated
Only 4 studies assessed the use of individual folic acid supplement intake
When analyzed as quintiles, we observed no association between dietary intake of vitamins A, C, and E and folate and ovarian cancer risk (table 3). The pooled age-adjusted and multivariate RRs were similar. When we excluded the two studies for which supplement use data were not available when the ovarian cancer database was finalized (the Canadian National Breast Screening Study and the Swedish Mammography Cohort) the pooled RRs were similar to those presented in table 3 (results not shown). To ensure that the RRs for dietary intake were not influenced by supplemental sources of the vitamins in the studies that assessed supplement intake, we examined dietary intake of each individual vitamin only among those women who did not consume supplemental vitamins (n=517 cases); the RRs were not greatly changed (results not shown). When the analyses of dietary folate were restricted to the North American studies, where participants were exposed to folate fortification from approximately 1997 onwards, the RRs were similar to those presented in table 3 (the pooled multivariate RR for highest versus the lowest quintile was 0.96 (95% CI: 0.80-1.15)). Among the North American studies, when follow-up was limited to the pre-fortification period (i.e. up to 1997), the pooled multivariate RR (95% CI) for the highest versus the lowest quintile of dietary folate intake was 0.99 (0.82-1.19).
Table 3.
Pooled relative risks (95% confidence intervals) of epithelial ovarian cancer for quintiles of vitamin intake
| Quintile of intake |
p-value, test for trend | p-value, test for between-studies heterogeneity, quintile 5 | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Dietary vitamin A | |||||||
| Number of cases | 366 | 379 | 393 | 426 | 409 | ||
| Age-adjusted | 1.00 (reference) | 1.01 (0.87-1.17) | 1.02 (0.88-1.17) | 1.08 (0.94-1.25) | 1.04 (0.90-1.20) | 0.37 | 0.83 |
| Multivariate1 | 1.00 (reference) | 1.01 (0.87-1.17) | 1.03 (0.89-1.19) | 1.08 (0.93-1.24) | 1.03 (0.89-1.19) | 0.51 | 0.67 |
| Total vitamin A2 | |||||||
| Number of cases | 251 | 224 | 274 | 226 | 282 | ||
| Age-adjusted | 1.00 (reference) | 0.83 (0.62-1.12) | 1.02 (0.83-1.25) | 0.85 (0.69-1.04) | 1.01 (0.85-1.20) | 0.42 | 0.52 |
| Multivariate1 | 1.00 (reference) | 0.83 (0.61-1.13) | 1.00 (0.80-1.25) | 0.82 (0.66-1.01) | 0.97 (0.80-1.17) | 0.83 | 0.33 |
| Dietary vitamin C | |||||||
| Number of cases | 376 | 402 | 421 | 390 | 384 | ||
| Age-adjusted | 1.00 (reference) | 1.08 (0.93-1.24) | 1.10 (0.95-1.27) | 1.00 (0.86-1.15) | 0.98 (0.85-1.13) | 0.44 | 0.74 |
| Multivariate1 | 1.00 (reference) | 1.08 (0.93-1.25) | 1.10 (0.95-1.28) | 1.00 (0.86-1.15) | 0.97 (0.84-1.13) | 0.40 | 0.55 |
| Total vitamin C2 | |||||||
| Number of cases | 251 | 232 | 260 | 257 | 257 | ||
| Age-adjusted | 1.00 (reference) | 0.89 (0.74-1.07) | 0.97 (0.82-1.16) | 0.95 (0.80-1.14) | 0.94 (0.78-1.14) | 0.98 | 0.36 |
| Multivariate1 | 1.00 (reference) | 0.88 (0.73-1.05) | 0.95 (0.80-1.13) | 0.92 (0.77-1.10) | 0.89 (0.74-1.08) | 0.54 | 0.36 |
| Dietary vitamin E | |||||||
| Number of cases | 399 | 406 | 385 | 388 | 395 | ||
| Age-adjusted | 1.00 (reference) | 1.00 (0.87-1.15) | 0.94 (0.77-1.16) | 0.95 (0.81-1.11) | 0.96 (0.82-1.12) | 0.24 | 0.29 |
| Multivariate1 | 1.00 (reference) | 1.00 (0.86-1.16) | 0.95 (0.77-1.17) | 0.94 (0.80-1.09) | 0.95 (0.80-1.12) | 0.24 | 0.23 |
| Total vitamin E2 | |||||||
| Number of cases | 257 | 240 | 235 | 250 | 275 | ||
| Age-adjusted | 1.00 (reference) | 0.91 (0.72-1.15) | 0.90 (0.75-1.08) | 0.95 (0.80-1.14) | 1.01 (0.85-1.20) | 0.49 | 0.58 |
| Multivariate1 | 1.00 (reference) | 0.90 (0.72-1.14) | 0.88 (0.73-1.05) | 0.92 (0.77-1.10) | 0.96 (0.81-1.14) | 0.70 | 0.57 |
| Dietary folate | |||||||
| Number of cases | 385 | 399 | 402 | 414 | 373 | ||
| Age-adjusted | 1.00 (reference) | 1.03 (0.87-1.22) | 1.01 (0.87-1.16) | 1.02 (0.89-1.18) | 0.91 (0.78-1.06) | 0.54 | 0.39 |
| Multivariate1 | 1.00 (reference) | 1.04 (0.87-1.24) | 1.00 (0.87-1.16) | 1.02 (0.89-1.18) | 0.90 (0.77-1.05) | 0.36 | 0.34 |
