Abstract
Background. Dengue viruses (DENV-1–4) pose a transfusion-transmission risk. This study estimated the dengue RNA detection period in asymptomatic blood donors and relationships between donor viremia and dengue incidence during a large epidemic.
Methods. Donor samples from the 2012 dengue transmission season in Rio de Janeiro, Brazil, were tested for DENV RNA by a transcription-mediated amplification (TMA) assay, with DENV types and viral loads determined by polymerase chain reaction. Samples collected during the first and last weeks of enrollment were tested for DENV immunoglobulin (Ig) G and IgM to estimate incidence during the study period, which was analyzed relative to nucleic acid amplification technology (NAT) yield to estimate the duration of NAT-detectable viremia and compared with reported clinical dengue cases in Rio.
Results. Samples from 16 241 donations were tested; 87 (0.54%) were confirmed as DENV-4 RNA positive. Dengue IgM-positive/IgG-positive reactivity increased from 2.8% to 8.8%, indicating a 6.2% incidence (95% confidence interval [CI], 3.2%–9.1%) during the study period. Based on these data, we estimated a 9.1-day period (95% CI, 4.4–13.9 days) of RNA detectable with TMA. With 100 475 reported cases of clinical dengue, 1 RNA-positive donation was identified per 800 DENV cases.
Conclusions. These parameters allow projections of dengue incidence from donor NAT yield data and vice versa, and suggest that viremic donations will be rare relative to clinical disease cases.
Keywords: dengue, transfusion-transmission, NAT, clinical symptoms
There has been a marked global reemergence of dengue in the last 25 years, with recent epidemics in >100 countries [1–3]. The 4 antigenically related dengue viruses (DENV-1–4) cause similar disease manifestations ranging from asymptomatic infection to dengue fever, consisting of fever plus ≥2 other concurrent symptoms, to severe dengue hemorrhagic fever and dengue shock syndrome [4, 5]. Although there is extensive cross-reactivity in serological tests, there is no long-term cross-protective immunity; hence, humans can have as many as 4 lifetime DENV infections, with evidence for increased risk of serious disease with secondary and subsequent infections [6, 7].
Concern over potential transfusion-transmission (TT) of dengue and other arboviruses increased after documentation of TT of West Nile virus (WNV) in US epidemics beginning in 2003 [8, 9]. The first case of probable TT of DENV was documented in 3 recipients in Hong Kong in 2002, and in 2008 transmission to 3 recipients was reported in Singapore [10, 11]. More recently, a case of dengue hemorrhagic fever was reported in a recipient of an RNA-positive transfusion identified through a look-back study in Puerto Rico [12], 2 cases of TT dengue infection were described in Brazil and Singapore [13, 14], and an additional case of probable TT dengue was reported from Puerto Rico [15]. Studies of blood donor samples from multiple endemic countries using nucleic acid amplification technology (NAT) assays have documented rates of viremia as high as 0.5% during outbreaks [12, 15–18], providing quantitative data on the potential magnitude of risk of TT infection and further highlighting concerns over blood safety [8, 9, 19, 20].
Brazil is the country with the largest number of dengue cases reported annually in the Americas [21–23]. We recently conducted a linked blood donor and transfusion recipient study in 2 cities in Brazil (Rio de Janeiro and Recife) to investigate rates of transmission by DENV RNA-positive donations and clinical symptoms of dengue infection in the transfused population during the first large-scale DENV-4 outbreak since its reintroduction to Brazil in 2010 [24]. We identified 6 probable cases of asymptomatic transfusion acquired infection after transfusions of RNA-positive blood components into 16 susceptible recipients (37.5% rate of transmission; 95% confidence interval [CI], 15.2%–64.6%). The current supplemental analyses were executed on the donor samples from that study that were collected in Rio de Janeiro (Rio) to estimate how long dengue RNA is detectable in asymptomatic blood donors and the relationships between detection of donor viremia, the incidence of asymptomatic dengue infections in blood donors, and the clinical case reports of dengue disease in the Rio population overall and the subset of the population eligible to donate blood. Characterizing these parameters provides the capacity to project dengue infection incidence from donor NAT-positive donation data and vice versa, as well as estimating the proportion of infections that result in reported dengue disease and the predicted rates and timing of detection of viremic blood donations relative to clinical disease case reports during epidemics.
