Abstract
Background. The elderly host is highly susceptible to severe disease and treatment failure in Clostridium difficile infection (CDI). We investigated how treatment with vancomycin in the aged host influences systemic and intestinal humoral responses and select intestinal microbiota.
Methods. Young (age, 2 months) and aged (age, 18 months) C57BL/6 mice were infected with VPI 10463 after exposure to broad-spectrum antibiotics. Vancomycin was given 24 hours after infection, and treatment was continued for 5 days. At select time points, specimens of serum and intestinal tissue and contents were collected for histopathologic analysis, to measure antibody levels and the pathogen burden, and to determine the presence and levels of select intestinal microbiota and C. difficile toxin.
Results. Levels of disease severity, relapse, and mortality were increased, and recovery from infection was slower in aged mice compared to young mice. Serum levels of immunoglobulin M, immunoglobulin A, and immunoglobulin G against C. difficile toxin A were depressed in aged mice, and vancomycin treatment reduced antibody responses in both age groups. While baseline levels of total bacterial load, Bacteroidetes, Firmicutes, and Enterobacteriaceae were mostly similar, aged mice had a significant change in the Firmicutes to Bacteroidetes ratio with vancomycin treatment.
Conclusions. Vancomycin treatment decreases the systemic humoral response to CDI. Increased mortality from and recurrence of CDI in the aged host are associated with an impaired humoral response and a greater susceptibility to vancomycin-induced alteration of intestinal microbiota.
Keywords: diarrhea, elderly, Clostridium difficile infection, antibiotic-associated colitis
Clostridium difficile is the most common cause of healthcare-associated infections, resulting in almost half a million cases in 2011, approximately 29 000 deaths [1, 2], and ≥1.0 billion dollars in healthcare costs in US hospitals [3]. People aged ≥65 years are more susceptible to infection and are more likely to have poor outcome [1]. The number of estimated cases of C. difficile infection (CDI) in people aged ≥65 years made up 57% of the total estimated CDI cases in 2011, but the same study found that 83% of total estimated deaths from CDI were from this same age group. In a separate review of the death registry from 2008, 92% of deaths from CDI occurred in people aged ≥65 years [4]. In a study of 336 patients with stool specimens positive for C. difficile in Brigham and Women's Hospital between 2004 and 2005, the odds ratio for severe disease was 3.35 for subjects aged ≥70 years [5]. Even when the analysis controlled for comorbidities by comparing patients with the same Charles comorbidity index, older patients did worse, although the difference was less notable when accounting for higher comorbidity scores [6].
There are a number of different possible etiologies for the higher susceptibility of elderly individuals to CDI. Immunosenescence, or the effect of aging on the immune system, may potentially affect the outcome of CDI. Aging is known to cause dysregulation of the innate immune system [7], as well as the adaptive immune response, including T-cell activity [8–11]. Both the percentage and number of naive human B cells in blood decrease with age [12, 13]. There is also an age-related contraction of the B-cell repertoire with age that correlates with poor health status [14]. The most significant change with aging in humoral immunity is impairment in the ability to produce high-affinity immunoglobulins and undergo immunoglobulin class switching, which allows the production of high-affinity immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin E (IgE) [15]. Serum IgA levels are elevated in elderly subjects as compared with younger controls, but these IgA antibodies are predominantly monomeric and are therefore not transported to the mucosal surface as secretory IgA [16]. These findings may be associated with the decrease in specific antibody responses in humans vaccinated against tetanus toxoid, encephalitis viruses, Salmonella organisms, Streptococcus pneumoniae, and influenza virus [10]. Antibody responses specific for C. difficile toxins A and B have been shown to be an important part of host immune response against CDI, which can be affected by aging [17, 18]. Furthermore, intestinal dysbiosis is a key element in the pathogenesis of CDI. Previous studies analyzing bacterial populations in the gut indicated alterations of the gut microbiota with aging [19, 20]. These findings suggest that aging may affect the intestinal microbiota in ways that make patients more susceptible to CDI.
Dissection of the underlying mechanisms of the increased susceptibility to and worse outcome of CDI among aging persons is challenging because of the complexity of the immune response in aging hosts. Multiple interacting factors, such as comorbidities, polypharmacy, and healthcare exposure, in addition to biological factors, may contribute to the observed outcomes of infection in the elderly population. In this study, we aimed to simulate CDI in elderly individuals, using an aged C57/BL mouse model, and to investigate the biological reasons behind severe disease and poor outcomes with aging.
METHODS
Murine Model of C. difficile Infection and Treatment
The infection model is a modification of the protocol published by Chen et al [21]. This protocol has been approved by the Center for Comparative Medicine at the University of Virginia. Male C57BL/6 mice (Charles River), 2 and 18 months old, were used in all experiments. Mice were pretreated with an antibiotic cocktail (vancomycin [0.0045 mg/g], colistin [0.0042 mg/g], gentamicin [0.0035 mg/g], and metronidazole [0.0215 mg/g] in drinking water for 3 days, followed by clindamycin [32 mg/kg] intraperitoneally 1 day prior to infection) as previously described [22]. Food and water were allowed ad libitum. Although each mouse or treatment group was housed in a separate cage, all mice were housed in the same biosafety level 2 containment pod in the vivarium. Infection was performed with VPI 10463 (ATCC) in an inoculum of 104 or 105 administered by oral gavage. One set of experiments was performed in which infected mice were treated with vancomycin (50 mg/kg) daily for 5 days and were observed for 14 days after infection. A clinical scoring system was developed on the basis of weight loss, diarrhea, activity level, and appearance of eyes and hair (each parameter was scored from 0 to 3, in which 0 is normal and 3 is the worst, with a maximum score of 20) [22]. Mice judged to be moribund or with a clinical score of ≥14, those with a weight loss of ≥25% from the day of infection, and all surviving mice at the end of the experiment were euthanized. Separate sets of experiments were performed for harvesting samples at days 4, 6, 7, and 13 or 14 after infection.
