Abstract
Background. Herpes simplex virus type 2 (HSV-2) reactivation is accompanied by a sustained influx of CD4+ and CD8+ T cells that persist in genital tissue for extended periods. While CD4+ T cells have long been recognized as being present in herpetic ulcerations, their role in subclinical reactivation and persistence is less well known, especially the role of CD4+ regulatory T cells (Tregs).
Methods. We characterized the Treg (CD4+Foxp3+) population during human HSV-2 reactivation in situ in sequential genital skin biopsy specimens obtained from HSV-2–seropositive subjects at the time of lesion onset up to 8 weeks after healing.
Results. High numbers of Tregs infiltrated to the site of viral reactivation and persisted in proximity to conventional CD4+ T cells (Tconvs) and CD8+ T cells. Treg density peaked during the lesion stage of the reactivation. The number of Tregs from all time points (lesion, healed, 2 weeks after healing, 4 weeks after healing, and 8 weeks after healing) was significantly higher than in control biopsy specimens from unaffected skin. There was a direct correlation between HSV-2 titer and Treg density.
Conclusions. The association of a high Treg to Tconv ratio with high viral shedding suggests that the balance between regulatory and effector T cells influences human HSV-2 disease.
Keywords: HSV-2, regulatory T cells, reactivation, herpes, viral shedding, T cells, Foxp3
(See the editorial commentary by Levin and Weinberg on pages 4–5.)
Herpes simplex virus type 2 (HSV-2) is the major causative agent of recurrent genital herpes. During primary infection, HSV-2 infects the genital skin epithelial cells and then further spreads to the sensory neurons and establishes latency [1]. During latency, HSV-2 reactivation episodes that occur in immunocompetent individuals can range from asymptomatic, subclinical episodes to clinical episodes with ulcerative lesions that last several days [2]. Within the same individual, some HSV-2 reactivations are contained rapidly (within 24 hours), and others spread and persist for several days [3]. The viral titer and length of shedding episodes correlate with clinical symptom severity [4]. Increasing evidence supports the notion that rapid containment of the virus is mediated by localized immunity to the virus.
HSV-2 reactivations are accompanied by a sustained influx of both CD4+ and CD8+ T cells [5, 6], which persist in genital tissue for extended periods after lesion healing. HSV-2–specific tissue-resident CD8+ T cells localize at the dermal epidermal junction (DEJ) contiguous to sensory nerve fibrils where they play a role in immunosurveillance and early containment of reactivation [5, 7, 8]. While conventional CD4+ T cells (Tconvs) have long been recognized as being present in herpetic ulcerations [6, 9], their roles, especially those of CD4+ regulatory T cells (Tregs), in HSV-2 containment or persistence are less well understood.
Tregs are a subset of CD4+ T cells that suppress immune responses and can be identified by expression of the markers interleukin 2 receptor α (IL-2RA; also known as CD25) and the transcription factor Foxp3 and the lack of CD127 expression. These cells play a major role as immune suppressors during acute disease and contribute to dampening immune responses to chronic viral infections.
Several studies in mice have analyzed the function of Tregs in primary HSV-2 infection [10–13]. However, HSV-2 does not undergo reactivation in the murine model and hence cannot directly address the role Tregs play in the major manifestation of HSV-2 in humans: recurrent reactivation. It has been shown that memory CD4+CD25+ Tregs from the peripheral blood of HSV-2–seropositive patients can suppress proliferation of HSV-2–specific CD4+ T cells at times of clinical quiescence [14]. Little else is known about the role of Tregs in individuals with chronic HSV-2 genital infection, and the paucity of data describing localized Treg suppression during clinical disease and asymptomatic periods demonstrates the incomplete understanding of HSV-2 pathogenesis in humans.
In this study, we monitored CD4+Foxp3+ T cells in situ in sequential biopsy specimens taken from genital lesions of HSV-2–positive patients throughout the course of healing. We describe the dynamics of Tregs during the immune response, in addition to their localization pattern at the site of reactivation. Our data indicate that a high Treg count at the time of genital lesions is associated with increased viral replication, providing the first evidence that the extent of tissue infiltration of Tregs correlates with the severity of recurrent genital HSV-2 lesions in the human host.