METHODS
Ethical committees in Brazil and institutional review boards in the United States approved this study. From 15 February through 14 June 2012, all donors from HemoRio, the major public blood bank in the city of Rio de Janeiro, were asked to participate in the study, and an extra Plasma Preparation Tube (Becton Dickinson) was collected from each consenting donor. The tubes were centrifuged within 6 hours of collection and frozen at −20°C for subsequent aliquoting of plasma in Brazil and batch testing for DENV RNA and antibodies in the United States.
One plasma aliquot from each consenting donor was tested using a transcription-mediated amplification (TMA) assay (Hologic/Grifols) with a 50% detection limit of approximately 5 RNA copies/mL for each of the 4 DENVs [17]. For TMA repeat-reactive samples, the second plasma aliquot was tested at a separate laboratory (Blood Systems Research Institute) with real-time polymerase chain reaction assays to confirm infection, define DENV type, and estimate viral load [17]. Plasma samples from DENV confirmed RNA-positive donations were also tested for DENV immunoglobulin (Ig) M and IgG antibodies (Focus Diagnostics).
Samples from the first 500 sequential TMA-nonreactive donations collected at the beginning of the study period (15 February through 5 March) and the last 500 sequential samples from the end of the study period (8–14 June) were tested for DENV IgG and IgM antibodies (Focus Diagnostics) to determine the background rate of DENV exposure in the donor population and to estimate the incidence of IgM and IgG seroconversion during the study accrual period. All IgM-positive/IgG-positive (n = 58) and 50 representative IgM-negative/IgG-positive samples were characterized for DENV-1-4 type-specific neutralizing activity using reporter viral particles (Integral Molecular) [25].
Based on the estimate for incidence of infection during the study period derived from the serosurveys and the NAT screening results (proportion RNA-positive donations) during the study period, we derived an estimate for the duration of viremia detectable by the DENV TMA assay, as follows: If PE is the prevalence of IgM-positive cases at the start of the observation interval (weeks 7–10 in 2012) and PL is the prevalence of IgM-positive cases at the end of the observation interval (weeks 23 and 24), then the probability of becoming IgM positive during the observation interval is (PL–PE)/(1–PE). If Ti is the proportion TMA-positive for dengue in week i (11 ≤ i ≤ 23), then the TMA detection interval of DENV RNA (also referred to as the detectable window period, W) is estimated as
Confidence limits for W were estimated using a delta method estimate of the variance of W [26].
The number of cases of clinical dengue reported in the Rio metropolitan area were obtained from the public health reporting system (Coordenação Geral do Programa Nacional de Controle da Dengue). The weekly case rate was calculated as total reported cases in a week divided by the size of the Rio population. The adjusted case rate was calculated by limiting the data to the age range of blood donors (16–67 years), as follows. Cases in Rio were reported to the public health system in 5-year age intervals. To obtain the case rate for those 16–67 years old, we estimated that 80% of the case patients and 80% of the Rio population in the 15–19-year age interval were 16–19 years old, and 60% of those in the 65–69-year interval were 65–67 years old. The weekly total and adjusted case rates were plotted against week of the year for comparison with the weekly rates of TMA-positive blood donations.
