Abstract
Background. Both wasting and obesity are associated with inflammation, but the extent to which body weight changes influence inflammation during human immunodeficiency virus infection is unknown.
Methods. Among a random virologically suppressed participants of the Prospective Evaluation of Antiretrovirals in Resource-Limited Settings trial, inflammatory markers were measured at weeks 0, 24, and 48 after antiretroviral therapy (ART) initiation. Associations between both baseline and change in body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) and changes in inflammation markers were assessed using random effects models.
Results. Of 246 participants, 27% were overweight/obese (BMI, ≥ 25), and 8% were underweight (BMI < 18.5) at baseline. After 48 weeks, 37% were overweight/obese, and 3% were underweight. While level of many inflammatory markers decreased 48 weeks after ART initiation in the overall group, the decrease in C-reactive protein (CRP) level was smaller in overweight/obese participants (P = .01), and the decreases in both CRP (P = .01) and interleukin 18 (P = .02) levels were smaller in underweight participants. Each 1-unit gain in BMI among overweight/obese participants was associated with a 0.02-log10 increase in soluble CD14 level (P = .05), while each 1-unit BMI gain among underweight participants was associated with a 9.32-mg/L decrease in CRP level (P = .001).
Conclusions. Being either overweight or underweight at ART initiation was associated with heightened systemic inflammation. While weight gain among overweight/obese persons predicted increased inflammation, weight gain among underweight persons predicted reduced inflammation.
Keywords: immune activation/inflammation, body mass index, HIV/AIDS, HAART clinical outcomes, noncommunicable diseases
Globally, there are 36.9 million persons living with human immunodeficiency virus (HIV), and a growing number are noted to be overweight or obese, paralleling the global epidemic of obesity [1–3]. As shown across multiple study populations, the majority of HIV-infected individuals gain weight with antiretroviral therapy (ART) initiation, with the greatest weight gains among individuals with the lowest CD4+ T-cell counts and highest viral loads [4–7]. Weight gain with ART can reflect a slowing resting energy expenditure with suppressed virus level, as well as an improvement in HIV-associated enteropathy, and often signals a return to health [6]. In some settings, obesity is viewed as an external manifestation of health and prosperity and, thus, a desirable outcome of HIV treatment [8–9]. Continued weight gain over time has resulted in an increased prevalence of obesity among HIV-infected persons in both resource-rich and resource-limited settings [2, 3, 10–13]. There is likely a tipping point at which weight gain begins to have a negative influence. Indeed, a recent analysis within the Veterans Aging Cohort found improved survival with weight gain in the first year of ART among underweight or normal-weight participants but not overweight or obese participants [6]. In contrast, weight loss or failure to gain weight with ART initiation is often a poor prognostic sign and may be a marker of concomitant infections, such as tuberculosis; severe wasting; or metabolic diseases, such as diabetes or adrenal insufficiency [14].
Both obesity and cachexia (or wasting) are associated with chronic inflammation [15], with heightened levels of multiple inflammatory cytokines, including interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and C-reactive protein (CRP) [15–19]. Although levels of inflammatory markers tend to decrease with suppressive ART, HIV infection is also associated with chronic low-level inflammation, with levels of several markers of inflammation or immune activation elevated even after years of therapy, comparison with levels in HIV-uninfected populations [20, 21]. This persistent, low-grade inflammatory state, even in treated HIV infection, has been associated with an increase in multiple non–AIDS-related comorbidities, including cardiovascular disease, cancer, osteoporosis, weakness, frailty, and death [22–27]. Multiple factors likely contribute to ongoing inflammation, including low-level HIV replication, chronic hepatitis B and C, other viral coinfections, microbial translocation, and lifestyle factors [28].
The degree to which a potentially modifiable and easily measurable condition such as change in body weight, particularly within the first year after ART initiation, influences changes in levels of inflammatory markers is unknown. An understanding of the impact of body weight changes on inflammation across diverse settings with a variety of diets and levels of physical activity may be particularly informative to the early recognition of individuals at increased risk for morbidity and mortality. Furthermore, higher baseline body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) and weight change after ART initiation predict CD4+ T-cell count recovery, and changes in levels of inflammatory markers may explain these findings [4–6]. We hypothesized that overweight/obese and underweight patients have a higher risk of persistent immune activation and inflammation as compared to normal-weight patients. The Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS) trial was a randomized international trial of ART initiation with a high proportion of both underweight and overweight participants and a nearly equal proportion of men and women, many with advanced HIV infection [29]. Using the PEARLS trial, we sought to investigate the association between baseline weight and weight gain with ART initiation on changes in markers of inflammation and immune activation.
