Abstract
There is a growing awareness of the chronic brain injury that results from the sepsis syndrome. We review experiments in several animal models of sepsis and show in one model, cecal ligation and puncture (CLP), that permanent structural pathology matures after the initial event. Specifically, we observed after exposure to CLP significant decreased spine density on the apical tree, but not the basal tree, of dendrites in the CA1 region of the dorsal hippocampus that was accompanied by a significantly diminished arbor of the apical dendrites, by 8 weeks, but not after 2 weeks. These novel data from dendritic arborizations elaborate information about a cohort of mice that had behaved in spatial memory tasks. These results raise questions about the relationship between long-term behavioral consequences and intervention strategies.
Keywords: Sepsis syndrome, Cecal ligation and puncture, CA1 hippocampus, Golgi analysis
Introduction
Sepsis syndrome includes organ dysfunction and may begin with an infection that activates the systemic inflammatory response, especially the innate immune system, that causes fever, tachycardia, tachypnea and increased white blood cell count [1]. Nearly simultaneously, sepsis syndrome activates the compensatory anti-inflammatory response mediated by a complex interaction of regulatory T cells and myeloid-derived suppressor cells. The balanced interplay necessary for a healthy outcome also depends on additional anti-inflammatory components including the vagus nerve secretion of acetylcholine, which activates the α7 nicotinic acetylcholine receptor on innate immune cells and downregulates inflammatory cytokines [2, 3]. While the invading pathogen initiates the inflammatory response, the subsequent tissue destruction is caused by the host response and likely contributes importantly to high mortality and persistent morbidity. This imbalance in the pro-inflammatory and anti-inflammatory response to pathogens cannot be underestimated for sepsis accounts for ~750,000 new patients each year, and ~200,000 deaths per year, in the USA alone [4, 5].
Compounding the failure of several trials that attempted to control the post-inflammatory component of the sepsis syndrome, the acute mortality rate in the 20–25 % range belies the problems that occur in those who survive [6]. In the 50–80 % of those patients who survive the acute event and exit the hospital, there is an increased disability burden perhaps best characterized by persistent cognitive impairment and emotional disturbances [7–9]. Few of these patients return to former lifestyle and the survivors’ burden of disability increases [7]. Age of the patient group has been proposed as an important factor in the poor long-term outcomes [10], but poor outcome after sepsis correlated only with low body mass among several geriatric conditions [11]. The neuropathy and myopathy that occur after prolonged intensive care unit hospitalization are well documented [12]; however, it is the study of brain injury after severe sepsis that strongly suggests direct injury to the central nervous system [9, 11, 13]. There is a dearth of neuropathological or neuroimaging data in clinical studies of severe sepsis in patients who sustained hypotension and cardiac events that might have led to cerebral ischemia and reperfusion injury. Despite the obvious clinical deterioration, the lack of straight forward clinical structural brain information suggests that the injury is subtle.
