Abstract
Purpose
To evaluate the accuracy of shear wave elastography (SWE) in the quantitative diagnosis of liver fibrosis severity.
Methods
The published literatures were systematically retrieved from PubMed, Embase, Web of science and Scopus up to May 13th, 2016. Included studies reported the pooled sensitivity, specificity, positive and negative predictive values, as well as the diagnostic odds ratio of SWE in populations with liver fibrosis. A bivariate mixed-effects regression model was used, which was estimated by the I2 statistics. The quality of articles was evaluated by quality assessment of diagnostic accuracy studies (QUADAS).
Results
Thirteen articles including 2303 patients were qualified for the study. The pooled sensitivity and specificity of SWE for the diagnosis of liver fibrosis are as follows: ≥F1 0.76 (p<0.001, 95% CI, 0.71–0.81, I2 = 75.33%), 0.92 (p<0.001, 95% CI, 0.80–0.97, I2 = 79.36%); ≥F2 0.84 (p = 0.35, 95% CI, 0.81–0.86, I2 = 9.55%), 0.83 (p<0.001, 95% CI, 0.77–0.88, I2 = 86.56%); ≥F3 0.89 (p = 0.56, 95% CI, 0.86–0.92, I2 = 0%), 0.86 (p<0.001, 95% CI, 0.82–0.90, I2 = 75.73%); F4 0.89 (p = 0.24, 95% CI, 0.84–0.92, I2 = 20.56%), 0.88 (p<0.001, 95% CI, 0.84–0.92, I2 = 82.75%), respectively. Sensitivity analysis showed no significant changes if any one of the studies was excluded. Publication bias was not detected in this meta-analysis.
Conclusions
Our study suggests that SWE is a helpful method to appraise liver fibrosis severity. Future studies that validate these findings would be appropriate.
Introduction
Liver fibrosis is a diffuse excessive deposition of extracellular matrix especially collagen material in the liver, which is a repair response mechanism after chronic liver injury of various causes [1]. Mild to moderate fibrosis is reversible while cirrhosis, the endstage outcome of fibrosis, is generally irreversible. Traditionally, although the liver biopsy remains as the gold standard to measure fibrosis as it offers precise diagnostic information, it could lead to various complications [2]. This could be affected by targeted sampling error, heterogeneity of liver fibrosis and limited sampling range. Some patients even can not accept the repeated sampling. Recently, emerging studies have depicted non-invasive ways to quantify the severity of liver fibrosis, such as serum markers, radiological imaging and elastography. Transient elastography (TE) is performed by using FibroScan, offering a quantifiable value of liver stiffness (kPa), however, the accuracy of TE evaluation of fibrosis severity is insufficient and the liver stiffness measurement (LSM) threshold of the different stages overlap. It is susceptible to abdominal effusion, obesity and breathing. Its performance in moderate liver fibrosis is especially low [3]. Acoustic radiation force impulse (ARFI) measures the average elasticity value in the region of interested (ROI) with the standard deviation, which can not provide elastic measurement in real time. Most imaging-based techniques do well at discerning patients at the extremes of fibrosis but could not exactly distinguish intermediate stages [4]. More recently, real-time shear wave elastography (SWE) that was first reported by Bercoff J et al in 2004, is a two-dimensional transient elastography technique based on the principle of Mach Cones for noninvasive evaluation of liver fibrosis [5, 6]. The key of the image is shear wave from radiation force generated by an amplitude modulated beam of focused ultrasound. These waves then are detected by a proper imaging modality [7]. The Young’s modulus is calculated via E = 3ρC2, where ρ is the density and the shear wave speed C is a time-of-flight estimation between two points during the shear wave propagation. The SWE mode shows a region of higher stiffness coded as a red area. Lesion margins are much better depicted on the elastography than on the ultrasound grayscale image [8]. Liver fibrosis also has a greater elastic coefficient than the normal hepatic tissue.
Despite its benefits, SWE also has some limitations. For example, SWE is difficult to be applied in skeletal muscle system due to the insufficient resolution, in which it has to depend on the legible two-dimensional images. There is a lack of large scale prospective studies on the application of SWE in the diagnosis of liver fibrosis and since there is limited number of studies, the effectiveness of this technique is still inconsistent [9–21]. Thereby, we conducted a meta-analysis to appraise the accuracy of SWE in them (≥F1; ≥F2; ≥F3 and F4).