METHODS
Study Design and Population
We used a random sample of persons who experienced virologic suppression within the AIDS Clinical Trials Group (ACTG) PEARLS trial (ClinicalTrials.gov registration NCT00084136) to address our objective. In brief, PEARLS was a randomized trial of ART initiation in 1571 treatment-naive adults from 9 countries (Brazil, Haiti, India, Malawi, Peru, South Africa, Thailand, United States, and Zimbabwe) [29]. Enrollment occurred between May 2005 and August 2007, and participants were followed through May 2010, with a primary outcome of HIV disease progression. Eligible participants had a CD4+ T-cell count of <300 cells/mm3 and no history of recent acute illness (ie, pneumonia, gastroenteritis, or pelvic inflammatory disease) or opportunistic infections. Participants were randomized to receive one of the following 3 HIV treatment regimens: (1) efavirenz + lamivudine + zidovudine (EFV+3TC+ZDV), (2) efavirenz+emtricitabine+tenofovir ((EFV+FTC+TDF), or (3) atazanavir+didanosine+emtricitabine (ATV+DDI+FTC). Further details of the PEARLS study design, participant characteristics, and the primary results have been previously published [29]. A New Works Concept Sheet (NWCS-319) was developed as a substudy to PEARLS study that had >80% power to assess the effect of inflammatory and nutritional markers on clinical end points. A sample of 270 individuals from PEARLS comprising 30 randomly selected individuals from each country was included in NWCS-319, and these data were used to assess the effect of BMI on inflammatory markers. We excluded 24 participants (8.9%) who did not achieve virologic suppression at weeks 24 and/or 48.
The human experimentation guidelines of the Department of Health and Human Services were followed, and the institutional review board or ethics committees at each site provided approval. Informed consent, including permission to use biological materials, was obtained from all participants.
Laboratory Testing
Serum and plasma specimens were obtained from all participants at weeks 0, 24, and 48 after ART initiation and were stored at −80°C in a repository until the time of testing. Each marker was batch tested at single centralized laboratories [30]. Plasma soluble CD14 (sCD14) and plasma CRP levels were measured using commercially available ELISA kits (R&D Systems, Minneapolis, Minnesota); plasma interferon γ (IFN-γ)–inducible protein 10 (CXCL-10; also referred to as IP-10) was measured using an electrochemiluminescent bridging immunoassay (Meso-Scale Discovery, Gaithersburg, Maryland). Plasma levels of the soluble inflammatory cytokines TNF-α, IL-6, and IFN-γ were measured using the Luminex multiplex cytokine platform (R&D Systems, Minneapolis, Minnesota). IL-18 was performed using ELISA (Platinum ELISA, eBiosciences, San Diego, California).
Analyses
Weight was measured at weeks 0, 24, and 48 and height at week 0. BMI was categorized as underweight (<18.5), normal (18.5–24.9), overweight (≥25–29.9), and obese (≥30) [31]. The overweight and obese categories were combined to increase the sample size for many of the analyses. The demographic covariates were summarized and compared between all categories of BMI. Skewed continuous variables were summarized using medians and interquartile ranges (IQRs) and were compared using a Mann–Whitney test. Nonskewed continuous variables were summarized using means and standard deviations (SDs) and were compared using an unpaired t test. Categorical variables were summarized using frequencies and were compared using a χ2 or Fisher exact test when applicable. Heat maps of the log10-transformed values of markers were designed to illustrate the overall trend of data variation according to baseline BMI status and week since ART initiation. Association between baseline BMI and BMI change from weeks 0 to 48 and longitudinal changes in immune activation and inflammatory markers, with adjustment for age, sex, country, study treatment arm [31], screening CD4+ T-cell count, plasma baseline log10 HIV RNA load, and prevalent tuberculosis, was assessed using random effects models. We further assessed the effect of an obese BMI at weeks 0, 24, or 48 on longitudinal changes in immune activation and inflammatory markers, using random effects models. All analyses were conducted in Stata, version 13.1, and JMP 12.0 (SAS Statistics, Cary, North Carolina).
RESULTS
Of the 246 participants, at baseline, 8% were underweight, 65% had a normal BMI, 20% were overweight, and 7% obese (Figure 1A). As shown in Table 1, baseline differences by country and race were seen: India had the highest proportion of underweight participants (31%), and the United States (58%), Brazil (41%), and South Africa (36%) had the highest proportions of overweight/obese participants (P < .001). None of the underweight participants were white (P = .004). Overweight/obese participants tended to be older (38 vs 35 years; P = .03) and underweight participants tended to have a lower hemoglobin level (10.5 vs 12.4 mg/dL; P = .001) and serum albumin level (3.6 vs 4 mg/mL; P = .01).
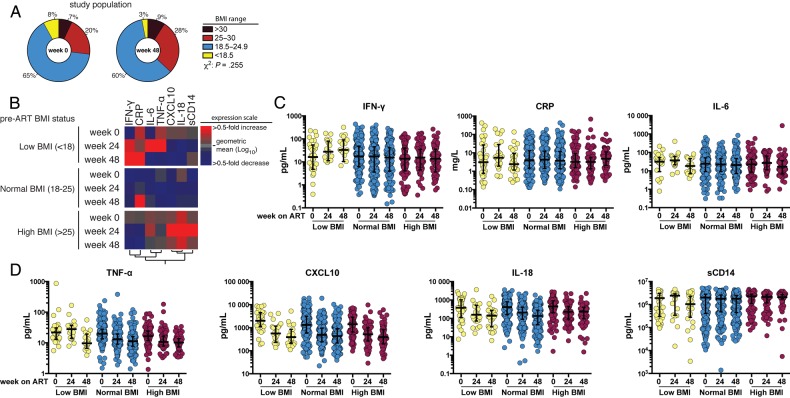
Figure 1.
