Abstract
Low drug delivery efficiency and drug resistance from highly heterogeneous cancer cells and tumor microenvironment represent major challenges in clinical oncology. Growth factor receptor, IGF-1R, is overexpressed in both human tumor cells and tumor associated stromal cells. The level of IGF-1R expression is further up-regulated in drug resistant tumor cells. We have developed IGF-1R targeted magnetic iron oxide nanoparticles (IONPs) carrying multiple anticancer drugs into human tumors. This IGF-1R targeted theranostic nanoparticle delivery system has an iron core for non-invasive MR imaging, amphiphilic polymer coating to ensure the biocompatibility as well as for drug loading and conjugation of recombinant human IGF-1 as targeting molecules. Chemotherapy drugs, Doxorubicin (Dox), was encapsulated into the polymer coating and/or conjugated to the IONP surface by coupling with the carboxyl groups. The ability of IGF1R targeted theranostic nanoparticles to penetrate tumor stromal barrier and enhance tumor cell killing has been demonstrated in human pancreatic cancer patient tissue derived xenograft (PDX) models. Repeated systemic administrations of those IGF-1R targeted theranostic IONP carrying Dox led to breaking the tumor stromal barrier and improved therapeutic effect. Near infrared (NIR) optical and MR imaging enabled noninvasive monitoring of nanoparticle-drug delivery and therapeutic responses. Our results demonstrated that IGF-1R targeted nanoparticles carrying multiple drugs are promising combination therapy approaches for image-guided therapy of stroma-rich and drug resistant human cancer, such as pancreatic cancer.
Keywords: Theranostic nanoparticles, IGF1R targeted cancer therapy, Pancreatic cancer, Patient derived xenografts, MRI, Image-guided cancer therapy, Tumor microenvironment
1. Introduction
Although various nanomaterials have been developed as drug delivery systems such as carbon nanotubes, polymeric and inorganic nanoparticles [1-3], magnetic iron nanoparticle (IONP) has attracted great attention due to its biocompatibility, ability of large scale production and surface modification, and the feasibility of monitoring nanoparticle-drug delivery using non-invasive MRI [4-6]. IONP drug carriers have been developed to deliver single or multiple therapeutic drugs for cancer treatment and their effects have been examined in preclinical animal tumor models. Currently, various targeting ligands have been used to direct therapeutic agents into tumors and improve therapeutic effect [7, 8].
Tumor stroma represents one of the major barriers for drug delivery, especially for nanoparticle drug carriers [9, 10]. Increasing evidence supports the importance of the development of cancer therapeutic agents that act on tumor cells as well as tumor microenvironment. Our group has developed insulin-like growth factor 1 (IGF1) conjugated theranostic IONPs that target to IGF1 receptor (IGF1R) highly expressed in many types of tumor cells, tumor stromal fibroblasts and macrophages [11]. For example, 40-90% of the pancreatic cancers highly express IGF1R in both epithelial cancer cells and tumor stromal components [12, 13]. In contrast, its expression in normal pancreas is relative low. Furthermore, IGF-1R is a good cell surface marker for targeting drug resistant tumor cells since the level of IGF1R expression is further increased in the drug resistant tumor cell population [14, 15]. All these features make IGF1R an ideal target for targeted cancer therapy.
At present, animal tumor models for evaluation of nanoparticle drug delivery and response to therapy are mainly from established tumor cell lines that often result in low success rate in the translation of cancer therapeutics into clinical. One reason is that tumor cell line derived xenografts usually fail to reproduce tumor cell heterogeneity and stromal microenvironment that are integral to tumor development and metastasis. Recently, patient tissue-derived tumor xenografts (PDXs), which are established from implanting surgically resected fresh tumor tissues of patients into immunecompromised mice, are considered to more accurately mimic human tumors with the high similarity in the tumor growth environment, intratumor heterogeneity, and histological characteristics seen in primary human tumors [16-18]. Therefore, PDXs have potential to offer relevant predictive insights into clinical outcomes for the evaluation of the efficacy of novel cancer therapies [19].
