Abstract
The role of antibody-mediated immunity in preventing newly acquired oral human papillomavirus (HPV) is not well understood. Among 1618 men participating in the HPV Infection in Men (HIM) Study, we evaluated oral rinses for HPV DNA and baseline sera for HPV-6, -11, -16, and -18 L1 antibodies. Thirty percent of men (486) were seropositive for ≥1 HPV type, and 25 men developed incident oral HPV infection (HPV-6 was detected in 7, HPV-11 in 0, HPV-16 in 17, and HPV-18 in 1). Cox models revealed that men with circulating antibodies to HPV-6, -11, -16, or -18 were not less likely to acquire type-specific oral HPV than men without antibodies (hazard ratio for the risk of acquiring HPV-6, -11, -16, or -18, 1.63; 95% confidence interval, .56–4.76).
Keywords: oral HPV, HPV16, antibodies, serology, men
Infection with human papillomavirus (HPV), particularly HPV-16, causes cancer at multiple anatomic sites, including a subset of cancers of the head and neck. Incidence rates of HPV-driven oropharyngeal cancer (OPC), comprising tumors of the tonsils, base of the tongue, and soft palate, have increased dramatically in the United States in recent decades [1], with the burden of OPC among men 3 times higher than that among women and higher than that of cervical cancer [2].
Following a natural HPV infection, a proportion of individuals develop immunoglobulin G (IgG) antibodies to the L1 capsid protein [3]. High circulating serum antibody titers are believed to provide partial protection against subsequent re-infection of the same or related HPV genotype at the cervix; however, this antibody response is slow to develop [4] and levels tend to be low [4]. Only a subset of women infected with genital HPV develop antibodies to the HPV L1 capsid protein and a smaller proportion of men with genital HPV seroconvert [5, 6].
It remains unknown whether circulating HPV antibodies offer protection against the acquisition of a new oral HPV infection. Epidemiological studies examining the protective role of naturally acquired immunity against oral HPV infection have been limited [7], largely due to the fact that infection at this anatomic site is rare. We hypothesized that serum HPV antibodies may translocate to the oral epithelium [8] offering local immune protection not observed at the genital or anal epithelium. This study aimed to assess whether naturally induced serum antibodies to HPV were associated with a reduced risk of subsequent oral HPV infection in a large, multinational cohort of otherwise healthy, immunocompetent men.
METHODS
Study Design and Population
This prospective study was nested within the HPV Infection in Men (HIM) Study, an ongoing natural history study of HPV infection among adult men in the United States, Mexico, and Brazil [9]. Details of the oral component of the HIM Study are published elsewhere [10]. Briefly, the HIM Study oral subcohort consists of 1626 men who provided oral samples that have been tested for HPV DNA. Men ranged in age from 18 to 73 years; reported no symptoms of or treatment for sexually transmitted infections, including HIV infection; and reported not having received the HPV vaccine. A total of 1618 men were included in the current analysis if they also had results of serum IgG antibody testing for the presence of HPV-6, -11, -16, and -18 from their first visit. The human subjects committees of all institutions approved all study procedures and all participants provided written informed consent [10].
Procedures
Participants completed a baseline visit, were enrolled on completion of their first follow-up visit (2 weeks after baseline), and then followed up every six months for up to 4 years. At each visit, participants completed a computer-assisted self-interviewing questionnaire including questions on sociodemographic and behavioral characteristics. Participants also underwent a clinical examination, during which they provided blood and oral gargle samples. Since the oral subcohort was created approximately 2 years after HIM Study enrollment began, the first oral gargle sample obtained was not necessarily collected during the participant's baseline HIM Study visit.
Using 15 mL of mouthwash, participants were asked to provide an oral gargle specimen. Methods for oral gargle processing, DNA extraction, and HPV genotyping have been described previously [10]. Briefly, oral cells underwent robotic DNA extraction and HPV genotyping for 37 HPV types, using Linear Array (Roche Molecular Diagnostics, Alameda, California).
At baseline, a 10-mL specimen of venous blood was collected for antibody testing. As described previously, serum antibodies to HPV-6, -11, -16, and -18 were evaluated using a type-specific HPV L1 virus-like particle–based enzyme-linked immunosorbent assay in the laboratory of R. P. V. [11]. Seroreactivity was measured in OD units.
Statistical Analysis
Two serology measures were used to estimate the association between antibodies and oral HPV infection: (1) seropositive versus seronegative, with seropositivity defined as ≥0.2, ≥0.3, ≥0.2, and ≥0.2 OD units for HPV types 6, 11, 16, and 18, respectively; and (2) log10-transformed antibody titer.