Body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) distributions and effects of baseline BMI on markers of inflammation and immune activation following antiretroviral (ART) initiation. A, Distribution of the study population according to indicated BMI status at weeks 0 and 48 after ART initiation. B, Heat map depicts the overall pattern of expression of markers of inflammation/immune activation. Participants are grouped in rows by baseline BMI, and differences in markers (columns) are seen across rows. Expression scale for each marker represents log10 fold-change from the geometric mean of the entire study population at each time point, with red hues indicating an increase and blue hues indicating a decrease. C and D, Scatterplots of the markers show the overall trend of variation in concentrations of each marker at weeks 0, 24, and 48 following ART initiation, separated by baseline BMI. The lines represent median values and interquartile ranges. Abbreviations: CRP, C-reactive protein; CXCL-10, interferon γ–inducible protein 10; IFN-γ, interferon γ; IL-6, interleukin 6; IL-18, interleukin 18; sCD14, soluble CD14; TNF-α, tumor necrosis factor α.
Table 1.
Baseline Characteristics, by Body Mass Index (BMI)
| Characteristic | Overall (n = 246) | Underweight (n = 19) | Normal Weight (n = 163) | Overweight/Obese (n = 64) | P Value |
|---|---|---|---|---|---|
| Female sex | 135 (50) | 11 (55) | 86 (48) | 38 (54) | .66 |
| Race | .004 | ||||
| Asian | 61 (23) | 10 (50) | 40 (22) | 11 (15) | |
| Black | 144 (53) | 10 (50) | 96 (54) | 38 (54) | |
| White | 29 (11) | 0 | 15 (8) | 14 (20) | |
| Other | 36 (13) | 0 | 28 (16) | 8 (11) | |
| Age, y | 35 (29–41) | 34 (30–37) | 34 (29–40) | 38 (31–43) | .03 |
| CD4+ T-cell count, cells/µL | 179 (90–229) | 118 (50–223) | 183 (87–227) | 175 (119–240) | .06 |
| Plasma HIV RNA load, log10 copies/mL | 5.07 (4.57–5.47) | 5.28 (5.00–5.58) | 5.07 (4.64–5.46) | 4.91 (4.34–5.57) | .09 |
| Hemoglobin level, mg/dL | 12.4 (10.9–13.8) | 10.5 (9.7–11.6) | 12.5 (11.1–13.8) | 12.5 (11.1–13.9) | .001 |
| Albumin level, mg/mL | 4.0 (3.6–4.3) | 3.6 (3.1–4.0) | 3.9 (3.6–4.2) | 4.1 (3.8–43.0) | .01 |
| ART regimen | .90 | ||||
| EFV + 3TC-ZDV | 99 (37) | 7 (35) | 69 (39) | 23 (32) | |
| EFV + FTC-TDF | 89 (33) | 7 (35) | 56 (31) | 26 (37) | |
| ATV + DDI + FTC | 82 (30) | 6 (30) | 54 (30) | 22 (31) | |
| Country | <.001 | ||||
| Brazil | 29 (12) | 1 (5) | 16 (10) | 12 (19) | |
| Haiti | 30 (12) | 5 (26) | 20 (12) | 5 (8) | |
| India | 26 (11) | 8 (42) | 14 (9) | 4 (6) | |
| Malawi | 26 (11) | 2 (11) | 19 (12) | 5 (8) | |
| Peru | 28 (11) | 0 | 24 (15) | 4 (6) | |
| South Africa | 28 (11) | 0 | 18 (11) | 10 (16) | |
| Thailand | 28 (11) | 1 (5) | 21 (13) | 6 (9) | |
| United States | 24 (10) | 0 (0) | 10 (6) | 14 (22) | |
| Zimbabwe | 27 (11) | 2 (11) | 21 (13) | 4 (6) |
Data are no. (%) of subjects or median value (interquartile range). BMI was calculated as the weight in kilograms divided by the height in meters squared and categorized as underweight (<18.5), normal (18.5–24.9), overweight (>25–29.9), and obese (≥30) [31].
Abbreviations: ART, antiretroviral treatment; ATV, atazanavir; DDI, didanosine; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus type 1; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine; 3TC, lamivudine.
Baseline BMI and Levels of Inflammatory Markers at Weeks 0, 24, and 48
At week 0 of ART, levels of the inflammatory markers IL-6, INF-γ, CXCL-10, IL-18, and sCD14 were comparable between participants who were either underweight, had a normal BMI, or were overweight/obese, with the exception of baseline TNF-α level (Supplementary Table 1). Compared with participants with a normal BMI at baseline, baseline TNF-α levels were significantly higher among underweight participants and lower among overweight or obese participants (20.6 pg/mL vs 24.6 pg/mL vs 15.9 pg/mL; P = .005).