Human PDX models provide excellent mouse models to evaluate the effect of IGF1R targeted nanoparticles on both tumor cells and tumor associated fibroblasts and macrophages. It is well know that pancreatic cancer is one of the most stroma-rich tumor types with 50% to 85% of tumor stroma components in tumor masses. Pancreatic cancer is one of the most aggressive cancer types with five-year survival of 5% and an estimated 39,590 deaths in the U.S. in 2014 [20]. Resistance to chemotherapy is the most common reason for the failure of pancreatic cancer treatment. The physical barriers created by an intensive tumor stromal component and disorganized vasculatures prevent a sufficient amount of therapeutic agents reaching tumor site [10]. Additionally, genetic alterations and dysfunctional signaling pathways in pancreatic tumor cells also lead to intrinsic drug resistance [21]. Therefore, the development of new therapeutics for effective treatment of pancreatic cancer requires novel approaches to break drug delivery barriers in the tumor stroma and increase the drug concentration delivered to tumor cells in order to overcome drug resistant mechanisms [9, 22]. We have established human pancreatic cancer PDXs and examined the effect of IGF1R targeted IONPs carrying doxorubicin (Dox) on targeted delivery, response to therapy and the ability of breaking tumor stroma in the PDXs in nude mice. The feasibility of using IGF1 as targeting ligand for pancreatic cancer has been demonstrated in vivo. By conjugating a NIR dye to the nanoparticles, the in vivo targeting effect can be monitored by both non-invasive optical imaging and MRI. The accumulation of IONPs in tumor was further confirmed by histological analysis and biodistribution studies. Systemic delivery of IGF1-IONP-Dox significantly reduced the growth of tumors in the pancreatic cancer PDX model. Targeted drug delivery and response to therapy could be monitored by MRI. Our results demonstrated that IGF1 conjugated theranostic IONP is a new and effective nanoparticle drug delivery system for improving targeted therapy of stromal rich pancreatic cancer.
2. Results and Discussion
2.1. Establishment of orthotopic human pancreatic cancer PDX models
To establish an orthotopic pancreatic PDX model, fresh pancreatic cancer specimens obtained from surgically resected tumor tissues from patients were implanted into the pancreas of SCID mice. Tumor growth was monitored twice a week until it reach a diameter of around 1 cm. Tumors were then collected and implanted into the pancreas of nude mice as passage 1 for testing the effect of receptor targeted theranostic IONPs (Figure 1a). Histological analysis of tumor tissues from the primary human tumor and PDX obtained from two pancreatic cancer patients as well as a pancreatic cancer cell line (MiaPaCa-2 cell line) derived xenograft by H&E and immnofluorescence staining (such as IGF1R) showed that the PDX tissues retained histopathological characteristics of primary tumor tissues of the patients. On the other hand, typical features of human pancreatic cancer tissues, such as infiltration ductal carcinoma, metaplastic carcinoma, and dense stroma, were not found in MiaPaca2 cell line derived tumor xenografts (Figure 1b). Pancreatic cancer PDX tissues, but not cell line derived tumor xenograft, had a level of IGF1R (Figure 1b). Therefore, our results showed that establishment of pancreatic cancer PDX model provided a unique opportunity for evaluation of the effect of targeted drug delivery, imaging and treatment response in heterogeneous human tumors. Furthermore, after conventional chemotherapy with either cisplatin or Dox, drug resistant tumor cells showed 1.5 to 1.6 fold increases in the level of IGF1R expression than tumors without any treatment, making IGF1R an ideal target for treatment of drug resistant tumor (Figure 1c and 1d).
Figure 1. Establishment and characterization of orthotopic human pancreatic PDX tumor models.
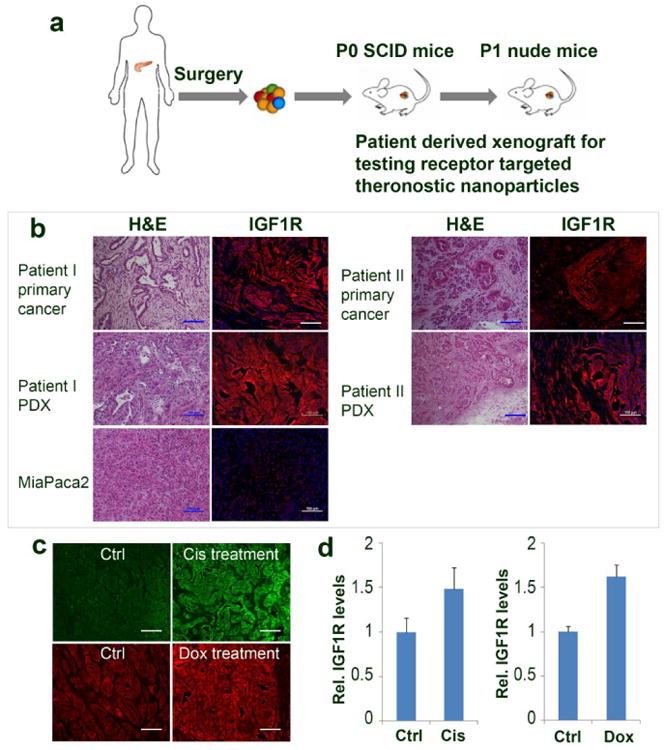
a) Schematic illustration of the protocol of establishment of an orthotopic pancreatic PDX tumor model. b) Comparison of histological characterization and IGF1R expression of surgically resected primary human pancreatic cancer, first passenger PDX-tumor from SCID mice, and human xenograft tumor derived from human pancreatic cancer MiaPaca-2 cells. c) Immuno-fluorescence labeling detects the levels of IGF1R expression in cisplatin (Cis) or doxorubicin (Dox) treated and no treatment control tumor tissues (green, Cis treated; red, Dox treatment); d) Relative levels of IGF1R expression in the no treatment control tumors and chemoresistant tumors determined by measuring fluorescence intensity in tumor tissue sections using Image J software. Scale bars are 100 μm.
