Abstract
Implementation of Human Papillomavirus (HPV) vaccination should be considered a key cervical cancer prevention strategy in Tunisia, where Pap smear screening is not efficient. This study aims to estimate the prevalence and to identify risk factors associated with HPV infection among women from Grand Tunis, Tunisia. We conducted a cross-sectional study, between December 2012 and May 2013. Eligible women for this study were those aged 18–65 years, sexually active, who sought medical attention at their primary health care centre or clinic in Grand Tunis, Tunisia and who gave written consent. A liquid-based Pap smear sample was obtained from all women using a cervical brush. Only women with betaglobin positive test were further analysed for HPV detection and typing. A nested-PCR of the L1 region was performed followed by reverse line blot hybridization to facilitate the specific detection of 31 HPV genotypes. Multiple logistic regression modeling was used for the analysis of associations between variables with some considered possible confounders after checking for interactions. A total of 391 women were enrolled in this study and 325 out of the 391 cervical samples were positive for the betaglobin test. Overall HPV prevalence was 13.2% [9.8%−17.5%], with the following most prevalent HPV genotypes: HPV6 (40%), HPV40 (14%), HPV16 (12%), HPV52 (9%), HPV31 and HPV59 (7%), followed by HPV68 (4%). Mean age of HPV positive women was 40.7±0.92 years. Independently associated risk factors of HPV infection were smoking (OR:2.8 [0.8–9.6]), low income (OR:9.6 [1.4–63.4), bad housing type (OR:2.5 [1–6.8]), partner with multiple sexual relationship (OR:4.5 [0.9–22.9]) and single women (widowed, divorced, separated, never married) (OR:6.9 [1.1–42.2]). This study provides the first national-based estimate of HPV prevalence in Tunisia. Our findings contribute to the evidence on the current burden of HPV infection, the critical role of sexual behaviour and socioeconomic status and call for increased support for the screening program in Tunisia to prevent cervical cancer. These results allow us to evaluate the cost-effectiveness of vaccine program implementation in Tunisia in future.
Introduction
Human Papillomavirus (HPV) is the most common cause of sexually transmitted diseases and causes a wide range of pathologies [1, 2]. Although the majority of HPV infections are asymptomatic and self-limiting, the persistent infection with a high-risk HPV (HR-HPV) may cause precancerous lesions that can progress to cancer [1, 3, 4].
In the 1980s, the link between cervical cancer (CC) and HPV was established [5]. During the 1990s, the causal role of HPV was established and accounts worldwide for almost 99% of CC [4–8]. Two vaccines are currently available (Bivalent (HPV16/18 and Quadrivalent HPV6/11/16/18) to protect from HR-HPV-16 and 18 with a good safety and efficacy and a cross-protection against other common HR- HPV types [2, 9–11]. These vaccines have been widely introduced into the national immunization programs in most medium and high- income countries [2, 12].
In Tunisia CC is the third cause of cancer in women resulting in an estimated 1,000 deaths per year often in young women [13]. It represents a major health problem where national screening programs have not shown efficiency [14]. Whereas both vaccines are available in Tunisia, they have not yet been included in the national vaccination program. Such a decision should be informed by estimates of the national HPV prevalence data and a better understanding of the main circulating strains. Three previous Tunisian studies are available reporting different estimates due to differences in the participant recruitment methods, regional variability, and differences in detection tests [15–18]. To our knowledge, no national -based study has previously been conducted in our country.
The present study is a part of a national pilot study. It aims to estimate the prevalence and distribution of HPV genotypes and identify related risk factors among women in the Grand-Tunis region (the capital and main surroundings).
Materials and Methods
Study Population
A cross-sectional descriptive study was conducted between December 2012 and May 2013. Eligible women were those aged 18 to 65 years old, sexually active resident in the Grand Tunis region, seeking medical attention at their local healthcare centre (CSB) or at a regional reproductive health centre (CRSR) and who gave written consent. The two health centres are considered as a first line healthcare in Tunisia. Selection of CSBs and CRSRs was made proportionally to the size of the governorates between December 2012 and May 2013. As shown in Fig 1, the Grand Tunis region contains four governorates: Tunis, Ariana, Mannouba and Ben Arous.
Fig 1. Map showing the 4 governorates of the Grand Tunis region.
The sample size (n) was calculated for each governorate (Table 1) using the formula for a simple random sample: () with a 2% accuracy (d = 2%), an 8% estimated HPV infection prevalence as shown in previous Tunisian studies [17,18], an α error risk of 5% () and a correction factor of dE = 1.5.
Table 1. Sample size of each governorate included in the study.
| TUNIS | ARIANA | BEN AROUS | MANOUBA | Total | ||
|---|---|---|---|---|---|---|
| CSB | 78 | 44 | 49 | 34 | 205 | |
| CRSR | 75 | 36 | 45 | 30 | 186 | |
| Total | 153 | 80 | 94 | 64 | 391 | |
Data and sample collection
Properly trained medical doctors and midwives conducted face-to-face interviews using a standardized questionnaire, collecting information on socio-demographic status, sexual behaviour of the woman and her partner, reproductive and socio-economic status and medical history. For all women who provided written informed consent, a liquid-based cervical sample was obtained using a cervix brush (Cervexbrush, Easyfix solution Labonord, Templemars, France). Samples were kept at 4°C and sent to the Laboratory of Human and Experimental Pathology at the Pasteur Institute of Tunis (which is the EMRO WHO HPV Reference LabnetLaboratory) in less than one week. Part of the sample was used for a Pap smear and the remaining content stored at -20 C for further use in genomic DNA extraction.