Material and Methods
2.1. Search strategy
This meta-analysis was performed on the basis of the PRISMA statement [22] (S1 and S2 Files). A systematic literature search was independently conducted by two individual investigators with the same method from PubMed, Embase, Web of science and Scopus up to May 13th, 2016, using the keywords “shear wave elastography”, “supersonic shear imaging”, “liver”, “hepar” and “hepatic” (S3 File). Data were obtained from the full-published paper and no language or race restriction was utilized. Furthermore, additional relevant published references were manually retrieved.
2.2. Selection criteria
The included studies had to meet the following criterias: (1) The study appraised the performance of SWE for the diagnosis of liver fibrosis. (2) Histopathological examination on a METAVIR fibrosis scale as the gold standard was applied to identify the classification of liver fibrosis. (3) Available data could be used to compute the true-positive, false-positive, true-negative and false-negative results of SWE for diagnosis of this disease. (4) Prospective and retrospective studies were included in this study. Researches with greater sample sizes were brought in when overlapping patient samples were recruited in more than one study.
2.3. Exclusion criteria
The exclusion criterias were as follows: (1) conference abstracts, case reports, review articles or inadequate data descriptions; (2) if the same study appeared in other publications, only studies with greater sample sizes were selected for this study.
2.4. Data extraction and quality assessment
According to the METAVIR scoring system, the histologic staging of fibrosis was classified into five stages: F0, no fibrosis; F1, early fibrosis (portal fibrosis without septa); F2, moderate fibrosis (portal fibrosis and few septa); F3, severe fibrosis (numerous septa without cirrhosis); and F4, cirrhosis [23]. Data were independently extracted by two investigators for these information, including the first author, date of publication, study design, population characteristics, country, male/female, age, BMI and cut-off value, with disagreements determined by consulting a third investigator. The quality assessment of diagnostic accuracy studies (QUADAS) questionnaire was applied to estimate the quality of the recruited articles, which was intended to estimate the internal and external validity of diagnostic accuracy studies included in the meta-analysis [24]. The QUADAS tool has 14 items appraising study design-related questions and the validity of the results in the study. Each item may be recorded as ‘‘yes”, ‘‘no” or ‘‘unclear”.
2.5. Statistical analysis
We computed the pooled sensitivity, specificity, positive and negative likelihood ratios, and the diagnostic odds ratio of SWE, and 95% CIs by a bivariate mixed-effects regression model. We can depict the sensitivity versus specificity and figure out the area under the curve (AUROC) through a summary receiver operating characteristic (SROC) curve [25]. We also estimated the level of between-study heterogeneity using the I2 test [26]. Additionally, publication bias was tested by regression of diagnostic odds ratio (lnDOR) against the inverse of the square root of the effective sample size (1/ESS1/2) and weighting by ESS. If P was less than 0.05 for the slope coefficient, it suggested marked asymmetry [27]. Threshold effects were evaluated by the Spearman correlation coefficient. Fagan nomograms were conducted as measures of post-test probabilities on the basis of the pooled sensitivity and specificity. All statistical analyses were performed by Stata 12.0 and MetaDiSc version 1.4 software.
Results
3.1. Characteristics of eligible studies
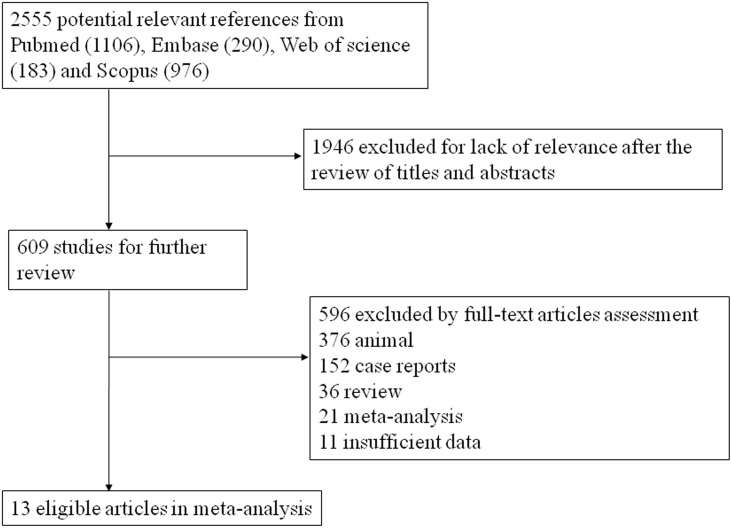
We identified 2303 cases (≥F1: 1226; ≥F2: 2073; ≥F3: 1836; F4: 1989) from 13 eligible studies up to May 13th, 2016 through the mentioned search strategies (Fig 1). The main characteristics of included studies for this meta-analysis are summarized in Table 1. They were all prospective cohort studies. The mean age of included patients was 36, and 57.1% were females. The most common underlying diseases were hepatitis B or C, and all patients underwent biopsy and the diagnosis was based on the histopathological examinations. The QUADAS scale showed that most of the studies were appraised as being of good quality (Table 2).
