Abstract
Brazil has a concentrated HIV epidemic and men who have sex with men (MSM) are disproportionately affected. Yet, no data is available on the HIV care cascade for this population. This study aimed to assess the HIV care cascade among MSM newly diagnosed through innovative testing strategies in Rio de Janeiro. Data from 793 MSM and travestites/transgender women (transwomen) tested for HIV at a non-governmental LGBT organization and a mobile testing unit located at a gay friendly venue were analyzed. A 12-month-after-HIV-diagnosis-censored cohort was established using CD4, viral load and combination antiretroviral therapy (cART) longitudinal data from those diagnosed with HIV. A cross-sectional HIV care cascade was built using this data. The relative risks of achieving each cascade-stage were estimated using generalized linear models according to age, self-declared skin-color, education, history of sexually transmitted diseases (STD), drug use and prior HIV testing. From Jan-2013 to Jan-2014, 793 MSM and transwomen were tested, 131 (16.5%) were HIV-infected. As of January 2015, 95 (72.5%) were linked to HIV care, 90 (68.7%) were retained in HIV care, 80 (61.1%) were on cART, and 50 (38.2%) were virally suppressed one year after HIV diagnosis. Being non-white (Relative risk [lower bound; upper bound of 95% confidence interval] = 1.709 [1.145; 2.549]) and having a prior HIV-test (1.954 [1.278; 2.986]) were associated with an HIV-positive diagnosis. A higher linkage (2.603 [1.091; 6.211]) and retention in care (4.510 [1.880; 10.822]) were observed among those who were older than 30 years of age. Using community-based testing strategies, we were able to access a high-risk MSM population and a small sample of transwomen. Despite universal care coverage and the test-and-treat policy adopted in Brazil, the MSM cascade of care indicates that strategies to increase linkage to care and prompt cART initiation targeted to these populations are critically needed. Interventions targeting non-white and young MSM should be prioritized.
Introduction
Globally, the HIV epidemic among men who have sex with men (MSM) and transgender women (TGW) are showing expanding trajectories [1,2]. Most of the new HIV cases in Latin America occur among these populations [3]. In Brazil, while the HIV prevalence in the general population is 0.6%, among MSM it is 14.2% [4], reinforcing that this population, especially the very young MSM [5], is disproportionately affected by HIV infection in the country [6].
Although TGW represent smaller population than MSM, they have extremely elevated HIV infection rates. A meta-analysis of data from 15 countries estimated an HIV prevalence of 17.7% (95% CI 15.6–19.8) in this population, with an odds ratio of 50.0 (95% CI 26.5–94.3) for HIV infection among TGW versus all adults of reproductive age in low- and middle-income countries [7].
In Brazil, HIV testing at no cost is available in public health care centers as well as in 383 voluntary counseling and testing (VCT) units across the country. Nevertheless, access to testing and services is markedly insufficient, especially for the most vulnerable population groups [8,9]. Studies have shown that about half of Brazilian MSM report prior HIV testing [4,10,11] with only 19% reporting HIV testing in the previous year [12]. Infrequent testing remains a barrier for maintaining accurate HIV serostatus awareness even among those who have previously been tested [13].
Routine HIV testing is critical for timely HIV diagnosis as it triggers the cascade of care for those who are HIV-positive while also providing an unique opportunity for risk management counseling for those who are HIV-negative, including referral for pre-exposure prophylaxis (PrEP) for those at higher risk of HIV acquisition [14]. For vulnerable groups such as MSM and TGW, diverse testing venues are needed to encourage those still unaware of their HIV status to seek testing. In this scenario, community-based testing using HIV rapid tests has been shown to be a valuable option [15].
As part of the activities to guarantee, expand and improve quality and access to sexually transmitted diseases (STD) and HIV prevention services in Brazil, in 2008, the pilot project called, Quero Fazer (Let’s Do It) funded by the United States Agency for International Development (USAID) was implemented by the non-governmental organization (NGO) Espaço de Prevenção e Atenção Humanizada (EPAH), in partnership with the Brazilian Ministry of Health (BMOH) and five STD/AIDS State programs (including Rio de Janeiro). The Quero Fazer Project offered free confidential community-based testing using HIV rapid tests to MSM and transvestites/TGW (herein called transwomen) in stigma free environments. A mobile unit operated by a local NGO and a testing outlet based in a local Lesbian, Gay, Bisexual and Transgender (LGBT) NGO were implemented at each of the 5 Brazilian States. Differently from the regular health services in Rio de Janeiro, these units operated after regular working hours. This study aims to assess the HIV care cascade among MSM and transwomen newly diagnosed with HIV infection through the Quero Fazer Project in Rio de Janeiro, Brazil.
