Abstract
Background. A novel translational pharmacology investigation was conducted by combining an in vitro efficacy target with mucosal tissue pharmacokinetic (PK) data and mathematical modeling to determine the number of doses required for effective human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP).
Methods. A PK/pharmacodynamic (PD) model was developed by measuring mucosal tissue concentrations of tenofovir, emtricitabine, their active metabolites (tenofovir diphosphate [TFVdp] and emtricitabine triphosphate [FTCtp], respectively), and competing endogenous nucleotides (dATP and dCTP) in 47 healthy women. TZM-bl and CD4+ T cells were used to identify 90% effective concentration (EC90) ratios of TFVdp to dATP and FTCtp to dCTP (alone and in combination) for protection against HIV. Monte-Carlo simulations were then performed to identify minimally effective dosing strategies to protect lower female genital tract and colorectal tissues.
Results. The colorectal TFVdp concentration was 10 times higher than that in the lower female genital tract, whereas concentrations of endogenous nucleotides were 7–11 times lower. Our model predicted that ≥98% of the population achieved protective mucosal tissue exposure by the third daily dose of tenofovir disoproxil fumarate plus emtricitabine. However, a minimum adherence to 6 of 7 doses/week (85%) was required to protect lower female genital tract tissue from HIV, while adherence to 2 of 7 doses/week (28%) was required to protect colorectal tissue.
Conclusions. This model is predictive of recent PrEP trial results in which 2–3 doses/week was 75%–90% effective in men but ineffective in women. These data provide a novel approach for future PrEP investigations that can optimize clinical trial dosing strategies.
Keywords: HIV, antiretroviral, translational medicine, dose response, population pharmacokinetics-pharmacodynamics, quantitative pharmacology, preexposure prophylaxis
A fixed-dose, combination tablet of tenofovir disoproxil fumarate (TDF) 300 mg and emtricitabine (FTC) 200 mg received Food and Drug Administration approval for human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) on the basis of findings from 2 trials. Partners PrEP demonstrated 75% efficacy among heterosexual serodiscordant couples [1], and iPrEx demonstrated 44% efficacy among men who have sex with men (MSM) and transgender women [2]. However, 2 subsequent trials investigating daily TDF, with or without FTC, in high-risk women (FEM-PrEP and VOICE) failed to demonstrate efficacy [3, 4]. Analysis of plasma drug concentrations from these studies revealed that only 24%–30% of these women exhibited evidence of recent product use. Yet in a cohort of male participants from the successful iPrEx trial, only 28% had evidence of recent product use [2]. Subsequent analysis revealed that drug exposure consistent with 2–3 doses/week achieved 75%–90% protection in MSM [5, 6]. These findings pose an important question: why are adherence requirements for effective PrEP less strict for men?
We recently demonstrated that exposure of TDF's active metabolite (tenofovir diphosphate [TFVdp]) is 100-fold higher in colorectal tissue relative to female genital tract (FGT) tissue [7]. This may partially explain different adherence requirements in men and women. Another potential factor is the concentration of host cells' endogenous 2′deoxynucleotides analogs, deoxyadenosine triphosphate (dATP) and deoxycytidine triphosphate (dCTP). The active intracellular phosphorylated metabolites of TFV and FTC (TFVdp and emtricitabine triphosphate [FTCtp]) compete with dATP and dCTP for incorporation into the proviral DNA strand to terminate chain elongation [8]. Since dATP and dCTP can be modulated by proinflammatory signaling molecules [9, 10] and because mucosal tissues may have environments with altered inflammatory states, it is possible that endogenous nucleotides differ between cervical, vaginal, and colorectal tissue and contribute to altered antiviral efficacy of TFVdp and FTCtp. To investigate mechanisms of efficacy within FGT and colorectal tissues, we used a quantitative systems pharmacology approach to predict the probability of protection against HIV with varying patterns of adherence to TDF, FTC, and TDF plus FTC.
METHODS
Study Design
We conducted a phase 1, single-dose, pharmacokinetic (PK) investigation of TDF and FTC at 50%, 100%, or 200% of the treatment dose (clinical trials registration NCT01330199). Blood specimens were collected at baseline and over 48 hours, and each participant provided 1 cervical, vaginal, and colorectal tissue sample at 6, 12, 24, or 48 hours after dose receipt. Drug concentrations were quantified in plasma specimens, peripheral blood mononuclear cells (PBMCs), and tissue specimens. The study was conducted in accordance with good clinical practices, and all participants provided written informed consent before study entry. Details about the clinical trial, sample processing, and analytical methods are provided in the Supplementary Methods.
In Vitro Concentration Versus Response
TZM-bl reporter cells (NIH AIDS Reagent Program, Germantown, MD) [11, 12] and human CD4+ T cells were used to determine HIV type 1 (HIV-1) infection in the presence of TFV and FTC. A total of 100 000 TZM-bl cells were plated in 6-well, flat-bottomed culture plates in 2 mL of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics to match the experimental conditions for drug quantification where >100 000 cells were needed. Cells were incubated in TFV (0.05–35 µM), FTC (0.03–30 µM), or their combination (NIH AIDS Reagent Program) for 24 hours at 37°C. A total of 5 µL of HIV-1JR-CSF (NIH AIDS Reagent Program; multiplicity of infection [MOI], 0.006) and 5 µL of DEAE-dextran were added to each well. This HIV-1JR-CSF dose was selected to ensure that infection, as measured by luminescence, was within linear range. After 48 hours, cells were lysed with 500 µL of GloLysis Buffer (Promega, Madison, Wisconsin), and relative light units were measured using a Luciferase Assay System kit (Promega) on a Veritas Microplate Luminometer (Turner Biosystems, Sunnyvale, California). Infectivity was normalized to infection in the absence of drug and reported as percentage inhibition.