Thus, studies of animal models of acute sepsis may provide clues to begin to understand the cryptic brain injury in the clinical situation. While each experimental paradigm, for example orthopedic trauma and chemical stress that mimicked infectious sepsis induction, demonstrated that activation of the immune system led to the rapid early serum elevation of one or more of the following candidate cytokines, IL-1β, TLR4, TNFα, or IL-6, multiple studies demonstrated that excessive serum activity of these candidate “cognitive” cytokines [14] caused a post-septic behavioral impairment [15–23]. These experiments focused on the singular effect of cytokine elevation in the circulation of mice on their subsequent performance in a variety of tasks. Furthermore, some of these experiments used genetic manipulation or cytokine blockade to alter the cytokine serum level and altered the behavioral abnormality. In other experiments that have additionally probed the neuropathological consequences of sepsis, the investigators used LPS injection as the inciting stress, and demonstrated increased TNFα and IL-1β, and the associated memory performance impairment was accompanied by the loss of neurons in the hippocampus and parietal cortex [24–26]. The finding that hippocampal and cortical regions of brain are vulnerable to the toxic effects of at least these candidate cytokines that rise after trauma or infectious stress is consistent with parts of the clinical outcome data. The recorded preclinical data suggest that cytokine induced damage to the hippocampus and diffusely throughout the cortex might go undetected in many clinical circumstances; revealed only by the most advanced neuroimaging techniques,
The CLP model seemed particularly advantageous because it replicated key aspects of human sepsis, such as 20–30 % acute mortality, early hypotension and organ failure [27]. CLP also mimicked the most frequent form of polymicrobial gram-negative sepsis that occurs in hospitalized patients [28–33]. Further, CLP permitted the study of the chronic condition since animals that survived the initial injury are long lived (we have followed animals for 24 weeks after the CLP exposure [34]). The time course permitted examination of some of the late responses of the immune system, which included the elevation of molecules like HMGB1 [35, 37], which may stay elevated in sepsis survivors even until discharge. This important relationship to the clinical condition reflects a failure of public health education, because often by the time sepsis is recognized clinically so that patients seek aid, the serum levels of cytokines, particularly TNFα, are no longer elevated [36].
Methods
Animal handling and the CLP procedure
Implementing the CLP model for behavioral and pathological analysis required animals be maintained on a reverse light schedule (dark period, 09:00–21:00), with ad libitum access to food and water. These male C57BL/6 mice (Charles River Laboratories) were 6- to 8 weeks old at the time of CLP surgery. Mice were anesthetized using ketamine (100 mg per kg) and xylazine (8 mg per kg) administered intramuscularly. Detailed surgical preparation has been described [34, 37]. Briefly, the cecum below the ileocecal valve was isolated and punctured, and the abdomen closed and the mouse was treated with saline and a single dose of antibiotic (Primaxin, Merck, 0.5 mg per mouse in 200 μL sterile saline injected subcutaneously) and resuscitative fluid. SHAM-operated animals had the cecum isolated and then returned to the peritoneal cavity, without being ligated or punctured, followed by comparable post op care. After a recovery period, the mice were ready for behavioral and neuropathological analysis.
Behavioral analysis
The behavioral analysis procedures have been described at length [38, 39]. In general, before testing, mice were handled frequently for 5 days in 10-min sessions. Handling and behavioral testing occurred during the dark period of the circadian cycle. Each mouse was subjected to a primary screen of their behaviors, adapted from well-standardized research [40, 41], such as open-field test measuring spontaneous locomotion, rota-rod test measuring motor coordination, a black-and-white alley test measuring anxiety and a navigational test measuring spatial working memory. These tests were separated by at least one day. Specialized tests of working memory or freezing behavior to measure spatial and flexible memory and fear conditioning to tone and context have been documented [34, 39, 42]. Recently, we have shown that CLP-exposed animals had normal standard neuropathological assessment yet display a sustained impairment of spatial memory [34]. Thus, the opportunity to explore the neuropathological associations with the CLP-exposed mouse turned to the use of the Golgi method of impregnating neurons for substructural analysis.
Pathological preparation
For Golgi impregnation, mice were saline perfused, and brains were removed and processed using the Rapid Golgi kit (FD Neurotechnologies, Ellicott City, MD) as previously described [43]. Brains were immersed in an impregnation solution (2 weeks), cut between the hemispheres in the sagittal plane and then cut 20° from the ventral horizontal plane so the hemisphere could be mounted on a cryostat chuck at an angle permitting the most advantageous sections of the dorsal hippocampus. On a cryostat, 100-μm sections were transferred to gelatin-coated slides. After drying, slides were stained and coverslipped. For the analysis of the CA1 region of the dorsal hippocampus, Z-stacks images (2.0 μm steps) of the neurons were acquired at 40X with a Zeiss Axio imager (Zen 2, Zeiss, Thornwood, NY). The images were transferred to an automated program (Neurolucida, MBF, Williston, VT) for counting and analysis. Quantitation of dendritic apical and basilar spine density of CA1 pyramidal cells was assessed. We only used dendrites that were whole and unbroken in the projection field [34, 43]. We used Scholl analysis to quantitate the dendritic arbors. CA1 apical and basal dendrites were measured. We present data that show the Sholl analysis in this hippocampal region for mice that survived 2 and 8 weeks after CLP.