RESULTS
Detection and Characterization of Dengue RNA-Positive Donations During the 2012 Rio Epidemic
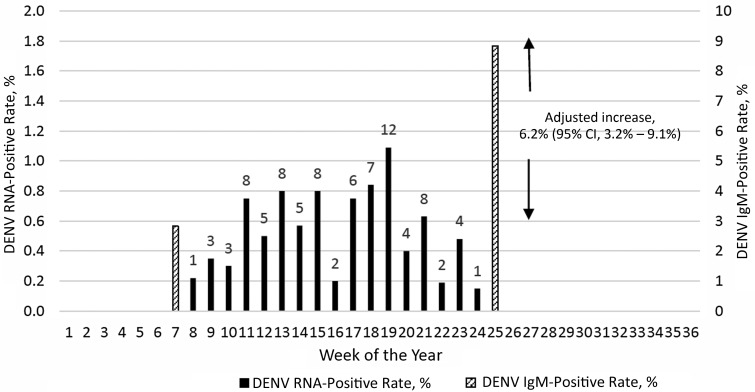
Of 23 412 donations collected by HemoRio during the study period, 16 241 donors (69%) consented to DENV RNA testing. These were tested by DENV TMA, and 87 (0.54%) were repeat reactive. All 87 TMA-reactive samples were confirmed as DENV RNA positive by polymerase chain reaction on an independent sample, and all were typed as DENV-4. Figure 1 displays the rate of detection of viremic donations over time. Of the 87 viremic donations, 11 (12.6%) tested positive for DENV IgM. Viral loads were significantly higher in IgM-negative than in IgM-positive donations (median pre-IgM and post-IgM viral loads were 3.5 and 2.3 Log10 copies/mL, respectively; P = .001).
Figure 1.
Proportions of dengue virus (DENV) transcription-mediated amplification (TMA) repeat-reactive donations during the study period (solid bars; left Y axis) and of DENV immunoglobulin (Ig) M–positive/IgG-positive donations at the beginning and end of the study period (hatched bars; right Y axis). All TMA repeat-reactive donations were confirmed as RNA positive by polymerase chain reaction testing and typed as DENV-4. Abbreviation: CI, confidence interval.
Serosurvey to Estimate Incidence of DENV Infection and the Individual Donation-NAT Detection Period
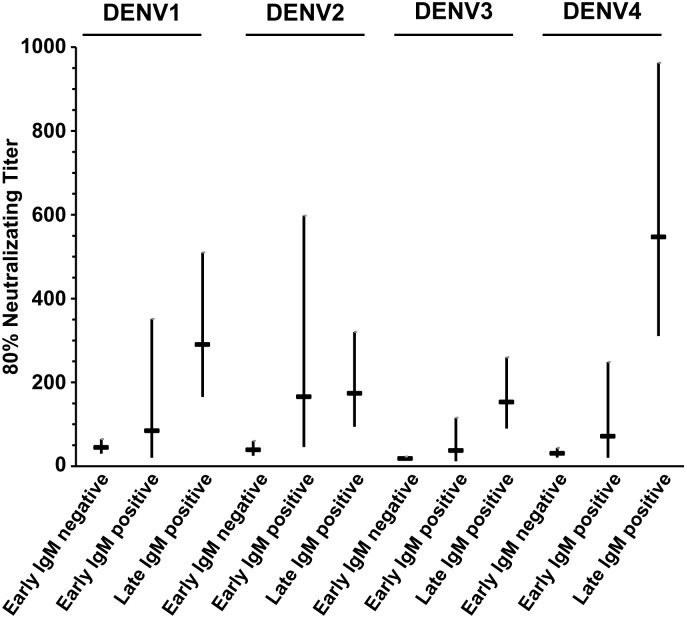
Table 1 summarizes the results of DENV IgG and IgM testing performed on approximately 500 consecutive early and late epidemic samples from consenting donors (several samples from each 2-week period did not yield valid antibody results). Dengue IgG-positive/IgM-positive reactivity increased from 2.8% of 494 donations in the early study period to 8.8% of 498 donations late in the epidemic; IgG reactivity also increased from 88% to 90%, indicating a very high rate of prior infection with dengue in the Rio population with only a small increment of primary infections during the 2012 epidemic. Analysis of the increase in the IgM positivity rate—[100(8.8 − 2.8)/(100 − 2.8)]—indicated that the incidence of DENV infection in the Rio blood donor population during the study period was approximately 6.2% (95% CI, 3.2%–9.1%), as illustrated in Figure 1. Reporter viral particle neutralization data of seroreactive donations demonstrated typical high-level cross-reactivity to all 4 DENVs early in the epidemic in IgG-positive/IgM-negative and IgG-positive/IgM-positive donations. The results from the late epidemic IgM-positive/IgG-positive samples indicated boosting of neutralizing activity to DENV-4 to a significantly higher level than the neutralizing antibodies to the other 3 DENVs, indicative of recent DENV-4 infection (Figure 2), consistent with detection of only DENV-4 in the RNA-positive donor samples. Based on statistical correlation of the incidence during the study period derived from the increase in IgM-reactivity and the temporal yield of DENV RNA-positive donations by NAT screening in Rio during the intervening study period (see Methods and Figure 1), we estimated a 9.1-day period (95% CI, 4.4–13.9 days) of detectable RNA with the TMA assay applied to individual donation samples.