PBMCs isolated from buffy coats obtained from the New York Blood Center (Long Island City, New York) were sorted for CD4+ T lymphocytes, using an EasySep negative selection kit (Stemcell Technologies, Vancouver, Canada). Typical cell yields using this kit demonstrate approximately 96% purity and >90% viability. CD4+ T cells were stimulated for 48 hours in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum, antibiotics, phytohemagglutinin (PHA; 5 µg/mL), and interleukin 2 (10 units/mL). A total of 1 × 106 CD4+ T cells/mL were resuspended and incubated in PHA-free RPMI medium as prepared above and with either TFV (0.3–10 µM) or FTC (0.03–30 µM) for 24 hours. Pseudotyped virus was generated using NL4-3.Luc.R-.E-backbone (NIH AIDS Reagent Program) [13, 14] and transfected with env expression plasmid from HIV-1JR-CSF, using X-tremeGENE HP (Roche Life Science, Indianapolis, Indiana). Virions were concentrated 10-fold using Lenti-X Concentrator (Clontech Laboratories, Mountain View, California). Cells were incubated for 48 hours in 25 µL of pseudotyped virus (MOI, 1) then lysed with 100 µL of GloLysis Buffer. Infection was reported as described above.
Ratios of the cellular active metabolite concentration to the endogenous nucleotide concentration were quantified with an identical set of uninfected TZM-bl or CD4+ cells. Cells were harvested at the time of HIV-1 challenge (24 after incubation in the drug), washed, counted, lysed in 70% methanol/water, and stored at −80°C for TFVdp, FTCtp, dATP, and dCTP quantification.
The following reagents were obtained through the AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: TZM-bl, from Dr John C. Kappes, Dr Xiaoyun Wu, and Tranzyme; HIV-1JR-CSF, from Dr Irvin Chen; and pNL4-3.Luc.R–.E–, from Dr Nathaniel Landau.
PK Analysis
Calculation of the area under curve over 48 hours (AUC0–48h) was performed individually, using the linear up/log down trapezoidal rule for plasma and PBMCs, with WinNonlin v6.3 (Pharsight, Sunnyvale, California). The AUC0–48h was calculated using sparsely sampled data and the linear trapezoid rule for tissue. Concentrations below the limit of detection or quantification were imputed as 10% or 50% of the lower limit of quantification, respectively.
Monte Carlo simulations were performed with a population PK model built using the clinical trial concentration data and NONMEMv7.4, ICON plc. Simulations were performed for the first 10 doses and at steady state with dosing intervals representing 1–7 doses/week. Time to steady-state was defined as the first dose at which the 24-hour concentration (Ctrough) was within 10% of the simulated steady-state Ctrough.
Statistical Analysis
Dose proportionality was assessed in tissues across a 4-fold dosing range to assist with PK modeling, using the Holder method [15], and was declared if the 90% confidence interval (CI) around the regression line slope () for the log-transformed dose versus concentration fit within 0.64–1.36. This analysis was conducted using Rv2.14 [16]. On the assumption of a percentage coefficient of variation of ≤45%, 8 women/dosing level provided 83% power to declare dose proportionality. Between-tissue comparisons of endogenous nucleotides were made using 1-way analysis of variance with the Bonferroni correction. Descriptive and comparative statistics were performed with SASv9.3 (SAS Institute, Cary, North Carolina).
Exposure versus response was described for the in vitro data using Emax regression (E0 fixed to 0, Emax fixed to 1, E = Response, MR = molar ratio) for active metabolite:endogenous nucleotide versus fraction inhibition of HIV-1 infection, as follows:
| (1) |
Negative values were fixed at 0. Synergy was assessed with the following pharmacodynamic (PD) interaction model () [17] for the ratios of TFVdp to dATP concentrations and FTCtp to dCTP concentrations in TZM-bl cells by fixing the 50% effective concentration (EC50) and Hill slope (H) parameters from equation 1:
| (2) |
A 3-dimensional goodness-of-fit plot was generated and visually inspected to evaluate model predictions for bias. The final fitted parameters (HTFV, HFTC, EC50TFV, EC50FTC, and ) were used to predict percentage inhibition from simulated concentrations, with an efficacy target of 90%.
RESULTS
Participant Demographic Characteristics and Safety
Between April 2012 and August 2013, 49 healthy female volunteers gave consent for the clinical trial. Participant demographic characteristics are summarized in Table 1 and stratified by treatment arm. Eight participants were enrolled into each dosing group except TDF 300 mg, in which 1 participant was unable to provide samples and was withdrawn and replaced. Samples from 1 participant who received FTC 200 mg were not analyzed because of improper storage. Single doses of TDF and FTC up to 200% of the licensed treatment dose were well tolerated with no adverse events above grade 1 severity (Table 1).
Table 1.