Cytological Diagnosis
Cytological diagnosis was performed blindly by two pathologists of the laboratory of Human and Experimental Pathology at the Pasteur Institute and the final conclusion was taken by consensus according to the 2001 Bethesda system [19].
DNA extraction
DNA was extracted from a 200μl aliquot of the suspended cell samples using the QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer’s instructions. The quality of extracted DNA was evaluated by betaglobin-specific primers PC04/GH20. Only samples positive for betaglobin were further analysed for HPV detection and genotyping.
HPV detection
HPV was detected using nested PCR with biotinylated PGMY09/11 primers for the first PCR and GP5+/GP6+ primers for the second PCR. Briefly, 50μl mixture containing 3mM MgCl2, 10 μmol of each primer, 1.5 mM of dNTP (dATP, dCTP, TTP, dGTP), 5μl of the Taq DNA polymerase buffer, 1U of Taq DNA Polymerase, and 10 μl of DNA preparation was aliquoted. The PCR cycling parameters were composed of a 10 minutes initial denaturation at 94°C, followed by 30 amplification cycles of 30 s at 94°C, 1 min at 50°C and 1 min at 72°C, and a final extension step for 7 minutes at 72°C. This reaction was followed by a nested PCR using 10μl of the PGMY PCR product in a reaction mixture containing 50 μmol of GP5+/GP6+ primers, 3mM MgCl2, 1.5 mM each of the dNTP, 1U of Taq DNA Polymerase and 5μl of the Taq DNA polymerase buffer. The PCR cycling parameters comprised of a 10 minutes initial denaturation at 94°C, followed by 40 amplification cycles of 1 minutes at 94°C, 2 minutes at 40°C and 1.5 minute at 72°C, and a final extension step for 7 minutes at 72°C.
HPV genotyping
Genotyping of positive samples was performed by Reverse Line Hybridization as described in the Human Papillomavirus Laboratory Manual published by the World Health Organization [20]. Briefly, 15μL of denatured PCR products were allowed to hybridize with oligonucleotide probes specific for 31 HPV types (HPV6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 44, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 69, 70, 73, 82, 83, and 84) that were immobilized on a Biodyne C membrane using the Miniblotter MN45. The hybridized DNA was detected using Streptavidin peroxydase and Enhanced chemiluminiscent ECL®.
Statistical analysis
Qualitative variables were described by the simple counts and percentages; quantitative variables by the mean ± SEM. The distribution of HPV genotypes was summarized using frequency distributions. The relation of HPV positivity with demographic, epidemiological and clinical differences was first examined by univariate analysis using the two-sample t test for normally distributed continuous data, the Mann-Whitney test for non-parametric data, the chi-squared test for frequencies and the Fisher’s test and the chi-square test for trend. Odds Ratios (ORs) and 95% confidence intervals (CI) were used to quantify the association between risk factors and positivity for HPV. Multivariable analysis was done using multiple logistic regression to study the relationship between HPV infection (HPV-positive vs. HPV-negative) and the explanatory variables, while adjusting for confounding factors and effect modification if needed. Model building was done using backward procedures. Only variables that retained statistically significant associations with the outcome variables were left in multivariate analyses. A p-value <0.05 was considered statistically significant. Data analysis was performed using SPSS version 22 software.
Ethical considerations
The study was approved by the Ethics Committee of Institut Pasteur de Tunis and conducted in accordance with Good Clinical Practice, ensuring confidentiality and anonymity. Written informed consent was obtained prior to enrolment of study participants.
Results
Three hundred ninty-one women were enrolled in this study. Sixty-six out of 391 samples collected were negative for betaglobin and were excluded. HPV detection and typing were performed on the remaining 325 samples. Of note, no significant differences were observed between excluded women and included women in this study (S1 Table).
Socio-demographic characteristics of study population
Socio-demographic characteristics of the participants are presented in Table 2. The mean age of participants was 40.7± 0.5 years and 54% were over 40 years old. The majority of the participants were married (95%), 17% had no formal education, 64% were unemployed and only 11% had ever smoked. Median age at first sexual intercourse was 23 years (IQR 20–23) and 27% had multiple sexual partners.
Table 2. Characteristics of the studied women in the Grand Tunis region, Tunisia.
| N | % | |
|---|---|---|
| Age group (years) | ||
| < = 30 | 35 | 10.8% |
| [30–40[ | 115 | 35.5% |
| [40–50[ | 127 | 39.2% |
| >50 | 47 | 14.5% |
| Governorate | ||
| Tunis | 127 | 39.4% |
| Ariana | 70 | 20.6% |
| Ben Arous | 81 | 25.5% |
| Mannouba | 47 | 14.5% |
| Marital status | ||
| Married | 308 | 94.8% |
| Widowed, divorced, separated, never married | 17 | 5.2% |
| Level of education | ||
| Illiterate | 56 | 17.2% |
| Primary level | 164 | 50.5% |
| Secondary and high level | 105 | 32.3% |
| Smoking | ||
| Yes | 37 | 11.4% |
| No | 287 | 88.6% |
| Mean age at sexual intercourse debut (years ±SD) | 24.1±5.7 | |
| Mean number of sexual partners | 1.7±7.0 | |
HPV prevalence and HPV genotype
HPV prevalence in 325 cases was 13.2% (95% CI, 9.8%-17.5%). Prevalence of HPV infection according to socio-demographic, clinical and behaviour characteristics is summarised in Table 3.