| Total folate2 | |||||||
| Number of cases | 224 | 255 | 260 | 250 | 268 | ||
| Age-adjusted | 1.00 (reference) | 1.09 (0.89-1.33) | 1.11 (0.93-1.33) | 1.06 (0.88-1.27) | 1.12 (0.94-1.34) | 0.33 | 0.48 |
| Multivariate1 | 1.00 (reference) | 1.08 (0.90-1.31) | 1.09 (0.91-1.30) | 1.02 (0.85-1.23) | 1.07 (0.89-1.28) | 0.68 | 0.52 |
Adjusted for parity (0,1,2,3+), oral contraceptive use (never, ever), menopausal status and postmenopausal hormone use (premenopausal, unknown menopausal status, postmenopausal never use, postmenopausal past use, postmenopausal current use), age at menarche (<13, 13, 14+ years), body mass index (<23, 23 to <25, 25 to <30, 30+ kg/m2), physical activity (low , medium, high), smoking status (never, past, current) and total energy intake (kcal/day, continuous); age in years and year of questionnaire return were included as stratification variables
The Netherlands Cohort Study was excluded from these analyses because folate was not included in their multivitamins, and because their prevalence of multivitamin use was much lower than in the other studies such that total intake of vitamins A, C and E in quintile 5 was not comparable to the intake levels in the other studies.
For total intake of vitamins A, C, and E and folate, the pooled age-adjusted and multivariate RRs indicated that there was no association with ovarian cancer risk with RRs ranging from 0.89 to 1.07 comparing the highest versus lowest quintile (table 3). For total folate intake among the North American studies, the pooled multivariate RR (95% CI) for the highest versus the lowest quintile of intake was 1.09 (0.89-1.34) when follow-up was limited to the pre-fortification period. When total vitamin intakes were analyzed according to categories based on identical absolute cut points across the studies, the observed associations were consistent with the analyses by categories of study-specific quintiles, and indicated no association (Supplementary table).
When dietary intakes of each vitamin were modeled as continuous variables, the pooled multivariate RRs were consistent with the analyses based on categories of intake and indicated no association with ovarian cancer risk, even when corrected for measurement error (results not shown). Similarly, the pooled multivariate RRs (95% CI) for total intake of each vitamin modeled as continuous variables were 1.02 (0.97-1.07) for each 1300 mcg/day increase in vitamin A, 1.01 (0.99-1.04) for each 400 mg/day increase in vitamin C, 1.02 (0.97-1.06) for each 130 mg/day increase in vitamin E and 1.01 (0.96-1.07) for each 250 mcg/day increase in folate. There was no evidence of statistically significant heterogeneity between studies for dietary (P >0.42) or total (P > 0.32) intake of any of the vitamins.
When comparing RRs among participants diagnosed before the age of 62 years with those diagnosed at age 62 years or later, a statistically significant interaction was observed for dietary vitamin E intake (P for interaction=0.01) and total vitamin E intake (P for interaction=0.04). The pooled multivariate RRs (95% CI) for each 3 mg/day increase in dietary intake of vitamin E was 1.06 (1.01-1.11) for participants diagnosed before 62 years of age and 0.96 (0.93-1.01) for those diagnosed at ages ≥62 years. For total vitamin E intake, the pooled multivariate RRs (95% CI) for each 130 mg/day increase in intake were 1.06 (0.99-1.14) for participants diagnosed before 62 years of age and 0.93 (0.83-1.05) for those diagnosed at ages ≥62 years. A similar pattern was generally observed for both dietary and total intakes of the other vitamins, but the differences were not statistically significant (results not shown).
For each vitamin, estimates did not differ greatly between analyses that were limited to the first 5 years of follow-up (n=731 cases and 622 cases, in the analyses of dietary and total intake, respectively) and those that included the follow-up period that occurred 5 years or more after baseline (n=1,242 cases and 843 cases, in the analyses of dietary and total intake, respectively; results not shown).