At weeks 24 and 48 after ART initiation, TNF-α level no longer differed by BMI categories, and levels of the inflammatory markers IL-6, INF-γ, CXCL-10, IL-18, and sCD14 remained comparable between BMI categories, with the exception of week 48 CRP level (Supplementary Table 1). At week 48 of ART, compared with participants with a normal BMI, the CRP levels were significantly higher among participants who were overweight/obese at baseline and significantly lower among those who were underweight at baseline (2.61 mg/L vs 5.70 mg/L vs 1.68 mg/L; P = .003).
Baseline BMI and Change in Levels of Inflammatory Markers Over 48 Weeks
Figures 1B–D illustrate the changes in all tested markers of inflammation and immune activation after ART initiation, by baseline BMI status. Among all participants, compared with baseline immune markers, TNF-α (P < .001), CXCL-10 (P < .001), and IL-18 (P < .001) levels decreased significantly over 48 weeks. INF-γ, CRP, IL-6, and sCD14 levels did not change significantly.
Among the normal BMI group, compared with baseline immune markers, CXCL-10 (P < .001) and IL-18 (P < .001) levels decreased significantly over 48 weeks; changes in TNF-α, IFN-γ, IL-6, sCD14, and CRP levels, however, did not meet statistical significance. Among individuals in the underweight group, compared with baseline levels, TNF-α (P < .001) and CXCL-10 (P < .001) levels decreased over 48 weeks, while levels of other markers did not change significantly. In the overweight/obese group, TNF-α (P < .001), CXCL-10 (P < .001), and IL-18 (P < .001) levels decreased; IL-6, CRP, sCD14, and IFN-γ levels did not change significantly.
We next assessed the association between baseline BMI and levels of immune markers, using a random effects model adjusted for age, sex, country, study treatment arm, screening CD4+ T-cell count, baseline plasma log10 HIV RNA load, and prevalent tuberculosis. In these adjusted analyses (Table 2), compared with individuals of normal weight, the decrease in IL-6, TNF-α, IFN-γ, CXCL-10, IL-18, and CRP levels over 48 weeks was smaller in participants who were overweight/obese at baseline; this only reached statistical significance for CRP (P = .01). Similarly, CRP (P = .02) and IL-18 (P = .02) levels declined significantly among those who were underweight at baseline (Table 2).
Table 2.
Association Between Baseline Body Mass Index (BMI) on Longitudinal Inflammatory and Immune Activation Markers
| Marker | Univariable Analysis |
Multivariable Analysisa |
||
|---|---|---|---|---|
| Difference,b Mean (95% CI) | P Value | Difference,b Mean (95% CI) | P Value | |
| IL-6 level in pg/mL | ||||
| Normal weight | Reference | Reference | ||
| Underweight | 5.8 (−17.8 to 29.3) | .63 | 14.2 (−9.5 to 37.9) | .24 |
| Overweight | −1.5 (−26.2 to 23.2) | .91 | −0.2 (−25.7 to 25.4) | .99 |
| TNF-α level in pg/mL | ||||
| Normal weight | Reference | Reference | ||
| Underweight | −1.5 (−6.6 to 3.6) | .57 | 0.9 (−4.0 to 5.9) | .71 |
| Overweight | −2.0 (−7.3 to 3.4) | .47 | −0.3 (−5.7 to 5.0) | .90 |
| INF-γ level in pg/mL | ||||
| Normal weight | Reference | Reference | ||
| Underweight | −23.1 (−43.1 to −2.9) | .02 | −6.9 (−26.0 to 12.2) | .48 |
| Overweight | −35.1 (−56.1 to −13.9) | .001 | −17.4 (−37.9 to 3.3) | .10 |
| CXCL-10 level in pg/mL | ||||
| Normal weight | Reference | Reference | ||
| Underweight | 52.4 (−525.6 to 630.4) | .86 | 82.3 (−496.3 to 661.0) | .78 |
| Overweight | 12.9 (−593.8 to 619.8) | .97 | −94.4 (−718.5 to 529.7) | .77 |
| IL-18 level in pg/mL | ||||
| Normal weight | Reference | Reference | ||
| Underweight | −172.9 (−314.3 to −31.5) | .02 | −175.4 (−321.0 to −29.9) | .02 |
| Overweight | −114.5 (−263.8 to 34.8) | .13 | −137.8 (−295.1 to 19.6) | .09 |
| CRP level in mg/L | ||||
| Normal weight | Reference | Reference | ||
| Underweight | −10.1 (−21.2 to .9) | .07 | −11.3 (−19.6 to −3.0) | .01 |
| Overweight | −6.7 (−18.4 to 5.0) | .26 | −10.4 (−19.5 to −1.4) | .02 |
| Log10 sCD14 level in pg/mL | ||||
| Normal weight | Reference | Reference | ||
| Underweight | 0.1 (−.02 to .3) | .10 | 0.1 (−.2 to .3) | .49 |
| Overweight | 0.3 (.2–.5) | <.001 | 0.2 (−.04 to .5) | .09 |
BMI was calculated as the weight in kilograms divided by the height in meters squared and categorized as underweight (<18.5), normal (18.5–24.9), overweight (>25–29.9), and obese (≥30) [31].
