Abstract
Chlamydia pneumoniae (Cpn), a common respiratory pathogen of humans, is associated with human cardiovascular disease and the acceleration of atherosclerosis in hyperlipidemic animal models. Our laboratory has demonstrated that murine norovirus (MNV), a prevalent infection of laboratory mice, can unpredictably alter atherosclerosis in hyperlipidemic Ldlr−/− and ApoE−/− mice. Given that MNV has a tropism for macrophages and may exacerbate atherogenesis, we investigated whether coinfection with MNV and Cpn might alter macrophage phenotypes in vitro and atherosclerosis in ApoE−/− mice. In the presence of oxidized low-density lipoprotein, coinfection of ApoE−/− bone marrow-derived macrophages (BMDM) with MNV and Cpn resulted in significant increases in gene expression of IL6, MCP1, iNOS, and TNFα compared with Cpn-monoinfected BMDM. On the basis of these findings, we hypothesized that concurrent MNV–Cpn infection might increase plaque lesion size in vivo. As expected, Cpn monoinfection of ApoE−/− mice increased mean plaque size by 62% compared with that in uninfected mice. However, MNV did not significantly alter plaque lesion size in MNV–Cpn-coinfected mice compared with Cpn-monoinfected mice. There were no differences in aortic cytokines locally at the site of plaque development or in peritoneal macrophages at 1 wk after infection in MNV–Cpn-coinfected mice compared with Cpn-monoinfected mice. MNV was not detected in the aortic tissue of MNV-infected mice at 1 or 8 wk after infection regardless of Cpn status. These data suggest that MNV infection does not appreciably alter plaque development in Cpn-accelerated atherosclerosis in ApoE−/− mice.
Abbreviations: ApoE−/−, apolipoprotein E knockout; Arg1, arginase 1; BMDM, bone marrow-derived macrophages; Cpn, Chlamydia pneumoniae; MNV, murine norovirus; MCP1, macrophage chemoattractant protein 1; iNOS, inducible nitric oxide synthase; MOI, multiplicity of infection; oxLDL, oxidized low-density lipoprotein
Cardiovascular disease is the leading cause of mortality in the United States, accounting for nearly 37% of all deaths.43 Cardiovascular disease encompasses a number of conditions including atherosclerosis, the hardening and narrowing of arteries as a result of plaque buildup that can progress to myocardial infarction, stroke, and peripheral vascular disease. Atherosclerosis involves chronic vascular inflammation and, although both genetic and environmental features have been identified as risk factors, these alone do not completely account for all incidences of disease.11 For this reason, much consideration has been given to the role of infectious pathogens in atherosclerosis. Many pathogens, both viral and bacterial, have been reported as having positive seroepidemiologic association with atherosclerosis.11,31,36,37,45
Chlamydia pneumoniae (Cpn), an obligate intracellular bacterium and prevalent respiratory pathogen in humans, is among the most studied agents implicated in atherosclerosis.1,5,6,23,30,36,37,45 In addition to a positive correlation between exposure to Cpn and cardiovascular disease, the organism has been localized to and cultured from human atherosclerotic plaques.6,23 Notably, the causality between this bacterium and disease has been demonstrated in various animal models, including hyperlipidemic rabbits, rats, and mice.1,5,6,15,30 In addition, viruses including cytomegalovirus, influenza A, and HIV, have been implicated in promoting vascular inflammation.2,3,16,28,31,45
These infectious agents are thought to affect atherosclerosis through both direct and indirect mechanisms. Direct effects result from pathogen residence at the site of plaque formation. Localization of the pathogen can induce and increase inflammation, cause dysfunction and proliferation of vascular cells, or alter lipid accumulation locally.36 However, it also has been proposed that pathogens can accelerate atherosclerosis without residing at the sites of plaque formation through their systemic effects.11,36 Such indirect mechanisms include changes in circulating cytokine levels and in immune cell phenotypes.10,21,36,38
Hyperlipidemic animal models have been useful tools in understanding the pathogenic mechanisms of these infectious agents in atherosclerosis.5,6 Typically these animals are maintained SPF, meaning they are free of bacteria and viruses that are known or suspected to interfere with research studies, but they may still be enzootically infected with agents of unknown effects. Murine norovirus (MNV), a highly infectious and highly prevalent RNA virus is an enzootic agent of laboratory mice for which the effects of infection in mouse models of disease is still under investigation.13,35 MNV infection typically is clinically silent and therefore has the potential to alter disease models without being noticed.13,42 Initial in vitro studies with MNV revealed that the virus had a tropism for macrophages and dendritic cells.44 For this reason, its effect on models of chronic inflammatory disease in which macrophages and dendritic cells play an important role—including obesity, inflammatory bowel disease, and atherosclerosis—has been investigated.17,18,20,25,26,32-34
Previously, our laboratory showed that MNV infection in mouse models of atherosclerosis may alter plaque development, but these effects might depend on genetics, timing of infection, or dietary fat content.17,32,34 For example, MNV infection in Ldlr−/− mice promoted atherosclerotic lesion development and was associated with increased macrophage content in the lesion.34 However, this effect was dependent on the timing of infection.32 In addition, MNV infection altered atherosclerosis in ApoE−/− mice fed a standard chow diet, but this effect was unpredictable.17 Given the varied effects of MNV on atherosclerosis in hyperlipidemic mice, we chose to investigate the potential of MNV to change Cpn-accelerated atherosclerosis in the ApoE−/− mouse model by both direct and indirect mechanisms. We hypothesized that concurrent infection with MNV would alter macrophage and monocyte phenotypes, cytokine production, and ultimately result in larger atherosclerotic plaques in ApoE−/− mice as compared with animals infected with Cpn alone. We found, however, whereas Cpn infection increased atherosclerotic plaque size as expected, concurrent infection with MNV did not alter the atherosclerosis induced by Cpn infection.