Demographic Characteristics and Adverse Events
| Variable | Tenofovir Disoproxil Fumarate (n = 25) | Emtricitabine (n = 24) |
|---|---|---|
| Demographic characteristic | ||
| Age, y | 27 (22.75–31.25) | 22 (21–27) |
| Weight, kg | 67.4 (60.4–76.8) | 62.8 (57.7–72.3) |
| BMIa | 24.1 (21.6–26.9) | 22.5 (20.8–26.5) |
| Race | ||
| White | 16 (64) | 18 (75) |
| African American | 8 (32) | 4 (16.7) |
| Asian American | 1 (4) | 1 (4.2) |
| American Indian | 0 (0) | 1 (4.2) |
| Adverse event | ||
| Headache | 1 (4) | 5 (20.8) |
| Nausea | 2 (8) | 0 |
| Early menses | 1 (4) | 0 |
| Fatigue | 1 (4) | 0 |
| Bowel disturbances | 1 (4) | 1 (4.2) |
| Elevated transaminase levels | 0 | 1 (4.2) |
| Pelvic cramps | 0 | 1 (4.2) |
| Postnasal drip | 0 | 1 (4.2) |
| Vaginal dryness | 0 | 1 (4.2) |
| Ear infection | 1 (4) | 0 |
| Viral pharyngitis | 1 (4) | 0 |
Data are median value (interquartile range) or no. (%) of subjects.
a Body mass index (BMI) is calculated as the weight in kilograms divided by the height in square meters.
Drug Concentrations and Dose Proportionality
Figure 1 depicts the area under the concentration-time curve versus dose for extracellular concentrations and intracellular metabolite concentrations in plasma, PBMCs, and tissues. Table 2 summarizes dose proportionality statistics. Plasma FTC concentrations increased dose proportionally. Plasma TFV concentrations did not meet dose proportionality criteria but exhibited dose linearity. PBMC concentrations of TFVdp but not FTCtp increased dose proportionally. Since no notable difference was observed between cervical and vaginal tissue concentrations, they were averaged for subsequent analyses. A single composite tissue AUC0–48h was calculated for each dosing group and tissue type. Only FGT FTC concentrations increased dose proportionally.
Figure 1.
Dose proportionality in blood plasma, peripheral blood mononuclear cells (PBMCs), and mucosal tissues. Mean (±standard error) areas under curve over 48 hours (AUC0–48h) for tenofovir (TFV; A), TFV diphosphate (TFVdp; B), emtricitabine (FTC; C), and FTC triphosphate (FTCtp; D) in female genital tract (FGT) tissue (Δ), colorectal tissue (○), and plasma (□) for tenofovir and emtricitabine and in PBMCs for TFVdp and FTCtp (□).
Table 2.
Dose Proportionality Statistics
| Matrix | Analyte | Slope, a (90% CI) | r2 | P | Dose Proportionalb |
|---|---|---|---|---|---|
| Plasma | Emtricitabine | 0.84 (.70–.97) | 0.85 | <.0001 | Yes |
| Plasma | Tenofovir | 0.77 (.63–.92) | 0.80 | <.0001 | No |
| PBMC | FTCtp | 0.19 (.012–.37) | 0.13 | .09 | No |
| PBMC | TFVdp | 1.07 (.91–1.24) | 0.83 | <.0001 | Yes |
| FGT | Emtricitabine | 0.91 (.78–1.05) | NAc | Yes | |
| FGT | FTCtp | 0.12 (−.29 to .53) | NAc | No | |
| FGT | Tenofovir | 0.77 (.46–1.09) | NAc | No | |
| FGT | TFVdp | 0.93 (.14–1.72) | NAc | No | |
| Colorectal | Emtricitabine | 1.24 (−.29 to 2.77) | NAc | No | |
| Colorectal | FTCtp | 0.22 (−.23 to .67) | NAc | No | |
| Colorectal | Tenofovir | 2.15 (.31–4.00) | NAc | No | |
| Colorectal | TFVdp | 0.35 (−1.10 to 1.80) | NAc | No |
Abbreviations: CI, confidence interval; FGT, female genital tract; FTCtp, emtricitabine triphosphate; PBMC, peripheral blood mononuclear cell; TFVdp, tenofovir diphosphate.
a denotes the regression line slope for the log-transformed area under curve over 48 hours versus the log-transformed dose.
b Perfect dose proportionality is denoted by . Therefore, dose proportionality is declared if the 90% CI is within .64 and 1.36.
c Not applicable (NA) because of sparsely sampled data.
Median dose-adjusted AUC0–48h for TFV and TFVdp were 10–45 times higher in colorectal tissue (38.5 µg*hr*g−1 and 2046.5 pmol*hr*g−1, respectively), compared with FGT tissue (0.83 µg*hr*g−1 and 188 pmol*hr*g−1, respectively). Although median dose-adjusted FTC AUC0–48h was higher in colorectal tissue (222.3 µg*hr*g−1) than FGT tissue (17.6 µg*hr*g−1), FTCtp values were 140 times higher in FGT tissue (15 094.3 pmol*hr*g−1) than colorectal tissue (108.2 pmol*hr*g−1). Concentration versus time profiles for blood and tissues are provided in Supplementary Figures 1 and 2, respectively.
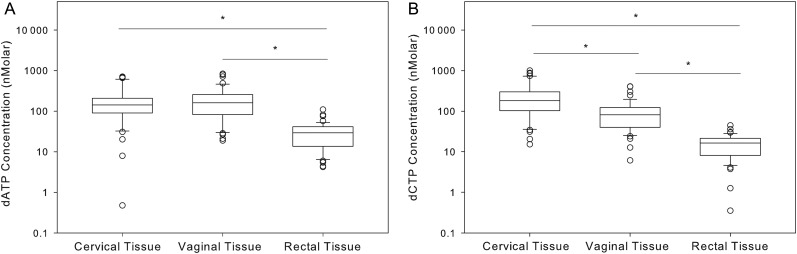
Human Mucosal Tissue Endogenous Nucleotides
Although dATP concentrations were similar in vaginal and cervical tissues (Figure 2A), dCTP concentrations were 50% lower in vaginal versus cervical tissue (Figure 2B). Colorectal tissue contained lower endogenous nucleotide pools as compared to the FGT: dATP concentrations were 5 times lower (P < .05), and dCTP concentrations were 5–11 times lower (P < .05). In all tissues, dATP and dCTP concentrations were highly correlated (cervical tissue, Pearson r = 0.84; vaginal tissue, Pearson r = 0.92; and colorectal tissue, Pearson r = 0.73; P < .0001). No differences in PBMC or tissue dATP/dCTP concentrations were noted between treatment arms, dosing groups, or sample collection time. In the female genital and colorectal tissues, ratios of the TFVdp concentration to the dATP concentration (TFVdp:dATP) ranged from 0.00074 to 0.14 and 0.0014 to 5.9, respectively, and ratios of the FTCtp concentration to the dCTP concentration (FTCtp:dCTP) ranged from 0.025 to 17 and 0.016 to 1.4, respectively.