Fig 1. Flow diagram of the study selection process.
Table 1. Summaries of the studies included.
| Author | Design | Population characteristics | Country | Male/female | Age (years) | BMI | Cut-off (Kpa) | Liver biopsy for fibrosis staging | Manufacturers of the instrument for SWE |
|---|---|---|---|---|---|---|---|---|---|
| Ferraioli G et al.2012 | Prospective cohort study | Patients with chronic hepatitis C | Italy | 87/34 | 44.8±11.9 | 25.4±3.8 | ≥F2:7.1;≥F3:8.7;F4:10.4 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Leung VY et al.2013 | Prospective cohort study | Patients with chronic hepatitis B | China | 214/183 | 48.8±12.3 | 24.2±18.6 | ≥F1:6.5;≥F2:7.1;≥F3:7.9;F4:10.1 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Cassinotto C et al.2014 | Prospective cohort study | Patients with 8 hepatitis C;33 hepatitis B;145 non-alcoholic steatohepatitis;8 viral reactivation post-liver transplantation;5 sclerosing cholangitis;16 autoimmune diseases;7 hepatitis E;7 primary biliary cirrhosis;13 drug-related hepatitis;3 hemochromatosis;2 overlap syndrome;31 unexplained chronic cytolysis | France | 188/161 | 54.8±14 | 27.4±6.4 | ≥F1:7.8;≥F2:8;≥F3:8.9;F4:10.7 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Zeng J et al.2014 | Prospective cohort study | Patients with chronic hepatitis B | China | a)169/37;b)82/22 | a)36.3±9.4;b)37.2±10.9 | a)22.3±3.2;b)22.1±3.4 | ≥F2:7.2;≥F3:9.1;F4:11.7 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Beland M et al.2014 | Prospective cohort study | Patients with 21 hepatitis C;15 elevated liver function tests;5 nonalcoholic steatohepatitis;3 cirrhosis;3 autoimmune hepatitis;2 hepatitis B;1 methotrexate therapy | USA | 25/25 | 52 | NA | ≥F2:10.49 (1.87m/s), using the conversion formula from kPa to m/s as √(kPa/3) | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Suh CH et al.2014 | Retrospective cohort study | Patients with 123 nonsteatotic;73 hepatic steatosis | Korea | 130/66 | 29.2±9.2 | 22.8±3.0 | ≥F1:6.2 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Deffieux T et al.2015 | Prospective cohort study | Patients with 44 hepatitis C;24 hepatitis B;11 healthy liver;11 non-alcoholic steatohepatitis;10 alcoholic liver;10 autoimmune diseases;2 hepatitis E;2 primary biliary cirrhosis;2 cryptogenic cirrhosis;2 steatosis;1 drug-related hepatitis;1 hepatocellular carcinoma | France | 86/34 | 46.2±14.3 | 24.2±4.09 | ≥F2:8.9;≥F3:9.1;F4:10.2 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Zheng J et al.2015 | Prospective cohort study | Patients with 9 hepatitis C;164 hepatitis B;7 alcoholic liver;3 autoimmune diseases;4 primary biliary cirrhosis;4 drug-related hepatitis;7 unclassified | China | 119/48 | Male:36.6±9.7;female:39.7±11.8 | 21.6±3.4 | NA | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Tada T et al.2015 | Retrospective cohort study | Patients with hepatitis C | Japan | 23/32 | 61 | 21.3 | ≥F2:8.8 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Samir AE et al.2015 | Prospective cohort study | Patients with 43 hepatitis C;8 hepatitis B;1 alcoholic liver;18 autoimmune diseases;1 hemochromatosis;1 HIV and HCV coinfection;60 elevated liver function test;4 elevated liver function test after transplantation; | USA | 70/66 | 49 | NA | ≥F2:7.29;≥F3:8.90;F4:9.59 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Yoneda M et al.2015 | Prospective cohort study | Patients