Results and discussion
Here we elaborate structural data by demonstrating the altered dendritic arbors in a Sholl analysis (using established methods for inducing CLP and for Golgi impregnation techniques [34]). CLP mice spend significantly more time solving spatial memory problems, and the partial report of the neuropathological changes showed decreased spine density in the apical, but not basilar, dendritic tree [34] and [59].
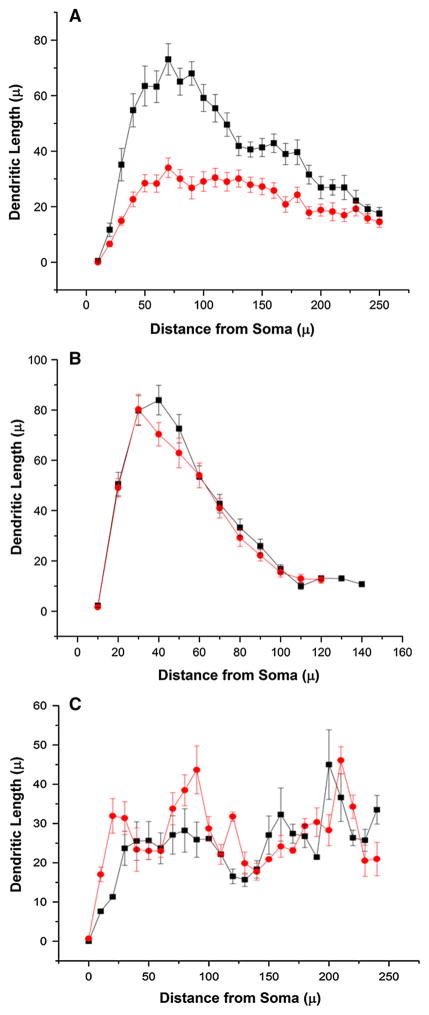
Essentially, mice exposed to CLP (Fig. 1a) demonstrated that mice 8 weeks after CLP exposure have significantly diminished apical dendrites in the CA1 region compared to sham mice (P < 0.01, Kolmogorov–Smirnov test). Analysis of 8-week survivors of CLP, however, demonstrated that the basal dendrites of the CA1 hippocampus were comparable to sham mice (Fig. 1b; ns, Kolmogorov–Smirnov test). Mice that survived two weeks also showed no difference in the apical dendritic tree of the CA1 hippocampus (ns, Kolmogorov–Smirnov test).
Fig. 1.
Sholl analysis of apical and basal dendrites in 2 and 8 weeks post-CLP-exposed mice and shams. a Sholl analysis of apical CA1 dendrites of the hippocampus demonstrates significant loss of arbors in animals 8 weeks post-CLP or sham (five mice in each group, neurons = 29 in each group; D = 0.5, P <0.01, Kolmogorov–Smirnov test). b Sholl analysis of basal CA1 dendrites demonstrates comparable arbors in animals 8 weeks post-CLP or sham (four mice in each group, neurons = 27 in sham and 26 in CLP; D = 0.1, NS, Kolmogorov–Smirnov test). c Sholl analysis of apical CA1 dendrites of the hippocampus demonstrate comparable arbors in animals 2 weeks post-CLP or sham (six mice in each group, neurons = 6; D = 0.3, NS, Kolmogorov–Smirnov test). Sham black, CLP red (Color figure online)
The analysis of the Sholl dendrite arbors is consistent with the spine data that we have already reported [34]; namely, the CA1 hippocampal apical spine density is diminished [by 4 weeks after CLP, the apical spine density was 0.51 ± 0.09 spines/μm compared to sham = 1.12 ± 0.14 spines/μm (P < 0.05), and 16 weeks after CLP the apical spine density was 0.48 ± 0.02 spines/μm compared to sham = 0.94 ± 0.07 spines/μm (P < 0.05)], yet the basal dendrite spine density was comparable across CLP and sham mice [59].