The RRs for ovarian cancer associated with dietary and total intake of each vitamin were not modified by parity (≤1 versus 2 or more, P, test for interaction> 0.14), oral contraceptive use (ever versus never use, P, test for interaction> 0.11), postmenopausal hormone use (never versus past versus current use, P, test for interaction > 0.26), or smoking status (never versus past versus current smoker, P, test for interaction > 0.09). In analyses stratified by alcohol consumption (drinker versus non-drinker) a marginally statistically significant interaction was observed only with dietary vitamin E intake (P, test for interaction=0.04 for dietary vitamin E; P, test for interaction>0.11 for the remaining vitamin variables). In both strata of alcohol consumption, non-significant associations were observed; the pooled multivariate RRs (95% CIs) for a 3 mg/day increase in dietary vitamin E intake were 0.97 (0.93-1.02) for drinkers (n=1,185 cases) and 1.04 (0.99-1.09) for non-drinkers (n=710 cases). Given that the association between folate intake and risk of other cancers has been found to be modified by alcohol intake [53, 54], we further stratified alcohol intake (non-drinkers, <1 drink/day, 1+ drinks/day) to determine if there were differences in associations for dietary and total folate intake by level of alcohol intake. We observed no statistically significant interactions (P, tests for interaction>0.78).
Associations between dietary intake of each of the vitamins with serous, endometrioid and mucinous ovarian cancers were not significantly different from each other (results not shown). When examining total vitamin intake, a statistically significant difference by histological type was observed for intakes of total vitamins A and C (P, test for differences by histological type ≤ 0.05), where a statistically significant positive association with endometrioid, but not serous and mucinous, ovarian cancers was observed (table 4). A similar pattern of differences was observed for folate intake, which was of borderline statistical significance.
Table 4.
Pooled multivariate relative risks (95% confidence intervals) of epithelial ovarian cancer for total vitamin intake, by histological type of ovarian cancer
| Increment unit1 | Serous n=728 | Endometrioid4 n=156 | Mucinous5 n=82 | p-value, test for differences by serous, endometrioid and mucinous cancers | ||||
|---|---|---|---|---|---|---|---|---|
| Multivariate RR2,3 (95% CI) | pheterogeneity6 | Multivariate RR2,3 (95% CI) | pheterogeneity6 | Multivariate RR2,3 (95% CI) | pheterogeneity6 | |||
| Total vitamin A | 1300 μg/day | 0.99 (0.91-1.07) | 0.22 | 1.16 (1.04-1.30) | 0.24 | 0.95 (0.76-1.17) | 0.92 | 0.05 |
| Total vitamin C | 400 mg/day | 0.95 (0.86-1.05) | 0.28 | 1.12 (1.06-1.20) | 0.76 | 0.84 (0.53-1.34) | 0.22 | 0.01 |
| Total vitamin E | 130 mg/day | 1.00 (0.94-1.07) | 0.81 | 1.10 (0.92-1.32) | 0.04 | 0.97 (0.75-1.25) | 0.51 | 0.56 |
| Total folate | 250 μg/day | 0.98 (0.90-1.06) | 0.89 | 1.15 (1.03-1.29) | 0.58 | 0.96 (0.73-1.27) | 0.89 | 0.06 |
The increment units are based on the mean of the standard deviation of the mean intake of each vitamin across studies
Adjusted for parity (0,1,2,3+), oral contraceptive use (never, ever), menopausal status and postmenopausal hormone use (pre-menopausal, unknown menopausal status, postmenopausal never use, postmenopausal past use, postmenopausal current use), age at menarche (<13, 13, 14+ years), body mass index (<23, 23 to <25, 25 to <30, 30+ kg/m2), physical activity (low, medium, high), smoking status (never, past, current) and total energy intake (kcal/day, continuous); age in years and year of questionnaire return were included as stratification variables
The Canadian National Breast Screening Study and Swedish Mammography Cohort were not included in these analyses because supplement use data at baseline were not available in these studies
The analysis of endometrioid ovarian cancers included the Breast Cancer Detection Demonstration Project, the Cancer Prevention Study II Nutrition Cohort, the low a Women's Health Study, the Netherland Cohort Study, Nurses' Health Study (a), Nurses' Health Study (b), and Nurses' Health Study II, as all of these studies had at least 10 cases
The analysis of mucinous ovarian cancers included the Cancer Prevention Study II Nutrition Cohort, the Iow a Women's Health Study, the Netherland Cohort Study, Nurses' Health Study (a) and Nurses' Health Study (b), as all of these studies had at least 10 cases
p-value, test for heterogeneity between studies
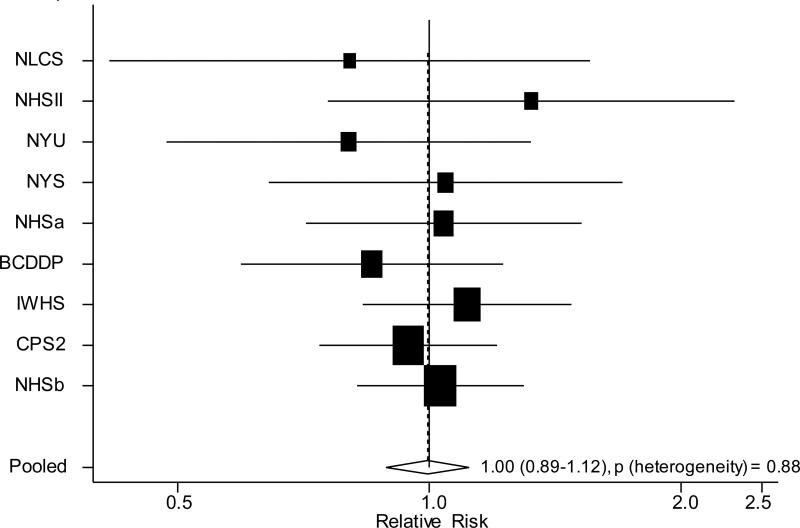
Given the importance of multivitamins as a source of total intake for each of the vitamins, we analyzed multivitamin use separately and observed a pooled multivariate RR (95% CI) of 1.00 (0.89-1.12) for ovarian cancer overall (Figure 1). When analyses were conducted by histology, the pooled multivariate RRs (95% CI) comparing multivitamin use to non-use were 0.96 (0.81-1.12) for serous cancers, 1.34 (0.95-1.89) for endometrioid cancers and 0.76 (0.43-1.32) for mucinous cancers. The difference between the RRs by histological type was not statistically significant (P, test for differences by histological type=0.13). We also examined separately use of supplemental intake of vitamins A, C and E, in categories defined by dose, in relation to ovarian cancer risk and did not observe any statistically significant associations (results not shown). In these analyses, which were adjusted for dietary intake of the relevant individual vitamin, the associations for dietary intake were virtually unchanged from the main analyses (results not shown).