Histopathologic Analysis
Upon euthanasia, cecal and colonic tissues were fixed in Bouin's solution overnight and then placed in 70% ethanol before being sent for paraffin embedding and hematoxylin and eosin (H/E) staining at the University of Virginia Histology Research Core. Histopathologic scoring was performed coded (C. A. W. and M. S. R.). H/E-stained tissues were coded and scored as we previously described in detail [22]. On a set of H/E-stained tissues, myeloperoxidase (MPO)–based immunohistochemical analysis was performed to determine MPO levels.
Quantification of C. difficile and Microbiota
Genomic DNA was extracted from the stool or cecal samples, using a modified protocol for the QIAamp DNA stool mini kit (Qiagen). Stool lysis buffer (400 µL) was added to a mixture of frozen stool sample and diluent (from an enzyme-linked immunosorbent assay [ELISA]). The subsequent DNA extraction was performed as described previously [22]. All quantitative polymerase chain reaction (qPCR) analyses were performed on the Bio-Rad CFX system, using primers to detect the gene encoding C. difficile toxin B (TcdB; forward, GGAGAGTCA TCCAACTTATATG; reverse, CCACCAATTTCTTTTAATGCAG), a Bacteroidetes common gene sequence from Bacteroides ovatus (forward, GGARCATGTGG TTTAATTCGATGAT; reverse, AGCTGACGACAACCATGCAG), a 16S gene sequence that amplifies a relatively conserved region between the V3 and V4 variable regions (forward, GGCTAACTCCGTGCCAGCA; and reverse, GCGCTTTACGCCCAGTAATTC), a Firmicutes common gene sequence from Lactobacillus acidophilus (forward, GGCAGCAGTRGGGAATCTTC; reverse, ACACYTAGYACTCATCGTT), and an Enterobacteriaceae common gene sequence from Escherichia coli (forward, GTGCCAGCMGCCGCGGTAA; reverse, GCCTCAAGGGCACAACCTCCAAG) [23]. Mixtures for qPCRs, in a final volume of 20 µL, consisted of 10 µL of QuantiFast SYBR green Supermix (Qiagen), 1 µL each of forward and reverse primers, 4 µL of sterile water, and 4 µL of sample DNA. PCR assays were performed in duplicate under the following cycling conditions: 3 minutes at 95°C, 10 seconds at 95°C, and 30 seconds at 57°C for C. difficile (59°C for all others) for 40 repeats and a melt curve between 65°C and 95°C for 5 seconds at 0.5°C intervals. For all primer sets, standard curves were generated from known concentrations of bacteria grown. Bacterial counts were expressed as counts or colonies per milligram of mouse stool assayed.
C. difficile Toxin Assay
C. difficile toxins A and B were detected using a modified protocol for the Tech Lab Toxin A/B II ELISA kit. Each stool sample was weighed, and the amount of diluent per sample was normalized to provide the same stool mass to diluent ratio for each sample. The diluent-sample mixtures were homogenized by grinding and vortexing, and 1:100 serial dilutions were made of the sample. A total of 100 µL of the 1:100 dilution of each sample was added to a precoated well provided in the kit. A negative control consisted of 100 µL of diluent, and a positive control consisted of 1 drop of the positive control toxin A-B mixture provided in the kit. One drop of conjugate was added to each well, and the plate was incubated at 37°C for 50 minutes. Each well was washed 3 times with 200 µL of a 1× dilution of the wash buffer provided in the kit. Two drops of substrate were added to each well. After 10 minutes, 1 drop of stop solution was added to each well. The plate was allowed to sit for 2 minutes before being read in an ELISA reader.
Anti–C. difficile Toxin A Antibody Assay
Anti–C. difficile toxin A antibody was detected using ELISA plates precoated with 0.1 µg of C. difficile toxin A blocked with bovine serum albumin blocking buffer. A total of 100 µL of a 1:10 dilution of plasma samples was added to the wells and incubated for 1 hour. Plates were then washed 3 times with a 1× dilution of the wash buffer from the Tech Lab Toxin A/B II ELISA kit, and 100 µL of goat anti-mouse IgG secondary antibody was added at a 1:1000 dilution for 1 hour. For separate sets of ELISAs, goat anti-mouse IgM and IgA were used at a 1:5000 dilution. The plates were then washed 3 times again, and 2 drops of substrate from the Tech Lab Toxin A/B II ELISA kit was added to each well. After 10 minutes, 1 drop of stop solution was added to each well. The plate was allowed to sit for 2 minutes before being read in an ELISA reader.
Measurement of Immunoglobulins by Enzyme-Linked Immunospot (ELISPOT) Analysis
ELISPOT assays to quantify IgG and IgA secretion were conducted as previously described (PMC2841050) [24], using cells isolated from the lamina propria of the cecum (PMC3811747) [25]. Briefly, 1 × 105 lamina propria cells from uninfected or infected age-matched mice (n = 3; 6 days after infection) were plated in triplicate, serially titrated, and incubated for 5 hours.
Flow Cytometry
Mesenteric lymph nodes were dissected from uninfected or infected age-matched mice (n = 3; 6 days after infection). Germinal center B cells (B220+, CD95+, GL7+) were measured as previously described (PMC2841050).