Abbreviations: CI, confidence interval; CRP, C-reactive protein; CXCL-10, interferon γ–inducible protein 10; HIV, human immunodeficiency virus; IFN-γ, interferon γ; IL-6, interleukin 6; IL-18, interleukin 18; sCD14, soluble CD14; TNF-α, tumor necrosis factor α.
a Longitudinal markers over 48 weeks were modeled using a random effects model. The multivariable model was adjusted for age, sex, country, study treatment arm, screening CD4+ T-cell count, baseline log10 HIV RNA load, and prevalent tuberculosis.
b Over 48 weeks.
BMI Change and Change in Levels of Inflammatory Markers Over 48 Weeks
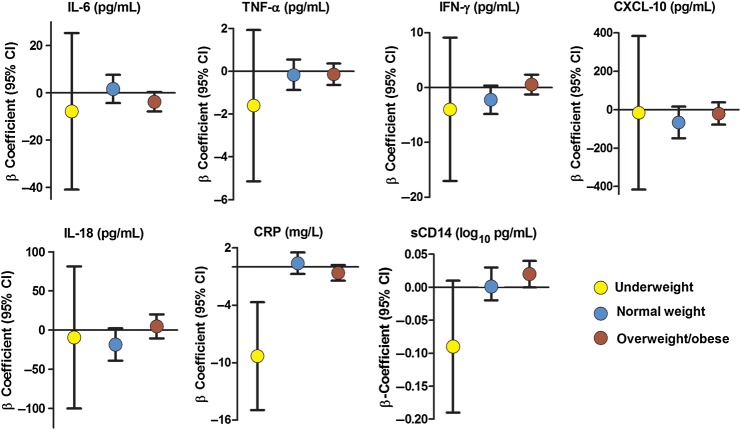
As shown in Figure 1A, between week 0 and week 48 of ART, the proportion of underweight participants decreased >2-fold from 8% to 3%, and the proportion of overweight/obese participants increased from 27% to 37%. A median 14% increase in BMI was observed among underweight participants, compared with a median 5% increase in both normal and overweight groups. We next examined the impact of this change in BMI from week 0 to week 48 on the change in inflammatory markers. When adjusted for sex, age, country, screening CD4+ T-cell count, baseline log10 plasma HIV RNA load, and study treatment arm in multivariable analyses, among obese or overweight participants, each further 1-unit increase in BMI was associated with an increased sCD14 level (β=0.02; P = .05; Figure 2). In contrast, among underweight participants, each incremental increase in BMI was associated with a decreased CRP level (β = −9.32 mg/L; P = .001). Similar trends were seen among several of the other inflammation/immune activation markers, but none reached statistical significance (P > .05 for all comparisons; Figure 2 and Supplementary Table 2).
Figure 2.
Change in immune activation markers over 48 weeks by change in body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) and antiretroviral treatment initiation. β-coefficients represent the incremental change in each inflammatory marker per increase in BMI. The underweight group is indicated by yellow, normal weight by blue, and overweight/obese groups by red. All analyses are adjusted for age, sex, country, study treatment arm, screening CD4+ T-cell count, plasma baseline log10 human immunodeficiency virus type 1 RNA load, and prevalent tuberculosis. Abbreviations: CI, confidence interval; CRP, C-reactive protein; CXCL-10, interferon γ–inducible protein 10; IFN-γ, interferon γ; IL-6, interleukin 6; IL-18, interleukin 18; sCD14, soluble CD14; TNF-α, tumor necrosis factor α.
Association Between Obesity and Change in Levels of Inflammatory Markers
Last, we investigated the impact of an obese BMI (vs a BMI <30) at baseline, week 24, or week 48 on baseline and change in inflammatory markers (Table 3). In multivariable analyses, having an obese BMI at any time point on the study was associated with a 0.19 log10 pg/mL greater sCD14 level, compared with participants who were never obese (P = .02). No significant associations were detected between obesity and other inflammatory markers.
Table 3.
Findings of Unadjusted and Adjusted Random Effects Models of the Effects of Obesity at Weeks 0, 24, or 48 on the Change in Levels of Inflammatory or Immune Activation Markers
| Biomarker | Unadjusted Analysis |
Adjusted Analysisa |
||
|---|---|---|---|---|
| Slope (95% CI) | P Value | Slope (95% CI) | P Value | |
| IL-6 level in pg/mL | −12.8 (−52.8 to 27.2) | .53 | −23.4 (−65.2 to 18.4) | .27 |
| TNF-α level in pg/mL | −1.3 (−6.2 to 3.5) | .60 | −1.6 (−6.5 to 3.3) | .52 |
| IFN-γ level in pg/mL | −1.5 (−19.8 to 16.7) | .87 | 4.8 (−12.9 to 22.5) | .59 |
| CXCL-10 level in pg/mL | −57.8 (−593.0 to 477.4) | .83 | −203.3 (−756.0 to 349.5) | .47 |
| IL-18 level in pg/mL | 131.0 (2.3–259.7) | .05 | 127.6 (−3.4 to 258.7) | .05 |
| CRP level in mg/L | −5.7 (−17.7 to 6.3) | .35 | −5.5 (−13.6 to 2.6) | .18 |
| Log10 sCD14 level in pg/mL | 0.2 (.05–.4) | .009 | 0.2 (.04–.4) | .02 |
Obesity was defined as a body mass index (calculated as the weight in kilograms divided by the height in meters squared) of >30.