Table 1.
Results of DENV IgG and IgM Antibody Testing of Early and Late Epidemic Samples From Blood Donors During the 2012 Rio de Janeiro Epidemica
| Samples | IgG Positive, No. (%) | IgM Positive, No. (%) |
|---|---|---|
| Early epidemic samples (n = 494) | 438 (88.7) | 14 (2.8) |
| Late epidemic samples (n = 498) | 453 (90.9) | 44 (8.8) |
Abbreviations: DENV, dengue virus; IgG, immunoglobulin G; IgM immunoglobulin M.
a Early epidemic samples were collected from 15 February to 5 March 2012; late epidemic samples, from 8 June to 14 June 2012. The adjusted seasonal increase in IgM-positive samples was 6.2% (95% confidence interval, 3.2%–9.1%).
Figure 2.
Dengue virus (DENV) type-specific neutralization analysis of early versus late epidemic immunoglobulin (Ig) M/IgG seroreactive donor samples indicating predominantly DENV-4 infections acquired during 2011/12 epidemic season. Differences in mean log titer between time subgroups (early negative, early positive, and late positive) varied among DENV subtypes (P < .001). Larger changes were observed in neutralizing titer for subtype 4 than for the other 3 subtypes (subtype 4 vs 1, P < .001; 4 vs 2, P < .001; 4 vs 3, P = .03).
Rates of Clinical Dengue Cases Relative to Seroincidence and NAT Yield Donations
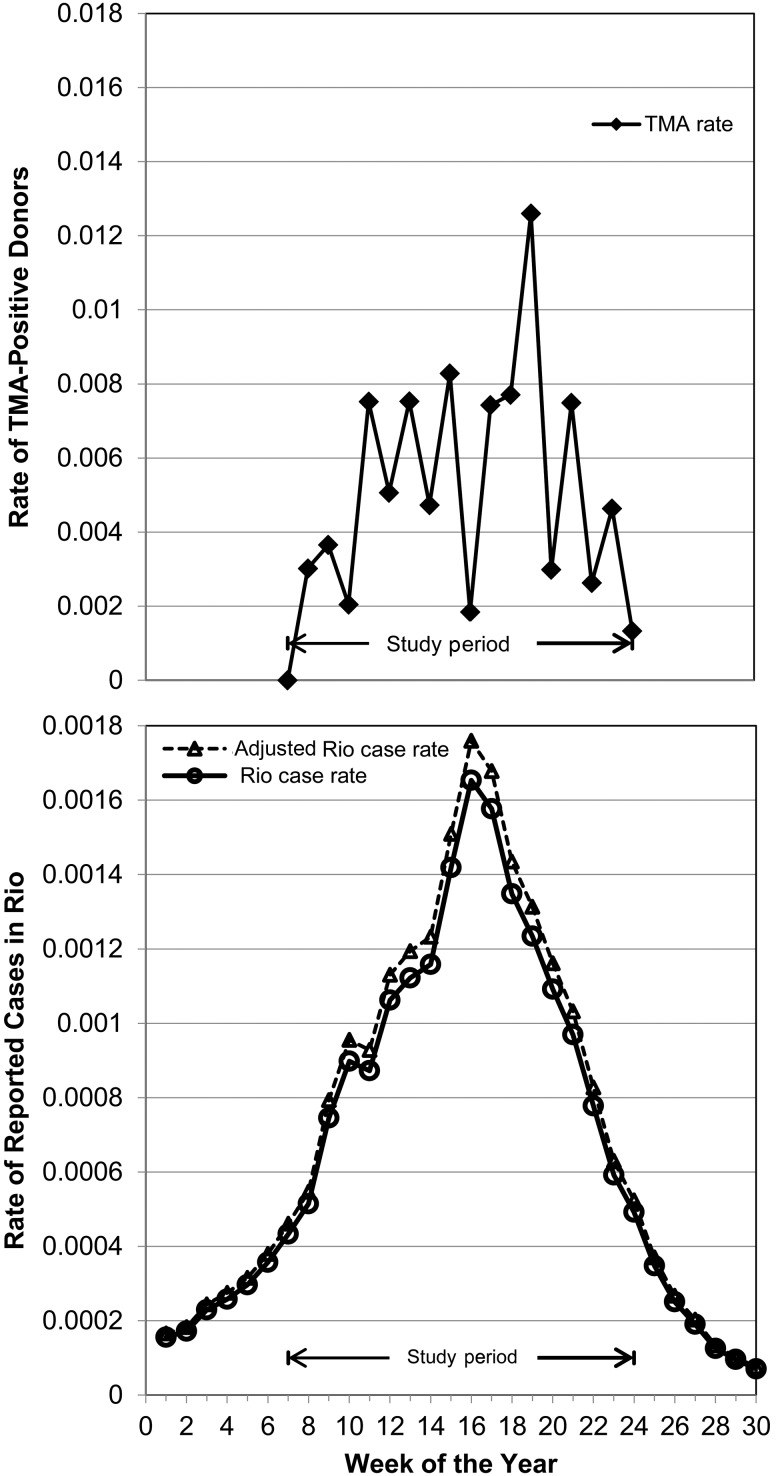
Figure 3 presents the weekly rate of reported dengue cases to the Rio public health system relative to the rate of confirmed viremic donations by donors to HemoRio detected in the REDS-III study from February through June 2012. The figure demonstrates that this donor NAT screening study was conducted during the 4 months that represented the height of the epidemic and that the rate of viremic donations generally paralleled the increase, peak and decline in clinical case reports. During the study period, 113 500 clinical cases of dengue were reported in Rio, representing 1.8% of 6.3 million inhabitants. When restricted to the 4.5 million Rio inhabitants in the age range eligible to donate blood (16–67 years old), there were 100 475 reported clinical cases of dengue, yielding a 2.3% estimated rate of clinical dengue cases. Juxtaposing the 2.3% rate of clinical case reports and the 6.2% seroincidence in asymptomatic donors, we inferred that approximately one-third of DENV-4 infections that occurred in this Rio epidemic resulted in reported disease (2.3% divided by 6.2%%)