Statistical Analysis
Results were expressed as means ± standard errors of the mean, as generated by GraphPad Prism, version 5.0 (GraphPad Software, San Diego, California). Differences between experimental groups were compared using analysis of variance (ANOVA) with the Bonferroni multiple-comparison test. Two-way ANOVA was used to compare experimental groups across intervals. Differences between 2 groups were analyzed using an unpaired Student t test, where appropriate. Mortality rates between treatment groups were analyzed with the log-rank (Mantel–Cox) test or the log-rank test for trend. Statistical significance was set at a P value of <.05.
RESULTS
Aged Mice Experience Increased Disease Severity During Acute CDI
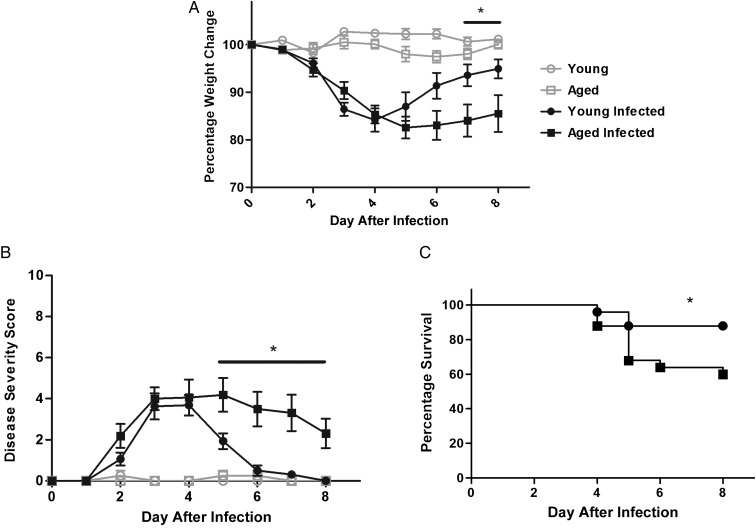
After pretreatment with a broad-spectrum antibiotic cocktail, both young (age, 2 months) and aged (age, 18 months) mice received a gavage of 105 of the same VPI10463 inoculum . Twenty-four hours after infection, both groups started to lose weight, with the peak of weight loss from days 4 to 5. After this period, surviving young infected mice regained weight. However, infected aged mice regained less weight than young mice, resulting in a 9%–10% weight difference by days 7 and 8 after infection (Figure 1A). Disease severity scores (which include diarrhea severity) peaked at days 3–5. While young infected mice recovered following this peak, aged mice continued to have significantly elevated disease severity scores from days 5 to 8 after infection (Figure 1B). The overall survival rates from 2 experiments were 88% and 60% in young and aged infected mice, respectively (P = .03; Figure 1C).
Figure 1.
Effect of Clostridium difficile (VPI10463, 105) infection in young C57BL/6 mice (age, 2 months) and aged C57BL/6 mice (age, 18 months). There were 4 mice per uninfected group and 13 mice per infected groups. *P < .05 between infected young mice and infected aged mice by 2-way analysis of variance with the Bonferroni correction or by the log-rank test. A, Weight change from baseline, prior to infection, up to the day of death. B, Disease severity scores among surviving mice. C, Survival curve (by the Mantel–Cox test).
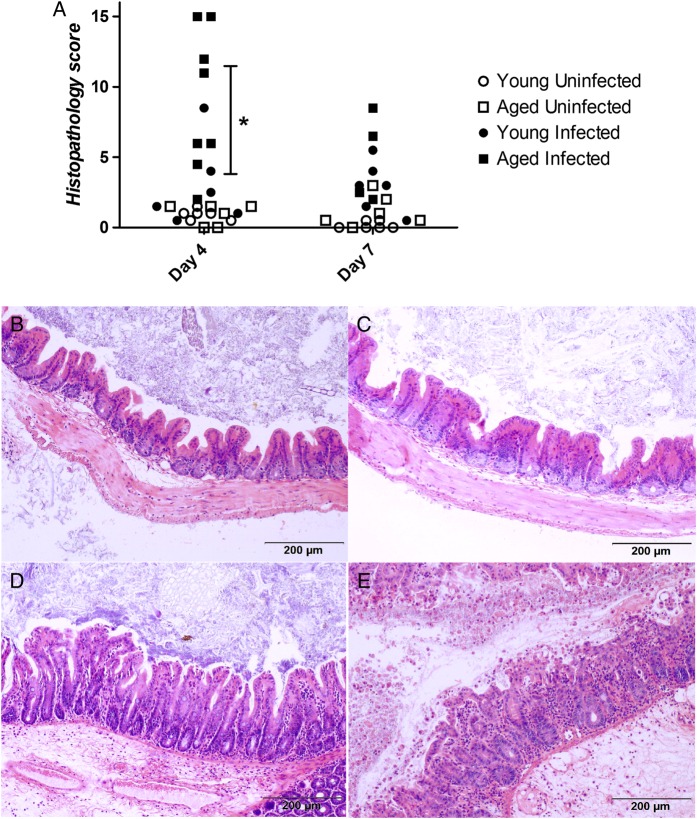
In a separate experiment, mice were euthanized at days 4 (peak of disease severity) and 7 (recovery period) to examine whether disease severity was associated with the degree of intestinal pathology. Cecal and colonic histopathology scores in aged mice were significantly increased as compared to those in young mice at day 4, and although this trend was found to be higher at day 7, it was not statistically significant (Figure 2A). Compared with young mice, cecal intestinal sections from aged mice had more cellularity, epithelial disruption, and submucosal edema at the peak of infection (Figure 2B–E).
Figure 2.