Figure 1.
Study-specific and pooled multivariate RRs and 95% CI of ovarian cancer comparing multivitamin users to nonusers1,2
1 BCDDP=Breast Cancer Detection Demonstration Project Follow-up Cohort, CPS II=Cancer Prevention Study II Nutrition Cohort, IWHS=Iowa Women's Health Study, NLCS =Netherland Cohort Study, NYSC = New York State Cohort, NYUWHS=New York University Women's Health Study, NHSa=Nurses’ Health Study (a), NHSb=Nurses’ Health Study (b), NHS II=Nurses’ Health Study II
2 The black squares and horizontal lines correspond to the study-specific multivariate RR and 95% CI, respectively. The area of the black square reflects the study-specific weight (inverse of the variance). The diamond represents the pooled multivariate RR and 95% CI. The solid vertical line indicates a RR of 1.0. The Canadian National Breast Screening Study and Swedish Mammography Cohort were not included in this analysis because data on multivitamin use was not available in these studies at baseline.
DISCUSSION
In this pooled analysis, the observed associations between dietary and total intake of vitamins A, C, and E and folate and ovarian cancer risk were generally null with relatively narrow confidence intervals. These null associations were observed when intakes were modeled as continuous variables and categories based on study-specific quintiles or common cut points of absolute intake. There was no statistical evidence of heterogeneity between studies in these analyses. While statistically significant differences between subgroups (i.e. vitamin E by age at diagnosis and by alcohol intake) were suggested in a few analyses, the observed RRs in those specific strata were modest and generally of marginal statistical significance. For the remaining analyses by levels of ovarian cancer risk factors, the results for each of the vitamin variables did not differ appreciably. In analyses by histological type, we observed some suggestion that greater vitamin intakes were associated with modestly higher risks of endometrioid tumors, but not other histological types.
Based on previous experimental evidence, we hypothesized that intake of these vitamins would be inversely associated with ovarian cancer risk. With respect to previous epidemiological evidence, between 6 and 16 studies, not including those that are in this pooled analysis [10-16], have previously reported on intake of vitamins A, C, and E and/or folate, whether from food, supplements or both, in relation to ovarian cancer risk. Only one of these studies used a prospective cohort design [6], and the reported RRs were near the null value for intakes of dietary, supplemental and total vitamin A, C and E, similar to our results. Among the case-control studies, relatively consistent results indicating an inverse association were observed only for vitamin E intake [55-62], where risk reductions of 20-59% were reported in 6 [55-60] out of 8 studies, which were statistically significant in 4 [55-57, 59]. Results from previous studies were generally consistent also for folate intake [7-9, 59, 62-64]; all suggested a null association, except one study that reported a statistically significant inverse association [9]. Among the 15 case-control studies on vitamin A intake [55-58, 60-62, 65-72] and 12 on vitamin C intake [55, 57-63, 65, 67-69], statistically significant inverse associations were reported in only a few [55, 57, 60-62], although inverse associations were suggested in some others [55, 56, 58, 63, 67, 69]. To our knowledge, only 3 studies have examined multivitamin use in relation to ovarian cancer risk and a null association was observed in all of these studies [56, 71, 73]. Although few observational studies have reported on associations with supplemental sources of vitamins, these relations are important to examine given that previous randomized controlled trials of supplemental vitamins have shown no significant benefit for cancer prevention and, in some cases, harmful effects [74, 75].