with 117 hepatitis C;15 hepatitis B;7 alcoholic liver;13 non-alcoholic steatohepatitis;4 autoimmune diseases;6 primary biliary cirrhosis;9 primary sclerosing cholangitis;5 others | USA | 115/59 | 57±12 | 30.1±4.1 | a)≥F1:6.2;≥F2:7.9;≥F3:9.3;F4:11.4;b)≥F1:7.4;≥F2:8.6;≥F3:10.9;F4:14.7 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Guibal A et al.2016 | Prospective cohort study | Patients with 30 non-alcoholic steatohepatitis;22 hepatitis B or C;17 alcoholic liver;4 autoimmune hepatitis;4 chronic biliary disease;14 others | France | 95/53 | 54.3±13.2 | 24.9±4.3 | ≥F2:8.8;≥F3:11.5;F4:18.1 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
| Verlinden W et al.2016 | Retrospective cohort study | Patients with 80 hepatitis C, including 26 coinfected with HIV | Belgium | 63/17 | 43±10.2 | NA | ≥F2:8.5;≥F3:10.4;F4:11.3 | METAVIR | Aixplorer,SuperSonic Imagine,Aix-en-Provence,France |
NA:not available
Table 2. Quality assessment for included studies by QUADAS.
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representative spectrum of patients | Clear description of selection criteria | Adequate RS | Short time period between RS and index test | All patient verified by RS | Same RS used | RS independent of index test | Adequate index test description | Adequate RS | Blinding for index test | Blinding for RS | Clinical data available | Report of uninterruptible test result | Description of withdrawals | |
| Ferraioli G et al. 2012 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA |
| Leung VY et al. 2013 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Cassinotto C et al. 2014 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Zeng J et al. 2014 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Yoneda M et al. 2015 | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y |
| Zheng J et al. 2015 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA |
| Deffieux T et al. 2015 | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | Y | N | Y |
| Samir AE et al. 2015 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | NA |
| Beland M et al. 2014 | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | NA | Y | N | NA |
| Suh CH et al. 2014 | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | NA | Y | N | NA |
| Tada T et al. 2015 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | NA |
| Guibal A et al. 2016 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | NA |
| Verlinden W et al. 2016 | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | NA | Y | N | NA |
NA: not available
3.2. Diagnostic accuracy results
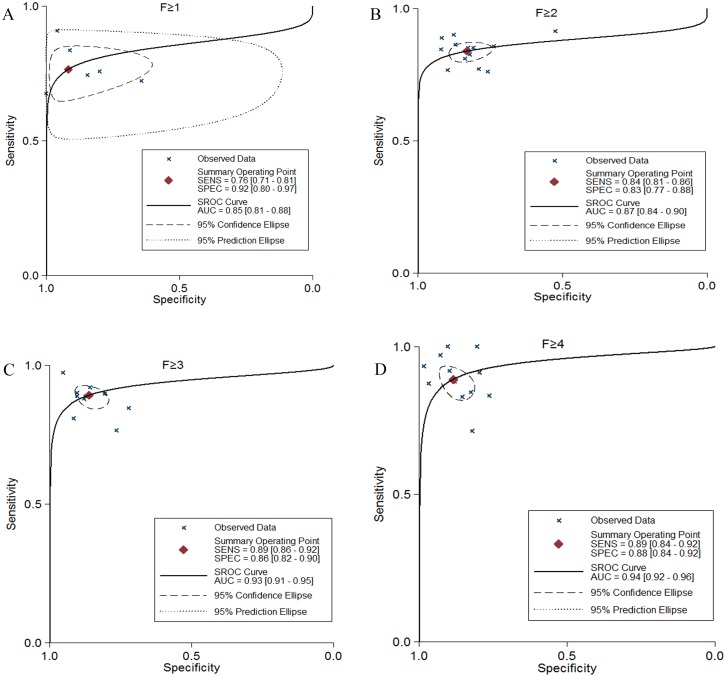
In the S1–S4 Figs, the pooled sensitivity, specificity, diagnostic odds ratio (DOR), positive, negative LRs and the area under the curve (AUC) of SWE for detecting accuracy of hepatic fibrosis severity were shown in Table 3. The summary area under the curve (AUC) was ≥F1 0.85 (0.81–0.88), ≥F2 0.87 (0.84–0.90), ≥F3 0.93 (0.91–0.95) and F4 0.94 (0.92–0.96) (Fig 2). According to the Spearman correlation coefficient, threshold effect was not found significant in ≥F1-4 stagings (≥F1–0.086, p = 0.872; ≥F2–0.011, p = 0.972; ≥F3–0.355, p = 0.284 and F4–0.406, p = 0.191). Fagan nomograms suggested that for all liver fibrosis severity, a positive test substantially increased the pre-test probability, while a negative test markedly reduced the pre-test probability (S4 Fig).