Table 3. HPV Prevalence by socio-demographic characteristics, medical history and behaviour.
| Demographic Characteristic | Sample Size | HPV Prevalence [95% CI] | P value |
|---|---|---|---|
| Overall Age | 324 | 13.2% [9.8%-17.5%] | |
| Center | |||
| CSB | 27 | 62.8%[55.6%-70%] | 0.126 |
| CRSR | 16 | 37.2%[29.5%-44.9%] | |
| Age group (years) | |||
| < = 30 | 35 | 20.0% [10.4%-33.7%] | 0.45 |
| [30–40[ | 115 | 12.2% [7.5%-18.6%] | |
| [40–50[ | 127 | 11.0% [6.8%-16.9%] | |
| >50 | 47 | 17.0% [9.2%-28.3%] | |
| Governorate | |||
| Tunis | 127 | 9.4 [5.6%-15.0%] | 0.34 |
| Ariana | 70 | 12.9 [7.0%-21.3%] | |
| Ben Arous | 81 | 17.3 [10.8%-25.9%] | |
| Mannouba | 47 | 17.0 [9.2%-28.3%] | |
| Education level | |||
| Illiterate | 56 | 12.5 [6.4%-21.9%] | 0.17 |
| Primary level | 164 | 16.5 [11.7%-22.4%] | |
| Secondary and high level | 105 | 8.6 [4.7%-14.5%] | |
| Marital status | |||
| Married | 308 | 11.0% [8.0%-14.7%] | <10−3 |
| Widowed, divorced, separated, never married | 17 | 52.9%[32.9%-72.2%] | |
| Income Index | |||
| Below Poverty | 74 | 6.8% [3.0%-13.3%] | 0.15 |
| Above Poverty Intermediate Index | 136 | 13.2% [8.7%-19.3%] | |
| Above Poverty High Index | 89 | 16.9% [10.7%-25.0%] | |
| Housing Type | |||
| Good | 97 | 18.6% [12.2%-26.6%] | 0.07 |
| Bad | 226 | 11.1% [8.0%-15.9%] | |
| Occupation | |||
| unemployed | 207 | 11.1% [7.6%-15.7%] | 0.11 |
| With regular job | 115 | 17.4% [11.7%-24.6%] | |
| Tobacco use | |||
| Yes | 37 | 29.7%[18.0%-44.1%] | 0.02 |
| No | 287 | 11.1%[8.1%-15.0%] | |
| Menopause | |||
| Yes | 51 | 15.7% [8.4%-26.3%] | 0.6 |
| No | 274 | 12.8% [9.4%-16.9%] | |
| Pregnancy | |||
| Yes | 9 | 33.3% [13.7%-66.0%] | 0.06 |
| No | 296 | 12.2% [9.0%-16.1%] | |
| Contraception | |||
| Yes | 88 | 14.8% [9.0%-22.6%] | 0.6 |
| No | 231 | 12.4% [8.9%-17.0%] | |
| Medical history of chronic disease | |||
| Yes | 102 | 10.8%[6.3%-17.3%] | 0.4 |
| No | 223 | 14.3%[10.4%-19.2%] | |
| Surgical history | |||
| Yes | 126 | 15.1%[10.0–21.6%] | 0.4 |
| No | 198 | 12.1%[8.3%-16.9%] | |
| casual sexual relation the last 12 months | |||
| Yes | 12 | 50.0%[27.7%-72.3%] | <10−3 |
| No | 288 | 11.5%[8.3%-15.3%] | |
| Sexually transmitted infection history | |||
| Yes | 87 | 16.1%[10.0%-24.2%] | 0.35 |
| No | 238 | 12.2%[8.7%-16.6%] | |
| Multiple sexual intercourse of partner | |||
| Yes | 29 | 31.0% [17.9%-47.3%] | 0.001 |
| No | 277 | 10.5% [9.1%-17.3%] | |
| age at first sexual intercourse | |||
| ≤ 18 years | 39 | 20.5% [17.8%-24.0%] | 0.13 |
| >18 years | 286 | 12.2% [11.2%-13.5%] | |
| Multiple sexual partners | |||
| Yes | 18 | 33.3% [29.1%-38.4%] | 0.015 |
| No | 236 | 11.0% [10.0%-12.3%] | |
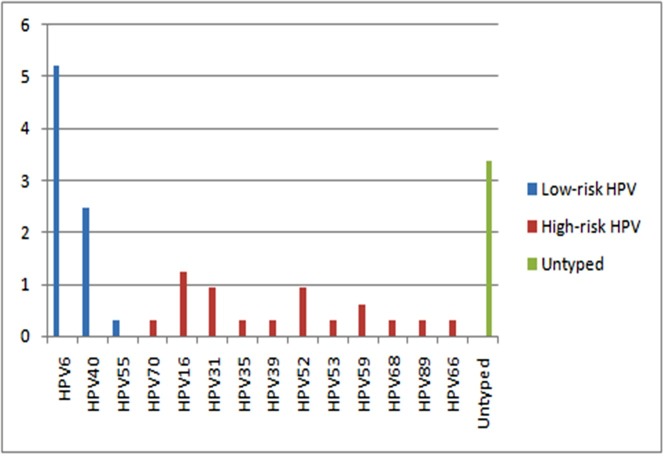