While we hypothesized that vitamin intakes would be inversely associated with ovarian cancer risk, we observed some positive associations, such as an increased risk with dietary and total vitamin E among women diagnosed at younger versus older ages. Similar differences by age at diagnosis were suggested with intake of the other vitamins, but the differences were not statistically significant. These observed increased risks among women diagnosed at younger ages may reflect the increased risks that we observed for the endometrioid histological type, as some past research suggests that age at diagnosis may be slightly younger for the endometrioid histological type (versus serous) [76, 77]. Data from the Pooling Project support a lower age at diagnosis for the endometrioid histological type (not shown). Associations by ovarian cancer histological type have been examined for vitamin A in one study [68] and for folate in three studies [7, 8, 56], with no significant differences between histological types reported in these studies, although case numbers of non-serous tumors were small. RRs specific to endometrioid ovarian cancers were not reported in any of these studies. Emerging research has offered a new paradigm to the classification of ovarian cancer that is based not only on histological type but also on grade and molecular markers [69]. Unfortunately, the only data we had available on tumor characteristics in the Pooling Project was on histological type. Thus, if there are differences in associations with vitamin intake according to ovarian cancer types classified using the new paradigm, our analyses by histology alone may not have captured these differences. Moreover, these findings may reflect chance given that the analyses of endometrioid cancers were based on very small numbers (n=156 cases, where in 6 out of the 7 studies in this analysis, there were <30 cases).
Our results strongly suggest no inverse association between dietary and total vitamin intake and ovarian cancer risk overall, even for vitamin E intake, for which the majority of previous case-control studies suggested an inverse association. In fact, a suggestive increased risk of endometrioid ovarian cancer was observed, highlighting the importance of examining associations by histological type. The potential for our results to have been influenced by confounding is minimal as we included as covariates several ovarian cancer risk factors, and importantly, we observed only weak evidence for confounding by these factors. Furthermore, we observed little evidence for modification of the association between vitamin intake and ovarian cancer risk by these factors. This pooled analysis was based on cohort studies with diet assessed before the onset of disease, which reduced the potential for recall and selection biases that may occur in case-control studies. On the other hand, our findings of null associations may reflect bias towards the null resulting from measurement error in the assessment of diet using a FFQ. As food composition databases are generally country-specific [78], and since each of the Pooling Project studies calculated vitamin intakes for their participants using their own databases, there was some variation in the food composition databases used by each study, which may have contributed to some misclassification. However, the use of country-specific food composition databases may also have resulted in improved accuracy because they take into account differences in food nutrient contents that may result from varying growing conditions or fortification practices [79, 80]. Nevertheless, the results for dietary intake of each of the vitamins were not appreciably different from the results after correction for measurement error in the assessment of intake.
Misclassification might also have been introduced by the way vitamin intakes were modeled. For instance, with the study-specific quantile approach, true differences in absolute intake cannot be accounted for, which may result in risk estimates for different intake levels being combined when pooling the study-specific results. On the other hand, in the categorical analyses based on identical absolute cut points across studies, misclassification may have occurred because there may have been differences in vitamin intakes across studies due to differences in food composition databases or questionnaire design. Nonetheless, our results were similar regardless of whether vitamin intakes were modeled as continuous variables, study-specific quintiles, or categories defined by absolute intakes.
Another potential source of misclassification of vitamin intake is from having used FFQ data collected at baseline only. Thus, we were unable to examine cumulative exposure which would account for changes in intake during follow-up. However, associations did not vary for cases diagnosed shortly after baseline versus after a longer follow-up, suggesting that measurement error occurring with lengthy follow-up and unmeasured changes in diet did not substantially influence the results. Indeed, given that the latency period of cancer is generally long, baseline diet may have better represented the pertinent exposure period. However, if vitamin intakes during childhood, adolescence, or early adulthood are more relevant, our analysis of adult diet might not have captured the pertinent exposure period.
We prospectively examined 10 cohorts from North America and Europe with a wide range of vitamin consumption. By conducting a pooled analysis, we were able to define and categorize vitamin intakes, as well as other covariates, in a standardized manner across studies and thus minimize heterogeneity between studies due to differences in exposure and covariate definitions. As well, our study included almost 2,000 cases of invasive epithelial ovarian cancer and thus had greater statistical power to analyze these associations compared to each individual cohort separately and the majority of the previous case-control studies. The large number of cases also provided the opportunity to explore potential differences in risk according to the main histological types of ovarian cancer, as well as by levels of other ovarian cancer risk factors.
In summary, our results suggest that consumption of vitamins A, C, and E and folate during adulthood is not associated with a decreased risk of ovarian cancer overall, although vitamin intake may play a role on specific ovarian cancer types. These results are consistent with what we observed in the Pooling Project for ovarian cancer risk with intakes of carotenoids [81], some of which can be converted to vitamin A, and fruits and vegetables [18], an important source of vitamins.