Histopathologic analysis of cecal and colonic tissue specimens obtained from Clostridium difficile–infected and control young and aged mice. A, Histopathology scores. *P < .001 between infected young mice and infected aged mice. B–E, Representative cecal tissue specimens (at 100× original magnification) from young uninfected mice (B), aged uninfected mice (C), young infected mice (D), and aged infected mice (E).
Aged Infected Mice Treated With Vancomycin Are More Susceptible Than Young Infected Mice to Relapse
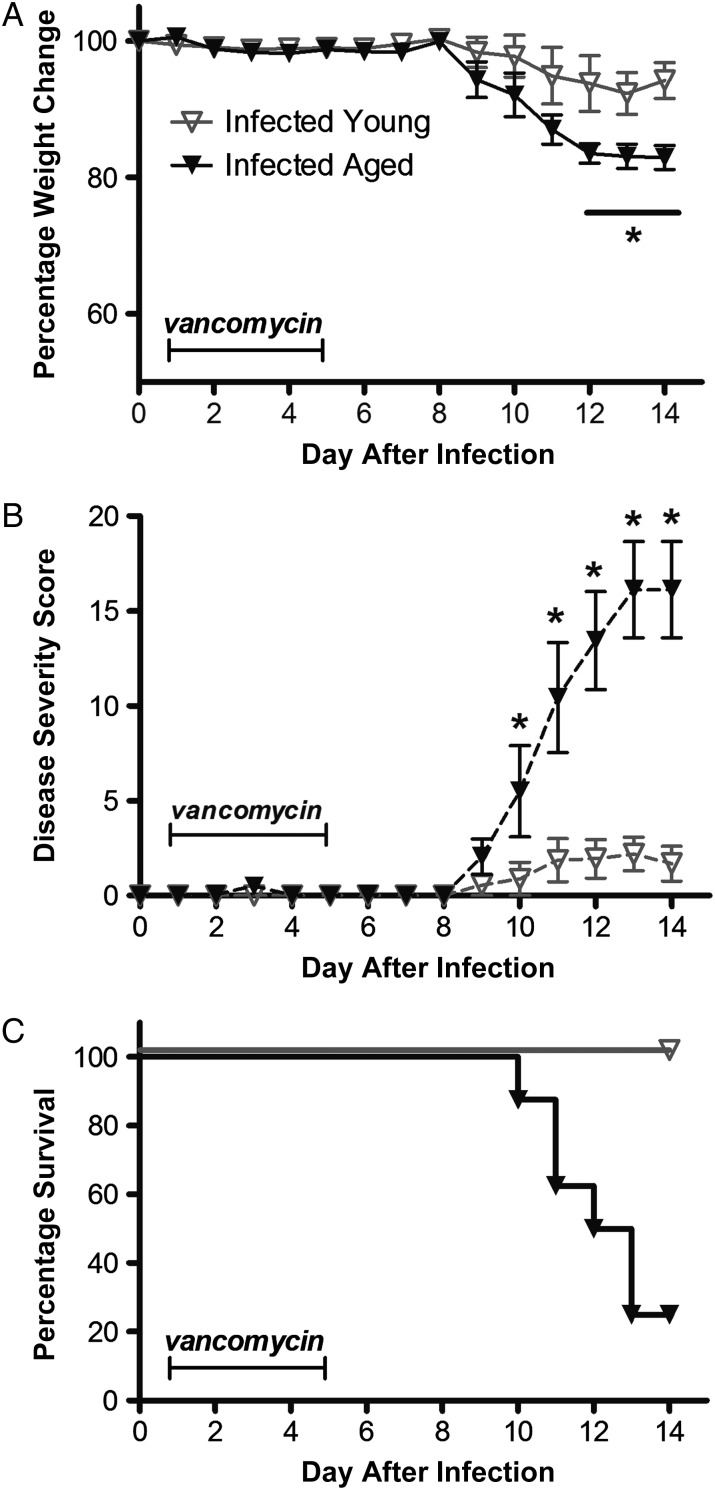
Next we interrogated the effect of vancomycin treatment on the outcomes of infection. Two groups of young and aged infected mice initiated vancomycin therapy 24 hours after infection. Compared with untreated infected groups, infected mice that received vancomycin for 5 days did not lose weight or experience diarrhea during treatment. However, 3–4 days after stopping vancomycin treatment, infected mice started having weight loss and diarrhea (Figure 3A). Weight loss and disease severity scores were higher in the aged infected mice, compared with the young infected mice (Figure 3B). Seventy five percent of the aged mice that experienced infection relapse eventually died, compared with none of the young mice (Figure 3C).
Figure 3.
Effect of treatment with vancomycin (50 mg/kg/day) for 5 days (days 1–5 after infection) in Clostridium difficile–infected young mice (age, 2 months) and infected aged mice (age, 18 months). Data are for 8 mice per group. A, Weight change from baseline, prior to infection, up to the day of death. *P < .05 by 2-way analysis of variance (ANOVA) with the Bonferroni correction. B, Disease severity scores of surviving mice. *P < .001 by 2-way ANOVA with the Bonferroni correction. C, Survival curve. P < .0001 by the log-rank (Mantel–Cox) test.