MATERIALS AND METHODS
Human Subjects and Specimens
Ten subjects who were culture- and serology-positive for recurrent genital HSV-2 infection were recruited to the study by the Virology Research Clinic at the University of Washington, Seattle (Supplementary Table 1). All subjects were human immunodeficiency virus type 1 and 2 seronegative, immunocompetent, and not receiving HSV suppressive therapy during the study period. The protocol was similar to our previously published studies [5, 7] and is outlined in Supplementary Table 2. Patients were enrolled at the onset of an acute, symptomatic recurrent lesion and seen by clinic staff 5–7 days later (ie, the “healed” time point) and at 2, 4 and 8 weeks after healing. At each genital exam visit, viral cultures and 3-mm punch biopsy specimens were obtained from the active lesion site as previously described. Punch biopsy specimens from inner arm skin (control) were obtained at the enrollment and the week 8 visit; 100 mL of blood was collected at both time points for peripheral blood mononuclear cell (PBMC) analysis. The biopsy tissue specimen was snap frozen in optimum cutting temperature compound (Tissue-Tek; Sakura Finetek USA, Inc., Torrance, California) and stored at −80°C. PBMCs were processed and frozen at −80°C. Biopsy specimens were stained for quantitative evaluation of CD4+ and CD8+ T cells, as well as for HSV-2 DNA, using validated real-time quantitative polymerase chain reaction (PCR) analysis [7]. The study protocol was approved by the University of Washington Institutional Review Board. Informed consent was received from all participants prior to inclusion in the study.
Immunofluorescence Staining
Frozen tissue specimens were divided into 6–8-µm sections and kept frozen until fixation and permeabilization with acetone for 20 minutes at −20°C. A detailed description of specimen preparation and antibody staining is provided in the Supplementary Methods. A list of primary and secondary antibodies is provided in Supplementary Table 3. Images were captured with the Axio Observer 2.1 (Zeiss, Pleasanton, California) microscope, using the 20X/0.8M27 Plan-Apochromat lens and the AxiocamMR3 camera, and were analyzed with Axiovision program (release 4.8.2, Zeiss).
Laser Capture Microdissection (LCM)
A detailed description of LCM is provided in the Supplementary Methods. Briefly, 6–8-μm sections of frozen biopsy specimens were adhered on membrane slides, fixed, and incubated with Foxp3-AF647 or CD4-AF647. Foxp3+ and CD4+ T cells were imaged and subjected to laser microdissection, using the PALM MicroBeam instrument (Zeiss). Sequential sections were stained for CD4 and Foxp3, and CD4 T cells were collected from biopsy areas with scarce Foxp3+ T cells. Between 75 and 120 cells were captured from each sample, and RNA was immediately extracted. Total RNA extraction and complementary DNA (cDNA) synthesis and amplification were conducted as described previously [8], except that 5 µL of the final 10 µL of pure RNA were added to the cDNA synthesis reaction.
Flow Cytometry
A total of 5 × 106 PBMCs were stained with the Live/Dead Fixable Dead Stain Cell kit (Molecular Probes, Grand Island, New York) according to the manufacturer's instructions. Cells were washed with phosphate-buffered saline (PBS) and then resuspended with either the Treg phenotype cocktail, containing antibodies to CD3, CD4, CD25, CD127, and Foxp3 (antibodies are listed in Supplementary Table 3), or the cocktail with the anti-Foxp3 antibody omitted (FMO) at room temperature for 20 minutes and then washed with fluorescence-activated cell-sorting buffer (2% fetal bovine serum in PBS). Intracellular staining was performed using the Foxp3/Transcription Factor Staining Buffer Set (eBiosciences, San Diego, California) according to the manufacturer's instructions. Cells were washed, resuspended in 2% FACS buffer, and analyzed on LSRII (Becton Dickinson, Franklin Lakes, New Jersey). Data were analyzed using FlowJo software.
Droplet Digital PCR (ddPCR)
ddPCR was used to quantify the expression of genes encoding Foxp3, CTLA-4, IL-2RA (CD25), and actin B (ACT B) in cDNA samples of the laser-captured Foxp3+ and CD4+ T cells. TaqMan primer/probe sets for each of the above genes (Applied Biosystems, Waltham, Massachusetts; Supplementary Table 4) were added to the cDNA, together with ddPCR Supermix for Probes (Bio-Rad, Hercules, California). The Bio-Rad QX100 system was used to partition the PCR mixture into the droplets, and Bio-Rad QuantaSoft software was used to analyze the ddPCR results. Details are provided in the Supplementary Methods.
Statistics
All analyses were performed by the University of Washington HSV Biometric Unit. The CD4+, CD8+, and Foxp3+ raw cell densities were skewed; therefore, to adjust for this, statistical analysis was performed on the natural log–transformed data. A paired t test was used to test whether there was a significant difference in the cell densities measured at biopsy specimen sections obtained at lesion and control time points. Details of statistical analyses are provided in Supplementary Methods.