Table 3. Pooled sensitivity, specificity, positive likelihood ratio (+LR), negative likelihood ratio (-LR) and diagnostic oddsratio (DOR) (95% CI).
| Pooled indexes | ≥F1 | ≥F2 | ≥F3 | ≥F4 |
|---|---|---|---|---|
| Sensitivity | 0.76(0.71–0.81) | 0.84(0.81–0.86) | 0.89(0.86–0.92) | 0.89(0.84–0.92) |
| I2 | 75.33 | 9.55 | 0 | 20.56 |
| P | <0.001 | 0.35 | 0.56 | 0.24 |
| Specificity | 0.92(0.80–0.97) | 0.83(0.77–0.88) | 0.86(0.82–0.90) | 0.88(0.84–0.92) |
| I2 | 79.36 | 86.56 | 75.73 | 82.75 |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
| Diagnostic OR | 36.07(12.76–101.96) | 25.16(17.40–36.38) | 50.69(30.42–84.47) | 60.89(31.26–118.61) |
| +LR | 9.28(3.57–24.08) | 4.92(3.65–6.61) | 6.40(4.76–8.62) | 7.68(5.30–11.13) |
| -LR | 0.26(0.20–0.32) | 0.20(0.17–0.23) | 0.13(0.10–0.17) | 0.13(0.09–0.18) |
| AUC | 0.85(0.81–0.88) | 0.87(0.84–0.90) | 0.93(0.91–0.95) | 0.94(0.92–0.96) |
DOR: Diagnostic OR
+LR: Positive LR
-LR: Negative LR
Fig 2. SROC curve of SWE in ≥F1-4 stagings (A, B, C and D) for liver fibrosis.
In addition, according to the fibrosis etiology and due to small sample size on fatty liver disease, we have not performed a sensitivity test on it but do this test in 7 articles on hepatitis. These results of sensitivity and specificity were indicated as follows: ≥F2 0.84 (p = 0.13, 95% CI, 0.79–0.88, I2 = 40.78%), 0.88 (p<0.001, 95% CI, 0.82–0.92, I2 = 72.09%); ≥F3 0.90 (p = 0.22, 95% CI, 0.84–0.94, I2 = 32.37%), 0.91 (p = 0.37, 95% CI, 0.88–0.93, I2 = 3.91%); F4 0.90 (p = 0.1, 95% CI, 0.82–0.95, I2 = 49.27%), 0.90 (p<0.001, 95% CI, 0.84–0.94, I2 = 84.61%), respectively. The detailed results were indicated in S1 Table.
3.3. Sensitivity analysis and publication bias
Sensitivity analysis was performed to assess the stability of the results and found no significant change if any one of the studies was excluded. The publication bias were not detected in our meta-analysis (≥F1: t = -0.89, p = 0.425; ≥F2: t = -1.18, p = 0.265; ≥F3: t = -0.28, p = 0.782; F4: t = 0.44, p = 0.667).
Discussion
Progressive fibrosis could result in serious consequences such as cirrhosis and hepatocellular carcinoma [28]. Assessment of the degree of liver fibrosis is vital for optimal therapeutic methods as well as the prognosis [29, 30]. Shear wave elastography, a new ultrasound-based elastographic method, is based on the traditional ultrasonography. It adds the data on tissue stiffness that may increase the accuracy of diagnosis. Depending on the present meta-analysis, we found that SWE may be an accurate technique in recognizing liver fibrosis.