The prevalence of HR-HPV and low-risk HPV (LR-HPV) types were 3.1% (95% CI, 1.5%-5.2%) and 6.5% (95% CI, 4.0%-9.5%), respectively. Multiple infections were detected in only 18% of positive samples (8/43) and single infections in 55.8% (24/43). Of the 8 multiple HPV infected samples, 5 were infected with 2 HPV types, 2 were infected with 3 HPV types, and 1 was infected with 4 HPV types. Multiple infections with both HR-HPV and LR-HPV types were the most common (62.5%). mixed infections with HR-HPVs were observed in 25% of cases while mixed infections with LR-HPVs were seen in 12.5% of cases. The most common HR-HPVs were HPV16 (1.2%; 95% CI, 1.0%-1.6%), HPV31 and HPV52 (0.9%; 95% CI, 0.8%-1.2%). HPV6 (5.2%; 95% CI, 4.6%-6.0%) and HPV40 (2.5%; 95% CI, 2.1%-3.0%) were the most common in LR-HPV. The prevalence of detected HPV types is shown in Fig 2.
Fig 2. HPV Type prevalence in Grand Tunis Region.
HPV distribution according to age
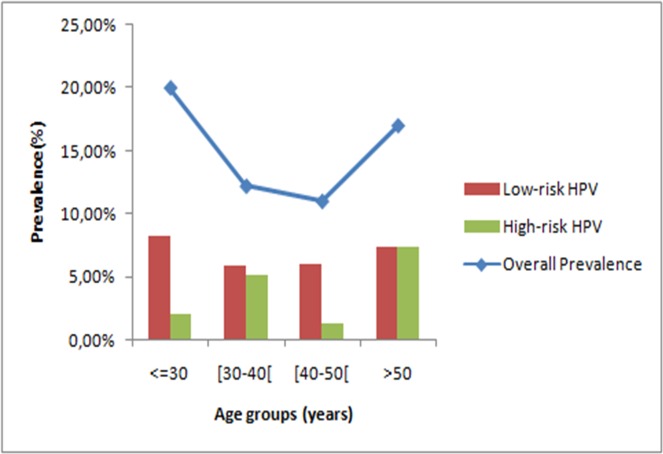
Prevalence of HPV infection was highest among women aged less than 30 years (20%; 95% CI, 10.4%-33.7%), followed by a non-significant decline in HPV prevalence in women aged 30 to 50 years and a new increase after 50 years of age (p = 0.45). There was a statistically significant difference between low- and high-risk HPV prevalence trends by age (Fig 3). Prevalence of HR-HPV types increased significantly with age (p = 0.04), while this was not the case with LR-HPV prevalence (p = 0.8) as showed in Fig 3.
Fig 3. HPV prevalence according to age groups.
The distribution of detected genotypes according to age groups showed that HPV 16 was the most common type in women aged 31–40 and over 50 years-old. HPV31 was present in all age groups except in women aged 41–50 year. Here HPV6, HPV40, and HPV35 were the major genotypes (Table 4).
Table 4. HPV type distribution according to age groups.
| Age groups | ||||
|---|---|---|---|---|
| HPV Genotypes | < = 30 | 31–40 | 41–50 | >50 |
| HPV6 | 11,8% | 41,2% | 35,3% | 11,8% |
| HPV40 | 37,5% | 25,0% | 25,0% | 12,5% |
| HPV55 | 0,0% | 0,0% | 0,0% | 100,0% |
| HPV70 | 0,0% | 0,0% | 100,0% | 0,0% |
| HPV16 | 0,0% | 50,0% | 0,0% | 50,0% |
| HPV31 | 33,3% | 33,3% | 0,0% | 33,3% |
| HPV35 | 0,0% | 0,0% | 100,0% | 0,0% |
| HPV39 | 0,0% | 100,0% | 0,0% | 0,0% |
| HPV52 | 33,3% | 33,3% | 0,0% | 33,3% |
| HPV53 | 0,0% | 100,0% | 0,0% | 0,0% |
| HPV59 | 0,0% | 50,0% | 0,0% | 50,0% |
| HPV68 | 0,0% | 0,0% | 100,0% | 0,0% |
| HPV89 | 0,0% | 0,0% | 100,0% | 0,0% |
| HPV66 | 0,0% | 0,0% | 100,0% | 0,0% |
HPV distribution according to cytology
A total of 27 (8.3%) cervical cytology samples were inadequate. For those with adequate results, Pap smear was normal for 115 women (35.4%, 95% CI, 33.4%-44.4%). It was atrophic or inflammatory for 141 women (43.4%, 95% CI, 41.9%-52.8%), Low-squamous intraepithelial lesions (LSIL) were seen for 38 women (11.7%, 95% CI, 9.5%-16.7%) and High-squamous intraepithelial lesion (HSIL) and Atypical glandular undetermined significance (AGUS) accounted for two cases (0.6%, 95% CI, 0.2%-1.9%). HPV prevalence was 16.5% (95% CI, 11.0%-23.6%) in normal smear, 10.6% (95% CI, 6.6%-16.1%) in atrophic or inflammatory smears and 18.4% (95% CI, 9.6%-31.3%) in LSIL. HPV infection in HSIL accounted for 50% of cases and all were single HPV31 infections. The 2 cases of AGUS were HPV negative.