Figure 2.
Tissue endogenous nucleotide concentrations plotted as median values (solid line within each box), 25th and 75th percentiles (box edges), and 10th to 90th percentiles (whiskers) for 47 women. *P < .05. A, Median deoxyadenosine triphosphate (dATP) concentrations are 85% lower in colorectal tissue, compared with cervical and vaginal tissues (P < .05; n = 47). B, Median deoxycytidine triphosphate (dCTP) concentrations in colorectal tissue are 90% lower than those in cervical tissue and 80% lower than those in vaginal tissue (P < .001; n = 47).
PK Modeling
An 8-compartment model with 7 gastrointestinal transit compartments (Supplementary Figure 3) best fit the clinical trial concentration data. Model parameters (Supplementary Table 1) and goodness of fit have been previously reported [18]. A 1000-subject Monte Carlo simulation was performed to simulate TFVdp:dATP and FTCtp:dCTP in FGT and colorectal tissues over the dosing interval for the first 10 daily doses and under steady-state conditions for each drug alone and in combination. TFVdp reached steady-state concentrations by dose 3 in FGT tissue and by dose 9 in colorectal tissue. FTCtp reached steady-state concentrations by dose 2 in FGT tissue and by dose 6 in colorectal tissue.
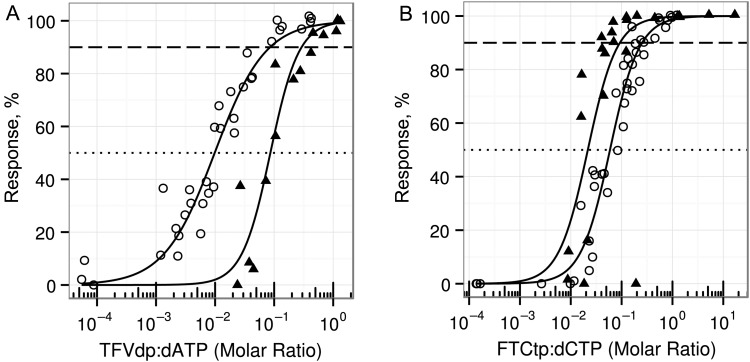
In Vitro Concentration Versus Response
We investigated targets for protective efficacy against HIV infection in both a cell line and primary human cells. Figure 3 illustrates the best fit Emax parameters for active metabolite to endogenous nucleotide concentration ratios and protection from HIV infection. The estimated TFVdp:dATP EC90 was 0.086 in TZM-bl cells) and 0.29 in CD4+ T cells. The estimated FTCtp:dCTP EC90 was 0.27 in TZM-bl cells and 0.07 in CD4+ T cells. Emax model parameters for culture medium drug concentration and intracellular active metabolite versus percentage inhibition are provided in Supplementary Table 2. The TFVdp:dATP and FTCtp:dCTP demonstrated synergy in TZM-bl cells ( ± standard error, 0.63 ± 0.074; P < .001, where indicates synergy). A synergistic interaction was also noted between TFV and FTC in CD4+ T cells (data not shown).
Figure 3.
In vitro concentration versus response for TZM-bl cells and CD4+ T cells. TZM-bl and stimulated, primary CD4+ T cells were incubated for 24 hours in 0.03–35 µM concentrations of tenofovir and emtricitabine. Intracellular active metabolite and endogenous nucleotide (EN) concentrations were quantified at the time of challenge with human immunodeficiency virus type 1 (HIV-1JR-CSF; for TZM-bl cells) or pseudovirus with HIV-1JR-CSF envelope (for CD4+ T cells). For TZM-bl cells, 34 samples were collected for tenofovir diphosphate (TFVdp):deoxyadenosine triphosphate (dATP) measurement and 41 for emtricitabine triphosphate (FTCtp):deoxycytidine triphosphate (dCTP) measurement across at least 2 independent experiments (values in 6 of 72 samples were below the limit of quantification [BLQ]). For CD4+ T cells, 14 samples were collected for TFVdp:dATP measurement and 27 for FTCtp:dCTP measurement across at least 2 independent experiments (values in 2 of 42 samples were BLQ). Solid lines represent the median regression line of the Emax model. The dashed reference line indicates 90% inhibition of human immunodeficiency virus (HIV) infection, and the dotted line represents 50% inhibition. The 50% effective concentration (EC50) ratio (±standard error [SE]) for TFVdp:dATP (A) was 0.010 ± 0.001 (P < .001), with a Hill slope (±SE) of 1.02 ± 0.09 (P < .001) in TZM-bl cells (○) and 0.086 ± 0.011 (P < .001) with a Hill slope of 1.81 ± 0.39 (P < .001) in CD4+ T cells (▴). B, The EC50 ratio (±SE) for FTCtp:dCTP was 0.059 ± 0.004 (P < .001) with a Hill slope (±SE) of 1.42 ± 0.11 (P < .001) in TZM-bl cells (○) and 0.022 ± 0.005 (P < .001) with a Hill slope of 1.88 ± 0.67 (P < .05) in CD4+ T cells (▴).