This meta-analysis showed that the pooled sensitivity and specificity of SWE for liver fibrosis were satisfactory. The odds ratio (OR) is a common statistic in epidemiology, representing the strength of correlation between exposures and diseases. Diagnostic OR is defined as the probability of having a positive detection in patients with a true histological stage of the disease in contrast to patients without the disease. They regulate the negative and curvilinear relations between sensitivities and specificities, as well as consider heterogeneity between studies about the different thresholds [31]. This efficiently helps doctors in offering treatment to patients with early stage of the disease (DOR = 25–61 times). If hepatic fibrosis happens, SWE is a dominant diagnostic test to examine the severity due to its high sensitivity and specificity. Besides these, SROC curves for SWE in F1-4 severity indicated that the AUC values were approximately close to 1 (more than 0.85). Thus, SWE is considered a good test to assess the severity of liver fibrosis. Comparing with other stages, SWE has higher diagnostic OR in ≥F4 staging, which means that it has better strengthen diagnosis of early-stage liver fibrosis. In comparison with previous meta-analysis by transient elastography [32] and ARFI [33], our study indicated that SWE has higher accuracy than TE and ARFI for assessing fibrosis severity. This resulted may because SWE recognized the diseased tissue hardness in real time. Threshold effect was not found significant in ≥F1-4 stagings. Thus heterogeneity in other severity may be due to some factors such as study population, trial condition and disease severity. The positive LR of a diagnostic test detected how well the test can correctly find a disease severity. The higher the positive LR, the better the diagnostic test in accurately recognizing the true disease state. The negative LR of a diagnostic test could be utilized to find how well the test correctly eliminates a disease severity. The lower the negative LR, the better the diagnostic test in declining a disease severity. SWE has a high positive LR and a low negative LR for all severity which suggests that SWE may perform better in diagnosing the correct histological severity of liver fibrosis. SWE can also be used in patients with ascites or obesity, which is not affected by gas as SWE is based on the integration of a radiation force generated in tissue by an ultrasonic beam and an ultrafast imaging sequence acquired in real time with the propagation of the resulting shear waves [8].
In recent analysis, there may be some explanations for the accuracy of SWE in evaluating liver fibrosis severity. Lu YP et al. showed that SWE well recognized the change in liver stiffness and the progression of liver fibrosis in rabbit fatty liver models [34]. SWE allowed real-time test of coagulation necrosis generated by radiofrequency in pigs and this would be applied to observe US-guided thermal ablation [35]. Hepatic stellate cells mainly come from extracellular matrix proteins in hepatic fibrosis, as seen in type I collagen.
Our results should be explained in view of several limitations. First, the heterogeneity of the meta-analysis must be stated because the justification for pooling the data could be susceptible. In this analysis, heterogeneity may come from the variation in study population characteristics and the prevalence of liver fibrosis. Second, the accuracy of SWE mainly relies on the operator's performance. Various fibrosis patterns among diseases could lead to heterogeneous liver elasticity measurements. The mean liver stiffness value measured by SWE was not associated with the size of the region of interest (ROI), age or BMI, but it was affected by the different segments of the liver, the detection depth and gender [36]. Lastly, the overall sampling size of included studies was relatively small.
In spite of these limitations, this study offers considerable information that could inform physicians the accuracy of SWE. Thereby, SWE would be an inexpensive technique with widespread availability, particularly in areas with insufficient healthcare resources. In addition, we meticulously retrieved all published literature relevant to the research question and then extracted the data in duplicate through the described protocols to guarantee high quality and consistency in the final data. Missing data were searched from the authors and studies results were statistically merged to support these estimates of SWE for the screening of liver fibrosis severity.
Conclusions
In conclusion, this meta-analysis indicated that SWE could be a promising tool to differentiate the severity of liver fibrosis. Future studies are also necessary to explore the potential confounding factors.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
This study was supported by the opening foundation of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital of Medical College, Zhejiang University, grant NO. 2015KF06.