RESULTS
Tregs Are Present in Substantial Numbers in Skin Lesions Caused by HSV-2 Reactivation
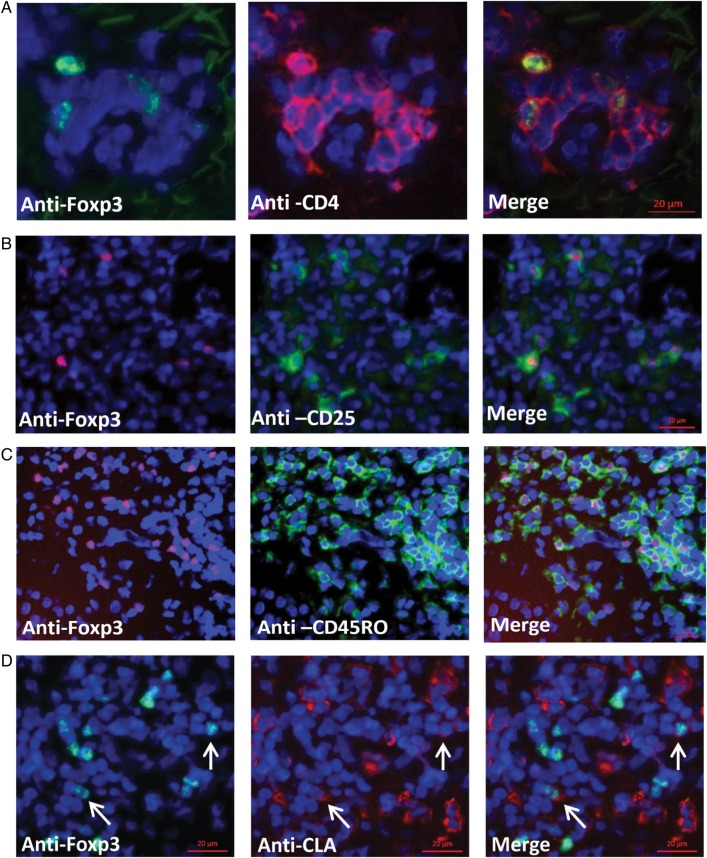
To determine whether Tregs are present in genital tissue during an acute HSV-2 reactivation episode, genital lesion biopsy specimens from all 10 patients were stained for Foxp3. Foxp3 localized to the nucleus of CD4+ T cells (Figure 1A). CD4+Foxp3+ T cells were also detected in control biopsy specimens obtained from the arm at the time of HSV-2 reactivation in 9 of 10 subjects. All Foxp3+ T cells detected in this representative field of lesional tissue were CD4+ T cells (Figure 1A).
Figure 1.
Characterization of Foxp3+ cells during human herpes simplex virus type 2 reactivation. A–D, Frozen genital skin biopsy specimens from an acute genital lesion were stained for Foxp3 (green) and CD4 (red; A); Foxp3 (red) and CD25 (green; B); Foxp3 (red) and CD45RO (green; C); and Foxp3 (green) and cutaneous lymphocyte antigen (CLA) (red; D). Arrows point to Foxp3+CLA+ cells. All sections were also counterstained with DAPI (blue). Scale bar = 20 µm.
Immunofluorescence microscopy of lesion biopsy specimens showed that CD4+ T cells coexpressed CD3 (Supplementary Figure 1A) and that Foxp3+ cells expressed CD25 but not CD127 (Figure 1B and Supplementary Figure 1B), phenotypes indicative of Tregs [15, 16]. Of interest, the Foxp3+ cells observed in the genital skin costained for CD45RO (Figure 1C), which is expressed on memory T cells [17], and a fraction of Foxp3+ cells expressed the skin-homing molecule cutaneous lymphocyte antigen (CLA) [18] (Figure 1D). CD4+Foxp3+ T cells did not express Ki67, a cell marker for proliferation, suggesting that they were not undergoing replication at the time of examination (Supplementary Figure 1C). Together, these results delineate the presence of Tregs in genital tissue during active HSV-2 lesional disease.
Expression of Treg Signature Genes by CD4+Foxp3+ T Cells in HSV-2 Lesions
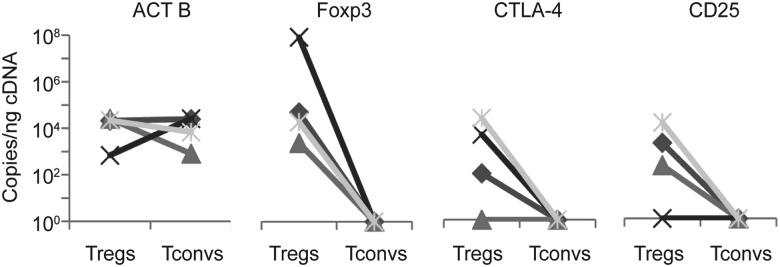
In addition to identifying protein expression indicative of Tregs, we analyzed the transcriptional profile of CD4+Foxp3+ T cells isolated from genital biopsy specimens from 4 patients, using LCM. CD4+Foxp3− (Tconv) cells were also collected by LCM from control biopsy specimens from the inner arm, and RNA from these cell-specific populations was tested for expression of Foxp3, CD25, CTLA-4, and ACT B by quantitative digital PCR [19]. In all 4 patients, transcriptional patterns showed an upregulation of Foxp3 in Tregs as compared to Tconvs (Figure 2). Tregs from 3 of 4 patients also expressed high levels of CD25 and CTLA-4 transcripts, whereas these genes were undetectable in Tconvs. ACT B expression was not significantly different between the 2 cell types. These data provide confirmatory evidence that the CD4+Foxp3+ T cells express Treg markers on both the protein and transcriptional levels.