2.2. Preparation of IGF1-IONP-Dox
The preparation of IGF1-IONPs and loading of Dox on IONPs are shown in Figure 2a. For all in vitro and in vivo studies, IGF1R targeted IONPs were produced by conjugation of a near infrared dye (NIR 830) [23] labeled human recombinant IGF1 to a 10 nm core size and amphiphilic polymer coated IONP. The molar ratio of IGF1 to IONPs for conjugation is 20:1. The size of an IGF1(8 KDa) was much smaller than an antibody (150 KDa), which allowed conjugation of higher numbers of targeting ligands compared to 2 to 3 antibody molecules that could be conjugated to each nanoparticle. Chemotherapy drug Dox, a hydrophobic molecule, was encapsulated in the hydrophobic space of the amphiphilic polymer layer on surface IONP and can be released in pH dependent fashion (Figure 2b), as amine group of Dox can be protonated to convert it into a hydrophilic molecule that releases from the nanoparticle [24, 25].
Figure 2. Production of IGF1-IONP-Dox.
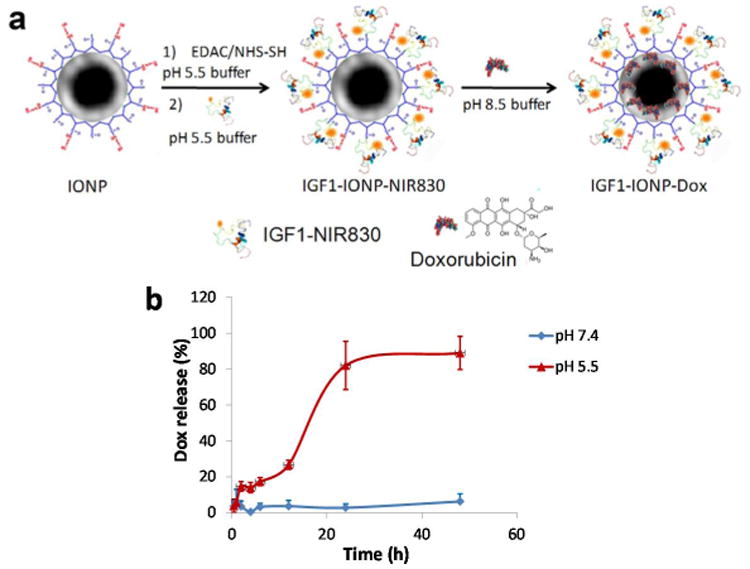
a) Schematic illustration of conjugation of NIR830-IGF1 to amphiphilic polymer coated IONPs and encapsulation of Dox to IGF1-IONPs. b) Release profile of Dox from IGF1-IONP-Dox in the borate buffer under different pH conditions.
2.3. In Vivo Targeting of IGF1-IONP in Human Pancreatic Cancer PDX models
To check the targeting effect and biodistribution of different IONPs, nude mice bearing orthotopic pancreatic cancer PDX with a diameter around 6 mm were administrated via the tail vein injection non targeted bovine serum albumin (BSA)-IONPs or IGF1R targeted IGF1-IONPs at an iron concentration of 20 mg/kg body weight, which was similar as the dose used for therapy study. NIR optical imaging was taken at 24 h after the IONP administration. Much higher signals were found in orthotropic tumor injected with IGF1-IONPs than the tumors in mice received non-targeted BSA-IONPs (Figure 3a). T2-weighted MRI was performed prior to and after administration of different iron IONPs (Figure 3b). The mean MRI contrast intensity was determined using Image J. About -20% of T2 signal decrease compared to pre-MR image was found in the tumor of the mice that received IGF1-IONP. However, no apparent signal change in the tumor received BSA-IONP injection. Furthermore, the accumulation of IGF1-IONPs but not BSA-IONPs in pancreatic cancer was also confirmed in tumor tissue sections by Prussian blue staining (Figure 3c).