Supplementary Material
Acknowledgment
We would like to thank the participants and staff of each of the cohorts for their valuable contributions and the organizations that funded the infrastructure for each cohort study. The centralization, checking, harmonization, and statistical analyses of the participant level data from each of the cohorts was funded by grant P01 CA55075 from the US National Cancer Institute and grant 20010 from the Fonds de recherche du Québec - Santé. Dr. Anita Koushik currently holds a New Investigator Award from the Canadian Institutes of Health Research.
Abbreviations used
- RR
relative risk
- CI
confidence interval
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–93. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 3.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011;51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br. Med. Bull. 1999;55:578–92. doi: 10.1258/0007142991902646. [DOI] [PubMed] [Google Scholar]
- 5.Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. World Cancer Research Fund/American Institute for Cancer Research; Washington DC: 2007. [Google Scholar]
- 6.Thomson CA, Neuhouser ML, Shikany JM, et al. The role of antioxidants and vitamin A in ovarian cancer: results from the Women's Health Initiative. Nutr. Cancer. 2008;60:710–9. doi: 10.1080/01635580802233983. [DOI] [PubMed] [Google Scholar]
- 7.Harris HR, Cramer DW, Vitonis AF, Depari M, Terry KL. Folate, vitamin B(6), vitamin B(12) , methionine and alcohol intake in relation to ovarian cancer risk. Int J Cancer. 2012;131:E518–29. doi: 10.1002/ijc.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb PM, Ibiebele TI, Hughes MC, et al. Folate and related micronutrients, folate metabolising genes and risk of ovarian cancer. Eur. J. Clin. Nutr. 2011;65:1133–40. doi: 10.1038/ejcn.2011.99. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Liu W, Hao Q, Bao L, Wang K. Folate intake and methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers for ovarian cancer risk. International journal of molecular sciences. 2012;13:4009–20. doi: 10.3390/ijms13044009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushi LH, Mink PJ, Folsom AR, et al. Prospective study of diet and ovarian cancer. Am J Epidemiol. 1999;149:21–31. doi: 10.1093/oxfordjournals.aje.a009723. [DOI] [PubMed] [Google Scholar]
- 11.Fairfield KM, Hankinson SE, Rosner BA, Hunter DJ, Colditz GA, Willett WC. Risk of ovarian carcinoma and consumption of vitamins A, C, and E and specific carotenoids: a prospective analysis. Cancer. 2001;92:2318–26. doi: 10.1002/1097-0142(20011101)92:9<2318::aid-cncr1578>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Kelemen LE, Sellers TA, Vierkant RA, Harnack L, Cerhan JR. Association of folate and alcohol with risk of ovarian cancer in a prospective study of postmenopausal women. Cancer Causes Control. 2004;15:1085–93. doi: 10.1007/s10552-004-1546-6. [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, Giovannucci E, Wolk A. Dietary Folate Intake and Incidence of Ovarian Cancer: The Swedish Mammography Cohort. J. Natl. Cancer Inst. 2004;96:396–402. doi: 10.1093/jnci/djh061. [DOI] [PubMed] [Google Scholar]
- 14.Navarro Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE. Dietary folate consumption and risk of ovarian cancer: a prospective cohort study. Eur. J. Cancer Prev. 2006;15:511–5. doi: 10.1097/01.cej.0000220627.54986.bf. [DOI] [PubMed] [Google Scholar]
- 15.Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE. Carotenoid, vitamin A, vitamin C, and vitamin E intake and risk of ovarian cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:395–7. doi: 10.1158/1055-9965.EPI-05-0835. [DOI] [PubMed] [Google Scholar]
- 16.Tworoger SS, Hecht JL, Giovannucci E, Hankinson SE. Intake of folate and related nutrients in relation to risk of epithelial ovarian cancer. Am J Epidemiol. 2006;163:1101–11. doi: 10.1093/aje/kwj128. [DOI] [PubMed] [Google Scholar]
- 17.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163:1053–64. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 18.Koushik A, Hunter DJ, Spiegelman D, et al. Fruits and vegetables and ovarian cancer risk in a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2005;14:2160–7. doi: 10.1158/1055-9965.EPI-05-0218. [DOI] [PubMed] [Google Scholar]
- 19.Genkinger JM, Hunter DJ, Spiegelman D, et al. Dairy products and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2006;15:364–72. doi: 10.1158/1055-9965.EPI-05-0484. [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ. Modern Epidemiology. Little Brown and Company; Boston: 1986. [Google Scholar]
- 21.Cho E, Spiegelman D, Hunter DJ, et al. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst. 2003;95:1079–85. doi: 10.1093/jnci/95.14.1079. [DOI] [PubMed] [Google Scholar]