Only the first-acquired type-specific HPV infection was considered; men who tested positive for type-specific HPV at the baseline oral visit were excluded. The cumulative risk of acquiring oral HPV was calculated from the baseline oral visit to the date of first HPV DNA detection, assuming that a new infection arose on the date of detection, or to the end of follow-up. The Kaplan–Meier method was used to estimate cumulative risk, stratified by baseline serostatus. Given the small sample sizes, the Cox F test was used in place of the log-rank test to compare cumulative incidence curves. The overall association between baseline serum antibodies and type-specific oral HPV incidence was further evaluated using the Wei–Lin–Weissfeld method for Cox proportional hazards regression.
Analyses were performed using SAS, version 9.3 (SAS Institute, Cary, North Carolina). All statistical tests were 2-sided and attained significance at an α level of 0.05.
RESULTS
The analyzed cohort consisted of 1618 men (499 from Brazil, 557 from Mexico, and 562 from the United States) who were followed for a median of 12.7 months (interquartile range [IQR], 12.1–14.7 months; range, 0.5–37.2 months) after the first oral sample was collected. The median age of participants at the time of the first oral gargle specimen collection was 32 years (IQR, 24–41 years). Sexual orientation and lifetime number of sex partners were significantly associated with HPV seropositivity (Supplementary Table 1). Men reporting high lifetime numbers of sex partners were more likely to be seropositive than men reporting low lifetime numbers of sex partners.
In this subcohort, 486 men (30%) were seropositive for at least 1 HPV type (ie, HPV-6, -11, -16, or -18) at baseline (Supplementary Table 1). Type-specific seropositivity was as follows: HPV-6, 8.8%; HPV-11, 15.3%; HPV-16, 11.6%; and HPV-18, 11.3%. Throughout the follow-up period, 25 men developed an incident oral HPV-6, -16, or -18 infection, with HPV-16 the most commonly detected type (in 17 men), followed by HPV-6 (in 7), and HPV-18 (in 1); no new HPV-11 infections were detected. Coinfection with ≥2 HPV types was not observed among men with incident oral HPV infection. Four men who acquired oral HPV were seropositive at baseline, whereas 21 were seronegative.
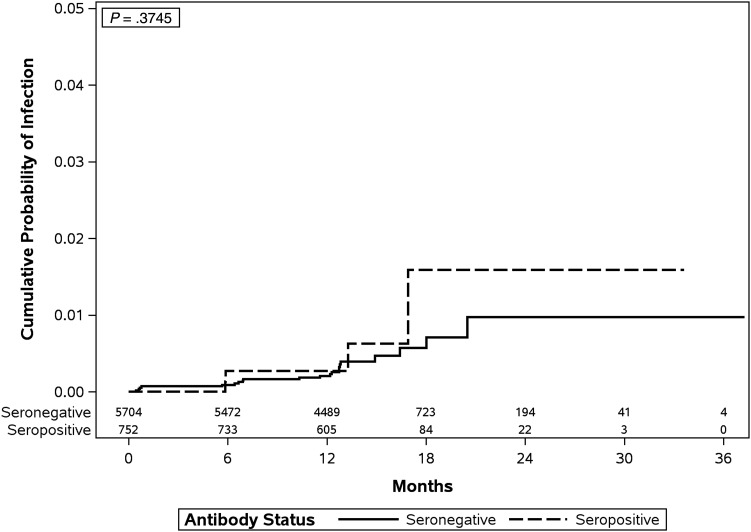
Use of the Kaplan–Meier method revealed that the cumulative risk of acquiring a new oral HPV infection over the entire study period was not significantly different between HPV-seropositive men and HPV-seronegative men (Cox F P = .375; Figure 1). Furthermore, using Cox models, we observed that men with circulating serum antibodies to HPV-6, -11, -16, or -18 were not at lower risk of acquiring oral HPV of the same type, regardless of the serology measurement used (Table 1). Acquisition of a new oral HPV infection appeared higher among men with low or high antibody titers, compared with those with no antibody titers (hazard ratio [HR], 1.63; 95% confidence interval [CI], .56–4.76); however, this association did not reach statistical significance. Similar results were observed after individual adjustment for lifetime numbers of sex partners (adjusted HR [aHR], 1.51; 95% CI, .49–4.69), sexual orientation (aHR, 1.58; 95% CI, .48–5.17), and age (aHR, 1.70; 95% CI, .60–4.83).
Figure 1.