Risk factors for HPV infection
Associated factors with HPV infection in univariate analysis were marital status, tobacco use, sexual behaviour and partner sexual behaviour (Table 5).
Table 5. Univariate analysis of associated factors with HPV infection.
| Characteristic | OR [95% CI] | P value* |
|---|---|---|
| Marital status | ||
| Married | Ref | |
| Widowed, divorced, separated, never married | 6.9 [1.1–42.2] | <10−3 |
| Income Index | ||
| Below Poverty Index | Ref | |
| Above Poverty Intermediate Index | 9.6 [1.4–63.4] | 0.019 |
| Above Poverty high Index | 22.1 [2.8–175.5] | 0.003 |
| Housing Type | ||
| Good | Ref | |
| Bad | 2.5 [1–6.8] | 0.05 |
| Smoking | ||
| Yes | 2.8 [0.8–9.6] | 0.003 |
| No | Ref | |
| Partner with multiple sexual encounters outside relationship | ||
| Yes | 4.5 [0.9–22.9] | 0.07 |
| No | ||
| Age at sexual intercourse debut | ||
| ≤ 18 years | 2.7 [0.8–9.5] | 0.12 |
| >18 years | Ref | |
* χ2 Wald
Multivariate analysis suggests that marital status, partner with multiple sexual encounters outside the stable relationship, housing type and income index were independently associated with HPV infection (Table 6). However, the age at sexual intercourse debut and the increasing number of sexual encounters of the partner were not significant in the multivariate model.
Table 6. Multivariable analysis of associated factors with HPV infection (N = 219).
| Characteristic | AOR [95% CI] | P value* |
|---|---|---|
| Marital status | ||
| Married | Ref | |
| Widowed, divorced, separated, never married | 6.9 [1.1–42.2] | <10−3 |
| Income Index | ||
| Below Poverty Index | Ref | |
| Above Poverty Intermediate Index | 9.6 [1.4–63.4] | 0.019 |
| Above Poverty high Index | 22.1 [2.8–175.5] | 0.003 |
| Housing Type | ||
| Good | Ref | |
| Bad | 2.5 [1–6.8] | 0.05 |
| Smoking | ||
| Yes | 2.8 [0.8–9.6] | 0.003 |
| No | Ref | |
| Partner with multiple sexual encounters outside relationship | ||
| Yes | 4.5 [0.9–22.9] | 0.07 |
| No | ||
| Age at sexual intercourse debut | ||
| ≤ 18 years | 2.7 [0.8–9.5] | 0.12 |
| >18 years | Ref | |
* χ2 Wald
Discussion
Baseline information on HPV prevalence and genotype distribution is crucial to evaluate the impact of HPV vaccines and inform the best approach for cervical cancer prevention. Our cross-sectional study estimated HPV prevalence in women residing in the Grand Tunis region and is a part of the first large-scale national-based epidemiological study on the prevalence of HPV infection and genotype distribution.
In this study, the prevalence of HPV infection in the Grand Tunis region was estimated as 13.2%. The few previous studies in Tunisia that focused on a small series of participants in cervical screening programs have shown divergent results, that is 43.8% among Tunisian female prostitutes [16], 7.8% in the reproductive health centre of Ariana [17] and 6.5% in Urban Tunis region [18]. Heterogeneity with regards to the methods for participant selection and the representativeness in the population and the lack of epidemiological and behavioural data make any comparison with the current study findings difficult. Several meta-analyses [21–23] confirmed that HPV infection varied by geographical area, age, life style and socio-economic status. Difference in HPV prevalence might also partly be attributed to the difference in sample population and methods used. The HPV prevalence estimated in our study is similar to that reported in a study in Morocco [24], but higher than that reported in other North African countries, namely 6.3% in Algeria [25] and 10.3% in Egypt [26].
In this study, HR-HPV accounted for about half of all HPV infections, with the most prevalent HR-HPV being HPV-16, 31, 52 and 59. Comparing with reported series, HPV16 was the most common genotype [24, 25,26] and HPV52 and 31 were the second most common genotypes in Africa and Europe, respectively [21–23]. Of note, HPV18 the second most frequently detected HR-HPV worldwide [21–23] was surprisingly scarce in our study population. In our study, almost four per cent of the samples could not be genotyped. This may be due to the fact that some samples had low viral load and/or some HPV types were not included in the tested probes [27].