Abbreviations
- SWE
shear wave elastography
- TE
transient elastography
- LSM
liver stiffness measurement
- ARFI
acoustic radiation force impulse
- ROI
region of interested
- QUADAS
quality assessment of diagnostic accuracy studies
- LR
likelihood ratio
- AUROC
the area under the ROC curve
- SROC
summary receiver operating characteristic
- OR
odds ratio
- AUC
area under the curve
Data Availability
Data are available from PubMed, Embase, Web of science and Scopus. Our search strategy can be found in the S3 File.
Funding Statement
This study was supported by the opening foundation of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital of Medical College, Zhejiang University, grant NO. 2015KF06. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. The Journal of biological chemistry. 2000;275(4):2247–50. . [DOI] [PubMed] [Google Scholar]
- 2.Sporea I, Lie I. Shear wave elastography. Ultraschall in der Medizin. 2012;33(4):393–4. [PubMed] [Google Scholar]
- 3.Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–50. . [DOI] [PubMed] [Google Scholar]
- 4.Hagan M, Asrani SK, Talwalkar J. Non-invasive assessment of liver fibrosis and prognosis. Expert review of gastroenterology & hepatology. 2015;9(10):1251–60. 10.1586/17474124.2015.1075391 . [DOI] [PubMed] [Google Scholar]
- 5.Sporea I, Sirli RL. Hepatic elastography for the assessment of liver fibrosis—present and future. Ultraschall in der Medizin. 2012;33(6):550–8. 10.1055/s-0032-1313011 . [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall in der Medizin. 2013;34(3):238–53. 10.1055/s-0033-1335375 . [DOI] [PubMed] [Google Scholar]
- 7.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound in medicine & biology. 1998;24(9):1419–35. . [DOI] [PubMed] [Google Scholar]
- 8.Tanter M, Bercoff J, Athanasiou A, Deffieux T, Gennisson JL, Montaldo G, et al. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound in medicine & biology. 2008;34(9):1373–86. 10.1016/j.ultrasmedbio.2008.02.002 . [DOI] [PubMed] [Google Scholar]
- 9.Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C, et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56(6):2125–33. 10.1002/hep.25936 . [DOI] [PubMed] [Google Scholar]
- 10.Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269(3):910–8. 10.1148/radiol.13130128 . [DOI] [PubMed] [Google Scholar]
- 11.Cassinotto C, Lapuyade B, Mouries A, Hiriart JB, Vergniol J, Gaye D, et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan(R). Journal of hepatology. 2014;61(3):550–7. 10.1016/j.jhep.2014.04.044 . [DOI] [PubMed] [Google Scholar]
- 12.Zeng J, Liu GJ, Huang ZP, Zheng J, Wu T, Zheng RQ, et al. Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. European radiology. 2014;24(10):2572–81. 10.1007/s00330-014-3292-9 . [DOI] [PubMed] [Google Scholar]
- 13.Beland MD, Brown SF, Machan JT, Taliano RJ, Promrat K, Cronan JJ. A pilot study estimating liver fibrosis with ultrasound shear-wave elastography: does the cause of liver disease or location of measurement affect performance? AJR American journal of roentgenology. 2014;203(3):W267–73. 10.2214/AJR.13.11718 . [DOI] [PubMed] [Google Scholar]
- 14.Suh CH, Kim SY, Kim KW, Lim YS, Lee SJ, Lee MG, et al. Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology. 2014;271(3):895–900. 10.1148/radiol.14131251 . [DOI] [PubMed] [Google Scholar]
- 15.Deffieux T, Gennisson JL, Bousquet L, Corouge M, Cosconea S, Amroun D, et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. Journal of hepatology. 2015;62(2):317–24. 10.1016/j.jhep.2014.09.020 . [DOI] [PubMed] [Google Scholar]
- 16.Zheng J, Guo H, Zeng J, Huang Z, Zheng B, Ren J, et al. Two-dimensional shear-wave elastography and conventional US: the optimal evaluation of liver fibrosis and cirrhosis. Radiology. 2015;275(1):290–300. 10.1148/radiol.14140828 . [DOI] [PubMed] [Google Scholar]
- 17.Samir AE, Dhyani M, Vij A, Bhan AK, Halpern EF, Mendez-Navarro J, et al. Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: determining accuracy and ideal site for measurement. Radiology. 2015;274(3):888–96. 10.1148/radiol.14140839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoneda M, Thomas E, Sclair SN, Grant TT, Schiff ER. Supersonic Shear Imaging and Transient Elastography With the XL Probe Accurately Detect Fibrosis in Overweight or Obese Patients With Chronic Liver Disease. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2015;13(8):1502–9 e5 10.1016/j.cgh.2015.03.014 . [DOI] [PubMed] [Google Scholar]