Figure 3. Detection of targeted delivery of IGF1-IONPs into pancreatic PDX tumors.
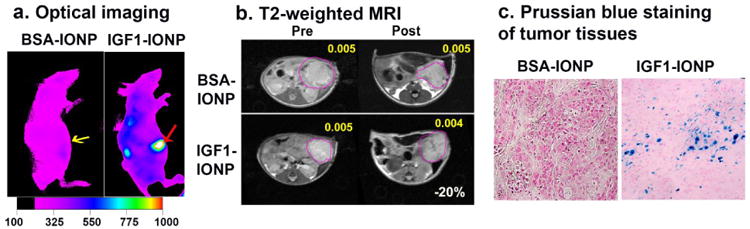
a) NIR optical imaging of mice receiving NIR830 labled IGF1-IONPs or non-targeted BSA-IONPs by tail vein injection. Arrows indicate orthotopic tumors. b) T2-weighted MRI. Yellow numbers indicate mean MRI intensity. Change of MRI T2 contrasts before and after injection is shown as white number. c) Prussian blue staining of tumor tissues. Tumor tissue sections showed IONP positive cells (blue) in the IGF1-IONP injected group but not in non-targeted BSA-IONP injected group.
2.4. Antitumor Effect of IGF1-NP-Dox in a Human Pancreatic Cancer Xenograft
The effect of systemic delivery of IGF1-NP-Dox theranostic nanoparticles on the growth of pancreatic cancer was examined in nude mice bearing human pancreatic PDXs in subcutaneous (s.c.) and orthotopic (pancreas) locations. The treatment started when the tumor reached an average diameter of 5 mm. Conventional free Dox, non-targeted IONP-Dox, and IGF1-IONP-Dox with equivalent dose of 5 mg/kg Dox were administered via the tail vein once per week for 6 times. Tumor volume and body weight were recorded every week. At the end of the study, one mouse from each group was chosen for MRI study. Five days after the final injection, mice were sacrificed and tumors were collected for histological analysis.
In comparison with no treatment control group, tumor bearing mice in all treated groups showed various degrees of tumor growth inhibition in both s.c. and orthotopic PDX tumors (Figure 4 a and 4d). In s.c. tumors, non-targeted IONP-Dox and IGF-1R targeted IGF1-IONP-Dox treated mice had marked tumor growth inhibition compared to no treatment control and free Dox treated mice. However, the effect of tumor growth inhibition of non-targeted IONP-Dox mouse group was not statistically significant (no treatment control vs. IONP-Dox: p = 0.22, Dox vs IONP-Dox: p = 0.068). On the other hand, there was a significant stronger anti-tumor effect in the tumor bearing mice treated with IGF1-IONP-Dox compared with no treatment control, Dox, or IONP-Dox (p < 0.05 for all groups, Figure 4a, left panel). At a Dox dosage of 5 mg/Kg, there was no apparent systemic toxicity in all treated group since there was no significant body weight change (Figure 4a, right panel). Examination of resistant residual tumors using non-invasive MRI revealed both selective accumulation of IGF1-IONP-Dox in s.c. tumor and a significant reduction in tumor size on mice receiving IGF1-IONP-Dox (Figure 4b), suggesting the feasibility of MRI-guided therapeutic response evaluation.
Figure 4. In vivo antitumor effect of IGF1-IONP-Dox theranostic nanoparticles in human pancreatic cancer PDXs following systemic delivery.
