Abstract
Objective
Creatine Transporter Deficiency (CTD) is an X-linked, neurometabolic disorder associated with intellectual disability that is characterized by brain creatine deficiency and caused by mutations in SLC6A8, the creatine transporter 1 protein gene. CTD is identified by elevated urine creatine/creatinine ratio or reduced creatine peak on brain magnetic resonance spectroscopy (MRS); the diagnosis is confirmed by decreased creatine uptake in cultured fibroblasts, and/or identification of a mutation in the SLC6A8 gene. Prevalence studies suggest this disorder may be underdiagnosed. We sought to identify cases from a well-characterized cohort of children diagnosed with neurodevelopmental disorders.
Method
Urine screening for CTD was performed on a cohort of 46 males with autism spectrum disorder (ASD) and 9 males with a history of non-ASD developmental delay classified with intellectual disability.
Results
We identified one patient with CTD in the cohort based on abnormal urine creatine/creatinine, and confirmed the diagnosis by the identification of a novel frameshift mutation in the SLC6A8 gene. This patient presented without ASD but with intellectual disability, and was characterized by a non-specific phenotype of early language and developmental delay that persisted into moderate-to-severe intellectual disability, consistent with previous descriptions of CTD.
Conclusion
Identification of patients with CTD is possible by measuring urine creatine and creatinine levels and the current case adds to the growing literature of neurocognitive deficits associated with the disorder affecting cognition, language and behavior in childhood.
Keywords: autism, creatine transporter deficiency, developmental disability, genetic disease, X-linked
INTRODUCTION
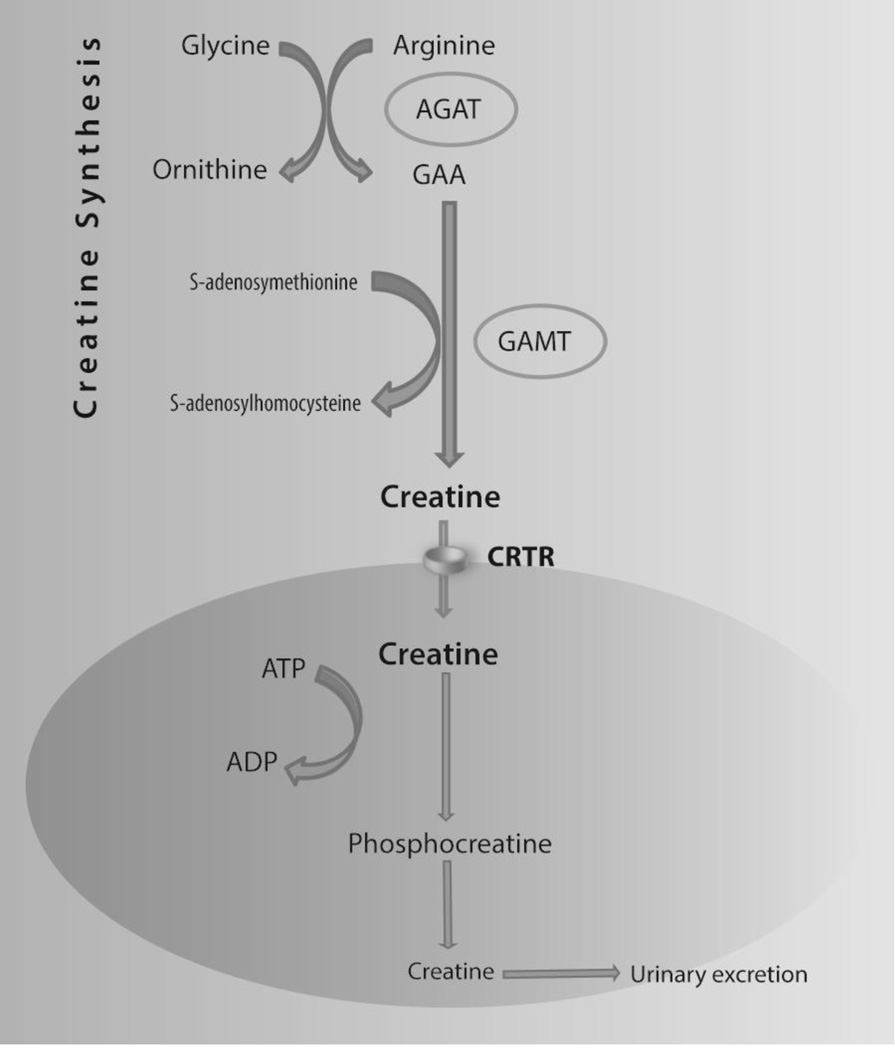
Creatine (Cr) plays an important role in the energy metabolism of cells and tissues with high and fluctuating energy demands such as the brain, as it facilitates the resynthesis of ATP from ADP through phosphocreatine (Figure 1) (1). Creatine Transporter Deficiency (CTD, OMIM #300352) is an X-linked, inborn error of metabolism caused by mutations in the creatine transporter SLC6A8 gene (2). The other two creatine deficiency syndromes are due to autosomal recessive defects of creatine synthesis: arginine:glycine amidinotransferase (AGAT; OMIM #612718) and guanidinoacetate methyltransferase (GAMT; OMIM #612736) (3). In all of these disorders, the common manifestations are developmental delay leading to intellectual disability, language delay, and decreased brain creatine detected by magnetic resonance spectroscopy (MRS) (2,3).