Figure 2.
Analysis of gene expression of Foxp3+ cells during human herpes simplex virus type 2 reactivation. Analysis of gene expression by digital droplet polymerase chain reaction from laser-captured Foxp3+ (regulatory T cell [Treg]) and CD4+Foxp3− (conventional CD4+ T cell [Tconv]) cells of actin B (ACT B), Foxp3, CTLA-4, and CD25 from 4 different participants. The copy number of the indicated genes was assessed in 1 ng of complementary DNA (cDNA). Each line represents 1 participant, and all reactions were performed on the same day.
Quantity and Kinetics of Treg Infiltrate During the Course of Genital Herpes
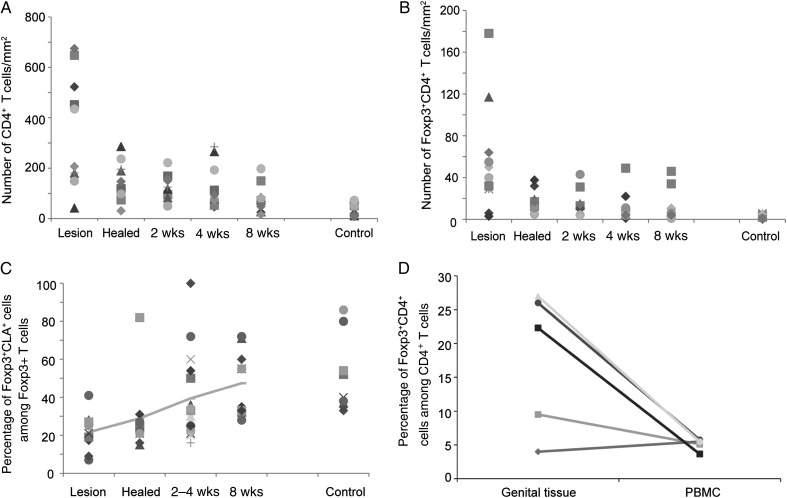
To determine the density of CD4+ and CD4+Foxp3+ T cells over the course of HSV-2 ulceration through the posthealing period, we calculated the number of cells in biopsy specimens for all time points. The mean number of CD4+ T cells during the time of lesion was 349 cells/mm2 (range, 42–615 cells/mm2), compared with 32.9 cells/mm2 (range, 7–73 cells/mm2) in control biopsy specimens (P < .001; Figure 3A). As previously described [7], the mean CD4+ T-cell counts decreased over the course of healing, with 127.3, 128.1, and 89.8 cells/mm2 detected 2, 4, and 8 weeks after healing, respectively (P < .01, compared with control biopsy specimens, for each time point, for natural log–transformed CD4+ T-cell densities; Figure 3A). More than 90% of the Foxp3+ cells detected in situ were CD4+ T cells. The mean number of CD4+Foxp3+ T cells peaked during acute ulceration (57 cells/mm2; range, 3–178 cells/mm2) and decreased over the course of healing (Figure 3B). The mean density of lesional CD4+Foxp3+ T cells was significantly greater than in controls (57 vs 2.6 cells/mm2); a 20-fold difference (P < .01), which equates to a difference in mean density of ln(2.98) cells/mm2 (95% confidence interval, ln(2.20)–ln(3.87) cells/mm2). In addition, CD4+Foxp3+ T-cell density in genital biopsy specimens from each time point was significantly higher than that of the control (lesion time point, P < .0001; healed time point, P = .002; 2 weeks after healing, P = .003; 4 weeks after healing, P = .003; and 8 weeks after healing, P = .015). CD4+Foxp3+ T cells were detected in all patients at all time points. Interestingly, at the lesion time point, >90% of CD4+Foxp3+ cells expressed CD45RO and 21% expressed CLA, which increased to a median of 45.6% 8 weeks after healing (P = .012 relative to the time of lesion onset, Figure 3C). CLA was detected in 40% of CD4+Foxp3+ cells from control biopsy specimens.
Figure 3.