Two peaks of infection by age appear clearly in our results. One peak in ages less than 30 years, which could represent the beginning of sexual activity, and another peak in ages greater than 50 years. This second peak may partly be explained by a relative lack of viral clearance and insufficient adaptive immune responses at this age caused by hormonal changes, contributing to HPV persistence or reactivation of latent HPV infections [28–31]. Our results were consistent with the meta-analysis conducted by Bruni et al. [22] wherein the age distribution of cervical HPV infection showed a bimodal curve with a first peak at younger ages (<25 years), that is just after sexual intercourse debut, a low prevalence plateau within the middle age groups (30–40 years), and a rebound at older ages (>45 years). Previous studies showed that the prevalence of multiple HPV infection was the highest in the oldest age group [32–33]. A possible explanation is that the immune response to HPV infection in older women is relatively low, and immunomodulation of the virus is suppressed [30–34]. These results indicate that women in the young and old age group need more clinical surveillance and management of HPV infection.
In this study HPV infection in normal Pap smears accounted for 16.5% (95% CI, 11.0%-23.6%). This prevalence is similar to reported series in Africa (7.3%-37.1%), America (4.6%-42.2%) and Europe (8.5%-22.7%) [22].
In our study, risk factors were shown to be specific: the better the income, the greater the risk of HPV infection. Several risk factors related to HPV infection and persistence, such as age, smoking, age at first sexual relation and multiple sexual partners, are reported in the world with differences due to the specificities of each studied population [1, 23, 25, 29]. In contradiction with our data, low-income level increases the risk of HPV infection, probably related to lack of access to proper care, which facilitates infection and persistence of HPV and an increasing risk of turning into cancer [35–37].
It is established that smoking increases the risk of HPV infection and persistence through a vulnerability of immune system which facilitates infection and persistence of HPV and an increasing risk of turning into cancer [38]. In our study, current smokers seem to be almost three times more at risk of getting HPV infection compared to non-smokers. However, the association is not significant, probably due to lack of power of a limited sample size. In the literature, it has been shown that smoking was associated with an increased prevalence of HPV [29, 38–40].
Various aspects of sexual behaviour were reported to be related to the acquisition of HPV infection [23, 29, 40]. In the present study, the marital status appears as a factor associated with HPV infection. Single or divorced women are more often infected than married ones (52.9% versus 11%). This could be explained by a different way of life that could lead them to have more than one sexual partner [40]. However, unlike previous studies [40–42], neither age at the first intercourse nor the number of sexual partner, were significantly associated with HPV infection. These findings need to be considered in light of the extremely sensitive nature of this question and possible inaccurate answer, leading to exposure misclassification bias.
Conclusion
HPV infection prevalence in the Grand Tunis region was 13.2% and is larger than previous estimates in Tunisia. HR-HPV types accounted for half of all infections and were mainly HPV-16, 31, 52 and 59. Smoking, sexual behaviour of partner and high level income were the main risk factors. This study is the pilot phase of a national survey which aims to assess the national HPV prevalence in the whole of Tunisia and provide baseline data to enhance the understanding of HPV infection. Those findings will inform recommendations to optimize HPV screening efforts for high-risk genotypes, the most affected age groups and to evaluate the cost-effectiveness of vaccine program implementation in Tunisia in future.
Supporting Information
(DOC)
Acknowledgments
The authors thank all the investigators, regional supervisors and data entry operator for their contribution to this study. We also thank Pride Linda for her critical review of the manuscript.
Data Availability
According to Tunisian Law N° 2004-63 of 27 July 2004, related to personal data in Tunisia, access to the database needs anterior authorization of the National Instance of protection of personal data. Moreover, the approval of the Ethics Committee did not involve transfer of data or public access to the database. A specific agreement is needed. Thus, we are unable to share our data publicly. However, data are available at the “Observatoire National des Maladies Nouvelles et Emergentes” and “Institut Pasteur de Tunis”. For any data requests, please contact: Pr. Nissaf Bouafif ONMNE (Email: nissafba@yahoo.fr) and/or Pr. Emna Ennaifer IPT (Email: Emna.Jerbi@pasteur.rns.tn).