PK/PD Simulations
EC90 ratios derived from the more biologically relevant activated CD4+ T-cell Emax model were used as target efficacy exposure for monotherapy simulations with TDF or FTC. The modeled a surface to describe all possible EC90 combination ratios when using TDF plus FTC. To quantify the difference in predictions produced by the 2 PD models, we simulated 1000 clinically relevant TFVdp:dATP and FTCtp:dCTP. The predicted proportion of the population achieving target exposure differed by ≤2% between the 2 models.
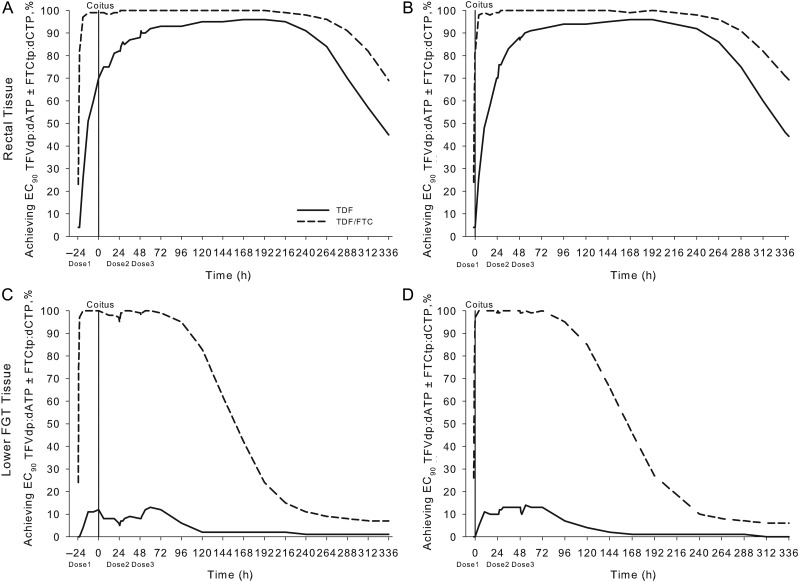
In colorectal tissue, the maximal proportion of the population (100%) achieved target exposure for efficacy after 3 daily doses of the fixed-dose combination (Figure 4A). In FGT tissue, the maximal proportion of the population (99%) achieved target exposure over the entire dosing interval after 3 daily doses of the fixed-dose combination (Figure 4C). In colorectal tissue, dosing twice/week with the fixed-dose combination achieved target exposure in >95% of the population (in FGT tissue, this dosing achieved target exposure in 65% of the population; Figure 4B and 4D).
Figure 4.
Time to protection and minimally effective preexposure prophylaxis (PrEP) dosing. Shown are pharmacokinetic (PK)/pharmacodynamic (PD) simulations for colorectal tissue (A and B) and lower female genital tract (FGT) tissue (C and D). A and C, The percentage of the simulated population achieving the 90% effective concentration (EC90) for tenofovir diphosphate (TFVdp):deoxyadenosine triphosphate (dATP), emtricitabine triphosphate (FTCtp):deoxycytidine triphosphate (dCTP), or combination ratios derived from the in vitro PK/PD relationship in CD4+ T cells is plotted over the dosing interval for the first 10 daily doses of tenofovir disoproxil fumarate (TDF; dashed line), emtricitabine (FTC; dotted line), or the fixed-dose combination (TDF plus FTC; solid line). B and D, The percentage of the population achieving these EC90 ratios at the end of the dosing interval under steady-state conditions with 1–7 doses/week of TDF, FTC, or TDF plus FTC are stratified by tissue. We predict that the maximal percentage of the population achieved EC90 ratios by the third daily dose of TDF plus FTC in colorectal and FGT tissues after beginning PrEP. Consistently using 7 doses/week of TDF plus FTC will achieve EC90 ratios in 100% of the population in FGT and colorectal tissues. Only 65% of the population using 2 doses/week of TDF plus FTC achieve target exposure in the FGT tissue, whereas ≥95% using 2 doses/week of either TDF or TDF plus FTC achieve target exposure in colorectal tissue.
To predict pericoital dosing effectiveness, we simulated the colorectal tissue exposure achieved by the Ipergay dosing regimen (2 doses 2–24 hours before coitus, 1 dose 24 hours after coitus, and 1 dose 48 hours after coitus) for TDF alone and with FTC. When administered 2 and 24 hours before coitus, TDF plus FTC achieved target exposure (at the time of coitus) in approximately 81% and 98% of the population, respectively (Figure 5A and 5B). Target exposure was sustained to 240 hours after coitus. In the FGT, when administered 2 and 24 hours before coitus, TDF plus FTC achieved target exposure at the time of coitus in 98% and 100% of the population, respectively (Figure 5C and 5D). The concentrations in the FGT were short-lived compared to colorectal tissue: <85% of the population had target exposure at 120 hours following coitus.
Figure 5.