Figure 3.
Upper panel, Comparison of rates of viremic donations detected during the study period. Lower panel, Rates of reported cases of clinical dengue for the Rio population as a whole (solid line) and among those 16–67 years old (dashed line) who are eligible to donate blood. Abbreviation: TMA, transcription-mediated amplification.
If DENV NAT screening had been performed on all 23 412 donations collected by HemoRio during the study period, we project that 125 DENV RNA-positive donations would have been detected (87 detected NAT RNA-positive donations, multiplied by 23 412 total donations, divided by 16 241 tested donations from consenting donors tested for DENV RNA). The ratio of the 100 475 clinical cases reported in the donor-age Rio population over the projected 125 RNA-positive donations detected during the study period indicates that there were approximately 800 cases of clinical dengue disease per identified NAT-positive donation.
DISCUSSION
During the 4-month study period within the large epidemic in Rio in 2012, we estimate that approximately 6.2% of donors acquired DENV infections, approximately 0.54% of blood donations tested RNA positive, and approximately 1.8% of the general and 2.3% of the donor-age population were reported as clinical case patients. This is among the highest rates of DENV infection documented in blood donors based on NAT screening [12, 15–18]. To our knowledge, ours is the first study to conduct a serosurvey of blood donor samples to allow for the correlation of the incidence of infection with NAT detection rates in donors. We were also able to correlate these observations in blood donors, with rates of clinical DENV cases reported in the corresponding metropolitan area during a large DENV-4 epidemic in a population that is hyperendemic for DENV infection, as evidenced by the 88% rate of IgG antibodies in the Rio donor population at the beginning of the study period.