- 22.Higginbotham S, Zhang ZF, Lee IM, Cook NR, Buring JE, Liu S. Dietary glycemic load and breast cancer risk in the Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2004;13:65–70. doi: 10.1158/1055-9965.epi-03-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terry PD, Miller AB, Jones JG, Rohan TE. Cigarette smoking and the risk of invasive epithelial ovarian cancer in a prospective cohort study. Eur J Cancer. 2003;39:1157–64. doi: 10.1016/s0959-8049(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 24.Mommers M, Schouten LJ, Goldbohm RA, van den Brandt PA. Consumption of vegetables and fruits and risk of ovarian cancer: results from The Netherlands Cohort Study. Cancer. 2005;104:1512–9. doi: 10.1002/cncr.21332. [DOI] [PubMed] [Google Scholar]
- 25.Bandera EV, Freudenheim JL, Marshall JR, et al. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States). Cancer Causes Control. 1997;8:828–40. doi: 10.1023/a:1018456127018. [DOI] [PubMed] [Google Scholar]
- 26.Larsson SC, Holmberg L, Wolk A. Fruit and vegetable consumption in relation to ovarian cancer incidence: the Swedish mammography cohort. Br. J. Cancer. 2004;90:2167–70. doi: 10.1038/sj.bjc.6601872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 28.Hannan LM, Leitzmann MF, Lacey JV, Jr., et al. Physical activity and risk of ovarian cancer: a prospective cohort study in the United States. Cancer Epidemiol Biomarkers Prev. 2004;13:765–70. [PubMed] [Google Scholar]
- 29.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: The New York University Women's Health Study. Nutr. Cancer. 1997;28:276–81. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- 30.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. World Health Organization; Geneva: 2000. [Google Scholar]
- 31.Willett WC, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 32.Feskanich D, Marshall J, Rimm EB, Litin LB, Willett WC. Simulated validation of a brief food frequency questionnaire. Ann. Epidemiol. 1994;4:181–7. doi: 10.1016/1047-2797(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 33.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. JAMA. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 34.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort food frequency questionnaire. Epidemiology. 2000;11:462–8. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Goldbohm RA, van den Brandt PA, Brants HAM, et al. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur. J. Clin. Nutr. 1994;48:253–65. [PubMed] [Google Scholar]
- 36.Jain M, Howe GR, Rohan T. Dietary assessment in epidemiology: comparison of a food frequency and a diet history questionnaire with a 7-day food record. Am J Epidemiol. 1996;143:953–60. doi: 10.1093/oxfordjournals.aje.a008839. [DOI] [PubMed] [Google Scholar]
- 37.Khani BR, Ye W, Terry P, Wolk A. Reproducibility and validity of major dietary patterns among Swedish women assessed with a food-frequency questionnaire. J Nutr. 2004;134:1541–5. doi: 10.1093/jn/134.6.1541. [DOI] [PubMed] [Google Scholar]
- 38.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136:192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 39.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 40.Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J Nutr. 1992;122:1792–801. doi: 10.1093/jn/122.9.1792. [DOI] [PubMed] [Google Scholar]
- 41.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 42.Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745–57. doi: 10.1007/s10552-010-9549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox DR. Regression models and life-tables. J R Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 44.SAS/STAT software: the PHREG procedure: preliminary documentation. SAS Institute; Cary: 1991. [Google Scholar]
- 45.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 46.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 47.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 48.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26:3735–52. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 49.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for measurement error: the case of multiple covariates measured with error. Am J Epidemiol. 1990;132:734–45. doi: 10.1093/oxfordjournals.aje.a115715. [DOI] [PubMed] [Google Scholar]
- 50.Stram DO. Meta-analysis of published data using a linear mixed-effects model. Biometrics. 1996;52:536–44. [PubMed] [Google Scholar]
- 51.Anderson TW. Introduction to multivariate statistics. John Wiley Sons; New York, NY: 1984. [Google Scholar]
- 52.Smith-Warner SA, Spiegelman D, Yaun SS, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA. 1998;279:535–40. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 53.Giovannucci E. Alcohol, one-carbon metabolism, and colorectal cancer: recent insights from molecular studies. J Nutr. 2004;134:2475S–81S. doi: 10.1093/jn/134.9.2475S. [DOI] [PubMed] [Google Scholar]
- 54.Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J. Clin. Oncol. 2010;28:4052–7. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M, Lee AH, Binns CW. Reproductive and dietary risk factors for epithelial ovarian cancer in China. Gynecol. Oncol. 2004;92:320–6. doi: 10.1016/j.ygyno.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 56.Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1521–7. [PubMed] [Google Scholar]