CD4+Foxp3+ T cells are abundant during acute herpes simplex virus type 2 reactivation episodes and persist in genital skin over time. A, Density of CD4+ and (B) CD4+Foxp3+ T cells in genital skin biopsy specimens (y-axis) from all 10 patients. The x-axis depicts the acute lesion time point (lesion), the recently healed time point (healed), and 2, 4, and 8 weeks after healing, as well as control biopsy specimens (control). C, The percentage of Foxp3+CLA+ cells among the total Foxp3+ cells in whole tissue-biopsy specimens. D, The percentage of CD4+Foxp3+ T cells among the total CD4+ T-cell population from a genital lesion biopsy specimen, compared with those found in peripheral blood mononuclear cells (PBMCs) isolated during the same time. The isolation of these cells by flow cytometry in depicted in Supplementary Figure 2.
To determine whether the percentage of CD4+Foxp3+/total CD4+ T cells in the genital tissue differed from that found in peripheral blood, we isolated PBMCs during the acute lesion time point from 5 patients and analyzed the cells by flow cytometry. PBMC Tregs were defined as CD3+CD4+CD25med–hiCD127dimFoxp3+. Isolation of this cell population from one patient is shown in Supplementary Figure 2. The median percentage of Tregs in the total CD4+ T-cell population in genital lesion biopsy specimens was 22.3%, compared with 5.47% for PBMCs taken during the same time point (Figure 3D), suggesting a specific recruitment or peripheral polarization of Tregs to the site of HSV-2 lesions.
Association Between Tregs and Viral Titer in HSV-2 Reactivation
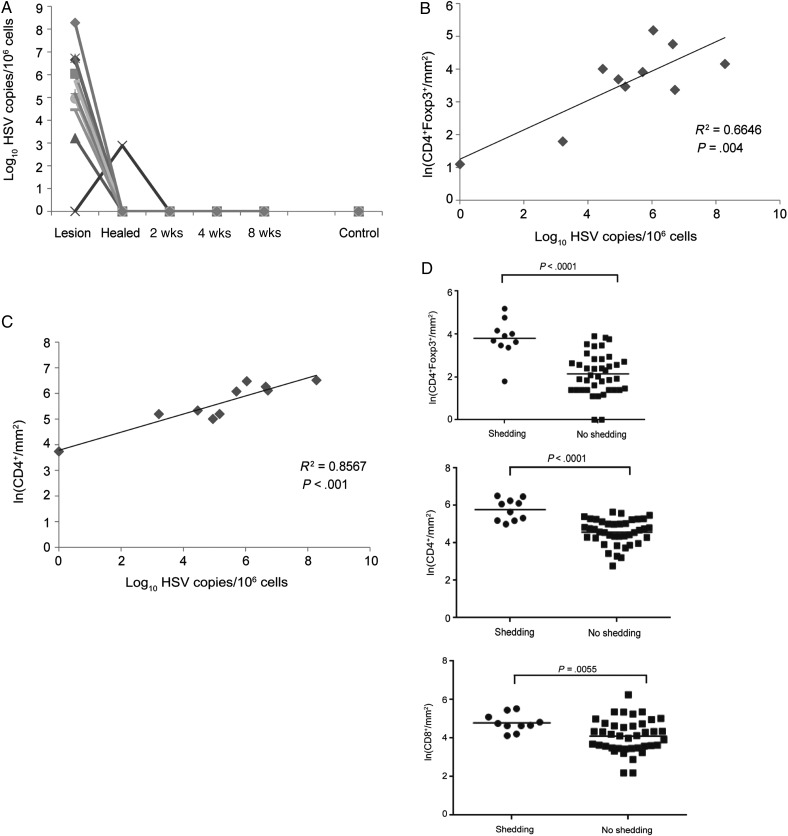
Quantitative real-time PCR was used to identify HSV-2 in biopsy specimens, and 9 of 10 patients tested positive for HSV-2 DNA at the acute lesion time point (Figure 4A). There was a direct correlation between viral titer and the number of CD4+Foxp3+ T cells in these biopsy specimens (r2 = 0.6646; P = .004); the mean Foxp3+ T-cell density was 0.448 ln cells/mm2 higher for each log10 increase in HSV-2 copies/million cells (P = .004; Figure 4B). We found a similar correlation between HSV-2 shedding and the influx of total CD4+ T cells, with the mean CD4+ T-cell density 0.353 ln cells/mm2 higher for each log10 increase in HSV-2 copies/million cells (r2 = 0.8567; P < .001; Figure 4C).
Figure 4.
Regulatory T-cell density is associated with virus shedding and titer. A, Herpes simplex virus type 2 (HSV-2) titer during acute lesion and posthealing time points. Each line depicts an individual patient. All subjects had no evidence of HSV-2 DNA after week 2. B and C, The correlation between viral titer (measured at the acute lesion stage) and CD4+Foxp3+ (B) and total CD4+ cells (C) by linear regression analysis. D, Association between CD4+Foxp3+ (top), CD4+ (middle), and CD8+ (bottom) T-cell density and HSV-2 shedding from all biopsy specimens in the data set. Natural log–transformed cell density is plotted according to viral shedding status: ●, shedding; ▪, no shedding. The horizontal line represents the mean log density. P values are shown.