- 19.Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Okuda S, et al. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: Comparison of liver fibrosis indices. Hepatology research: the official journal of the Japan Society of Hepatology. 2015;45(10):E122–9. 10.1111/hepr.12476 . [DOI] [PubMed] [Google Scholar]
- 20.Guibal A, Renosi G, Rode A, Scoazec JY, Guillaud O, Chardon L, et al. Shear wave elastography: An accurate technique to stage liver fibrosis in chronic liver diseases. Diagnostic and interventional imaging. 2016;97(1):91–9. 10.1016/j.diii.2015.11.001 . [DOI] [PubMed] [Google Scholar]
- 21.Verlinden W, Bourgeois S, Gigase P, Thienpont C, Vonghia L, Vanwolleghem T, et al. Liver Fibrosis Evaluation Using Real-time Shear Wave Elastography in Hepatitis C-Monoinfected and Human Immunodeficiency Virus/ Hepatitis C-Coinfected Patients. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine. 2016. 10.7863/ultra.15.08066 . [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine. 2009;6(7):e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–93. 10.1002/hep.510240201 . [DOI] [PubMed] [Google Scholar]
- 24.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC medical research methodology. 2003;3:25 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of clinical epidemiology. 2006;59(12):1331–2; author reply 2–3. 10.1016/j.jclinepi.2006.06.011 . [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of clinical epidemiology. 2005;58(9):882–93. 10.1016/j.jclinepi.2005.01.016 . [DOI] [PubMed] [Google Scholar]
- 28.El-Serag B. H. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36(Supplement 1):S74–83. [DOI] [PubMed] [Google Scholar]
- 29.Gerber L, Kasper D, Fitting D, Knop V, Vermehren A, Sprinzl K, et al. Assessment of Liver Fibrosis with 2-D Shear Wave Elastography in Comparison to Transient Elastography and Acoustic Radiation Force Impulse Imaging in Patients with Chronic Liver Disease. Ultrasound in medicine & biology. 2015;41. [DOI] [PubMed] [Google Scholar]
- 30.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142(6):1293–302 e4 10.1053/j.gastro.2012.02.017 . [DOI] [PubMed] [Google Scholar]
- 31.Cleophas TJ, Zwinderman AH. Meta-analyses of diagnostic studies. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2009;47(11):1351–4. 10.1515/CCLM.2009.317 . [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Huang YS, Wang ZZ, Yang ZR, Sun F, Zhan SY, et al. Systematic review with meta-analysis: the diagnostic accuracy of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B. Alimentary pharmacology & therapeutics. 2016;43(4):458–69. 10.1111/apt.13488 . [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Fu J, Hong R, Liu L, Li F. Acoustic Radiation Force Impulse Elastography for the Non-Invasive Evaluation of Hepatic Fibrosis in Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review & Meta-Analysis. PloS one. 2015;10(7):e0127782 10.1371/journal.pone.0127782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu YP, Wei J, Xu LR, Tang YY, Yuan Y, Zhang Y, et al. Assessment of fibrosis during the development of fatty liver in rabbits using real-time shear-wave elastography. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2014;34(6):921–8. 10.1007/s11596-014-1375-0 . [DOI] [PubMed] [Google Scholar]
- 35.Mariani A, Kwiecinski W, Pernot M, Balvay D, Tanter M, Clement O, et al. Real time shear waves elastography monitoring of thermal ablation: in vivo evaluation in pig livers. The Journal of surgical research. 2014;188(1):37–43. 10.1016/j.jss.2013.12.024 . [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Zheng J, Zeng J, Wang X, Wu T, Zheng R. Normal liver stiffness in healthy adults assessed by real-time shear wave elastography and factors that influence this method. Ultrasound in medicine & biology. 2014;40(11):2549–55. 10.1016/j.ultrasmedbio.2014.05.008 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
Data are available from PubMed, Embase, Web of science and Scopus. Our search strategy can be found in the S3 File.