Methods
This analysis was performed using data from the Quero Fazer Project in Rio de Janeiro, one of the epicenters of the Brazilian HIV epidemic. In Rio de Janeiro, community-based out of health-care testing using HIV rapid tests were offered in two venues: 1) once a week, on Wednesday evenings, at a mobile testing unit located in front of a unique popular LGBT venue; 2) four days a week, from Monday to Thursday, after working hours, at the site of Arco-Íris Group, a well-known LGBT NGO. Potential participants were offered voluntary counseling and testing through trained peers and counselors. All MSM and transwomen tested at both venues during the study period (from January 2013 to January 2014) were included in the analysis. Data were analyzed anonymously with participants’ records/information de-identified prior to analysis. The research was approved with waiver of informed consent by the Instituto de Pesquisa Clínica Evandro Chagas Institutional Review Board (CAEE: 40010014.0.0000.5262).
Data collected at HIV testing sites included: date and venue of the test; date of birth; age; city of residence; state of residence; gender; sexual orientation; skin color; level of education; history of sexually transmitted diseases (STD) in the prior 12 months; substance use in the prior 12 months; prior HIV testing; if previously test in NGO; date of receipt of the test result; and HIV test result.
Time-dependent CD4 cell counts, HIV viral load (VL) results, cART regimens with date of drug dispensation were provided by the BMOH from the national databases, which contain all information related to antiretroviral drug dispensation and CD4 and viral load monitoring. The final dataset was processed censoring all individual information (that is, all CD4, VL and cART dispensation information) 12 months after the date of the HIV diagnosis.
A cross-sectional cascade of HIV care was built including the following stages: HIV-diagnosed (HIV-infected at testing); linked to HIV care (at least one record of CD4 or VL or dispensation of antiretroviral drugs); retained in HIV care (at least two records >90 days apart of CD4 or VL or dispensation of antiretroviral drugs); on cART (at least one record of dispensation of antiretroviral drugs); and virally suppressed (at least on viral load ≤ 50 copies).
We used generalized linear models with logit link function in R software version 3.1.1 [16] to estimate the relative risk and 95% confidence intervals (RR [CI95%]) of reaching a particular stage of the cascade among those MSM who were in the predecessor cascade-stage. Variables explored in regression models as independent predictors of reaching a particular stage of the cascade included age, race/ethnicity, education and history of: STD, drug use and HIV trials. The final model included all variables found to independently predict each of the outcomes. Adjusted risk ratios were given for each covariate while adjusting for the variables found to independently predict each outcome.
Results
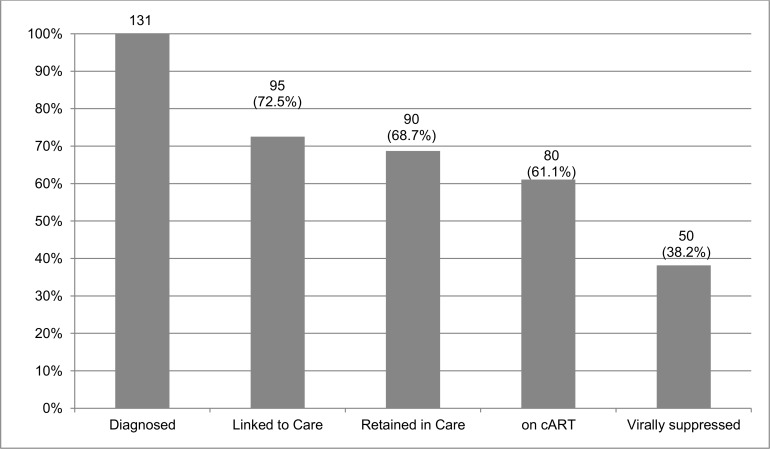
From January 2013 to January 2014, 756 MSM and 37 transwomen were tested (Table 1), 214 (27.0%) in the mobile testing unit and 579 (73.0%) at Arco-Íris Group. Overall, 131 (16.5%) were HIV-infected, 123 MSM (16.3% prevalence) and 8 transwomen (21.6% prevalence). This difference in the prevalence for MSM and transwomen was not statistically significant (p = 0.53 [Chi squared]). Forty-three (32.8%) were HIV-diagnosed at the mobile unit, while 88 (67.2%) were diagnosed at Arco-Íris Group. Mean age for the HIV-infected MSM and transwomen were 28.1 and 29.3, respectively. For those diagnosed with HIV infection, baseline mean CD4 cell count and viral load were 493 cells/mm3 (CI 95% 443–542) and 77.793 copies/ml (CI 95% 31.116–124.429). As of January 2015, 95 (72.5%) participants were linked to HIV care, 90 (68.7%) were retained in care, 80 (61.1%) were on cART, and 50 (38.2%) were virally suppressed (Fig 1).