Abbreviations: CI, confidence interval; CRP, C-reactive protein; CXCL-10, interferon γ–inducible protein 10; HIV, human immunodeficiency virus; IFN-γ, interferon γ; IL-6, interleukin 6; IL-18, interleukin 18; sCD14, soluble CD14; TNF-α, tumor necrosis factor α.
a Adjusted for age, sex, country, study treatment arm, screening CD4+ T-cell count, baseline log10 HIV RNA load, and prevalent tuberculosis.
DISCUSSION
Within the context of a randomized trial of ART initiation, we have uncovered important differences in the inflammatory/immune activation response to ART based on both the BMI prior to ART initiation and the BMI changes experienced in the first 48 weeks of ART. Similar to data from high-income settings [20, 21], we observed a decrease in many markers of inflammation and immune activation among participants in low- and middle-income settings after ART initiation. However, the declines in levels of some key markers were not as steep among either baseline underweight or overweight/obese participants. In addition, we observed that further weight gain among overweight and obese participants after ART initiation was associated with an increase in sCD14 level despite adequate virologic suppression, while weight gain among underweight participants was associated with a greater decrease in CRP level. Interestingly, a recent study from the Veterans Aging Cohort demonstrated a reduced mortality with weight gain among underweight patients but not overweight or obese patients in the first year after ART initiation [6]. Furthermore, the study found that each 1-unit gain in BMI among participants with normal BMI resulted in an increase of approximately 18%–20% in the risk of cardiovascular events [33]. These observed associations between clinical events and weight gain with ART initiation emphasize the clinical importance of both the pre-ART BMI and the changes in BMI with ART initiation and support a potential mechanistic link with heightened inflammation.
Another notable finding from our study is that over one third of our participants were overweight or obese after 48 weeks of ART. While the subset in this analysis may not be a truly representative sample from the participating countries, we found a relatively high prevalence of overweight or obese participants (39%) from Brazil and South Africa, while <20% of participants from India, Thailand, and Malawi were overweight or obese. With improved economic conditions, dynamic nutrition transitions to a diet higher in fat and refined carbohydrates, combined with less daily physical activity, obesity is becoming a worldwide public health problem, with the number of overweight individuals exceeding with the number who are underweight [34].
Why levels of sCD14, IL-18, and CRP were elevated in HIV-infected overweight/obese patients instead of IL-6 and TNF-α, as typically seen among HIV-uninfected obese patients, is an intriguing observation that deserves further investigation. It may be that adipocytes that appear to function differently in HIV-infected persons, ongoing microbial translocation, or ongoing coinfections could also account for differences in inflammatory profiles [15, 32, 35, 45, 47]. Notably, a similar association between obesity and both CRP level and sCD14 level has been observed among HIV-infected US adults [47].
sCD14 has been linked to cardiovascular disease risk and mortality among HIV-infected adults [35–38]; thus, the further increase in sCD14 in the overweight/obese groups is of particular relevance. Importantly, some studies have suggested that the level of sCD14 is relatively resistant to therapeutic interventions [28, 39, 40]. Following weight-reduction surgery among HIV-uninfected persons, a significant decrease in sCD14 level was only observed among those with marked weight loss of >30% [41]. Similarly, although sCD14 level was significantly lower among HIV-uninfected men as compared to HIV-infected men, little difference was seen between participants who were and those who were not receiving effective ART [21]. Both IL-18 and CRP have been associated with cardiovascular disease, obesity, and mortality [27, 34, 42–45]. As nonspecific markers of inflammation, elevated CRP and IL-18 levels among underweight participants at baseline may have been markers of coinfection [34, 46]; nearly all underweight participants were enrolled from sites in India or Africa, where the incidence of tuberculosis is among the highest in the world. Similarly, the significant decrease in CRP level among underweight participants who gained weight was suggestive of a return to health. Although weight gain among overweight participants did not exacerbate CRP or IL-18 levels, the lack of reduction despite effective ART may imply an ongoing, heightened cardiovascular disease risk. Importantly, previous studies have demonstrated that similar magnitude of change in sCD14, CRP, and IL-18 levels observed in our study was independently associated with increased mortality, clinical failure, and tuberculosis-associated immune reconstitution inflammatory syndrome, underscoring the clinical relevance of our findings [34, 46, 48].