Figure 1. Creatine Synthesis and Transport.
Note: Creatine (methyl guanidinoacetic acid) is derived from diet and biosynthesis, mainly in the liver, pancreas, and kidney, from arginine and glycine. Creatine is transported via the bloodstream to tissues with high and/or fluctuating energy demands, such as muscle, heart, and brain, where it is actively transported against a concentration gradient into cells by CRTR. Creatine is converted to phosphocreatine (PCr), which acts as a reservoir for high-energy phosphate, and is crucial for intracellular energy metabolism.
CTD is characterized by moderate-to-severe intellectual disability, severe language delay, behavioral problems, seizures, gastrointestinal problems, normal plasma creatine and GAA levels, and elevated urine creatine/creatinine ratio (Cr/Crn) (3,4); the diagnosis is confirmed by impaired creatine uptake in fibroblasts (5, 6) or identification of hemizygous mutations in SLC6A8 (6).
CTD is estimated to be present in 0.2–3.5% of males with intellectual disabilities (ID) or autism spectrum disorder (ASD) (7, 8, 9, 10, 11, 12), with average rates varying between 0.4% and 1.4% (13). However, very few studies have examined rates specifically in children with ASD (13). Since it is X-linked, some researchers suggest CTD should be included in the differential diagnosis of males with ID or ASD (14).
We describe the identification of CTD in a patient from a well-characterized cohort of males with ID and/or ASD. This patient presented with features similar to previously reported patients, namely ID, early language delay, neurological abnormalities, and hypotonia. Neurocognitive data collected from this patient over the ages of 5 to 10 years are presented.
MATERIALS AND METHODS
We evaluated male subjects who participated in an IRB-approved longitudinal natural history study of children with ASD or with a history of non-ASD developmental delay (DD) that led to ID (NIH protocol 06-M-0102). One of the aims of this study was to evaluate medical problems related to ASD and ID. Informed consent for all aspects of this study was obtained from the parents/guardians of all participants. Male patients were evaluated for CTD if a urine sample was available. Prior testing of this cohort included urine organic acids, plasma amino acids, comparative genomic hybridization (CGH) and fragile X testing.