The mean density of CD4+Foxp3+ T cells in biopsy specimens in which HSV-2 DNA was detected (shedding) was 5 times higher than in biopsy specimens with no evidence of viral DNA (no shedding; P < .0001; Figure 4D) and 3 times higher for CD4+ T cells between biopsy specimens with shedding and biopsy specimens with no shedding (P < .0001; Figure 4D). CD8+ T-cell density in the same biopsy specimens were 2 times higher during HSV-2 shedding (P = .0055; Figure 4D).
Spatial Localization of Tregs
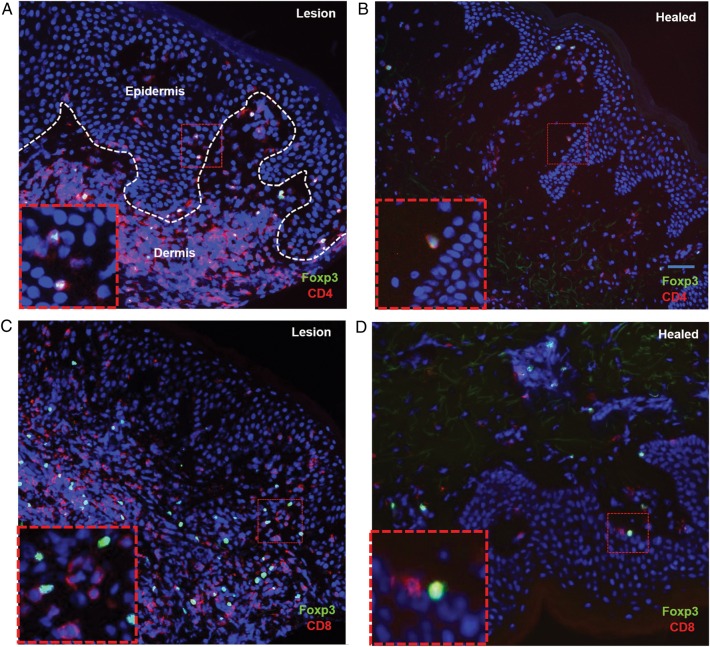
At the time of herpetic ulcerations, CD4+ and CD8+ T cells are present in both the epidermal and dermal layers of the genital skin. Over the course of healing, the CD4+ T cells mainly localize in the upper dermis, near blood vessels [8, 10]. A similar spatial pattern was observed for Foxp3+ T cells. Clusters of CD4+Foxp3+ T cells were seen in the upper dermis and rarely in the epidermis during acute lesions (Figure 5A). The cells were also largely localized to the upper dermis in biopsy specimens obtained at the healed time point but in close proximity to the DEJ, often in contact with epidermal cells (Figure 5B). Double staining for CD8+ and Foxp3+ demonstrated that Foxp3+ and CD8+ T cells were often localized to the same area in biopsy specimens obtained at both the lesion and healed time points, with some instances of cell-to-cell contact (Figure 5C and 5D).
Figure 5.
The spatial localization of regulatory T cells (Tregs) during herpes simplex virus type 2 reactivation. Micrographs depict Treg localization and persistence during the lesion time point (A and C) and healed time point (B and D). Biopsy specimens were stained for CD4+ (red) and Foxp3+ (green; A and B) and CD8+ (red) and Foxp3+ (green; C and D). Total Foxp3+ cells are noted in both the epidermis and dermis during acute lesion reactivation (lesion time point) and within 8–10 days (healed time point). CD4+Foxp3+ cells are reduced in number and found only in the dermis, usually below the dermal epidermal junction (DEJ) during healing (B). Insets are higher-magnification views of red hatched boxes (inset scale bar, 10 µm). The hatched white line in panel A outlines the DEJ. Scale bar = 50 µm.
Ratio of Tregs to CD4+ and CD8+ Tconvs Correlates With Viral Shedding
To compare the number of Tregs to effector T cells in genital tissue during viral shedding, we defined the Treg to Tconv ratio as the number of CD4+Foxp3+ T cells over the number of CD4+Foxp3− T cells, calculated as follows: [(number of CD4+Foxp3+ T cells)/(number of CD4+ T cells − number of CD4+Foxp3+ T cells)]. The mean Treg to Tconv ratio was 1.6 times higher at the time of viral reactivation than during virological quiescence (P = .0322; Figure 6A). To quantitate the ratio between Tregs and CD8+ T cells at the DEJ, we divided the number of CD4+Foxp3+ T cells by the number of CD8+ T cells. The Treg to CD8+ T-cell mean ratio was 2.6 higher in HSV-2–shedding positive samples compared with negative samples across all time points (P < .001; Figure 6B).