Our study does have limitations. First, considering the diversity of the population, the sample size is relatively small and limits the number of analyses and adjustments we could perform. Similarly, the proportions of underweight and overweight/obese participants were small and limited the power to detect some associations. Dual-energy absorptiometry or computed tomography findings were not available to further delineate whether changes in BMI were due to changes in subcutaneous fat, visceral fat, or muscle. The distribution of weight gain among these different compartments would be expected to have differing effects on the inflammatory profile. Analyses were not corrected for multiple comparisons; thus, given the marginally significant P values for many comparisons, our findings should be considered exploratory. Although we adjusted for country, we were unable to account for between-country differences in nutrition that may have played a role in weight and inflammatory changes. Last, we did not have measures of low-level HIV replication, chronic hepatitis B virus and hepatitis C virus infection, physical activity, or dietary intake to provide further insight into mechanisms for the weight changes. Despite these limitations, the multiple inflammatory markers before and after randomized ART initiation in a population with diverse ethnic, racial, lifestyle habits, and resources, balanced by sex, adds to the clinical relevance of these data and the generalizability to multiple settings.
In summary, our findings highlight the potential prognostic value in monitoring body weight in the course of ART. Although not applicable to all settings, an inability to gain weight among underweight persons may be a poor prognostic sign and signal a need for nutritional intervention, a need for evaluation of disease progression, or development of an opportunistic infection. In contrast, among those who are already overweight or obese, further weight gain appears to increase inflammation (notably the sCD14 level). Further research is needed to understand the potential barriers to weight maintenance and to test models for effective early nutritional counseling and lifestyle modifications as adjunctive therapy to ART.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank study coordinators, study team members, site staff, and participants, for their participation; and Veronique Franco and Sara Wendel, for their assistance in performing the sCD14 assays.
V. M., K. M. E., N. G., and A. G. conceived and drafted the manuscript. D. M. A., T. B. C., L. S., N. K., J. H., B. S., C. R., M. C. H., P. S., R. I., S. P., S. W. C., S. T., N. M., and S. B. implemented the parent study and collected data and assisted manuscript preparation. N. G., V. M., K. M. E., B. B. A., and A. G. led the data analyses. A. B., D. L. T., and R. C. B. assisted manuscript preparation. A. G. obtained funding for the study.
Financial support. This work was supported by the Statistical and Data Management Center, AIDS Clinical Trials Group (under National Institute of Allergy and Infectious Diseases [NIAID] grant 1 U01 AI068634); the NIAID (grants AI68636 and AI069450; and grant R01 AI45462 to A. G.) and National Institute on Aging (NIA K23 AG050260 to K. M. E.), National Institutes of Health (NIH); the Johns Hopkins University Center for AIDS Research (NIH/NIAID fund 1P30AI094189-01A1 to V. M. and A. G.): the NIAID grant to A. B. (Ro1 DA 016078); Boehringer-Ingelheim; Bristol-Myers Squibb; Gilead Sciences; and GlaxoSmithKline.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization: 2015 Global Health Observatory data. http://www.who.int/gho/hiv/en/ Accessed 8 March 2015.
- 2.Crum-Cianflone N, Roediger MP, Eberly L et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS 2000; 22:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shikuma CM, Zackin R, Sattler F et al. Changes in weight and lean body mass during highly active antiretroviral therapy. Clin Infect Dis 2004; 39:1223–30. [DOI] [PubMed] [Google Scholar]
- 5.Erlandson KM, Kitch D, Tierney C et al. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013; 27:2069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuh B, Tate J, Butt AA et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS 2003; 17:971–9. [DOI] [PubMed] [Google Scholar]
- 8.Hurley E, Coutsoudis A, Giddy J, Knight SE, Loots E, Esterhuizen TM. Weight evolution and perceptions of adults living with HIV following initiation of antiretroviral therapy in a South African urban setting. S Afr Med J 2011; 101:645–50. [PubMed] [Google Scholar]
- 9.Maia Leite LH, De Mattos Marinho Sampaio AB. Progression to overweight, obesity and associated factors after antiretroviral therapy initiation among Brazilian persons with HIV/AIDS. Nutr Hosp 2010; 25:635–40. [PubMed] [Google Scholar]
- 10.Boodram B, Plankey MW, Cox C et al. Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the Women's Interagency HIV Study. AIDS Patient Care STDS 2009; 23:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchacz K, Baker RK, Palella FJ Jr. et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther 2013; 18:65–75. [DOI] [PubMed] [Google Scholar]
- 12.Wand H, Ramjee G. High prevalence of obesity among women who enrolled in HIV prevention trials in KwaZulu-Natal, South Africa: healthy diet and life style messages should be integrated into HIV prevention programs. BMC Public Health 2013; 13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontbonne A, Cournil A, Cames C et al. Anthropometric characteristics and cardiometabolic risk factors in a sample of urban-dwelling adults in Senegal. Diabetes Metab 2011; 37:52–8. [DOI] [PubMed] [Google Scholar]
- 14.Sudfeld CR, Isanaka S, Mugusi FM et al. Weight change at 1 mo of antiretroviral therapy and its association with subsequent mortality, morbidity, and CD4T cell reconstitution in a Tanzanian HIV-infected adult cohort. Am J Clin Nutr 2013; 97:1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6:772–83. [DOI] [PubMed] [Google Scholar]