Table 1. Baseline characteristics of all tested individuals disaggregated by gender identity (MSM and transwomen), Rio de Janeiro, Brazil.
| MSM (n = 756)* | Transwomen (n = 37)* | p-value** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV- | HIV+ | All MSM | HIV- | HIV+ | All transwomen | HIV- vs. HIV+ | MSM vs. transwomen | ||
| Age | <30-year-old | 378 (59.72) | 72 (58.54) | 450 (59.52) | 20 (68.97) | 7 (87.5) | 27 (72.97) | 1.000 | 0.122 |
| > = 30-year-old | 255 (40.28) | 51 (41.46) | 306 (40.48) | 9 (31.03) | 1 (12.5) | 10 (27.03) | |||
| Skin Color | White | 283 (44.71) | 41 (33.33) | 324 (42.86) | 11 (37.93) | 2 (25) | 13 (35.14) | 0.029 | 0.006 |
| Non-white | 349 (55.13) | 82 (66.67) | 431 (57.01) | 16 (55.17) | 6 (75) | 22 (59.46) | |||
| Unknown | 1 (0.16) | 0 (0) | 1 (0.13) | 2 (6.90) | 0 (0) | 2 (5.41) | |||
| Education | High School or Less | 273 (43.13) | 61 (49.59) | 334 (44.18) | 25 (86.21) | 7 (87.5) | 32 (86.49) | 0.325 | <0.001 |
| Some College or Higher | 355 (56.08) | 62 (50.41) | 417 (55.16) | 4 (13.79) | 1 (12.5) | 5 (13.51) | |||
| Unknown | 5 (0.79) | 0 (0) | 5 (0.66) | 0 (0) | 0 (0) | 0 (0) | |||
| Exposure to HIV | Sexual intercourse | 626 (98.89) | 123 (100) | 749 (99.07) | 29 (100) | 8 (100) | 37 (100) | 1.000 | 1.000 |
| Others | 3 (0.47) | 0 (0) | 3 (0.40) | 0 (0) | 0 (0) | 0 (0) | |||
| Unknown | 4 (0.63) | 0 (0) | 4 (0.53) | 0 (0) | 0 (0) | 0 (0) | |||
| STD (12 m Hist.) | No | 567 (89.57) | 105 (85.37) | 672 (88.89) | 24 (82.76) | 5 (62.5) | 29 (78.38) | 0.099 | 0.064 |
| Yes | 66 (10.43) | 18 (14.63) | 84 (11.11) | 5 (17.24) | 3 (37.5) | 8 (21.62) | |||
| Drug use (12 m Hist.) | No | 550 (86.89) | 111 (90.24) | 661 (87.43) | 16 (55.17) | 3 (37.5) | 19 (51.35) | 0.913 | <0.001 |
| Yes | 78 (12.32) | 11 (8.94) | 89 (11.77) | 12 (41.38) | 5 (62.5) | 17 (45.95) | |||
| Unknown | 5 (0.79) | 1 (0.81) | 6 (0.79) | 1 (3.45) | 0 (0) | 1 (2.70) | |||
| History of HIV test | Previously untested | 514 (81.20) | 87 (70.73) | 601 (79.50) | 23 (79.31) | 4 (50) | 27 (72.97) | 0.004 | 0.405 |
| Previously tested | 119 (18.80) | 36 (29.27) | 155 (20.50) | 6 (20.69) | 4 (50) | 10 (27.03) | |||
| Baseline measures*** | Mean CD4 | - | 493.12 450.44; 538.50] | - | - | 490.83 [448.52; 536.41]**** | - | - | 0.691 |
| Mean Viral Load | - | 76645.21 [76103.34; 77189.56] | - | - | 93662.00 [93063.12; 94263.78]**** | - | - | 0.255 | |
* results are shown as: number (% of all MSM or transwomen)
** Fisher’s exact test was used for all categorical variables and Wilcoxon’s for numeric
*** mean [Poisson CI 95%] for baseline CD4 and VL, available only for patients retained in HIV care
**** only 6 HIV+ transwomen individuals were retained in HIV care.
Fig 1. Cascade of HIV care for Brazilian MSM in Rio de Janeiro, Brazil.
Results expressed in number and percentage of all HIV-diagnosed MSM for each cascade-stage.
Being non-white (1.709 [1.145; 2.549]) and having a prior HIV-test (1.954 [1.278; 2.986]) were associated with HIV-positive diagnosis (Table 1). A higher linkage (2.603 [1.091; 6.211] and retention in care (4.510 [1.880; 10.822]) were observed among those who were older than 30 year-old. (Tables 2 and 3).