These results together with our previous and submitted data [34, 59] support the idea that exposure to sepsis using the CLP model caused altered behavior. The association with reproducible and focal pathological changes reflected by the chronic loss of dendrites confined to the apical region of the CA1 hippocampus in this work describes the pathology at a new level.
Our past behavioral results based on chronically surviving animals show that behavioral abnormalities develop weeks after CLP experience. While most cytokine bursts peak by 48–72 h, it is the late rising cytokine HMGB1 that may be implicated in neurological destruction and behavioral abnormality. Indeed we have shown that HMGB1 injections alone disrupt memory performance and that treatment, within a week of the CLP exposure, spares some of the spine alterations in the CA1 apical dendrites [34]. This result is consistent with other experiments that also implicate increased HMGB1 over-activity in behavioral abnormalities of mice [44, 45]. While HMGB1 rises late in a septic episode, its potential contribution to the chronic neuronal alterations needs further investigation. There is evidence that immune mediators, and cytokines in particular, alter brain synaptic function [14, 46, 47], so it is not surprising that recent information shows the regulation by TNF and IL-1 of the surface density and activity of glutamate receptors [48]. These results suggest that late rising cytokines and HMGB1, in particular, and potential blockade of HMGB1 may hold clues for the late treatment of neurotoxic structural changes and behavioral impairments that result from severe sepsis. Alternatively, HMGB1 has been demonstrated to play a role in tissue repair [49], and whether blockade of HMGB1 and the timing of this blockade are ultimately beneficial needs to be explored.
The density, size and shape of spines, and the sufficient combination of spines and dendritic arbor that comprise the fundamental computing unit, compose a central issue that extends the activity of the neuron to neural networks [48]. Thus, a quantitative analysis of these structural neuronal components in disease models may be important. Much of the focus of synapse and dendrite stability is on the signaling pathways that lead to the formation and maintenance of spines and the interaction between the spines and the arbors [50, 51]. There is evidence in paradigms that test the effects of different molecules, like hormones or drugs of abuse, or learning and memory experiments, that altered spine density plays an important mechanistic role in the studied behaviors [52–54]. Reports in preclinical experiments demonstrate that acquired disorders of the nervous system, including autoimmune disorders, can alter neuronal structural integrity, particularly dendrite arbors [55, 56]. There is also precedent for alterations in spine density and dendritic arbors in various psychiatric and genetic disorders [50, 57, 58]. Thus, the present results demonstrate the potential usefulness of the CLP model to contribute to an understanding of the sepsis syndrome by pointing out potential regions of anatomic vulnerability. The mechanism of that damage awaits further experiments.
Acknowledgments
This work was supported by a Grant from the NIH, 1P01AI102852-01A1, and the Feinstein Institute for Medical Research.