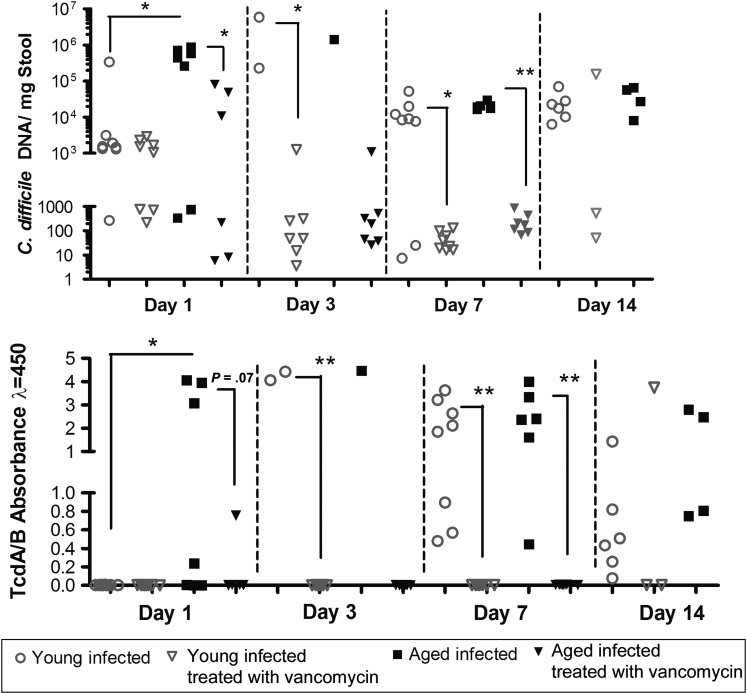
To determine the degree of intestinal infection, clostridial shedding and toxin levels were measured in the stool. Within 24 hours after infection, aged mice had a significantly elevated C. difficile fecal burden and toxin levels, compared with young mice, but these differences were lost at days 3 and 7 (Figure 4). Fecal clostridial shedding and toxin levels in treated infected mice that were able to provide stool specimens suggested that vancomycin was able to suppress bacterial proliferation and toxin production during treatment until day 7.
Figure 4.
Clostridium difficile bacterial and toxin burden in 8 infected young mice (age, 2 months) and 8 infected aged mice (age, 18 months) that were able to provide stool specimens for testing. Statistical analysis not performed on days 3 and 14 for aged mice because of an insufficient quantity of stool specimens. *P < .05 and **P < .001 by the unpaired Student t test. TcdA/B, C. difficile toxins A and B.
Antibody Production Is Depressed in Aged Infected Mice, and Vancomycin Treatment Is Associated With a Blunted Humoral Response
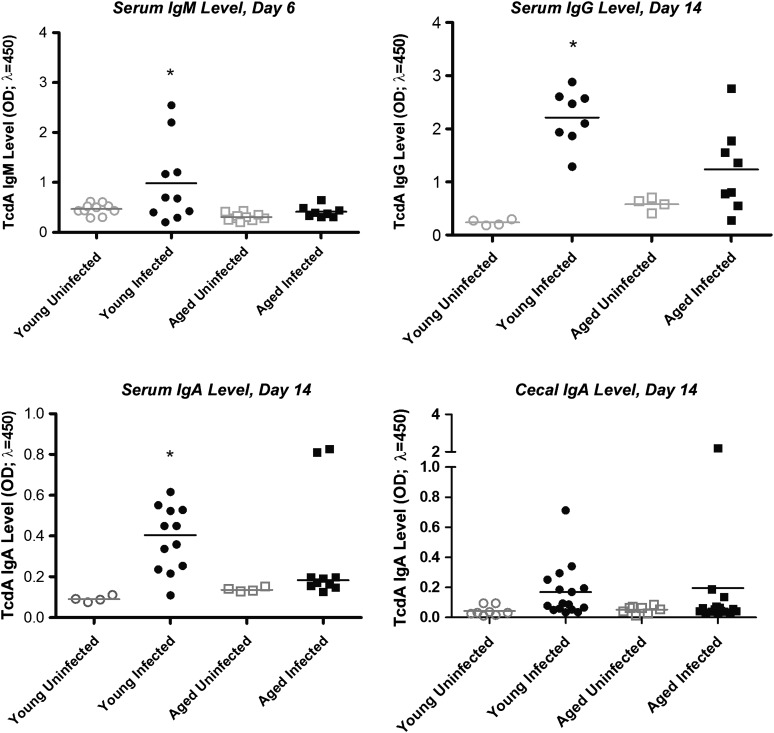
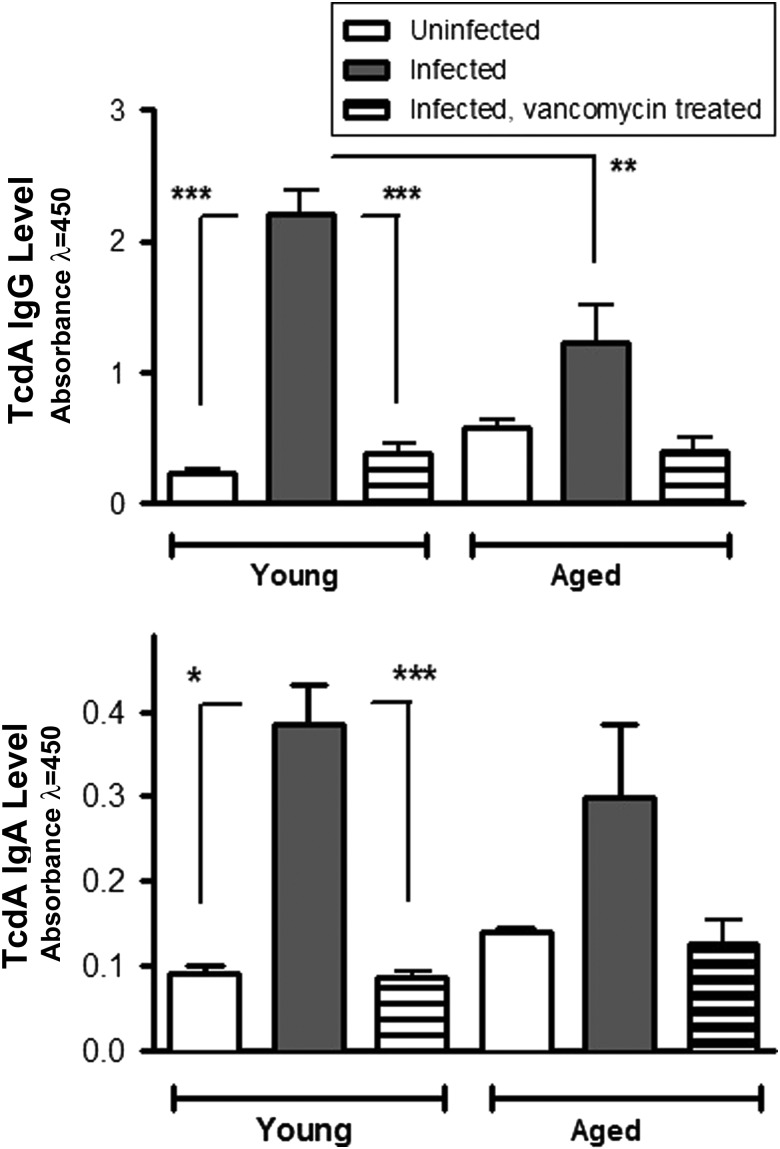
To assess whether inadequate humoral responses in aging mice may be involved in increased susceptibility to disease relapse after treatment, we measured levels of C. difficile toxin A (TcdA) antibody in sera (IgA, IgM, and IgG) and cecal contents (IgA) at specific time points after infection. Serum TcdA-specific IgM levels at day 6 and IgG levels at day 14 were significantly elevated in young mice, compared with those in aged infected mice (Figure 5). Serum but not cecal TcdA IgA levels were significantly higher in young infected mice, compared with those in infected aged mice, at day 14 after infection. Interestingly, treatment with vancomycin suppressed both IgA and IgG production in infected mice, irrespective of age group (Figure 6).
Figure 5.
Clostridium difficile toxin A (TcdA) antibody levels in infected young mice and infected aged mice. Data are for 2–4 mice in uninfected groups and 4–8 mice in infected groups. Each sample was evaluated in duplicate. A, TcdA immunoglobulin M (IgM) level in sera at day 6. *P < .01 between young infected mice vs young uninfected mice and aged infected mice. B, TcdA immunoglobulin G (IgG) level in sera at day 14. *P < .001 between young infected mice vs young uninfected mice and aged infected mice. C, TcdA immunoglobulin A (IgA) level in sera at day 14. *P < .01 between young infected mice vs young uninfected mice. D, TcdA IgA level in cecal contents at day 14. Statistical analyses were performed by 1-way analysis of variance with the Bonferroni correction.
Figure 6.