Table 2. HIV-diagnosed MSM among all tested MSM in Rio de Janeiro, Brazil.
| Categories | No | Yes | UnadjustedRR [IC95%]; p-value | Adjusted*RR [IC95%]; p-value | |
|---|---|---|---|---|---|
| Overall | 662 (83.48%) | 131 (16.52%) | |||
| Age | <30 | 398 (50.19%) | 79 (9.96%) | reference | reference |
| > = 30 | 264 (33.29%) | 52 (6.56%) | 0.992 [0.677; 1.455]; 0.969 | 0.949 [0.639; 1.410]; 0.797 | |
| Skin Color | White | 294 (37.07%) | 43 (5.42%) | reference | reference |
| Non-white | 365 (46.03%) | 88 (11.10%) | 1.648 [1.11; 2.449]; 0.013* | 1.709 [1.145; 2.549]; 0.009** | |
| Education | High School or Less | 298 (37.58%) | 68 (8.58%) | reference | reference |
| Some College or Higher | 359 (45.27%) | 63 (7.94%) | 0.769 [0.528; 1.120]; 0.171 | 0.811 [0.552; 1.194]; 0.289 | |
| 12m hist. STD | no | 591 (74.53%) | 110 (13.87%) | reference | reference |
| yes | 71 (8.95%) | 21 (2.65%) | 1.589 [0.938; 2.694]; 0.085 | 1.629 [0.955; 2.778]; 0.074 | |
| 12m hist. drug use | no | 566 (71.37%) | 114 (14.38%) | reference | reference |
| yes | 90 (11.35%) | 16 (2.02%) | 0.883 [0.500; 1.558]; 0.667 | 0.906 [0.508; 1.616]; 0.739 | |
| History of HIV test | Previously untested | 537 (67.72%) | 91 (11.48%) | reference | reference |
| Previously tested | 125 (15.76%) | 40 (5.04%) | 1.888 [1.241; 2.873]; 0.003* | 1.954 [1.278; 2.986]; 0.002** | |
| Strategy | Mobile unit | 171 (21.56%) | 43 (5.42%) | reference | reference |
| NGO | 491 (61.92%) | 88 (11.10%) | 0.713 [0.476; 1.068]; 0.100 | 0.787 [0.520; 1.191]; 0.258 |
* adjusted for: Skin Color, 12m hist. STD, and History of HIV test, when applicable
** p-value lower than 0.05.
Table 3. Linked to care among all HIV-diagnosed MSM in Rio de Janeiro, Brazil.
| Categories | No | Yes | UnadjustedRR [IC95%]; p-value | Adjusted*RR [IC95%]; p-value | |
|---|---|---|---|---|---|
| Overall | 36 (27.48%) | 95 (72.52%) | |||
| Age | <30 | 27 (20.61%) | 52 (39.69%) | reference | reference |
| > = 30 | 9 (6.87%) | 43 (32.82%) | 2.603 [1.091; 6.211]; 0.031* | 2.603 [1.091; 6.211]; 0.031** | |
| Skin Color | White | 16 (12.21%) | 27 (20.61%) | reference | reference |
| Non-white | 20 (15.27%) | 68 (51.91%) | 2.015 [0.910; 4.459]; 0.084 | 2.137 [0.946; 4.828]; 0.068 | |
| Education | High School or Less | 18 (13.74%) | 50 (38.17%) | reference | reference |
| Some College or Higher | 18 (13.74%) | 45 (34.35%) | 0.900 [0.418; 1.939]; 0.788 | 0.723 [0.309; 1.692]; 0.455 | |
| 12m hist. STD | No | 31 (23.66%) | 79 (60.31%) | reference | reference |
| Yes | 5 (3.82%) | 16 (12.21%) | 1.256 [0.424; 3.722]; 0.681 | 1.517 [0.490; 4.698]; 0.470 | |
| 12m hist. drug use | No | 31 (23.66%) | 83 (63.36%) | reference | reference |
| Yes | 4 (3.05%) | 12 (9.16%) | 1.120 [0.336; 3.737]; 0.853 | 1.413 [0.409; 4.880]; 0.584 | |
| History of HIV test | Previously untested | 26 (19.85%) | 65 (49.62%) | reference | reference |
| Previously tested | 10 (7.63%) | 30 (22.9%) | 1.200 [0.514; 2.802]; 0.673 | 1.177 [0.490; 2.825]; 0.715 | |
| Strategy | Mobile unit | 14 (10.69%) | 29 (22.14%) | reference | reference |
| NGO | 22 (16.79%) | 66 (50.38%) | 1.448 [0.651; 3.223]; 0.364 | 1.381 [0.599; 3.181]; 0.449 |
* adjusted for: Age, and Skin Color, when applicable;
** p-value lower than 0.05.
In the bivariate analysis, individuals on cART had a lower mean CD4 count (460.26) than those who were not on cART (604.99) (p = 0.05 [Wilcoxon test]). In generalized linear models, there were no statistically significant predictors of use of cART among those MSM who were retained in care (S1 Table). Viral suppression among those who were on cART was suggestively lower (0.306 [0.089; 1.043]) for those MSM with a history of STD (S2 Table).
Discussion
In this study, a community-based testing strategy in LGBT friendly settings was able to access hard-to-reach MSM and transwomen populations with a very high HIV prevalence. Alternative testing strategies, particularly those in partnership with the civil society, such as described in our study and in resource rich settings [17] are pivotal for the expansion of HIV testing among the most vulnerable. Among those individuals identified as HIV-infected, a low level of viral suppression after 12 months of follow up was observed. To our knowledge, this is the first study to evaluate the cascade of care continuum in the setting of a community based testing program for MSM and transwomen in a middle-income country.