Figure 6.
Regulatory T cell (Treg) to effector cell ratio and viral shedding. The natural log of the cell ratio for Treg to (A) CD4+ T conventional cells and (B) CD8+ cells are plotted for every measurement in the data set (n = 48) according to viral shedding status. ●, shedding; ▪, no shedding. The horizontal line represents the mean log density. P values are shown.
DISCUSSION
In this article, we present a comprehensive description of the dynamics and localization of regulatory T cells during human HSV-2 reactivation. To our knowledge, this is the first study examining the role of Tregs in human subjects in situ during the course of natural reactivation of a chronic viral infection. We found a dramatic infiltration of Tregs at sites of HSV-2 reactivation during the acute lesion time point, which was associated with the presence of HSV-2 DNA. These lesional Tregs appear to have a memory phenotype, as a high proportion expressed CD45RO. We also observed a spatial relationship between CD4+Foxp3+ T cells and tissue memory CD8+ T cells localized to the DEJ after lesion healing, implying a role for this CD4+ T-cell subset in controlling the immune response to the virus. We have demonstrated before that the DEJ is the anatomical location where sensory neural termini are found and where HSV-2 accesses the skin. In addition, we described an association between the Treg to Tconv ratio and the Treg to CD8+ T-cell ratio and HSV-2 shedding. Our previous publications described an immunosurveillance function for the persisting CD8+ T cells [5, 7, 8], and our current observations suggest that maintaining a balance between Tregs and conventional CD4+ and CD8+ T cells is important for immunosurveillance and for the control of reactivation.
The percentage of Tregs in the total T-cell population in nongenital skin was in agreement with findings from other studies that looked at skin tissue–resident Treg levels in humans [18, 20]. An extensive analysis of T cells localized to genital tissue during the acute HSV-2 lesion time point, the healing time point, and 2, 4, and 8 weeks after healing identified that Tregs are found in a high frequency in ulcerative lesions and that the Treg density is greater during the lesion phase of infection, compared with the healed phase. After the elimination of virus from lesions, Treg levels decrease dramatically and persist for weeks in the genital skin throughout the posthealing period at a relatively constant level. Forty percent of these Tregs express CLA, suggesting the importance of skin homing in Treg persistence. In contrast, Tregs found in acute lesions express low levels of CLA, suggesting that this population is mainly induced in the periphery.
Foxp3+ T cells were often found in clusters with CD4+Foxp3− T cells, perhaps exerting suppression through direct cell-to-cell contact, as described before [21]. CD4+Foxp3+ cells occasionally colocalized with CD8+ T cells in genital tissue, and while in proximity the 2 cell types were not always in direct contact, suggesting that Tregs could mediate suppression via secretion of cytokines and/or other factors to the extracellular milieu. In support of our data, a recent study demonstrated that skin-resident memory Tregs (Foxp3+CD25+CD127− cells) block proliferation of autologous skin resident effector memory cells upon antigen stimulation [19] and that human skin–resident Tregs from psoriatic lesions exhibited an inhibitory cytokine profile distinct of activated T cells [22].
Little is known about the role Tregs play in human HSV infection. One study found that Tregs (CD4+CD25+) obtained from the peripheral blood of HSV-2–seropositive patients suppressed recall of proliferative HSV-2–specific CD4+ T-cell responses [14]. These results support our observation that Tregs dampen the antiviral immune response that controls HSV-2. Much of what we currently know about Treg immunity comes from the murine model of primary HSV-1 and HSV-2 infection, in which the depletion of Tregs prior to experimental infection with HSV altered disease progression depending on the route of infection and Treg depletion method used [10–13]. Depletion of CD4+CD25+ T cells by using an anti-CD25 monoclonal antibody in mice prior to cornea primary infection with HSV-1 resulted in more-severe lesions and suggested that Tregs suppress inflammatory responses and limit pathological damage by limiting the infiltration of effector cells to infected tissue [10]. A different Treg depletion method that used diphtheria toxin receptor (DTR) in mice prior to primary vaginal infection with HSV-2 resulted in faster disease progression and higher viral loads at the site of infection and in the central nervous system, indicating an early protective role in resolution and a delay in immune effector cell migration to the site of the infection [12]. Similar results were observed when depleting Tregs prior to ocular HSV-1 infection in DTR mice [11]. These 3 studies suggested that Tregs were beneficial to resolution of the infection, which is contradictory to our findings; however, they only studied Tregs during primary acute infection. Treg depletion in mice prior to cutaneous infection with TK-deficient HSV-2 decreases the severity of secondary skin lesions (considered sites of ganglionic spread), suggesting that Tregs compromise host immunity by altering effector homing and limit CD8+ T-cell responses in the ganglia [13]. Together, murine studies suggest that higher Treg activity may influence the severity of local replication and influence episode severity. However, because HSV-1 and HSV-2 undergo spontaneous reactivation in mice only under artificial circumstances, this animal model cannot be used to analyze the immune response to recurrent disease or subclinical viral shedding—the hallmarks of human HSV-2 disease. These discrepancies may relate to differences in pathogenesis between primary and recurrent infection and demonstrate the complexity of HSV-2 disease.