Urine samples were obtained from 46 males with ASD and nine males with ID. Urine was frozen at −80°C shortly after collection and stored for 2–42 months. Urine creatine (Cr), creatinine (Crn), and guanidinoacetic acid (GAA) were measured by liquid chromatography and tandem mass spectrometry (clinical sensitivity 99%) using a CLIA-certified method widely available at biochemical genetics laboratories. Because false positives may occur due to creatine supplements or excess meat consumption (7), patients with positive results were asked to abstain from intake of meat or fish for 24 hours and creatine supplements for 72 hours before another urine sample was obtained for confirmation. DNA sequence analysis of SLC6A8 was performed on samples from individuals with two positive urine tests.
RESULTS
Screening for CTD
As shown in descriptives presented in Table 1, an increased urine Cr/Crn ratio was observed in three (5%) of the 55 patients screened. In one patient, the urine Cr normalized from 1114 to 745 (normal range for age: 24–890 mmol/mol Crn) after a 48-hour diet devoid of excess meat. In another patient, the ratio normalized from 1475 to 271 (normal range for age: 24–890 mmol/mol Crn) after stopping creatine supplements. The third patient, who was in the ID group, had a Cr of 2153 (normal range for age: 23–1500 mmol/mol Crn) in urine that had been collected at 86 months of age and stored at −80°C for 35 months. A second urine sample was freshly obtained at 126 months of age following 48 hours of creatine intake restriction. The urine Cr ratio remained elevated at 2025 (normal range for age: 24–890 mmol/mol Crn). To confirm the diagnosis, sequencing of SLC6A8 was performed and revealed a hemizygous frameshift mutation, c.1146_1150dup; p.Leu384Argfs*14, which results in a premature insertion of a stop codon. Genetic testing on his mother identified no SLC6A8 mutation, suggestive of a de novo mutation, although somatic or gonadal mosaicism could not be ruled out (14). Genetic counseling was provided to the family.
Table 1.
Sample Description
| Autism | ID | |
|---|---|---|
| Subjects with Unremarkable Results | ||
| n | 44 | 8 |
| Age at urine sample (years), M ± SD | 7.26 ± 1.62 | 5.86 ± 1.29 |
| Urine creatine result3, M ± SD | 347.32 ± 309.96 | 359.25 ± 226.61 |
| Median | 259 | 334.50 |
| Range | 17 – 1265 | 17 – 125 |
| Urine creatinine result, M ± SD | 7158.80 ± 3536.68 | 7791.13 ± 2663.35 |
| Median | 7011.30 | 7449.90 |
| Range | 1204.90 – 19293.30 | 3890.10 – 12570.50 |
| Urine GAA result, M ± SD | 58.86 ± 21.01 | 58.38 ± 26.64 |
| Median | 58.5 | 59 |
| Range | 15 – 114 | 17 – 94 |
| Age of first developmental concern (months)1, M ± SD | 14.02 ± 6.54 | 7.67 ± 2.96 |
| Nonverbal Ratio IQ2, M ± SD | 58.36 ± 25.92 | 60.6 ± 10.73 |
| Vineland Adaptive Behavior Composite, M ± SD | 67.93 ± 11.66 | 73.88 ± 8.94 |
| Subjects with Remarkable Results | ||
| n | 2 | 1 |
| Age at urine sample (years) | 5.87, 7.19 | 7.19 |
| Urine creatine result | 1475, 1114 | 2153 |
| Urine creatinine result | 6342.4, 8538 | 7678 |
| Urine GAA result | 87, 57 | 85 |
| Age of first developmental concern (months) | 12, 12 | 8 |
| Nonverbal Ratio IQ | 85, 44.95 | 28.39 |
| Vineland Adaptive Behavior Composite | 81, 60 | 62 |
Clinical judgment of the age when developmental abnormalities were first noticed by parents, based on Autism Diagnostic Interview-Revised.
Nonverbal Ratio IQ was derived from either the Differential Ability Scales, 2nd edition or the Mullen Scales of Early Learning.