References
- 1.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40(4):463–75. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, et al. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15(5):496–7. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9(6):418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306(23):2614–5. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 5.Quartin AA, et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277(13):1058–63. [PubMed] [Google Scholar]
- 6.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–4. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazosky A, et al. Quality of life after septic illness. J Crit Care. 2010;25(3):406–12. doi: 10.1016/j.jcrc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Semmler A, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84(1):62–9. doi: 10.1136/jnnp-2012-302883. [DOI] [PubMed] [Google Scholar]
- 10.Rubenfeld GD. Does the hospital make you older faster? Am J Respir Crit Care Med. 2012;185(8):796–8. doi: 10.1164/rccm.201202-0267ED. [DOI] [PubMed] [Google Scholar]
- 11.Iwashyna TJ, et al. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–41. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131(5):1541–9. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 13.Pandharipande PP, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–66. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Cibelli M, et al. Role of interleukin-1β in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–8. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham C, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65(4):304–12. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham C, et al. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godbout JP, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19(10):1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 19.Laflamme N, Lacroix S, Rivest S. An essential role of interleukin-1β in mediating NF-κB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. 1999;19(24):10923–30. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teeling JL, et al. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun. 2007;21(6):836–50. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Terrando N, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70(6):986–95. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuon L, et al. Imipramine reverses the depressive symptoms in sepsis survivor rats. Intensive Care Med. 2007;33(12):2165–7. doi: 10.1007/s00134-007-0804-y. [DOI] [PubMed] [Google Scholar]
- 23.Weberpals M, et al. NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci. 2009;29(45):14177–84. doi: 10.1523/JNEUROSCI.3238-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semmler A, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204(2):733–40. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Semmler A, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflamm. 2008;5(1):38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semmler A, et al. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30(2–3):144–57. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Dejager L, et al. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19(4):198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Bone RC. Gram-negative sepsis. Background, clinical features, and intervention. Chest. 1991;100(3):802–8. doi: 10.1378/chest.100.3.802. [DOI] [PubMed] [Google Scholar]
- 29.Boomer JS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunkhorst FM, et al. Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307(22):2390–9. doi: 10.1001/jama.2012.5833. [DOI] [PubMed] [Google Scholar]
- 31.Dellinger RP, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 32.Kreger BE, Craven DE, McCabe WR. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980;68(3):344–55. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- 33.Martin GS, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 34.Chavan SS, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–7. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angus DC, et al. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35(4):1061–7. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 36.Xiao W, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang EH, Rigotti A, Huerta PT. Age-related influence of the HDL receptor SR-BI on synaptic plasticity and cognition. Neurobiol Aging. 2009;30(3):407–19. doi: 10.1016/j.neurobiolaging.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huerta PT, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103(3):678–83. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irwin S. Comprehensive observational assessment: ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13(3):222–57. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 41.Rogers DC, et al. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8(10):711–3. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- 42.Kowal C, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21(2):179–88. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Wallace M, et al. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126(1):176–82. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 44.Mazarati A, et al. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and receptor for advanced glycation end products. Exp Neurol. 2011;232(2):143–8. doi: 10.1016/j.expneurol.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, et al. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1768–73. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 46.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11(8):973–84. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 47.McCoy M, Tansey M. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflamm. 2008;5(1):45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 49.Venereau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14(8):536–50. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–78. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frankfurt M, Luine V. The evolving role of dendritic spines and memory: interaction(s) with estradiol. Horm Behav. 2015;74:28–36. doi: 10.1016/j.yhbeh.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinrichs SC, et al. Dendritic structural plasticity in the basolateral amygdala after fear conditioning and its extinction in mice. Behav Brain Res. 2013;248:80–4. doi: 10.1016/j.bbr.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 55.Chang EH, et al. Selective impairment of spatial cognition caused by autoantibodies to the N-methyl-D-aspartate receptor. EBio-Medicine. 2015;2(7):755–64. doi: 10.1016/j.ebiom.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frauenknecht K, et al. Mice with experimental antiphospholipid syndrome display hippocampal dysfunction and a reduction of dendritic complexity in hippocampal CA1 neurones. Neuropathol Appl Neurobiol. 2015;41(5):657–71. doi: 10.1111/nan.12180. [DOI] [PubMed] [Google Scholar]
- 57.Goellner B, Aberle H. The synaptic cytoskeleton in development and disease. Dev Neurobiol. 2012;72(1):111–25. doi: 10.1002/dneu.20892. [DOI] [PubMed] [Google Scholar]
- 58.Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50(1):10–20. doi: 10.1016/j.mcn.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Huerta PT, Robbiati S, Frankfurt M, Volpe BT. Overwhelming sepsis disrupts the neural system that encodes contextual fear memory. Mol Med. doi: 10.2119/molmed.2015.00201. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]