Protection with pericoital preexposure prophylaxis (PrEP) dosing. Shown are pharmacokinetic (PK)/pharmacodynamic (PD) simulations for the Ipergay dosing regimen (2 tablets 2–24 hours before coitus [solid vertical line], 1 tablet 24 hours after coitus, and 1 tablet 48 hours after coitus) for tenofovir disoproxil fumarate (TDF; solid line) and the fixed-dose combination of TDF plus emtricitabine (FTC; dashed line). The percentage of the simulated population achieving the 90% effective concentration (EC90) of tenofovir diphosphate (TFVdp):deoxyadenosine triphosphate (dATP), with or without FTC triphosphate (FTCtp):deoxycytidine triphosphate (dCTP), derived from the in vitro PK/PD relationship in CD4+ T cells over 14 days following a single act of coitus in colorectal (A and B) and lower female genital tract (FGT) tissue (C and D). PK/PD simulations are reported on the assumption that the first dose was administered 24 hours (A and C) or 2 hours (B and D) before coitus. We predict the maximal percentage of the population achieving EC90 ratios in colorectal tissue over a 240-hour postcoital window is achieved by initiating the Ipergay dosing 24 hours before coitus. Dosing at 24 hours or 2 hours before coitus did not appear to alter the percentage of the population achieving target exposure in the FGT tissue over a 72-hour postcoital window.
DISCUSSION
Phase 3 clinical trials evaluating daily oral TDF, with or without FTC, for HIV PrEP have demonstrated mixed results in heterosexual women [1, 3, 4, 19]. To understand this, we generated comprehensive PK and biological data in vulnerable mucosal tissues and evaluated effective drug exposure with different dosing strategies. To our knowledge, this is the first biologically complete model to predict effective PrEP dosing. This analysis generated several important findings: (1) confirmation that TFV and FTC have different tissue distribution characteristics, in which TFV favors colorectal tissue and FTC favors FGT tissue; (2) characterization of the ratio of the intracellular active metabolite concentration to endogenous nucleotide concentration as an accurate PD target for nucleoside reverse transcriptase inhibitors (NRTIs), whereby endogenous nucleotides were found in higher concentration in FGT tissue; and (3) evidence that this PK/PD approach can be used to develop optimized TDF, with or without FTC, PrEP dosing strategies and predict clinical trial outcomes with different adherence patterns prior to the trials being conducted.
Previously, Patterson et al described 100-fold higher TFVdp concentrations in colorectal tissue, compared with FGT tissue, with a single dose of TDF plus FTC [7]. Here, we investigated 50%–200% of the licensed treatment doses to develop a robust PK model of TFVdp and FTCtp distribution. Our findings are consistent with those of this previous report: TFVdp concentrations overall were 10 times higher in colorectal tissue, while FTCtp concentrations were 140 times higher in FGT tissue. Between-tissue differences in drug transport systems may explain differential concentrations, yet blood flow to rectal and vaginal mucosa is similar [20]. Drug transport by lymph fluid into tissues has not been fully characterized. The lipid-rich lymph fluid may not favorably transport hydrophilic compounds like TFV and FTC [21, 22]. However, the leaky lymphatic endothelium could provide an alternative mode of absorption in colorectal tissue [23], possibly explaining the delayed peak we observed in this tissue. We also found that dATP and dCTP concentrations were 7 and 11 times lower, respectively, in colorectal tissue as compared with FGT tissue. Therefore, not only were TFVdp concentrations much higher in colorectal tissue, but the colorectal intracellular environment favors TFV's prophylactic activity.
A significant limitation in determining PrEP concentration targets for efficacy is the inability to easily measure the PK/PD relationship in humans. HIV prevention models rely on extrapolating data from cell culture systems, human tissue explants, or animal studies, each of which has limitations [24, 25]. Primate models use simian immunodeficiency virus or simian/human immunodeficiency virus, which may not mimic HIV's response to antiretrovirals [26]; antiretroviral exposure in humanized mouse models may not match humans [26]; and endogenous nucleotides in ex vivo tissue explants deteriorate by ≥80% within 24 hours of isolation [27]. Although a homogenous cell population fails to represent the 3-dimensional architecture and diverse microanatomy involved in transmission events, these models can be infected with HIV, can be exposed to clinically relevant antiretroviral concentrations, and can sustain endogenous nucleotide pools [28]. Since active metabolite concentrations in isolated mononuclear rectal cells strongly correlate with tissue homogenate concentrations [29], we believe that using isolated target cells to generate concentration-effect relationships for NRTI prevention strategies is reasonable.
A number of previous studies have highlighted the importance of endogenous nucleotides in NRTI efficacy [30–32]. Therefore, we postulated significant differences in tissue concentrations of endogenous nucleotide may affect the PK/PD relationships for HIV prevention. Using primary (human CD4+ T cells) and immortalized (TZM-bl cells) culture systems with a clinically relevant HIV-1 challenge, we identified efficacy targets for TFVdp and FTCtp relative to their competing endogenous nucleotides (dATP and dCTP). Differences in the concentration-response relationship between in vitro systems were noted. However, normalizing active metabolite to endogenous nucleotide concentrations reduced the difference between TZM-bl cell and CD4+ T cell EC90 ratio values by 70% (Supplementary Table 2) and resulted in a difference of <2% in clinical predictions between these model systems, highlighting the importance of the endogenous nucleotide exposure.
Combining the mathematical PK mucosal tissue model with the cellular target ratios, we determined that the maximal proportion of the population achieved effective FGT and colorectal concentrations after 3 doses of TDF plus FTC. Our data are consistent with those of the Cell-PrEP study [33]. These data differ from the Centers for Disease Control and Prevention guidelines, which are built on extrapolations from PBMC data [34]. However, since they are based on tissue PK data, we believe they better inform clinical usefulness. Importantly, we found that 2 doses/week of TDF, with or without FTC, achieved effective colorectal concentrations in >95% of the population, which emulate iPrEx-OLE results (90% protection with 2–3 dose/week of TDF plus FTC) [6].