Among subjects with unremarkable results, all subjects were aged 4–10 years except for one AUT subject (Creatine value = 17; GAA value = 34; Creatinine value = 10907.2). References ranges for creatine are as follows: Less than 4 years 23 – 1500 mmol/mol, 4–10 years 24 – 890 mmol/mol, and 11–50 10 – 370 mmol/mol.
Description of Patient with CTD
This patient, conceived by intracytoplasmic sperm injection, was the first child of unrelated parents. The delivery was complicated by breech position and fetal distress. The patient was delivered via cesarean section at term with a birth weight of 3.03 kg. Apgar scores were 8 and 9. During infancy, gastroesophageal reflux, irritability, irregular sleep pattern, and hypotonia were identified. He had a history of chronic loose stools, poor appetite and sensory aversion to texture. Developmental delay was first suspected by his parents about 12 months of age. He had delayed motor skills, crawling at 16 months, and walking at 3 years of age. He had balance and coordination problems and an abnormal gait.
As documented by medical records, the child had a history of weight and height at or below the 10th percentile (birth, 3.03 kg [10th percentile] and 46 cm [5th percentile]; 2 months, 4.11 kg [5th percentile] and 56 cm [10th percentile]; 4 months, 5.64 kg [10th percentile] and 60.8 cm [10th percentile]; 23 months, 10.90 kg [5th percentile] and 81.96 cm [5th percentile]). At initial presentation to the study (age 5), the child’s height was 95.6 cm and his weight was 13.18 kg (both <3rd percentile), and his head circumference was 50 cm (10–25th percentile). Exam at age 5 was significant for mild dysmorphism including cupped and mildly posteriorly rotated ears, upturned nasal tip, mouth with a cupid bow appearance, a mildly pointed chin and a broad forehead, hyperextensible fingers, a few fetal pads and mild fifth finger clinodactyly bilaterally. The patient had bilateral flat feet and generalized hypotonia.
Laboratory work-up included decreased serum creatinine: 0.32 mg/dL at 60 months, 0.30 mg/dL at 75 months, <0.2 mg/dL at both 86 months and 94 months of age (normal range: 0.34–0.55 mg/dL). Plasma amino acids and urine organic acids were normal. MRS was not performed on this patient. Genetic testing for Fragile X, FISH for Williams syndrome, karyotype (46 XY) and comparative genomic hybridization (CGH) revealed no abnormalities. The patient remained without a genetic etiology until the cohort was screened for CTD.
Global developmental delays were evident by both parent report and observation. Prior to acquisition of speech, his parents attempted to use sign language to communicate but he did not produce signs; he would attempt to speak but words were not intelligible. Single words were not used until 3 to 4 years, with phrase speech beginning at age 5 years. At 10 years his language was characterized as consisting of two-to-three word phrases with consistent articulation errors. As such, the patient was enrolled in special education programming, and received speech, occupational, and physical therapy. He was treated for attention deficit hyperactivity disorder (diagnosed at age 10) and had a history of severe sleep issues and self-injurious behavior that started at approximately age 4. He was partially toilet trained at 9 years.
Neuropsychological testing was performed as part of the natural history study on five occasions between the ages of 5 and 10 years; evaluations indicated developmental delay that led to an intellectual disability diagnosis (See Table 2). IQ was tested at the first visit using the Mullen Scales of Early Learning (19) and later using the Differential Ability Scales, Second edition (20) and the Stanford-Binet Intelligence Test (21). Scores were in the moderate range of impairment across all visits. The Adaptive Behavior Composite of the Vineland Scales of Adaptive Behavior, 2nd edition (VABS-II; 16) was mostly stagnant, with an unremarkable 8-point increase in the standard score from age 5 to age 10, attributed to improvements in daily living skills and socialization. While age equivalents at age 10 did not exceed 4 years 6 months for any skill, communication skills and gross motor skills had the lowest age equivalents (2 years 11 months for receptive language, 2 years 10 months for expressive language, and 2 years 6 months for gross motor).