Kaplan–Meier estimate of the cumulative incidence of oral human papillomavirus (HPV) infection, by baseline serostatus (for HPV-6, -11, -16, or -18), among men in the HPV Infection in Men Study (participants were recruited in Tampa, FL, Cuernavaca, Mexico, and São Paulo, Brazil). Because of small sample sizes, cumulative incidence curves were compared using a Cox P value derived from a Cox proportional hazards model.
Table 1.
Association Between Baseline Human Papillomavirus (HPV) Serum Antibody Level and Risk of Incident Type-Specific Oral HPV Infection Among Men Participating in the HPV Infection in Men Study
| Serostatus at Baseline | Any HPV Typea |
HPV-6 |
HPV-16 |
||||
|---|---|---|---|---|---|---|---|
| Infections, No. | Univariate HR (95% CI) | Multivariableb aHR (95% CI) | Infections, No. | Univariate HR (95% CI) | Infections, No. | Univariate HR (95% CI) | |
| Seronegative | 21 | 1.00 | 1.00 | 6 | 1.00 | 14 | 1.00 |
| Seropositivec | 4 | 1.63 (.56–4.76) | 1.51 (.49–4.69) | 1 | 1.78 (.21–14.78) | 3 | 1.70 (.49–5.90) |
| Antibody titer, log10 | 25 | 1.81 (.57–5.74) | 1.66 (.52–5.23) | 7 | 3.02 (.43–21.25) | 17 | 1.29 (.30–5.62) |
All analyses are HPV type specific. Participants were recruited in Tampa, Florida, Cuernavaca, Mexico, and São Paulo, Brazil.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio.
a Defined as HPV-6, −11, −16, and −18.
b Adjusted for lifetime number of sex partners (female and male).
c Defined as ≥0.2, ≥0.3, ≥0.2, and ≥0.2 OD units for HPV-6, -11, -16, and -18, respectively.
Seventeen men reported receiving at least 1 dose of the HPV vaccine after study enrollment; all were seronegative at baseline, and none acquired oral HPV. A sensitivity analysis was conducted to determine whether including these men influenced the modeling results. No meaningful difference was observed in the HR when these men were excluded from the analysis (data not shown); therefore, men who claimed to have been vaccinated remained in the analysis.
DISCUSSION
In our study, healthy adult men with antibodies induced by natural HPV infection were not protected against acquisition of a new oral infection with the same HPV genotype. The risk of acquiring oral HPV was not significantly different between men with type-specific antibodies and men with no circulating antibodies. The results were similar after adjustment for lifetime number of sex partners. HPV antibody levels appear to reflect a man's history of sexual behaviors and partnerships. In the HIM Study, men with detectable serum antibodies to HPV at baseline reported having had more sexual activity and more same-sex partners. Thus, men who mounted an antibody response may have had a greater number of HPV exposures, including oral HPV, in their lifetimes. Similar findings were reported in the only other published study to examine antibody protection against oral HPV infection. Beachler et al [7] observed that HPV-16 L1 seropositivity did not reduce the risk of a subsequent oral HPV-16 infection among HIV-positive and at-risk HIV-negative women and men who have sex with men.
Growing evidence suggests that antibody-mediated immunity does not play a protective role against acquisition of anogenital HPV among men. In a separate HIM Study analysis, Lu et al [11] observed no protection of serum antibodies against a subsequent or persistent penile HPV-16 infection. Similarly, in a recent study conducted by Mooij et al [12] among men who have sex with men, baseline HPV seropositivity was not associated with a decreased risk of acquiring penile or anal HPV. In contrast, numerous studies have demonstrated antibody protection against subsequent cervical HPV infection among women [5], although the use of different cutoffs and lack of an international reference makes comparisons across studies difficult [13].
Sex-based differences in the host immune response [14] following HPV infection may explain differences in antibody protection observed between men and women. Women consistently demonstrate a higher prevalence of antibody against HPV-6 and HPV-16 than men, despite having lower genital and oral HPV DNA prevalence [5, 6]. Although only a subset of individuals with HPV infection produce detectable antibodies, a larger proportion of women seroconvert to HPV-16 than men. Within 2 years of a newly acquired infection, 60% of young women developed an antibody response to HPV-16 [15], compared with only 4%–13% of men [5]. Compared with men, women tend to exhibit more-robust cell-mediated and humoral immune responses to infectious diseases in general [15]. One potential explanation is that high estrogen levels promote antibody production, which thereby increases immunocompetence, whereas androgens such as testosterone, suppress immune function [14].