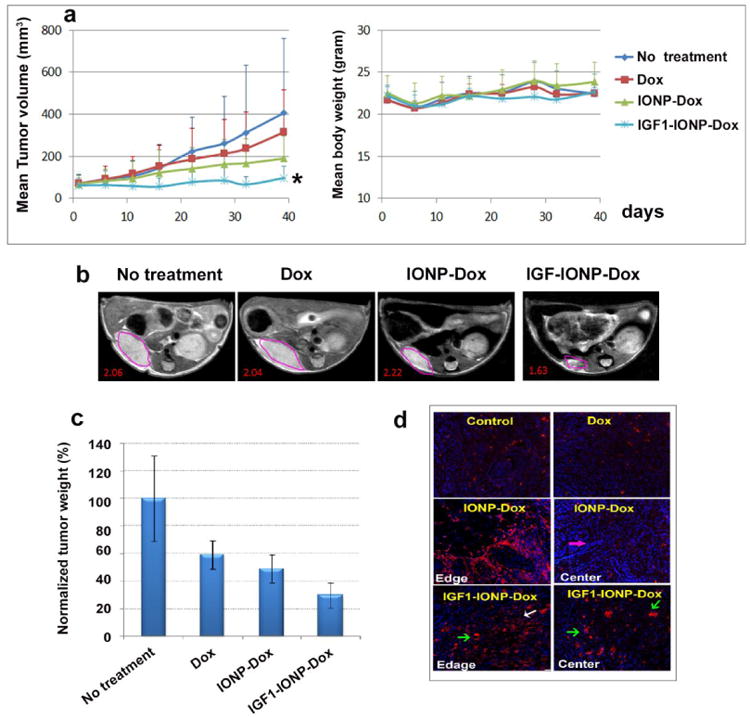
a) Tumor growth inhibition in s.c. PDX tumors. Left panel: tumor growth curve during different treatments. Dox dose: 5 mg/Kg, once per week for six tail vein injections. n = 4 mice/group. Mean tumor volume of each group was shown in the figure. * indicates P < 0.05 compared all other groups. Right panel: body weight change during treatment. Mean body weight of each group is shown. b) T2-weighted MRI of s.c. PDX tumor by the end of six treatments. 3T MRI scanner was used. Pink line circled areas: s.c. PDX tumors. Red number: mean MRI contrast intensity. c) Effect of systemic delivery of IGF1-IONP-Dox theranostic nanoparticles on orthotopic pancreatic PDX tumors. The percentage of tumor growth inhibition calculated using the mean tumor weight of no treatment control mouse group as 100%. The bar figure showed the mean tumor growth inhibition of two independent in vivo studies using PDX tumor models derived from two pancreatic cancer patients. n = 6 mice/group. d) Immunofluorescence labeling for apoptotic cell death (active caspase-3, red) in tumor tissue sections from orthotopic tumors after treatment.
In mice bearing orthotopic tumors, IGF1-IONP-Dox treated group had the smallest residual tumors among all experimental groups following the treatment with an approximately 70% tumor growth inhibition (Figure 4c). Statistically significant differences in the averaged tumor weight were observed as analyzed by student's t test (no treatment control vs IGF1-NP-Dox: p < 0.04, Dox vs IGF1-IONP-Dox: p < 0.005). Our results demonstrated that IGF1R targeted delivery of Dox using IGF1-NP-Dox theranostic nanoparticle is a more effective therapeutic approach for the treatment of human pancreatic cancer compare with conventional Dox treatment. Histological analysis of tumor tissue sections using an anti-active caspase-3 antibody and immunofluorescence labeling showed that IGF1R targeted delivery nanoparticle-Dox led to the induction of massive tumor cell death in both tumor edge and tumor center while non-targeted nanoparticle Dox carriers resulted in cell death in the peripherial tumor areas but not in the tumor center (Figure 4d). Results of this study indicated that IGF1R targeted delivery of nanoparticle drug carrier was able to overcome the tumor stromal barrier and deliver therapeutic agents into cancer cells in the tumor center.
3. Conclusions
In summary, a class of novel IGF1R targeted theranostic nanoparticles for both imaging and targeted delivery of Dox has been developed for the treatment of pancreatic cancer. Human pancreatic PDX models, which have high similarity to human patients' tumors in terms of intratumor heterogeneity, histological characteristics and tumor microenviroment, were established and used to evaluate the effect of the targeted theranostic nanoparticles. The effect of using a native growth factor IGF1 as a targeting ligand for targeted therapy of pancreatic cancer has been demonstrated in vivo using the pancreatic PDX tumors by NIR optical images, MRI and histological analysis. Systemic delivery of IGF1-IONP-Dox theranostic nanoparticles significantly reduced the growth of tumors in s.c. or orthotopic locations in pancreatic cancer PDX models as compared with that treated with free Dox or non-targeted IONP-Dox. Therefore, IGF1R targeted theranostic nanoparticles are a promising receptor targeted drug delivery system for further development of image-guided therapeutic approaches for effective treatment of stromal rich pancreatic cancer.