Funding Statement
The project of "National HPV prevalence in Tunisia" has been granted by the "African Development bank" (http://www.afdb.org/en/) under the grant number: 55001550022552. Grant recipient is Pr. N. Bouafif, Director of the "Observatoire National des Maladies Nouvelles et Emergentes de Tunis". The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sudenga S, Shrestha S. Key considerations and current perspectives of epidemiological studies on human papillomavirus persistence, the intermediate phenotype to cervical cancer. International Journal of Infectious Diseases. 2013; 17(4):e216–e220. 10.1016/j.ijid.2012.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Human papillomavirus vaccines: WHO position paper. Wold Health Organisation; 2014. [Google Scholar]
- 3.Doorbar J, Quint W, Banks L, Bravo I, Stoler M, Broker T, et al. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine. 2012; 30: F55–F70. 10.1016/j.vaccine.2012.06.083 [DOI] [PubMed] [Google Scholar]
- 4.Zur Hausen H. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events?. The Lancet. 1982; 320(8312):1370–1372. [DOI] [PubMed] [Google Scholar]
- 5.Walboomers J, Jacobs M, Manos M, Bosch F, Kummer J, Shah K, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 6.Dillner J. Prevention of Human Papillomavirus–Associated Cancers. Seminars in Oncology. 2015;42(2):272–283. 10.1053/j.seminoncol.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 7.IARC working group. Human papillomavirus. IARC Monographson the evaluation of carcinogenics risk to humans Vol.64 Lyon: International agency for research on cancer; 1995. [Google Scholar]
- 8.Bosch F, Lorincz A, Munoz N, Meijer C, Shah K. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology. 2002;55(4):244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Einstein M, Baron M, Levin M, Chatterjee A, Edwards R, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix ™ and Gardasil ® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Human Vaccines. 2009;5(10):705–719. [DOI] [PubMed] [Google Scholar]
- 10.Villa L. Prophylactic HPV vaccines: Reducing the burden of HPV-related diseases. Vaccine. 2006;24:S23–S28. [DOI] [PubMed] [Google Scholar]
- 11.De Vincenzo R, Ricci C, Conte C, Scambia G. HPV vaccine cross-protection: Highlights on additional clinical benefit. Gynecologic Oncology. 2013;130(3):642–651. 10.1016/j.ygyno.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 12.Jumaan A, Ghanem S, Taher J, Braikat M, Awaidy S, Dbaibo G. Prospects and Challenges in the Introduction of Human Papillomavirus Vaccines in the Extended Middle East and North Africa Region. Vaccine. 2013;31:G58–G64. 10.1016/j.vaccine.2012.06.097 [DOI] [PubMed] [Google Scholar]
- 13.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127(12):2893–2917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 14.Dimassi K, Hleili W, Saidi O, Ben Alaya N, Ben Romdhane H. Knowledge and uptake of genital cancer screening methods among Tunisian women. International Journal of Gynecology & Obstetrics. 2015;128(3):268–269. [DOI] [PubMed] [Google Scholar]
- 15.De Marco F, Houissa-Kchouk F, Khelifa R, Marcante M. High-risk HPV types in Tunisia. A pilot study reveals an unexpectedly high prevalence of types 58 and 82 and lack of HPV 18 among female prostitutes. J Med Virol. 2006;78(7):950–953. [DOI] [PubMed] [Google Scholar]
- 16.Ennaifer E, Tounsi H, Ben Aissa R, Kalai K, Fehri E, Laassili T. Screening of cervical HPV infection at the reproductive health centre of ariana. Tunis Med. 2014. April;92(4):253–7. [PubMed] [Google Scholar]
- 17.Guettiti H, Ennaifer E, Attia L, Chelly D, Alaya N, Aissa R, et al. Pre-vaccination prevalence and genotype distribution of human papillomavirus infection among women from urban Tunis: a cross-sectional study. Asian Pacific Journal of Cancer Prevention. 2014;15(21):9361–9365. [DOI] [PubMed] [Google Scholar]
- 18.Hassen E1, Remadi S, Chouchane L. Detection and molecular typing of human papillomaviruses: prevalence of cervical infection in the Tunisian central region. Tunis Med. 1999. Oct;77(10):497–502. [PubMed] [Google Scholar]
- 19.Bethesda System 2001. Acta Cytologica. 2001;45(6):1077–1078. [Google Scholar]
- 20.World Health Organization (2009): Human papillomavirus laboratory manual. 1st eds Unger E. R, Dillner J, Zhou T. Geneva, Switzerland.
- 21.Clifford G, Gallus S, Herrero R, Muñoz N, Snijders P, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. The Lancet. 2005;366(9490):991–998. [DOI] [PubMed] [Google Scholar]
- 22.Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch F, de Sanjosé S. Cervical Human Papillomavirus Prevalence in 5 Continents: Meta‐Analysis of 1 Million Women with Normal Cytological Findings. The Journal of Infectious Diseases. 2010;202(12):1789–1799. 10.1086/657321 [DOI] [PubMed] [Google Scholar]
- 23.Ogembo R, Gona P, Seymour A, Park H, Bain P, Maranda L, et al. Prevalence of Human Papillomavirus Genotypes among African Women with Normal Cervical Cytology and Neoplasia: A Systematic Review and Meta-Analysis. PLOS ONE. 2015;10(4):e0122488 10.1371/journal.pone.0122488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Mzibri M, Alhamany Z, Kharbach A, Malihy A, Abouqal R, Jaddi H, et al. Prevalence of human papillomavirus genotype among Moroccan women during a local screening program. J Infect Dev Ctries. 2010;4(11). [DOI] [PubMed] [Google Scholar]