Effect of vancomycin treatment on Clostridium difficile toxin A (TcdA) antibody levels in sera of infected young mice and infected aged mice at day 14 after infection. There were 2–4 mice in uninfected groups and 2–8 mice in infected groups. Each sample was evaluated in duplicate. The changes in sera antibody levels in aged mice after infection, with or without treatment, were not statistically significant. *P < .01, **P < .001, and ***P < .0001 by 1-way analysis of variance with the Bonferroni correction. Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G.
We also examined whether differences in the humoral response may be secondary to decreases in antibody-producing cells in the intestinal tissue 3 days after infection. There was no significant difference in the number of IgG-producing cells in the lamina propria between uninfected and infected mice within each age group, although at baseline, numbers of IgG-producing cells were lower in intestinal tissues of aged mice (Supplementary Figure 1). Furthermore, aged infected mice had significantly increased numbers of total IgG-producing cells in the intestinal tissues, compared with young mice. In contrast, IgA-producing cells were significantly increased in the cecal lamina propia from young mice during infection. The numbers of germinal center B cells in mesenteric lymph nodes were similarly increased by infection in both age groups.
Fecal Flora in Aged Infected Mice Is More Susceptible Than That in Young Infected Mice to Antibiotic-Induced Alteration
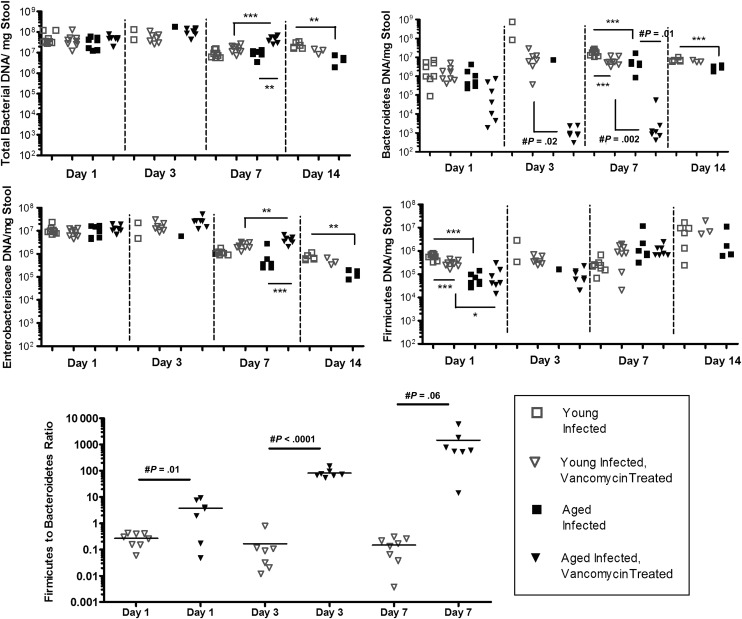
To determine whether posttreatment relapse may be explained by collateral damage to intestinal microbiota by anti–C. difficile antibiotic (ie, vancomycin) treatment, we assessed select phyla within stool samples. Total bacteria levels measured by 16S ribosomal RNA, Bacteroidetes level, and Enterobacteriaceae levels were significantly lower in infected aged mice, compared with infected young mice, at days 14, 7, and 14, respectively (Supplementary Figure 2). The total bacterial burden was similar between untreated infected young and aged mice at days 1, 3, and 7, but the aged mice had lower Bacteroidetes, Enterobacteriaceae, and Firmicutes levels at days 7 and 14, day 14, and day 1, respectively (Figure 7). In vancomycin-treated infected animals, aged mice had even lower fecal Bacteroidetes levels at days 3 and 7, lower Firmicutes levels at day 1, and higher Enterobacteriaceae levels at day 7. Because an elevated Firmicutes to Bacteroidetes ratio has been suggested by others to correlate with susceptibility to CDI, we compared this ratio between infected young and aged mice that received vancomycin treatment [26]. At days 1, 3, and 7, the Firmicutes to Bacteroidetes ratio was significantly increased in aged mice, compared with that in young infected mice. The ratio was not compared at day 14 because of inadequate specimens available in aged mice.