Being non-white and having a prior HIV test were associated with an HIV diagnosis. For the Brazilian scenario, unlike in the United States of America, the association between HIV infection and race/skin color is not yet properly established. In most studies enrolling HIV-infected individuals as well as health outcomes studies enrolling the general population, this association loses its statistical significance when the model is controlled by sociodemographic variables such as education, income, and access to consumer goods. The variable race/skin color was only included in 1996 in Brazilian’s most important health information systems, as the ones used for vital statistics (Mortality Information System—SIM—and Live Birth Information System—SINASC), and, only in 2000, in the System for the Reporting of Notifiable Conditions (SINAN). Consequently, a more consistent analysis of the race/skin color -related vulnerability to HIV remains limited in our setting, and the results of the present work should be interpreted in light of these limitations. In relation to health services discrimination, young men self-declared as black reported more discriminative experiences in the context of hospitalization compared to white individuals [18].
Having a prior HIV test may represent a personal risk-reduction strategy or a consequence of a sustained high-risk behavior [18,19]. A large contemporary study investigating the relationship between HIV risk behavior and HIV testing among MSM in the United States found that MSM who screened repeatedly for HIV reported higher sexual risk behavior at study entry than MSM who only screened once and a repeated HIV testing was associated with an increase of risk behavior [20]. No prior studies in our setting have evaluated such association. It is crucial to better understand the dynamics of risk-taking behavior in order to adequately frame the counseling messages. Tailored prevention efforts should be developed and evaluated for MSM who repeatedly screen for HIV.
Young (< 30 year-old) individuals showed a lower likelihood for linkage and retention to HIV-care, in agreement with studies from high-income settings [21–24] and also from a clinical cohort in our city [25]. A significant and growing population of young MSM are acquiring and living with HIV in Brazil, with those aged 15 to 24 years accounting for the largest number of incident HIV infections in the country [6]. More than one in twenty MSM aged under 24 years old were HIV-infected in a time-location sampling survey [11]. Although late presentation to care was found to be associated with older ages [26–28], this is still a common finding among HIV-infected youth. In addition, suboptimal cART adherence and high risk sexual practices synergistically amplify HIV transmission [29,30]. Thus, the evaluation of novel strategies to enhance linkage and retention in care among young MSM is critical in our setting. When looking at our results vis a vis the overall Brazilian care continuum cascade [6], among the HIV-diagnosed individuals, a lower proportion of individuals were linked (72.5% vs. 91.1%, respectively; p < 0.01 [Chi squared]) and retained in care (68.7% vs. 76.1%, respectively; p = 0.04 [Chi squared]). Both cascades of care indicate that at each level, important percentages of those living with HIV fall out of the care continuum.
Robust benefits of early initiation of combination antiretroviral therapy (cART) in resource limited and resource rich settings have emerged in the past decade [31,32]. Moreover, preliminary results of the PARTNER Study and of the Opposites Attract Study have provided evidence that the effect of ART in preventing HIV sexual transmission is similarly present among MSM serodiscordant couples [33]. For the benefits of cART to be fully realized both at the individual and population-level, HIV infected individuals must be fully engaged in the “HIV continuum of care”. Incomplete engagement at any of the stages will compromise the impact of cART at the individual and community levels, leading to persistent HIV transmission and increased morbidity and mortality [22,34]. Data from developed and developing countries demonstrate that a substantial reduction in patient retention occurs between each stage of the HIV treatment continuum from diagnosis, initiation of cART, retention in care and viral suppression [35–39]. Stigma and discrimination may pose additional barriers to the utilization of health services leading to poorer health outcomes among specific groups of MSM and transwomen [40,41]. Moreover, stigma and discrimination, impose further delays in seeking care after HIV-infection diagnosis and poor ART adherence have been well documented in other settings [42–45] and may also explain the poorer linkage and retention to care in our population. Comparing again our results with the overall Brazilian care continuum cascade [6], despite a similar proportion of individuals having initiated cART (61.1% vs. 60.3%, respectively; p = 0.85 [Chi squared]), viral suppression among those under cART in our study was suggestively lower (62.5% vs. 71.8%, respectively; p = 0.06 [Chi squared]). Further studies are needed to assess the barriers related to retention to care and viral suppression in this population.
This manuscript has limitations. Foremost, the criteria we used for the cascade-construction allowed us to include a patient into a cascade-stage even if he did not necessarily pass through the immediately prior stage [46]. Additionally, given the small number of transwomen enrolled with a HIV-prevalence that was not statistically significant different from the MSM, we decided to aggregate in the analysis data from transwomen and MSM. However, potentially different risk factors may compromise the extrapolation of the findings for the transwomen population. The comparison between the present MSM cascade of HIV care and the overall Brazilian cascade is limited due to methodological reasons. Our cascade involves a smaller number of hard-to-reach individuals, with follow up restricted to the one-year after the HIV-positive test date. In contrast, the overall Brazilian cascade was constructed using only the information of individuals prescribed cART in the last 100 days of a given calendar year. Finally, individuals who have a private health insurance may have opted to use the private sector, which could potentially lead to an underestimation of linkage, retention to care and viral suppression levels due to underrepresentation of their CD4 and VL results in the national database. Nevertheless, it is unlikely that a considerable number of such individuals with access to private health-care would have been tested for HIV at the project locations, thus decreasing the likelihood of the underestimation of linkage, retention to care and viral suppression levels.