Based on the estimated 6.2% incidence derived from the incremental IgM seroconversion rate and the observed rate of DENV RNA-positive donations during the study period, we estimated that a sensitive dengue NAT assay can detect viremia in asymptomatic or presymptomatic donors for approximately 9.1 days (95% CI, 4.4–13.9 days). However, our estimate of infection incidence may have underestimated the true incidence. Recent data from follow-up of DENV NAT yield donors in Puerto Rico indicate that 5%–10% of DENV RNA-positive donors with preexisting IgG fail to develop detectable IgM after testing of serial follow-up samples (personal communication, Susan Stramer. American Red Cross, and Marion Lanteri, Blood Systems Research Institute); this observation is consistent with findings in studies of clinical dengue cases indicating that approximately 10% of patients fail to develop detectable IgM antibodies or have very transient IgM responses after secondary DENV infections [7]. Because approximately 90% of infections in donors in Rio in 2012 were secondary infections, we need to consider the impact of lack of or transient IgM seroconversion on our estimates for the incidence of infection and the NAT detection period. A 10% underestimate of the IgM seroconversion rate during the approximately 4-month study period would increase our point estimate for inferred incidence from 6.2% to 6.8% and reduce our estimated period of detectable RNA from 9.1 to 8.2 days. This adjusted estimate for duration of asymptomatic viremia is generally consistent with periods of dengue viremia reported elsewhere [27–29].
During the study period, we estimated that 1.8% of 6.3 million Rio inhabitants and 2.3% of the 4.5 million Rio inhabitants in the age range eligible to donate blood were reported to have clinical cases of dengue. The higher rate of clinically diagnosed disease in adults relative to the total population that includes children probably reflects increased rates of secondary infections in adults, who have a higher probability of serious symptomatic manifestations because of immune enhancement after secondary infections. Given the 2.3% rate of clinical case reports in the donor-age population and the 6.2%–6.8% seroincidence in Rio donors during the study period, we estimated that approximately one-third of DENV-4 infections that occurred in this Rio epidemic resulted in reported disease. This estimate of disease penetrance is consistent with other estimates for the proportion of secondary DENV infections that result in clinical disease in adults [3–5]. In contrast with WNV, which can cause outbreaks similar in scale to the 2012 Rio dengue epidemic, with 1%–10% seasonal incidence rates [30, 31], <1 in 200 cases of infection result in clinically reported neuroinvasive disease [32]. On the other end of the arbovirus spectrum, chikungunya virus can infect 25%–50% of populations during single-season epidemics and up to 80% of infections are estimated to cause severe febrile illness with polyarthralgia [9, 33, 34].
By correlating the number of clinical cases reported in the donor-age Rio population (100 475) with the projected number of RNA-positive donations that would have been detected during the study period had all donations been screened by NAT (125 donations), we estimated that there were 800 cases of clinical dengue per NAT-positive donation identified. This high ratio reflects the combined impact of capturing all clinically reported cases of dengue in the population, whereas <3% of the Brazilian population donates blood each year [35], and the fact that infected donors are detected only in the 8–9 day period (95% CI, 4–14 days) of asymptomatic viremia. In contrast with WNV, which has a much lower disease penetrance in the general population but a comparable period of detectable viremia in donors, there are 2–3 reported cases of neuroinvasive disease relative to each NAT yield donation [30, 32, 36, 37]. Thus, when clinical cases of WNV are observed and reported to the public health system in a region experiencing an incipient epidemic, there is a high probability that viremic blood donations will occur; consequently minipool NAT is used in the United States to detect outbreaks, with individual donation-NAT triggered after detection of minipool-NAT–positive donations in a local geographic region. In contrast very large numbers of clinical DENV infection cases would have to be observed to expect detection of viremic blood donations.
It is important to acknowledge that this study was conducted in a hyperendemic setting during a large epidemic with recently introduced DENV-4. Further research is needed to establish the generalizability of our findings to other endemic and to nonendemic settings with different scale outbreaks of primary or secondary DENV infections. Moreover, the DENV infection rates we documented in asymptomatic blood donors may underestimate infection rates in the larger population because the donor population might be at lower risk than those living in conditions most conducive to dengue transmission; consequently, our estimate of underreporting of clinical dengue cases may be too conservative. However, we believe the relationships we documented between infection rates, NAT yield rates, and clinical case rates provide useful insights for dengue disease surveillance and, most specifically, for blood donor screening policy formulation.