Figure 7.
Effect of vancomycin treatment on total bacterial content and select intestinal microbiota in infected young mice and infected aged mice. There were 6–8 mice per group. Statistical analysis not performed on days 3 and 14 for aged mice because of an insufficient quantity of stool specimens. *P < .01, **P < .001, and ***P < .0001 by 1-way analysis of variance with the Bonferroni correction; #by the unpaired Student t test.
DISCUSSION
Advanced age is a risk factor for symptomatic CDI and is associated with worse outcome from initial infection and recurrent disease after treatment [27]. We have shown that, similar to humans, C. difficile disease severity is increased in infected aged mice, compared with that in infected young mice. Infected aged mice had significantly more weight loss, more severe diarrhea, and a decreased survival rate following initial infection. Consistent with the clinical presentation, histopathology scores for infected intestinal tissues from aged mice were also higher than scores determined for young mice. These findings also confirm what we have reported in aged gnotobiotic mice, in which infection with toxigenic C. difficile resulted in rapid fulminant disease, although no comparison with young mice was attempted previously [28].
We have previously reported that treatment with vancomycin resulted in recurrent disease a few days after discontinuation of antibiotic therapy [22]. In our current study, aged mice treated with vancomycin were much more susceptible than young mice to relapse, with a dramatic difference in mortality. In humans, higher rates of recurrence were also seen in older subjects in a post hoc analysis of a randomized clinical trial comparing fidaxomicin and vancomycin [29]. Each additional decade of life raised the risk of recurrence by 17% and decreased the likelihood of clinical cure by 13%. An increased risk of recurrence may be associated with the decreased capacity of the aging host to elicit a specific antibody response against C. difficile toxins. Indeed, production of antitoxin antibodies in serum and stool during or after infection correlated with protection from recurrent infections [17, 18]. In a prospective study to evaluate risk factors for recurrent CDI, the inability of the host to mount an adequate IgM and IgG response to an initial infection was associated with a higher likelihood of a recurrence [17]. After adjustment for disease severity, a low anti-TcdA IgM value (on day 3) and a low anti-TcdA IgG level (on day 12) were each associated with an increased risk of recurrence. Lower anti-TcdB IgA levels at day 0 and lower anti-TcdA and anti-TcdB IgA levels at days 18–21 were associated with a higher risk of recurrence [18]. Direct association between age and antibody production was either not examined or revealed no significant effect in previous human studies [17, 18], but the results from our mouse study suggests a likely effect of aging on antibody production against C. difficile toxins. Serum IgM, IgG, and IgA levels against TcdA were all lower in aged mice, compared with young mice. The low antibody titers observed in infected aged mice did not appear to be secondary to decreased levels of IgG-producing or IgA-producing cells in the intestinal lamina propia. Although IgG-producing cells were lower in aged mice, compared with young mice, at baseline, infection stimulated significant increases in levels of these cells, suggesting a nonspecific response. Likewise, levels of IgA-producing cells were not depressed with aging, although in contrast to findings for young infected mice, these cells did not increase in number in aged infected mice. These findings suggest that alteration in antibody responses, especially a lack of specific responses to either TcdA or TcdB, may be involved. Interestingly, we have shown that even in young infected mice, treatment with vancomycin was associated with significantly decreased serum TcdA IgG and IgA levels. These findings may be related to the decreases in C. difficile bacterial and toxin burden during treatment. However, these data also suggest the importance of the intestinal microbiota in antibody production against C. difficile.
Colonization resistance against CDI is afforded by a diverse intestinal microbiota. Lower bacterial diversity is seen in individuals with CDI and is even lower in patients with recurrent CDI, compared wit findings during an initial CDI [30]. The diversity of total gut bacteria is decreased, as well, in older humans [20]. Stool specimens from elderly subjects were found to have a smaller inhibitory effect on C. difficile than stool specimens from young subjects [31]. Although we did not perform an intensive microbiome study, our results show that the intestinal microbiota in aged animals is different, which may affect susceptibility to CDI. Infected aged mice had a lower total bacterial load, Enterobacteriaceae level, and Bacteroidetes level at days 14, 7, and 14, respectively. In a study looking at young and elderly healthy subjects and elderly patients with CDI, patients with CDI had lower numbers of Bacteroidetes and higher number of Clostridia [32]. Among healthy subjects, total counts of Bacteroides were higher in young adults but greater diversity was observed in elderly individuals. However, among elderly patients with CDI, only 1 Bacteroides species was isolated. Indeed, an increased Firmicutes to Bacteroidetes ratio, as we have consistently observed in infected aged mice, has also been associated with susceptibility to CDI even in children, a group that was traditionally considered at low risk [26]. Interestingly, our study showed that the microbiota of the aged host is also highly susceptible to further alteration during treatment with vancomycin for the treatment of CDI itself, potentially enhancing its susceptibility to reinfection or relapse.