An important limitation of the current study is that it uses convenience sampling; all individuals had been tested for HIV. Given that lower socioeconomic status [47] and fewer years of education [47,48] are associated with low levels of HIV-testing, the sampling method used in our study may account for the observed higher level of education among the study participants. The convenience sampling method used in the current study was compared with the respondent driven sampling method in an earlier study [47]; the proportion of MSM with similar levels of education (some College, or higher), and prior test for HIV was higher using the convenience sampling method (53.2% vs. 36.4%, respectively; p < 0.001 [Chi squared]). However, in interpreting this difference the following two reasons should also be considered. First, in the five years between the studies (2008/2009 and 2013/2014), access to higher education (College) in Brazil has improved with 26.8% more people enrolled in presential graduation courses in 2014 when compared to 2009 [49]. Secondly, while Brito et al. [47] used self-reported information of prior HIV testing, the current study collected data from HIV-tested individuals using implemented community-based testing strategies.
Conclusions
In conclusion, community-based testing strategy was effective in reaching a high-risk sample of MSM and a small sample of transwomen. Such strategy can be useful to identify high-risk individuals who can then be linked to prevention and care services. Despite the universal care coverage and the test-and-treat policy adopted in Brazil, our results indicate that strategies to increase linkage to care and prompt cART initiation are critically needed. Interventions for promoting engagement in HIV-care targeting non-white and young MSM should be prioritized.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
The authors acknowledge all the people that were involved in the implementation of the community-based testing strategies.
Data Availability
Data cannot be made publicly available for ethical reasons, public availability would compromise patient confidentiality or participant privacy. Anonymized and/or aggregated participant data will be available for researchers that meet our institutional review board (IRB) criteria for confidential data. The contact name and email where interested researchers can request this data can be the corresponding author (Rodolfo Castro, e-mail: rodolfo.castro@ini.fiocruz.br).
Funding Statement
The authors have no support or funding to report.
References
- 1.Baral SD, Grosso A, Holland C, Papworth E. The epidemiology of HIV among men who have sex with men in countries with generalized HIV epidemics: Curr Opin HIV AIDS. 2014;9: 156–167. 10.1097/COH.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 2.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. The Lancet. 2012;380: 367–377. 10.1016/S0140-6736(12)60821-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Boni R, Veloso VG, Grinsztejn B. Epidemiology of HIV in Latin America and the Caribbean: Curr Opin HIV AIDS. 2014;9: 192–198. 10.1097/COH.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 4.Kerr LRFS, Mota RS, Kendall C, Pinho A, Mello MB, Guimarães MDC, et al. HIV among MSM in a large middle-income country. AIDS Lond Engl. 2013;27: 427–435. 10.1097/QAD.0b013e32835ad504 [DOI] [PubMed] [Google Scholar]
- 5.Mello A, Chinaglia M. Assessment of risk factors for HIV infection among men who have sex with men in the metropolitan area of Campinas City, Brazil, using respondent-driven sampling Washington DC: Population Council; 2008. [Google Scholar]
- 6.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Boletim Epidemiológico—Aids e DST—Ano III—n° 01. Brasília: MS; 2014.
- 7.Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13: 214–222. 10.1016/S1473-3099(12)70315-8 [DOI] [PubMed] [Google Scholar]
- 8.Grangeiro A, Escuder MM, Wolffenbüttel K, Pupo LR, Nemes MIB, Monteiro PHN. Technological profile assessment of voluntary HIV counseling and testing centers in Brazil. Rev Saúde Pública. 2009;43: 427–436. 10.1590/S0034-89102009000300006 [DOI] [PubMed] [Google Scholar]
- 9.Grangeiro A, Escuder MM, Veras MA, Barreira D, Ferraz D, Kayano J. Voluntary counseling and testing (VCT) services and their contribution to access to HIV diagnosis in Brazil. Cad Saúde Pública. 2009;25: 2053–2063. 10.1590/S0102-311X2009000900019 [DOI] [PubMed] [Google Scholar]
- 10.Salani Mota RM, Sansigolo Kerr LRF, Kendall C, Pinho A, de Mello MB, Dourado I, et al. Reliability of self-report of HIV status among men who have sex with men in Brazil. J Acquir Immune Defic Syndr 1999. 2011;57 Suppl 3: S153–156. 10.1097/QAI.0b013e31821e9c10 [DOI] [PubMed] [Google Scholar]
- 11.de Sousa Mascena Veras MA, Calazans GJ, de Almeida Ribeiro MCS, de Freitas Oliveira CA, Giovanetti MR, Facchini R, et al. High HIV Prevalence among Men who have Sex with Men in a Time-Location Sampling Survey, São Paulo, Brazil. AIDS Behav. 2015;19: 1589–1598. 10.1007/s10461-014-0944-3 [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS. Global AIDS Response. Progress Reporting. Narrative Report. Brazil. Geneva: UNAIDS/WHO; 2014.