Notes
Acknowledgments. Dengue virus (DENV) neutralization assays were performed by Kai Lu, and kits were kindly provided by Integral Molecular.
Study group. The Recipient Epidemiology and Donor Evaluation Study-III, International Component Brazil, is the responsibility of the following persons: Cesar de Almeida Neto and Alfredo Mendrone-Junior (Fundação Pró-Sangue Hemocentro de São Paulo, Brazil); Anna Bárbara Carneiro-Proietti (Fundação Hemominas, Belo Horizonte, Brazil); Divaldo de Almeida Sampaio and Paula Loureiro (Fundação Hemope, Recife, Brazil); Clarisse Lobo and M. E. L. (Hemorio, Rio de Janeiro); João Eduardo Ferreira, Marcio Oikawa, Pedro Losco Takecian, and Cláudia Di Lorenzo Oliveira (University of São João Del-Rei, Brazil); E. C. S. and L. C. (University of São Paulo); B. C., M. P. B., Shannon Kelly, and Thelma T. Gonçalez (Blood Systems Research Institute, San Francisco/University of California, San Francisco); D. B. and C. M. (RTI International); and Simone A. Glynn (National Heart, Lung, and Blood Institute, National Institutes of Health).
Author contributions. Each author attests to the following: substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support. The work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (Recipient Epidemiology and Donor Evaluation Study-III contracts HHSN268201100001I and HHSN268201100007I); and Fundação de Amparo a Pesquisa do Estado de São Paulo (grant 2011/18955-1).
Potential conflicts of interest. J. M. L. is an employee of Hologic, the manufacturer of one of the DENV RNA tests used in this study. M. P. B. has served as a scientific advisor to Hologic. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the International Component of the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), Cesar de Almeida Neto, Alfredo Mendrone-Junior, Anna Bárbara Carneiro-Proietti, Divaldo de Almeida Sampaio, Paula Loureiro, Clarisse Lobo, Maria Esther Lopes, Ester Cerdeira Sabino, Ligia Capuani, João Eduardo Ferreira, Marcio Oikawa, Pedro Losco Takecian, Cláudia Di Lorenzo Oliveira, Brian Custer, Michael P. Busch, Shannon Kelly, Thelma T. Gonçalez, Donald Brambilla, Christopher McClure, and Simone A. Glynn
References
- 1.Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 2003; 3:19–28. [DOI] [PubMed] [Google Scholar]
- 2.Laughlin CA, Morens DM, Cassetti MC et al. Dengue research opportunities in the Americas. J Infect Dis 2012; 206:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004; 10:S98–109. [DOI] [PubMed] [Google Scholar]
- 4.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998; 11:480–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Dengue haemorrhagic fever, diagnosis, treatment, prevention and control. 2nd ed Geneva: World Health Organization, 1997. [Google Scholar]
- 6.Dejnirattisai W, Jumnainsong A, Onsirisakul N et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OhAinle M, Balmaseda A, Macalalad AR et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 2011; 3:114ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco C. Dengue and chikungunya viruses in blood donations: risks to the blood supply? Transfusion 2008; 48:1279–81. [DOI] [PubMed] [Google Scholar]
- 9.Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang 2010; 98:495–503. [DOI] [PubMed] [Google Scholar]
- 10.Chuang V, Wong TY, Leung YH et al. Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Med J 2008; 14:170–7. [PubMed] [Google Scholar]
- 11.Tambyah PA, Koay ES, Poon ML, Lin RV, Ong BK. Dengue hemorrhagic fever transmitted by blood transfusion. N Engl J Med 2008; 359:1526–7. [DOI] [PubMed] [Google Scholar]
- 12.Stramer SL, Linnen JM, Carrick JM et al. Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion 2012; 52:1657–66. [DOI] [PubMed] [Google Scholar]
- 13.Levi JE, Nishiya A, Felix AC et al. Real-time symptomatic case of transfusion-transmitted dengue. Transfusion 2015; 55:961–4. [DOI] [PubMed] [Google Scholar]
- 14.Oh HB, Muthu V, Daruwalla ZJ, Lee SY, Koay ES, Tambyah PA. Bitten by a bug or a bag? transfusion-transmitted dengue: a rare complication in the bleeding surgical patient. Transfusion 2015; 55:1655–61. [DOI] [PubMed] [Google Scholar]