- 16.Lihn AS, Richelsen B, Pedersen SB et al. Increased expression of TNF-alpha, IL-6, and IL-8 in HALS: implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab 2003; 285:E1072–80. [DOI] [PubMed] [Google Scholar]
- 17.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007; 92:1023–33. [DOI] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seelaender MC, Batista ML. Adipose tissue inflammation and cancer cachexia: the role of steroid hormones. Horm Mol Biol Clin Investig 2014; 17:5–12. [DOI] [PubMed] [Google Scholar]
- 20.De Pablo-Bernal RS, Ruiz-Mateos E, Rosado I et al. TNF-alpha levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J Antimicrob Chemother 2014; 69:3041–6. [DOI] [PubMed] [Google Scholar]
- 21.Wada NI, Jacobson LP, Margolick JB et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenorio AR, Zheng Y, Bosch RJ et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tien PC, Choi AI, Zolopa AR et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010; 55:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33:2244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford KW, Li X, Xu X et al. Lipodystrophy and inflammation predict later grip strength in HIV-infected men: the MACS Body Composition substudy. AIDS Res Hum Retroviruses 2013; 29:1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erlandson KM, Allshouse AA, Jankowski CM et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013; 208:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuller LH, Tracy R, Belloso W et al. Inflammatory and coagulation markers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erlandson KM, Campbell TB. Inflammation in chronic HIV infection: what can we do? J Infect Dis 2015; 212:339–42. [DOI] [PubMed] [Google Scholar]
- 29.Campbell TB, Smeaton LM, Kumarasamy N et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenforde MW, Gupte N, Dowdy DW et al. C-reactive protein (CRP), interferon gamma-inducible protein 10 (IP-10), and lipopolysaccharide (LPS) are associated with risk of tuberculosis after initiation of antiretroviral therapy in resource-limited settings. PLoS One 2015; 10:e0117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NHLBI Obesity Education Initiative. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. NIH publication no. 98-4083 Bethesda, MD: Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute, 1998. [Google Scholar]
- 32.Erlandson KM, Taejaroenkul S, Smeaton L, Gupta A, Singini IL et al. A Randomized Comparison of Anthropomorphic Changes With Preferred and Alternative Efavirenz-Based Antiretroviral Regimens in Diverse Multinational Settings. Open Forum Infect Dis 2015; 2:ofv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achhra AC, Mocroft A, Reiss P et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med 2015. In press. [DOI] [PubMed] [Google Scholar]
- 34.Imamura F, Micha R, Khatibzadeh S et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health 2015; 3:e132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koethe JR, Blevins M, Nyirenda C et al. Nutrition and inflammation serum markers are associated with 12-week mortality among malnourished adults initiating antiretroviral therapy in Zambia. J Int AIDS Soc 2011; 14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longenecker CT, Jiang Y, Orringer CE et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funderburg NT, Zidar DA, Shive C et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120:4599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva EF, Charreau I, Gourmel B et al. Decreases in inflammatory and coagulation markers levels in HIV-infected patients switching from enfuvirtide to raltegravir: ANRS 138 substudy. J Infect Dis 2013; 208:892–7. [DOI] [PubMed] [Google Scholar]
- 40.Lake JE, McComsey GA, Hulgan T et al. Switch to raltegravir decreases soluble CD14 in virologically suppressed overweight women: the Women, Integrase and Fat Accumulation Trial. HIV Med 2014; 15:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manco M, Fernandez-Real JM, Equitani F et al. Effect of massive weight loss on inflammatory adipocytokines and the innate immune system in morbidly obese women. J Clin Endocrinol Metab 2007; 92:483–90. [DOI] [PubMed] [Google Scholar]
- 42.Redd AD, Eaton KP, Kong X et al. C-reactive protein levels increase during HIV-1 disease progression in Rakai, Uganda, despite the absence of microbial translocation. J Acquir Immune Defic Syndr 2010; 54:556–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulware DR, Hullsiek KH, Puronen CE et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilarrasa N, Vendrell J, Sanchez-Santos R et al. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-alpha receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol 2007; 67:679–86. [DOI] [PubMed] [Google Scholar]
- 45.Vilarrasa N, Vendrell J, Maravall J et al. IL-18: relationship with anthropometry, body composition parameters, leptin and arterial hypertension. Horm Metab Res 2006; 38:507–12. [DOI] [PubMed] [Google Scholar]
- 46.Tan HY, Yong YK, Andrade BB et al. Plasma interleukin-18 levels are a biomarker of innate immune responses that predict and characterize tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2015; 29:421–31. [DOI] [PubMed] [Google Scholar]
- 47.Conley LJ, Bush TJ, Rupert AW et al. Obesity is associated with greater inflammation and monocyte activation among HIV-infected adults receiving antiretroviral therapy. AIDS 2015; 29:2201–7. [DOI] [PubMed] [Google Scholar]
- 48.Shivakoti R, Yang WT, Berendes S, Mwelase N et al. Persistently elevated C-reactive protein level in the first year of antiretroviral therapy, despite virologic suppression, is associated with HIV disease progression in resource-constrained settings. J Infect Dis 2016; 213:1074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.