Table 2.
Longitudinal Neurocognitive Findings of the CTD Patient
| Age (years, months) | 5y,0m | 6y,1m | 7y,2m | 7y,10m | 10y,6m |
|---|---|---|---|---|---|
| Nonverbal IQ (mean=100, SD=15) | 43 | 38 | 35 | 34 | 42 |
| Verbal IQ (mean=100, SD=15) | 39 | 39 | 42 | 49 | 43 |
| Vineland ABC (mean=100,SD=15) | 52 | 52 | 62 | 64 | 60 |
| ADOS Calibrated Severity Score (range=1–10) | 1 | 2 | 2 | 2 | - |
Note: Nonverbal and Verbal IQ at age 5 and 6 were ratio scores from the Mullen Scales of Early Learning, at 7 were standard scores derived from the Differential Ability Scales, 2nd edition, and at age 10 were standard scores from the Stanford Binet Intelligence Scales, Fifth Edition. Adaptive Behavior Composite was measured from the Vineland Adaptive Behavior Scales, 2nd edition and ADOS=Autism Diagnostic Observation Schedule. The Calibrated Severity Score ranges from 1–10, with scores of 1–2 falling in the range of “minimal-to-no evidence of autism-related symptoms,” 3–4 “low level of autism spectrum-related symptoms,” 5–7 “moderate level of autism spectrum-related symptoms” and 8–10 “high level of autism spectrum-related symptoms.”
According to the protocol, the Autism Diagnosis Interview-Revised (16) was administered to rule out ASD. This measure, along with repeated testing on the Autism Diagnostic Observation Schedule (18), consistently produced scores in the asymptomatic range.
DISCUSSION
A patient with CTD was identified by urine screening in a small cohort of males with ASD with or without ID, or non-ASD ID. CTD is a rare, X-linked, neurodevelopmental disorder caused by mutations in the creatine transporter (SLC6A8) gene. The phenotype of CTD may be nonspecific, given that the patient we report had previously undergone extensive evaluation without detection. However, similarities among patients are observed, as the phenotype of our patient was consistent with characteristics described in a retrospective review of 101 males with CTD (4). In that study, behavioral concerns were described in 85% of patients and commonly included attention deficit and hyperactivity. Hypotonia was found in 40% of those patients, as well as gastrointestinal symptoms in 35%.
A prompt diagnosis may be difficult if attempted solely on clinical grounds. Currently, the most convenient, non-invasive method to screen for creatine transporter deficiency is the determination of urine Cr/Crn (and guanidinoacetic acid) (4, 14, 22) leading directly to mutation testing of the proper candidate gene (SLC6A8, GATM or GAMT). False positive results can be easily addressed by re-testing after a diet devoid of meat, fish, and creatine supplements (22); to avoid having false negatives using this approach, it is recommended that control values are obtained after similar diet restrictions. Screening studies using this strategy had a much higher positive predictive value (23) and the methods have been implemented in large-scale screening campaigns (24). In addition, continuous age- and sex-adjusted reference intervals of large cohorts can be used to reduce the risk of false-positive and false-negative events (25). The method, performed often by LC-MS/MS, also measures guanidinoacetate (GAA), allowing the detection of the treatable disorders GAMT and AGAT (3). In our study, the Cr/Crn remained abnormal after restriction of exogenous creatine sources in the patient confirmed by genetic testing but normalized in the patients found to have no mutation on the SCL6A8 gene.
Diagnosis is confirmed by identifying a disease-causing mutation in SLC6A8, the gene encoding creatine transporter (CT1; CRTR). Our patient harbored a novel frameshift mutation, c.1146_1150dup; p.Leu384Argfs*14, which results in the premature insertion of a stop codon, adding to the increasing list of pathogenic mutations causing CTD. De novo mutations occur in approximately 30% of cases (4), as appears to have happened in our patient. Mothers of affected sons with a de novo mutation should be counseled about a recurrence risk in further pregnancies due to the possibility of low level somatic or germline mosaicism (4).