- 15.Matos D, Tomashek KM, Perez-Padilla J et al. Probable and possible transfusion-transmitted dengue associated with NS1 antigen-negative but RNA confirmed-positive red blood cells. Transfusion 2016; 56:215–22. [DOI] [PubMed] [Google Scholar]
- 16.Dias LL, Amarilla AA, Poloni TR, Covas DT, Aquino VH, Figueiredo LT. Detection of dengue virus in sera of Brazilian blood donors. Transfusion 2012; 52:1667–71. [DOI] [PubMed] [Google Scholar]
- 17.Linnen JM, Vinelli E, Sabino EC et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion 2008; 48:1355–62. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed H, Linnen JM, Munoz-Jordan JL et al. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion 2008; 48:1348–54. [DOI] [PubMed] [Google Scholar]
- 19.Teo D, Ng LC, Lam S. Is dengue a threat to the blood supply? Transfus Med 2009; 19:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilder-Smith A, Chen LH, Massad E, Wilson ME. Threat of dengue to blood safety in dengue-endemic countries. Emerg Infect Dis 2009; 15:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honorio NA, Nogueira RM, Codeco CT et al. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis 2009; 3:e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunes MR, Faria NR, Vasconcelos HB et al. Phylogeography of dengue virus serotype 4, Brazil, 2010–2011. Emerg Infect Dis 2012; 18:1858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teixeira Mda G, Costa Mda C, Barreto ML, Mota E. Dengue and dengue hemorrhagic fever epidemics in Brazil: what research is needed based on trends, surveillance, and control experiences? Cad Saude Publica 2005; 21:1307–15. [DOI] [PubMed] [Google Scholar]
- 24.Sabino EC, Loureiro P, Lopes ME et al. Transfusion-transmitted dengue and associated clinical symptoms during the 2012 epidemic in Brazil. J Infect Dis 2016; 213:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattia K, Puffer BA, Williams KL et al. Dengue reporter virus particles for measuring neutralizing antibodies against each of the four dengue serotypes. PLoS One 2011; 6:e27252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oehlert GW. A note on the delta method. Am Stat 1992; 46:27–29. [Google Scholar]
- 27.Chan M, Johansson MA. The incubation periods of dengue viruses. PLoS One 2012; 7:e50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A. Viraemia in patients with naturally acquired dengue infection. Bull World Health Organ 1981; 59:623–30. [PMC free article] [PubMed] [Google Scholar]
- 29.Wang WK, Chen HL, Yang CF et al. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis 2006; 43:1023–30. [DOI] [PubMed] [Google Scholar]
- 30.Carson PJ, Borchardt SM, Custer B et al. Neuroinvasive disease and West Nile virus infection, North Dakota, USA, 1999–2008. Emerg Infect Dis 2012; 18:684–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervantes DT, Chen S, Sutor LJ et al. West Nile virus infection incidence based on donated blood samples and neuroinvasive disease reports, Northern Texas, USA, 2012. Emerg Infect Dis 2015; 21:681–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect 2013; 141:591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen LR, Epstein JS. Chikungunya virus: new risk to transfusion safety in the Americas. Transfusion 2014; 54:1911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen LR, Stramer SL, Powers AM. Chikungunya virus: possible impact on transfusion medicine. Transfus Med Rev 2010; 24:15–21. [DOI] [PubMed] [Google Scholar]
- 35.Carneiro-Proietti AB, Sabino EC, Sampaio D et al. Demographic profile of blood donors at three major Brazilian blood centers: results from the International REDS-II study, 2007 to 2008. Transfusion 2010; 50:918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busch MP, Kleinman SH, Tobler LH et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis 2008; 198:984–93. [DOI] [PubMed] [Google Scholar]
- 37.Busch MP, Wright DJ, Custer B et al. West Nile virus infections projected from blood donor screening data, United States, 2003. Emerg Infect Dis 2006; 12:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]