- 57.McCann SE, Moysich KB, Mettlin C. Intakes of selected nutrients and food groups and risk of ovarian cancer. Nutr. Cancer. 2001;39:19–28. doi: 10.1207/S15327914nc391_3. [DOI] [PubMed] [Google Scholar]
- 58.Fleischauer AT, Olson SH, Mignone L, Simonsen N, Caputo TA, Harlap S. Dietary antioxidants, supplements, and risk of epithelial ovarian cancer. Nutr. Cancer. 2001;40:92–8. doi: 10.1207/S15327914NC402_3. [DOI] [PubMed] [Google Scholar]
- 59.Bidoli E, La Vecchia C, Montella M, et al. Nutrient intake and ovarian cancer: an Italian case-control study. Cancer Causes Control. 2002;13:255–61. doi: 10.1023/a:1015047625060. [DOI] [PubMed] [Google Scholar]
- 60.Tung KH, Wilkens LR, Wu AH, et al. Association of dietary vitamin A, carotenoids, and other antioxidants with the risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:669–76. doi: 10.1158/1055-9965.EPI-04-0550. [DOI] [PubMed] [Google Scholar]
- 61.Cramer DW, Kuper H, Harlow BL, Titus-Ernstoff L. Carotenoids, antioxidants and ovarian cancer risk in pre- and postmenopausal women. Int J Cancer. 2001;94:128–34. doi: 10.1002/ijc.1435. [DOI] [PubMed] [Google Scholar]
- 62.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Hernandez-Avila M. Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology. 2002;63:151–7. doi: 10.1159/000063814. [DOI] [PubMed] [Google Scholar]
- 63.McCann SE, Freudenheim JL, Marshall JR, Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr. 2003;133:1937–42. doi: 10.1093/jn/133.6.1937. [DOI] [PubMed] [Google Scholar]
- 64.Pelucchi C, Mereghetti M, Talamini R, et al. Dietary folate, alcohol consumption, and risk of ovarian cancer in an Italian case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:2056–8. doi: 10.1158/1055-9965.EPI-05-0192. [DOI] [PubMed] [Google Scholar]
- 65.Byers T, Marshall J, Graham S, et al. A case-control study of dietary and nondietary factors in ovarian cancer. J Natl Cancer Inst. 1983;71:681–6. [PubMed] [Google Scholar]
- 66.La Vecchia C, Decarli A, Negri E, et al. Dietary factors and the risk of epithelial ovarian cancer. J Natl Cancer Inst. 1987;79:663–9. [PubMed] [Google Scholar]
- 67.Slattery ML, Schuman KL, West DW, et al. Nutrient intake and ovarian cancer. Am J Epidemiol. 1989;130:497–502. doi: 10.1093/oxfordjournals.aje.a115363. [DOI] [PubMed] [Google Scholar]
- 68.Shu XO, Gao YT, Yuan JM, et al. Dietary factors and epithelial ovarian cancer. Br. J. Cancer. 1989;59:92–6. doi: 10.1038/bjc.1989.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tzonou A, Hsieh CC, Polychronopoulou A, et al. Diet and ovarian cancer: a case-control study in Greece. Int J Cancer. 1993;55:411–4. doi: 10.1002/ijc.2910550314. [DOI] [PubMed] [Google Scholar]
- 70.Risch HA, Jain M, Marrett LD, Howe GR. Dietary fat intake and risk of epithelial ovarian cancer. J Natl Cancer Inst. 1994;86:1409–15. doi: 10.1093/jnci/86.18.1409. [DOI] [PubMed] [Google Scholar]
- 71.Bertone ER, Hankinson SE, Newcomb PA, et al. A population-based case-control study of carotenoid and vitamin A intake and ovarian cancer (United States). Cancer Causes Control. 2001;12:83–90. doi: 10.1023/a:1008985015927. [DOI] [PubMed] [Google Scholar]
- 72.Bidoli E, La Vecchia C, Talamini R, et al. Micronutrients and ovarian cancer: a case-control study in Italy. Ann. Oncol. 2001;12:1589–93. doi: 10.1023/a:1013124112542. [DOI] [PubMed] [Google Scholar]
- 73.Neuhouser ML, Wassertheil-Smoller S, Thomson C, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women's Health Initiative cohorts. Arch. Intern. Med. 2009;169:294–304. doi: 10.1001/archinternmed.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 75.Kristal AR, Darke AK, Morris JS, et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106:djt456. doi: 10.1093/jnci/djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storey DJ, Rush R, Stewart M, et al. Endometrioid epithelial ovarian cancer : 20 years of prospectively collected data from a single center. Cancer. 2008;112:2211–20. doi: 10.1002/cncr.23438. [DOI] [PubMed] [Google Scholar]
- 77.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol. 2010;171:45–53. doi: 10.1093/aje/kwp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merchant AT, Dehghan M. Food composition database development for between country comparisons. Nutr J. 2006;5 doi: 10.1186/1475-2891-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis DR, Epp MD, Riordan HD. Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J. Am. Coll. Nutr. 2004;23:669–82. doi: 10.1080/07315724.2004.10719409. [DOI] [PubMed] [Google Scholar]
- 80.Csizmadi I, Kahle L, Ullman R, et al. Adaptation and evaluation of the National Cancer Institute's Diet History Questionnaire and nutrient database for Canadian populations. Public health nutrition. 2007;10:88–96. doi: 10.1017/S1368980007184287. [DOI] [PubMed] [Google Scholar]
- 81.Koushik A, Hunter DJ, Spiegelman D, et al. Intake of the major carotenoids and the risk of epithelial ovarian cancer in a pooled analysis of 10 cohort studies. Int J Cancer. 2006;119:2148–54. doi: 10.1002/ijc.22076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.