To predict colorectal protection with pericoital oral dosing, we evaluated the Ipergay dosing regimen [35]. When a double dose of TDF plus FTC is taken 24 hours before coitus and 2 doses are taken over 48 hours following coitus, we demonstrate that >95% of the population achieves effective concentrations in colorectal tissue at the time of and for ≥240 hours after coitus. When administered 2 hours before coitus, concentrations in approximately 17% of the population are below the target for the first 4 hours after coitus. However, this is likely an inconsequential delay, since it takes 4 hours for reverse transcription to occur after HIV binds to cellular membranes [36]. These findings reasonably emulate clinical trial data for this dosing regimen, which demonstrated 86% efficacy in MSM [35].
Our data show that daily use of TDF plus FTC is required for 100% of the population to be protected from HIV infection within the FGT. Our efficacy predictions are in agreement with the Partners PrEP trial, which demonstrated 92% risk reduction (95% CI, 19%–99%) when women taking TDF plus FTC for PrEP had plasma drug concentrations consistent with very high adherence [37]. We also simulated the Ipergay regimen for the FGT. Using this approach, >95% of the population achieve target exposure in FGT tissue at the time of and for 72 hours after coitus. However, the effect is comparatively short lived, as by 120 hours following coitus <85% are still at target exposure. Administering TDF plus FTC 2 or 24 hours before coitus did not change the proportion achieving target exposure in the FGT tissue across this time frame. To match the percentage of the population achieving target exposure in colorectal tissue with Ipergay dosing, women would need to take 9 daily doses of TDF plus FTC following coitus. These data suggest this may not be a strongly protective regimen for women vaginally exposed to HIV and are consistent with recent preliminary reports from clinical trials in which more women using intermittent dosing acquired HIV as compared to those using daily dosing [38].
Our approach has certain limitations. First, our in vitro methods necessitated the use of PHA/interleukin 2 stimulation, which may increase the phosphorylation of NRTIs and endogenous nucleotides in CD4+ T cells [9]. However, direct measurement of active metabolites and normalization to endogenous nucleotides helped minimize confounding. Although the exposure-response relationship was defined under conditions of static drug exposure, given the long intracellular half-lives of TFVdp and FTCtp (approximately 48 and 39 hours, respectively) [39, 40] marked fluctuation of active metabolite concentrations in tissues is not expected and has not been identified. The concentration data used to build the PK model was obtained over 48 hours. However, there is precedent for making steady-state drug exposure predictions from similarly developed models [41]. Further, our predicted steady-state plasma concentrations were consistent with literature values, and our predicted time to steady state in colorectal tissue (9 days for TFVdp) was consistent with findings from previous reports [33]. The use of homogenates of tissue biopsy specimens to measure mucosal tissue drug concentrations assumes uniform distribution of drug and endogenous nucleotide concentrations across the biopsy specimen, which is unlikely [42]. However, isolating HIV target cells from vaginal and cervical tissue biopsy specimens has resulted in incomplete PK data sets, owing to small, inconsistent cell yields [39], and previous publications have demonstrated linear relationship between isolated mucosal cells and tissue homogenate concentrations [29].
Although we used PK data collected only from women, parent and metabolite colorectal drug concentrations overlapped with previously published data in men [7], suggesting that mucosal tissue differences are driven primarily by the drugs' distribution characteristics rather than by inherent sex differences. Since the risk of HIV transmission per act of unprotected receptive anal intercourse does not differ between MSM and heterosexuals [43], it is unlikely that biological differences alter HIV infectability in male versus female colorectal tissue. Thus, we believe it is reasonable to substantiate our colorectal model predictions with PrEP clinical trials results from MSM-only populations. Last, our population PK model was generated using data from healthy volunteers in the absence of sexually transmitted diseases or conditions causing inflammation in the lower gastrointestinal tract and FGT. Active metabolite and endogenous nucleotide concentrations may be altered during mucosal tissue inflammation [44], along with altered susceptibility to HIV infection, and warrant further investigation.
In summary, this study is the first of its kind for HIV-associated oral PrEP. By pairing phase 1 tissue concentration data with in vitro systems of HIV infection, we created a predictive population PK/PD model for PrEP that is consistent with clinical trial outcomes. Here we demonstrate proof of concept that a more complete characterization of the achievable and effective concentrations can be used to generate PK/PD models to optimize drug dosing prior to the initiation of phase 3 trials and to quantify the effect of incomplete adherence on protective efficacy. We believe this approach can be applied to other drugs and combinations to inform clinical trial design and optimize resources within the HIV prevention field.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr John Schmitz of the UNC Center for AIDS Research Virology, Immunology, and Microbiology Core, for his work isolating CD4+ T lymphocytes from PBMCs.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the supporting agencies listed below.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant U01 AI095031), the Centers for AIDS Research (grant CFAR P30 AI050410), and the National Institute of General Medical Sciences (grant T32 GM086330).