- 13.Centers for Disease Control and Prevention (CDC). HIV prevalence, unrecognized infection, and HIV testing among men who have sex with men—five U.S. cities, June 2004-April 2005. MMWR Morb Mortal Wkly Rep. 2005;54: 597–601. [PubMed] [Google Scholar]
- 14.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. The Lancet. 2008;372: 669–684. 10.1016/S0140-6736(08)60886-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Consolidated guidelines on HIV testing services Geneva: WHO; 2015. [PubMed] [Google Scholar]
- 16.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available: http://www.R-project.org/
- 17.Lorente N, Preau M, Vernay-Vaisse C, Mora M, Blanche J, Otis J, et al. Expanding Access to Non-Medicalized Community-Based Rapid Testing to Men Who Have Sex with Men: An Urgent HIV Prevention Intervention (The ANRS-DRAG Study). PLoS ONE. 2013;8: e61225 10.1371/journal.pone.0061225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leaity S, Sherr L, Wells H, Evans A, Miller R, Johnson M, et al. Repeat HIV testing: high-risk behaviour or risk reduction strategy? AIDS Lond Engl. 2000;14: 547–552. [DOI] [PubMed] [Google Scholar]
- 19.Matković Puljić V, Kosanović Ličina ML, Kavić M, Nemeth Blažić T. Repeat HIV Testing at Voluntary Testing and Counseling Centers in Croatia: Successful HIV Prevention or Failure to Modify Risk Behaviors? PLoS ONE. 2014;9 10.1371/journal.pone.0093734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoenigl M, Anderson CM, Green N, Mehta SR, Smith DM, Little SJ. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med. 2015;13 10.1186/s12916-015-0458-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agwu AL, Lee L, Fleishman JA, Voss C, Yehia BR, Althoff KN, et al. Aging and loss to follow-up among youth living with human immunodeficiency virus in the HIV Research Network. J Adolesc Health Off Publ Soc Adolesc Med. 2015;56: 345–351. 10.1016/j.jadohealth.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulett KB, Willig JH, Lin H-Y, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDs. 2009;23: 41–49. 10.1089/apc.2008.0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10: 299–305. 10.1310/hct1005-299 [DOI] [PubMed] [Google Scholar]
- 24.Tripathi A, Youmans E, Gibson JJ, Duffus WA. The impact of retention in early HIV medical care on viro-immunological parameters and survival: a statewide study. AIDS Res Hum Retroviruses. 2011;27: 751–758. 10.1089/AID.2010.0268 [DOI] [PubMed] [Google Scholar]
- 25.Silva DS, De Boni RB, Lake JE, Cardoso SW, Ribeiro S, Moreira RI, et al. Retention in Early Care at an HIV Outpatient Clinic in Rio de Janeiro, Brazil, 2000–2013. AIDS Behav. 2015; 10.1007/s10461-015-1235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacCarthy S, Brignol S, Reddy M, Nunn A, Dourado I. Making the invisible, visible: a cross-sectional study of late presentation to HIV/AIDS services among men who have sex with men from a large urban center of Brazil. BMC Public Health. 2014;14: 1313 10.1186/1471-2458-14-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickson N, McAllister S, Sharples K, Paul C. Late presentation of HIV infection among adults in New Zealand: 2005–2010. HIV Med. 2012;13: 182–189. 10.1111/j.1468-1293.2011.00959.x [DOI] [PubMed] [Google Scholar]
- 28.Shivaji T, Diniz A, Cortes-Martins H. Characteristics of late presentation of HIV infection in MSM and heterosexual adults in Portugal 2011–2013. J Int AIDS Soc. 2014;17: 19690 10.7448/IAS.17.4.19690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magidson JF, Li X, Mimiaga MJ, Moore AT, Srithanaviboonchai K, Friedman RK, et al. Antiretroviral Medication Adherence and Amplified HIV Transmission Risk Among Sexually Active HIV-Infected Individuals in Three Diverse International Settings. AIDS Behav. 2015; 10.1007/s10461-015-1142-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer KH, Skeer MR, O’Cleirigh C, Goshe BM, Safren SA. Factors Associated with Amplified HIV Transmission Behavior among American Men who have Sex with Men Engaged in Care: Implications for Clinical Providers. Ann Behav Med Publ Soc Behav Med. 2014;47: 165–171. 10.1007/s12160-013-9527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14: 281–290. 10.1016/S1473-3099(13)70692-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373: 795–807. 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodger A, Bruun T, Cambiano V, Pietro Vernazza, Estrada V, Lunzen JV, et al. HIV Transmission Risk Through Condomless Sex If HIV+ Partner On Suppressive ART: PARTNER Study. Oral late breaker abstract 153LB. Boston: 21st CROI; 2014.