- 25.Hammouda D, Clifford G, Pallardy S, Ayyach G, Chékiri A, Boudrich A, et al. Human papillomavirus infection in a population-based sample of women in Algiers, Algeria. International Journal of Cancer. 2010;128(9):2224–2229. [DOI] [PubMed] [Google Scholar]
- 26.Shaltout M, Sallam H, AbouSeeda M, Moiety F, Hemeda H, Ibrahim A, et al. Prevalence and type distribution of human papillomavirus among women older than 18 years in Egypt: a multicenter, observational study. International Journal of Infectious Diseases. 2014;29:226–231. 10.1016/j.ijid.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 27.Milutin Gašperov N, Sabol I, Matovina M, Spaventi Š, Grce M. Detection and Typing of Human Papillomaviruses Combining Different Methods: Polymerase Chain Reaction, Restriction Fragment Length Polymorphism, Line Probe Assay and Sequencing. Pathology & Oncology Research. 2008;14(4):355–363. [DOI] [PubMed] [Google Scholar]
- 28.Mbulawa Z, Coetzee D, Williamson A. Human papillomavirus prevalence in South African women and men according to age and human immunodeficiency virus status. BMC Infect Dis. 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan P, Ho W, Wong M, Chang A, Chor J, Yu M. Epidemiologic risk profile of infection with different groups of human papillomaviruses. J Med Virol. 2009;81(9):1635–1644. 10.1002/jmv.21575 [DOI] [PubMed] [Google Scholar]
- 30.Caiyan X, Weiyuan Z, Minghui W, Songwen Z. Prevalence and risk factors of lower genital tract infections among women in Beijing, China. Journal of Obstetrics and Gynaecology Research. 2011;38(1):310–315. 10.1111/j.1447-0756.2011.01624.x [DOI] [PubMed] [Google Scholar]
- 31.Brismar-Wendel S, Froberg M, Hjerpe A, Andersson S, Johansson B. Age-specific prevalence of HPV genotypes in cervical cytology samples with equivocal or low-grade lesions. Br J Cancer. 2009;101(3):511–517. 10.1038/sj.bjc.6605165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grainge M, Seth R, Guo L, Neal K, Coupland C, Vryenhoef P, et al. Cervical Human Papillomavirus Screening among Older Women. Emerg Infect Dis. 2005;11(11):1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez P, Hildesheim A, Rodriguez A, Schiffman M, Porras C, Wacholder S, et al. Behavioral/Lifestyle and Immunologic Factors Associated with HPV Infection among Women Older Than 45 Years. Cancer Epidemiology Biomarkers & Prevention. 2010;19(12):3044–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith J, Melendy A, Rana R, Pimenta J. Age-Specific Prevalence of Infection with Human Papillomavirus in Females: A Global Review. Journal of Adolescent Health. 2008;43(4):S5.e1–S5.e62. [DOI] [PubMed] [Google Scholar]
- 35.Hildesheim A, Gravitt P, Schiffman M, Kurman R, Barnes W, Jones S, et al. Determinants of Genital Human Papillomavirus Infection in Low-Income Women in Washington, D.C. Sexually Transmitted Diseases. 1993;20(5):279–285. [DOI] [PubMed] [Google Scholar]
- 36.Bauer H, Hildesheima A, Schiffman M, Glass A, Rush B, Scott D, et al. Determinants of Genital Human Papillomavirus Infection in Low-Risk Women in Portland, Oregon. Sexually Transmitted Diseases. 1993;20(5):274–278. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell S, Sekikubo M, Biryabarema C, Byamugisha J, Steinberg M, Jeronimo J, et al. Factors associated with high-risk HPV positivity in a low-resource setting in sub-Saharan Africa. American Journal of Obstetrics and Gynecology. 2014;210(1):81.e1–81.e7. [DOI] [PubMed] [Google Scholar]
- 38.Moralejo D. Smoking increased risk of cervical cancer, independent of infection with high-risk HPV types. Evidence-Based Nursing. 2009;12(4):122–122. 10.1136/ebn.12.4.122 [DOI] [PubMed] [Google Scholar]
- 39.Coker A, Bond S, Williams A, Gerasimova T, Pirisi L. Active and passive smoking, high-risk human papillomaviruses and cervical neoplasia. Cancer Detection and Prevention. 2002;26(2):121–128. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Liu W, Liu Y, Ye X, Chen S. Multiple Sexual Partners as a Potential Independent Risk Factor for Cervical Cancer: a Meta-analysis of Epidemiological Studies. Asian Pacific Journal of Cancer Prevention. 2015;16(9):3893–3900. [DOI] [PubMed] [Google Scholar]
- 41.Ojiyi E, Dike I, Okeudo C, Ejikem C, Nzewuihe A, Agbata A. Local risk factors in genital human papilloma Virus Infection in cervical smears. Annals of Medical and Health Sciences Research. 2013;3(4):529 10.4103/2141-9248.122082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi R, Devarakonda S, Liu L, Taylor H, Mills G. Factors associated with genital human papillomavirus infection among adult females in the United States, NHANES 2007–2010. BMC Research Notes. 2014;7(1):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
According to Tunisian Law N° 2004-63 of 27 July 2004, related to personal data in Tunisia, access to the database needs anterior authorization of the National Instance of protection of personal data. Moreover, the approval of the Ethics Committee did not involve transfer of data or public access to the database. A specific agreement is needed. Thus, we are unable to share our data publicly. However, data are available at the “Observatoire National des Maladies Nouvelles et Emergentes” and “Institut Pasteur de Tunis”. For any data requests, please contact: Pr. Nissaf Bouafif ONMNE (Email: nissafba@yahoo.fr) and/or Pr. Emna Ennaifer IPT (Email: Emna.Jerbi@pasteur.rns.tn).