Potential conflicts of interest. A. K. and her laboratory are part of the study teams for CAPRISA 004 and 008, FACTS 001, MTN 006, HPTN 066, FEM-PrEP, and CONRAD 113, 114, and 117; and she and her institution have received grants from Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson PL, Glidden DV, Liu A et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Anderson PL, McMahan V et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson KB, Prince HA, Kraft E et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011; 3:112re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother 2011; 66:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao WY, Shirasaka T, Johns DG, Broder S, Mitsuya H. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Invest 1993; 91:2326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins BL, Wilcox CK, Fridland A, Rodman JH. Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy 2003; 23:695–701. [DOI] [PubMed] [Google Scholar]
- 11.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 1998; 72:2855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei X, Decker JM, Liu H et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 2002; 46:1896–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 1995; 69:6705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 1995; 206:935–44. [DOI] [PubMed] [Google Scholar]
- 15.Holder DJ, Hsuan F, Dixit R, Soper K. A method for estimating and testing area under the curve in serial sacrifice, batch, and complete data designs. J Biopharm Stat 1999; 9:451–64. [DOI] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2011: Vienna, Austria: ISBN 3-900051-07-0 http://www.R-project.org/. Accessed 13 March 2016. [Google Scholar]
- 17.Chakraborty A, Jusko WJ. Pharmacodynamic interaction of recombinant human interleukin-10 and prednisolone using in vitro whole blood lymphocyte proliferation. J Pharm Sci 2002; 91:1334–42. [DOI] [PubMed] [Google Scholar]
- 18.Yang K, Cottrell M, Sykes C, Prince HA, Patterson KB, Kashuba ADM. Multi-Compartment Population PK Model of Tenofovir (TFV) and Emtricitabine (FTC) for HIV Prevention. Abstract W052. In: American Conference on Pharmacometrics 5 2014: Las Vegas, Nevada October 12–15. [Google Scholar]
- 19.Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 20.Emmanuel AV, Kamm MA, Beard RW. Reproducible assessment of vaginal and rectal mucosal and skin blood flow: laser doppler fluximetry of the pelvic microcirculation. Clin Sci (Lond) 2000; 98:201–7. [PubMed] [Google Scholar]
- 21.Fletcher CV, Staskus K, Wietgrefe SW et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 2014; 111:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trevaskis NL, Kaminskas LM, Porter CJ. From sewer to saviour - targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov 2015; 14:781–803. [DOI] [PubMed] [Google Scholar]
- 23.Porter CJ, Charman WN. Intestinal lymphatic drug transport: an update. Adv Drug Deliv Rev 2001; 50:61–80. [DOI] [PubMed] [Google Scholar]
- 24.Shaw GM, Hunter E. HIV transmission. Cold Spring Harb Perspect Med 2012; 2:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arien KK, Kyongo JK, Vanham G. Ex vivo models of HIV sexual transmission and microbicide development. Curr HIV Res 2012; 10:73–8. [DOI] [PubMed] [Google Scholar]
- 26.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol 2012; 10:852–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicol MR, Emerson CW, Prince HM et al. Models for Predicting Effective HIV Chemoprevention in Women. J Acquir Immune Defic Syndr 2015; 68:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao WY, Johns DG, Mitsuya H. Anti-human immunodeficiency virus type 1 activity of hydroxyurea in combination with 2′,3′-dideoxynucleosides. Mol Pharmacol 1994; 46:767–72. [PubMed] [Google Scholar]
- 29.Yang KH, Hendrix C, Bumpus N et al. A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate. PLoS One 2014; 9:e106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer S, Shafer RW, Merigan TC. Hydroxyurea enhances the activities of didanosine, 9-[2-(phosphonylmethoxy)ethyl]adenine, and 9-[2-(phosphonylmethoxy)propyl]adenine against drug-susceptible and drug-resistant human immunodeficiency virus isolates. Antimicrob Agents Chemother 1999; 43:2046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis DM, Kewn S, Coull JJ et al. The addition of mycophenolate mofetil to antiretroviral therapy including abacavir is associated with depletion of intracellular deoxyguanosine triphosphate and a decrease in plasma HIV-1 RNA. J Acquir Immune Defic Syndr 2002; 31:45–9. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Lerma JG, Aung W, Cong ME et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol 2011; 85:6610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seifert SM, Glidden DV, Meditz AL et al. Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis 2015; 60:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC). Preexposure prophylaxis for the prevention of hiv infection in the United States. A clinical practice guideline. Atlanta: CDC, 2014:1–67. [Google Scholar]
- 35.Molina JM, Capitant C, Spire B et al. On Demand PrEP with Oral TDF/FTC in MSM Results of the ANRS Ipergay Trial. Abstract 23LB. In: Conference on Retroviruses and Opportunistic Infections 2015: Seattle, Washington February 23–26. [Google Scholar]
- 36.Kim SY, Byrn R, Groopman J, Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol 1989; 63:3708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnell D, Baeten JM, Bumpus NN et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekker LG, Hughes J, Amico R et al. HPTN067/ADAPT background and methods and Cape Town results [abstract MOSY0103]. In: 8th IAS Conference on HIV Pathogenesis, Treatment, and Prevention, Vancouver, Canada, 19–22 July 2015. [Google Scholar]
- 39.Louissaint NA, Cao YJ, Skipper PL et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013; 29:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang LH, Begley J, St Claire RL III, Harris J, Wakeford C, Rousseau FS. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses 2004; 20:1173–82. [DOI] [PubMed] [Google Scholar]
- 41.Reed RC, Dutta S, Liu W. Once-daily dosing is appropriate for extended-release divalproex over a wide dose range, but not for enteric-coated, delayed-release divalproex: evidence via computer simulations and implications for epilepsy therapy. Epilepsy Res 2009; 87:260–7. [DOI] [PubMed] [Google Scholar]
- 42.Shen Z, Fahey JV, Rodriguez-Garcia M, Bodwell JE, Kashuba AD, Wira CR. Female sex hormone regulation of tenofovir-diphosphate in human female reproductive tract (FRT) cells in culture. AIDS Res Hum Retroviruses 2014; 30(suppl 1):A149–50. [Google Scholar]
- 43.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol 2010; 39:1048–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rollenhagen C, Lathrop MJ, Macura SL, Doncel GF, Asin SN. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol 2014; 7:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.