While this study was too limited to adequately address prevalence in either ID or ASD, the estimated prevalence of CTD in 0.2–3.5% of males with intellectual disabilities or autism suggests that CTD should be considered in the genetic and metabolic evaluation of males with developmental delay, particularly if severe speech delay and static developmental delay are prominent features (21, 22).
Potential therapeutic candidates for CTD, such as creatine analogs that do not rely on the activity of SLC6A8 to cross the blood brain barrier are being studied. One recent study showed preclinical evidence that cyclocreatine, a creatine analog that crosses the blood brain barrier, showed improvement in cognition in a mouse model of CTD (26). Lipophilic creatine derivatives and lipid nanocapsules are also being considered (27). Given that potential therapies for CTD have been identified and are in the process of entering clinical trials, accurate diagnosis may have a significant impact on patient outcomes (26, 27, 28).
Acknowledgments
This research was supported by the Intramural Programs of the National Institute of Mental Health (NIMH) (Protocol 06-M-0102, NCT 00298246, 1ZIAMH002868), and the National Center for Advancing Translational Sciences (NCATS), all of the National Institutes of Health (NIH). We wish to thank the families that participated in this study, as well as John C. McKew and Margarita Raygada for their contributions to this manuscript.
Footnotes
CONFLICTS OF INTEREST
We report no conflicts of interest from any of the authors.
REFERENCES
- 1.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 2.Salomons GS, van Dooren SJ, Verhoeven NM, et al. X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet. 2001;68(6):1497–1500. doi: 10.1086/320595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo N, Ardon O, Vanzo R, et al. Disorders of creatine transport and metabolism. Am J Med Genet C Semin Med Genet. 2011;157(1):72–78. doi: 10.1002/ajmg.c.30292. [DOI] [PubMed] [Google Scholar]
- 4.van de Kamp JM, Betsalel OT, Mercimek-Mahmutoglu S, et al. Phenotype and Genotype in 101 Males with X-Linked Creatine Transporter Deficiency. J Med Genet. 2013;50(7):463–472. doi: 10.1136/jmedgenet-2013-101658. [DOI] [PubMed] [Google Scholar]
- 5.Ardon O, Amat di San Filippo C, et al. Creatine transporter deficiency in two half-brothers. Am J Med Genet A. 2010;152A(8):1979–1983. doi: 10.1002/ajmg.a.33551. [DOI] [PubMed] [Google Scholar]
- 6.Salomons GS, van Dooren SJ, Verhoeven NM, et al. X-linked creatine transporter defect: an overview. J Inherit Metab Dis. 2003;26(2–3):309–318. doi: 10.1023/a:1024405821638. [DOI] [PubMed] [Google Scholar]
- 7.Arias A, Corbella M, Fons C, et al. Creatine transporter deficiency: prevalence among patients with mental retardation and pitfalls in metabolite screening. Clin Biochem. 2007;40(16–17):1328–1331. doi: 10.1016/j.clinbiochem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Clark AJ, Rosenberg EH, Almeida LS, et al. X-linked creatine transporter (SLC6A8) mutations in about 1% of males with mental retardation of unknown etiology. Hum Genet. 2006;119(6):604–610. doi: 10.1007/s00439-006-0162-9. [DOI] [PubMed] [Google Scholar]
- 9.Lion-Francois L, Cheillan D, Pitelet G, et al. High frequency of creatine deficiency syndromes in patients with unexplained mental retardation. Neurology. 2006;67(9):1713–1714. doi: 10.1212/01.wnl.0000239153.39710.81. [DOI] [PubMed] [Google Scholar]