References
- 1.Chanda N, Kattumuri V, Shukla R, Zambre A, Katti K, Upendran A, et al. Bombesin Functionalized Gold Nanoparticles Show in Vitro and in Vivo Cancer Receptor Specificity. Proc Natl Acad Sci U S A. 2010;107:8760–8765. doi: 10.1073/pnas.1002143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng H, Zhao Y, Dong JY, Xue M, Lin YS, Ji ZX, et al. Two-Wave Nanotherapy To Target the Stroma and Optimize Gemcitabine Delivery To a Human Pancreatic Cancer Model in Mice. ACS Nano. 2013;7:10048–10065. doi: 10.1021/nn404083m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kam NWS, O'Connell M, Wisdom JA, Dai HJ. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie J, Liu G, Eden HS, Ai H, Chen X. Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc Chem Res. 2011;44:883–892. doi: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Delivery Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M, et al. Theranostic Nanoparticles with Controlled Release of Gemcitabine for Targeted Therapy and MRI of Pancreatic Cancer. ACS Nano. 2013;7:2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu MK, Park J, Jon S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, et al. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2:14–21. [Google Scholar]
- 9.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The Role of Stroma in Pancreatic Cancer: Diagnostic and Therapeutic Implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 10.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The Pancreas Cancer Microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Qian W, Uckun FM, Wang L, Wang YA, Chen H, et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano. 2015;9:7976–7991. doi: 10.1021/acsnano.5b01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, et al. Epidermal Growth Factor Receptor and Insulin-Like rowth Factor 1 Receptor Expression Predict Poor Survival in Pancreatic Ductal Adenocarcinoma. Cancer. 2012;118:3484–3493. doi: 10.1002/cncr.26661. [DOI] [PubMed] [Google Scholar]
- 13.Ouban A, Muraca P, Yeatman T, Coppola D. Expression and Distribution of Insulin-Like Growth Factor-1 Receptor in Human Carcinomas. Hum Pathol. 2003;34:803–808. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 14.Peled N, Wynes MW, Ikeda N, Ohira T, Yoshida K, Qian J, et al. Insulin-Like Growth Factor-1 Receptor (IGF-1R) as a Biomarker for Resistance to the Tyrosine Kinase Inhibitor Gefitinib in Non-Small Cell Lung Cancer. Cell Oncol. 2013;36:277–288. doi: 10.1007/s13402-013-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suda K, Mizuuchi H, Sato K, Takemoto T, Iwasaki T, Mitsudomi T. The insulin-like growth factor 1 receptor causes acquired resistance to erlotinib in lung cancer cells with the wild-type epidermal growth factor receptor. Int J Cancer. 2014;135:1002–1006. doi: 10.1002/ijc.28737. [DOI] [PubMed] [Google Scholar]
- 16.Hidalgo M, Von Hoff DD. Translational Therapeutic Opportunities in Ductal Adenocarcinoma of the Pancreas. Clin Cancer Res. 2012;18:4249–4256. doi: 10.1158/1078-0432.CCR-12-1327. [DOI] [PubMed] [Google Scholar]
- 17.Kopetz S, Lemos R, Powis G. The Promise of Patient-Derived Xenografts: The Best Laid Plans of Mice and Men. Clin Cancer Res. 2012;18:5160–5162. doi: 10.1158/1078-0432.CCR-12-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siolas D, Hannon GJ. Patient-Derived Tumor Xenografts: Transforming Clinical Samples into Mouse Models. Cancer Res. 2013;73:5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-Derived Tumour Xenografts as Models for Oncology Drug Development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel R, Ma JM, Zou ZH, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 21.Bergers G, Hanahan D. Modes of Resistance to Anti-Angiogenic Therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 23.Satpathy M, Wang L, Zielinski R, Qian W, Lipowska M, Capala J, et al. Active Targeting Using HER-2-Affibody-Conjugated Nanoparticles Enabled Sensitive and Specifi c Imaging of Orthotopic HER-2 Positive Ovarian Tumors. Small. 2014;10:544–555. doi: 10.1002/smll.201301593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, et al. Multifunctional Polymeric Micelles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 25.Burke TG, Morin MJ, Sartorelli AC, Lane PE, Tritton TR. Function of the Anthracycline Amino Group in Cellular Transport and Cytotoxicity. Mol Pharmacol. 1987;31:552–556. [PubMed] [Google Scholar]