Our findings in this article may have broader translational implications. An impaired immune response to infection or vaccine candidates influences disease or treatment outcomes in the aging host. The use of animal models that mirror age-specific changes in older individuals may be critical in studies investigating pathogenesis, disease mechanisms, and treatment responses in elderly individuals.
In summary, aging leads to severe disease and increased relapse in the mouse model of CDI, reflecting what is seen in human disease. The results of our studies suggest that an inadequate humoral response during infection and an increased susceptibility to antibiotic-induced alteration of the intestinal microbiota are potential underlying reasons that warrant further investigations. The aged mouse model may facilitate understanding the biological causes of worse outcome of CDI and development of therapeutic approaches targeted at elderly individuals.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was partially supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01 AI094458 [subcontract] to C. A. W. and UO1 AI075526).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lessa FC, Mu Y, Bamberg WM et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W et al. Multistate point-Prevalence survey of health care–Associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55(suppl 2):S88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep 2011; 59:1–126. [PubMed] [Google Scholar]
- 5.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile–associated disease. Emerg Infect Dis 2009; 15:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ Can Med Assoc J J Assoc Medicale Can 2005; 173:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 2013; 13:875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolich-Žugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8T cell homeostasis and immunity to infection. Semin Immunol 2012; 24:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8T cell repertoire of aged mice. J Immunol Baltim Md 1950 2009; 182:784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 2013; 14:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. Eur J Immunol 2002; 32:1567–73. [DOI] [PubMed] [Google Scholar]
- 12.Ademokun A, Wu Y-C, Dunn-Walters D. The ageing B cell population: composition and function. Biogerontology 2010; 11:125–37. [DOI] [PubMed] [Google Scholar]
- 13.Dunn-Walters DK, Ademokun AA. B cell repertoire and ageing. Curr Opin Immunol 2010; 22:514–20. [DOI] [PubMed] [Google Scholar]
- 14.Gibson KL, Wu Y-C, Barnett Y et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009; 8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frasca D, Landin AM, Lechner SC et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol Baltim Md 1950 2008; 180:5283–90. [DOI] [PubMed] [Google Scholar]
- 16.Penn ND, Purkins L, Kelleher J, Heatley RV, Mascie-Taylor BH. Ageing and duodenal mucosal immunity. Age Ageing 1991; 20:33–6. [DOI] [PubMed] [Google Scholar]
- 17.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001; 357:189–93. [DOI] [PubMed] [Google Scholar]
- 18.Bauer MP, Nibbering PH, Poxton IR, Kuijper EJ, van Dissel JT. Humoral immune response as predictor of recurrence in Clostridium difficile infection. Clin Microbiol Infect 2014; 20:1323–8. [DOI] [PubMed] [Google Scholar]
- 19.Claesson MJ, Cusack S et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 2011; 108(suppl 1):4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwielehner J, Liszt K, Handschur M, Lassl C, Lapin A, Haslberger AG. Combined PCR-DGGE fingerprinting and quantitative-PCR indicates shifts in fecal population sizes and diversity of Bacteroides, bifidobacteria and Clostridium cluster IV in institutionalized elderly. Exp Gerontol 2009; 44:440–6. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Katchar K, Goldsmith JD et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology 2008; 135:1984–92. [DOI] [PubMed] [Google Scholar]
- 22.Warren CA, van Opstal EJ, Riggins MS et al. Vancomycin treatment's association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob Agents Chemother 2013; 57:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamousé-Smith ES, Tzeng A, Starnbach MN. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS One 2011; 6:e27662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jørgensen TN, Alfaro J, Enriquez HL et al. Development of murine lupus involves the combined genetic contribution of the SLAM and FcgammaR intervals within the Nba2 autoimmune susceptibility locus. J Immunol 2010; 184:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Auria KM, Kolling GL, Donato GM, Warren CA, Gray MC, Hewlett EL, Papin JA. In vivo physiological and transcriptional profiling reveals host responses to Clostridium difficile toxin A and toxin B. Infect Immun 2013; 81:3814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling Z, Liu X, Jia X et al. Impacts of infection with different toxigenic Clostridium difficile strains on faecal microbiota in children. Sci Rep 2014; 4:7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen SH, Gerding DN, Johnson S et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 28.Pawlowski SW, Calabrese G, Kolling GL et al. Murine model of Clostridium difficile infection with aged gnotobiotic C57BL/6 mice and a BI/NAP1 strain. J Infect Dis 2010; 202:1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louie TJ, Miller MA, Crook DW et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc 2013; 61:222–30. [DOI] [PubMed] [Google Scholar]
- 30.Chang JY, Antonopoulos DA, Kalra A et al. Decreased Diversity of the Fecal Microbiome in Recurrent Clostridium difficile –Associated Diarrhea. J Infect Dis 2008; 197:435–8. [DOI] [PubMed] [Google Scholar]
- 31.Borriello SP, Barclay FE. An in-vitro model of colonisation resistance to Clostridium difficile infection. J Med Microbiol 1986; 21:299–309. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol 2002; 51:448–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.