- 10.Mercimek-Mahmutoglu S, Muehl A, Salomons GS, et al. Screening for X-linked creatine transporter (SLC6A8) deficiency via simultaneous determination of urinary creatine to creatinine ratio by tandem mass-spectrometry. Mol Genet Metab. 2009;96(4):273–275. doi: 10.1016/j.ymgme.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Puusepp H, Kall K, Salomons GS, et al. The screening of SLC6A8 deficiency among Estonian families with X-linked mental retardation. J Inherit Metab Dis. 2010;33(Suppl 3):S5–S11. doi: 10.1007/s10545-008-1063-y. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg EH, Almeida LS, Kleefstra T, et al. High prevalence of SLC6A8 deficiency in X-linked mental retardation. Am J Hum Genet. 2004;75(1):97–105. doi: 10.1086/422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Angley MT, Sorich MJ, et al. Is there a role for routinely screening children with autism spectrum disorder for creatine deficiency syndrome? Autism Res. 2010;3:268–272. doi: 10.1002/aur.145. [DOI] [PubMed] [Google Scholar]
- 14.Newmeyer A, Cecil M, Schapiro JF, et al. Incidence of brain creatine transporter deficiency in males with developmental delay referred for brain magnetic resonance imaging. J Dev Behav Pediatr. 2005;26(4):276–282. doi: 10.1097/00004703-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Betsalel OT, van de Kamp JM, Martinez-Munoz C, et al. Detection of low-level somatic and germline mosaicism by denaturing high-performance liquid chromatography in a EURO-MRX family with SLC6A8 deficiency. Neurogenetics. 2008;9(3):183–190. doi: 10.1007/s10048-008-0125-5. [DOI] [PubMed] [Google Scholar]
- 16.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. Second. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- 17.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 18.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 19.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 20.Elliott CD. Manual for the Differential Ability Scales. Second. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- 21.Roid GH. Stanford-Binet intelligence scales. 5th. Itasca, IL: Riverside; 2003. [Google Scholar]
- 22.Arias A, Corbella M, Fons C, et al. Creatine transporter deficiency: prevalence among patients with mental retardation and pitfalls in metabolite screening. Clinical biochem. 2007;40(16–17):1328–1331. doi: 10.1016/j.clinbiochem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Lion-Francois L, Cheillan D, et al. High frequency of creatine deficiency syndromes in patients with unexplained mental retardation. Neurology. 2006;67(9):1713–1714. doi: 10.1212/01.wnl.0000239153.39710.81. [DOI] [PubMed] [Google Scholar]
- 24.Cheillan D, Joncquel-Chevalier Curt M, Briand G, et al. Screening for primary creatine deficiencies in French patients with unexplained neurological symptoms. Orphanet. 2012;7:96. doi: 10.1186/1750-1172-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morkrid L, Rowe AD, Elgstoen KB, et al. Continuous age- and sex-adjusted reference intervals of urinary markers for cerebral creatine deficiency syndromes: a novel approach to the definition of reference intervals. Clin Chem. 2015;61(5):760–768. doi: 10.1373/clinchem.2014.235564. 2015. [DOI] [PubMed] [Google Scholar]
- 26.Kurosawa Y, Degrauw TJ, Lindquist DM, et al. Cyclocreatine treatment improves cognition in mice with creatine transporter deficiency. J Clin Invest. 2012;122:2837–2846. doi: 10.1172/JCI59373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trotier-Faurion A, Passirani C, Béjaud J, et al. Dodecyl creatine ester and lipid nanocapsule: a double strategy for the treatment of creatine transporter deficiency. Nanomedicine (Lond) 2015;10:185–191. doi: 10.2217/nnm.13.205. [DOI] [PubMed] [Google Scholar]
- 28.van de Kamp JM, Mancini GM, Salomons GSJ, et al. X-linked creatine transporter deficiency: clinical aspects and pathophysiology. Inherit Metab Dis. 2014;37(5):715–733. doi: 10.1007/s10545-014-9713-8. [DOI] [PubMed] [Google Scholar]