Our prior studies have shown that a high viral titer directly correlates with a longer lesion duration and greater severity [23]. As such, altering the number or function of Tregs in genital lesions could offer a new opportunity to shorten the course of an episode. We found that the shedding-positive biopsy specimens had significantly higher ratios of Tregs to CD4+ and CD8+ Tconvs than the biopsy specimens that lacked viral shedding, suggesting that a balance between the conventional T-cell population and the Treg population may affect the clinical presentation of disease. An increase in the level of Tregs can result in insufficient effector function and, therefore, an inability to control the HSV-2 leakage at nerve endings, which can ultimately result in lesion formation. We cannot however exclude the possibility at this stage that the increased infiltration of Tregs is a response to the high virus titer during reactivation and that Tregs are preferentially induced or attracted to areas of greater infection. Defining these issues will require further investigation.
In summary, our data suggest that there is a correlation between Tregs and the titer and, hence, severity of HSV-2 reactivation among immunocompetent patients. That acute ulcerative lesions are associated with a high frequency of CD4+Foxp3+ cells and that the density of Tregs is greater during the acute versus healed phases of infection illustrates the importance of Tregs in HSV-2 disease, especially the chronic, subclinical inflammatory response associated with recurrent genital herpes. Studies to evaluate the mechanism of their persistence and role in HSV-2 reactivation may offer novel insights on immunotherapeutic approaches for controlling HSV-2 infection.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Mindy Miner for technical editing of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants R37AI042528, R01AI04252815, R01AI111780-01, P01AI030731, and R56AI093746) and the James B. Pendleton Charitable Trust.
Potential conflicts of interest. D. M. K. is a consultant to EISAI, is co-inventor on patents owned by the University of Washington concerning herpes simplex virus (HSV) vaccines, and receives research funding from Sanofi Pasteur. A. M. consults for AiCuris. L. C. reports holding stock in Immune Design and being a co-inventor on several patents associated with the development of an HSV-2 vaccine. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med 2008; 59:381–95. [DOI] [PubMed] [Google Scholar]
- 2.Koutsky LA, Stevens CE, Holmes KK et al. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N Engl J Med 1992; 326:1533–9. [DOI] [PubMed] [Google Scholar]
- 3.Johnston C, Saracino M, Kuntz S et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 2012; 379:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mark KE, Wald A, Magaret AS et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008; 198:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J, Hladik F, Woodward A et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 2009; 15:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham AL, Diefenbach RJ, Miranda-Saksena M et al. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis 2006; 194(suppl 1):S11–8. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Koelle DM, Cao J et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 2007; 204:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Peng T, Johnston C et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 2013; 497:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest 1998; 101:1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol 2004; 172:4123–32. [DOI] [PubMed] [Google Scholar]
- 11.Veiga-Parga T, Suryawanshi A, Mulik S et al. On the role of regulatory T cells during viral-induced inflammatory lesions. J Immunol 2012; 189:5924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science 2008; 320:1220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez MA, Yu U, Zhang G et al. Treg depletion attenuates the severity of skin disease from ganglionic spread after HSV-2 flank infection. Virology 2013; 447:9–20. [DOI] [PubMed] [Google Scholar]
- 14.Diaz GA, Koelle DM. Human CD4+ CD25 high cells suppress proliferative memory lymphocyte responses to herpes simplex virus type 2. J Virol 2006; 80:8271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Putnam AL, Xu-Yu Z et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. [PubMed] [Google Scholar]
- 17.Clement LT. Isoforms of the CD45 common leukocyte antigen family: markers for human T-cell differentiation. J Clin Immunol 1992; 12:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Clark RA, Chong B, Mirchandani N et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol 2006; 176:4431–9. [DOI] [PubMed] [Google Scholar]
- 19.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood 2007; 109:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vukmanovic-Stejic M, Rustin MH, Nikolich-Zugich J, Akbar AN. Immune responses in the skin in old age. Curr Opin Immunol 2011; 23:525–31. [DOI] [PubMed] [Google Scholar]
- 21.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30:636–45. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez Rodriguez R, Pauli ML, Neuhaus IM et al. Memory regulatory T cells reside in human skin. J Clin Invest 2014; 124:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston C, Zhu J, Jing L et al. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 2014; 88:4921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.