To our knowledge, this is the largest prospective study to examine the role of naturally induced antibodies on the subsequent risk of oral HPV infection among men. However, the following limitations must be considered. Although the HIM Study represents one of the largest and longest running prospective HPV natural history studies, it was not specifically designed to assess the role of serologic antibody responses on oral HPV acquisition. Thus, the current study was not adequately powered to detect a protective effect. Larger studies examining oral HPV infection incidence would be needed to provide a more robust test of this association. In approximately 40% of participants, serum antibodies were measured ≥6 months prior to the collection of the first oral gargle specimen. Since serostatus was not evaluated at the same visit as the first oral gargle specimen, we were unable to compare whether the time to the first oral HPV infection differed by serostatus among men. However, given that HPV-16 seroconversion occurs slowly [4], we would not expect the antibody status to change substantially over a period of 6 months. By only including men who tested negative for HPV at the baseline oral visit, we hope to have captured truly incident infections. However, if the viral load was below the lower limit of detection of the assay, we may have misclassified men who had a prevalent infection with a low copy number as being HPV negative. Although participants were asked to report changes in HIV status throughout the study, it is possible that some study participants may have unknowingly become HIV positive throughout the 4-year study period. Last, although we included men from a broad age range and multiple countries, the results from this study may not be generalizable to all healthy, adult men.
Despite the highly immunogenic properties of the oral cavity, our data show that systemic antibodies produced in response to a natural HPV infection do not protect against a newly acquired oral infection among men. Given the increasing burden of HPV-driven OPC in countries such as the United States [1] and the lack of clinically proven methods to prevent or detect early OPC, HPV vaccine trials testing efficacy against oral HPV are needed.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the HIM Study participants and teams in the United States (Moffitt Cancer Center, Tampa, Florida), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer do Estado de São Paulo, Ludwig Institute for Cancer Research, São Paulo), and Mexico (Instituto Mexicano del Seguro Social, Instituto Nacional de Salud Pública, Cuernavaca); and the Biostatistics Core and Tissue Core Facilities, H. Lee Moffitt Cancer Center and Research Institute (a National Cancer Institute (NCI)–designated Comprehensive Cancer Center, supported under National Institutes of Health (NIH) grant P30 CA076292), for invaluable assistance.
Disclaimer. The contents of the report do not necessarily represent the official views of the NCI or the NIH, and the opinions expressed are those of the authors and do not necessarily represent those of Merck Sharp and Dohme.
Financial support. This work was supported by the NCI, NIH (grant R01 CA098803 to A. R. G.); the National Cancer Institute Intramural Program (to A. R. K.); the Investigator-Initiated Studies Program, Merck Sharp and Dohme (to A. R. G.); and the American Cancer Society (postdoctoral fellowship PF-13-222-01–MPC to C. M. P. C.).
Potential conflicts of interest. A. R. G. receives research funding from, serves on the advisory board for, and is a consultant on human papillomavirus vaccine trials for Merck Sharp and Dohme. L. L. V. serves as a consultant on human papillomavirus vaccine trials for Merck Sharp and Dohme. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Simard EP, Dorell C et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013; 105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter JJ, Koutsky LA, Wipf GC et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis 1996; 174:927–36. [DOI] [PubMed] [Google Scholar]
- 4.Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AR, Nyitray AG, Kreimer AR et al. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015; 136:2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliano AR, Viscidi R, Torres BN et al. Seroconversion following anal and genital HPV infection in men: The HIM Study. Papillomavirus Res 2015; 1:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beachler DC, Viscidi R, Sugar EA et al. A longitudinal study of human papillomavirus 16 l1, e6, and e7 seropositivity and oral human papillomavirus 16 infection. Sex Transm Dis 2015; 42:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berneman A, Belec L, Fischetti VA, Bouvet JP. The specificity patterns of human immunoglobulin G antibodies in serum differ from those in autologous secretions. Infect Immun 1998; 66:4163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giuliano AR, Lee JH, Fulp W et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet 2011; 377:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreimer AR, Pierce Campbell CM, Lin HY et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet 2013; 382:877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu B, Viscidi RP, Wu Y et al. Prevalent serum antibody is not a marker of immune protection against acquisition of oncogenic HPV16 in men. Cancer Res 2012; 72:676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mooij SH, Landen O, van der Klis FR et al. No evidence for a protective effect of naturally induced HPV antibodies on subsequent anogenital HPV infection in HIV-negative and HIV-infected MSM. J Infect 2014; 69:375–86. [DOI] [PubMed] [Google Scholar]
- 13.Scherpenisse M, Schepp RM, Mollers M et al. Comparison of different assays to assess human papillomavirus (HPV) type 16- and 18-specific antibodies after HPV infection and vaccination. Clin Vaccine Immunol 2013; 20:1329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter JJ, Koutsky LA, Hughes JP et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 2000; 181:1911–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.