- 34.Mugavero MJ, Norton WE, Saag MS. Health Care System and Policy Factors Influencing Engagement in HIV Medical Care: Piecing Together the Fragments of a Fractured Health Care Delivery System. Clin Infect Dis. 2011;52: S238–S246. 10.1093/cid/ciq048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayala G. HIV Treatment Cascades that Leak: Correlates of Drop-off from the HIV Care Continuum among Men who have Sex with Men Worldwide. J AIDS Clin Res. 2014;5: 331 10.4172/2155-6113.1000331 [DOI] [Google Scholar]
- 36.Nosyk B, Montaner JSG, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14: 40–49. 10.1016/S1473-3099(13)70254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner EM, Young B. The HIV care cascade through time. Lancet Infect Dis. 2014;14: 5–6. 10.1016/S1473-3099(13)70272-X [DOI] [PubMed] [Google Scholar]
- 38.Mugavero MJ, Amico KR, Horn T, Thompson MA. The State of Engagement in HIV Care in the United States: From Cascade to Continuum to Control. Clin Infect Dis. 2013;57: 1164–1171. 10.1093/cid/cit420 [DOI] [PubMed] [Google Scholar]
- 39.Nachega JB, Uthman OA, Rio C del, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ Heel in the HIV Care Continuum for the Success of a Test-and-Treat Strategy to Achieve an AIDS-Free Generation. Clin Infect Dis. 2014;59: S21–S27. 10.1093/cid/ciu299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mimiaga MJ, Reisner SL, Bland S, Skeer M, Cranston K, Isenberg D, et al. Health system and personal barriers resulting in decreased utilization of HIV and STD testing services among at-risk black men who have sex with men in Massachusetts. AIDS Patient Care STDs. 2009;23: 825–835. 10.1089/apc.2009.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pachankis JE, Hatzenbuehler ML, Hickson F, Weatherburn P, Berg RC, Marcus U, et al. Hidden from health: structural stigma, sexual orientation concealment, and HIV across 38 countries in the European MSM Internet Survey. AIDS. 2015;29: 1239–1246. 10.1097/QAD.0000000000000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peretti-Watel P, Spire B, Pierret J, Lert F, Obadia Y, VESPA Group. Management of HIV-related stigma and adherence to HAART: evidence from a large representative sample of outpatients attending French hospitals (ANRS-EN12-VESPA 2003). AIDS Care. 2006;18: 254–261. 10.1080/09540120500456193 [DOI] [PubMed] [Google Scholar]
- 43.Peretti-Watel P, Spire B, Obadia Y, Moatti J-P, VESPA Group. Discrimination against HIV-infected people and the spread of HIV: some evidence from France. PloS One. 2007;2: e411 10.1371/journal.pone.0000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logie C, Gadalla TM. Meta-analysis of health and demographic correlates of stigma towards people living with HIV. AIDS Care. 2009;21: 742–753. 10.1080/09540120802511877 [DOI] [PubMed] [Google Scholar]
- 45.Dowshen N, Binns HJ, Garofalo R. Experiences of HIV-related stigma among young men who have sex with men. AIDS Patient Care STDs. 2009;23: 371–376. 10.1089/apc.2008.0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? J Acquir Immune Defic Syndr 1999. 2013;63 Suppl 2: S228–232. 10.1097/QAI.0b013e318298721b [DOI] [PubMed] [Google Scholar]
- 47.Brito AM, Kendall C, Kerr L, Mota RMS, Guimaraes MDC, Dourado I, et al. Factors Associated with Low Levels of HIV Testing among Men Who Have Sex with Men (MSM) in Brazil. PloS One. 2015;10: e0130445 10.1371/journal.pone.0130445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pines HA, Goodman-Meza D, Pitpitan EV, Torres K, Semple SJ, Patterson TL. HIV testing among men who have sex with men in Tijuana, Mexico: a cross-sectional study. BMJ Open. 2016;6: e010388 10.1136/bmjopen-2015-010388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Instituto Nacional de Estudos e Pesquisas Educacionais Anísio Teixeira. Sinopses Estatísticas da Educação Superior—Graduação [Internet]. Brasília: MEC; 2014. Available: http://portal.inep.gov.br/superior-censosuperior-sinopse
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
Data cannot be made publicly available for ethical reasons, public availability would compromise patient confidentiality or participant privacy. Anonymized and/or aggregated participant data will be available for researchers that meet our institutional review board (IRB) criteria for confidential data. The contact name and email where interested researchers can request this data can be the corresponding author (Rodolfo Castro, e-mail: rodolfo.castro@ini.fiocruz.br).