Materials and Methods
Animals.
Male B6.129P2-Apoetm1Unc/J (ApoE−/−) mice (age, 6 wk) were purchased from The Jackson Laboratory (Bar Harbor, ME) and acclimated for 2 wk prior to study. Mice were certified by the vendor to be free of specific rodent pathogens including ectromelia virus, Theiler virus, Hantaan virus, K virus, LDH elevating virus, lymphocytic choriomeningitis, mouse hepatitis virus, mouse minute virus, mouse norovirus, mouse parvovirus, mouse thymic virus, pneumonia virus of mice, polyoma virus, reovirus 3, rotavirus, Sendai virus, Bordetella spp., CAR bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium bovis, Corynebacterium kutscheri, Helicobacter spp., Mycoplasma pulmonis, Pasteurella spp., Salmonella spp., Streptobacillus moniliformis, Klebsiella spp., Pneumocystis murina, Proteus mirabilis, Pseudomonas spp., Staphylococcus aureus, Streptococcus spp., Encephalitozoon cuniculi, and ectoparasites and endoparasites including fleas, lice, mites, tapeworms, and pinworms. Mice were maintained under SPF conditions (except for experimental infections with Cpn and MNV) according to sentinel surveillance. All mice were fed irradiated rodent chow (Picolab Rodent Diet 20 number 5053, PMI Nutrition, Brentwood, MO), provided autoclaved acidified water in bottles, and were housed by infection status in IVC (Allentown, Allentown, NJ) containing autoclaved corncob bedding (The Andersons, Maumee, OH). Standard operating procedures were used to restrict MNV and Cpn infection to only those mice experimentally infected with each or both agents. These procedures included handling cages in a class II biosafety cabinet (NuAire, Plymouth, MN) disinfected with chlorine dioxide (dilution, 1:18:1; Clidox S, Pharmacal Research Laboratories, Naugatuck, CT) prior to handling cages and in between infection groups. In addition, gloves were changed between experimental groups. By using fecal RT-PCR, MNV infection status was confirmed prior to and at the end of each in vivo study.18 All animal procedures were approved by the University of Washington's IACUC.
Infection of bone marrow-derived macrophages (BMDM).
Bone marrow was harvested from ApoE−/− mice (age, 9 to 12 wk), pooled, and differentiated as previously described.18 Differentiated macrophages were plated at a density of 1 to 1.4 × 106 cells per well (6-well plates) in RPMI 1640 media containing 1% Nutridoma-SP (Roche, Indianapolis, IN), 1% penicillin–streptomycin, 20 µg/ mL gentamicin, and 10 µg/mL oxidized LDL (oxLDL). For each treatment group, triplicate wells were infected and evaluated: (1) uninfected, (2) MNV monoinfected, (3) Cpn monoinfected, and (4) MNV and Cpn coinfected. BMDM were inoculated with Cpn AR39 at a multiplicity of infection (MOI) of 5 in RPMI 1640 medium or mock-infected (groups 1 and 2) by using RPMI 1640 media alone through centrifugation at 700 to 800 × g and incubated for 1 h at 37 °C in 5% CO2. The inoculum from all samples was removed after this 1-h incubation and replaced with RPMI 1640 media. At 24 h after Cpn infection, cells were infected with MNV at a MOI of 0.2 or mock-infected (groups 1 and 3) by using clarified RAW 264.7 cell lysates free of MNV. Cells were allowed to incubate for an additional 24 h All samples were harvested at 48 h after initial Cpn infections for RNA extraction (RNeasy kit, Qiagen, Valencia, CA).
Infections and tissue collection.
To evaluate changes in atherosclerotic plaque development, ApoE−/− mice were assigned to 1 of 4 treatment groups: (1) uninfected control mice (n = 21), (2) MNV-monoinfected mice (n = 21), (3) Cpn-monoinfected mice (n = 18), or (4) MNV–Cpn-coinfected mice (n = 16). For Cpn infections, 8-wk-old ApoE−/− mice were intranasally dosed with Cpn strain AR39. Mice were anesthetized with ketamine–xylazine and administered 3 × 107 inclusion-forming units of Cpn in 20 μL sterile PBS or sham-infected with 20 μL sterile PBS, as previously described.1,5 This procedure was repeated at 9 and 10 wk of age to establish a persistent infection. Two weeks later, mice were orally gavaged with 1 × 106 pfu MNV4, passage 7, in 200 μL RAW 264.7 cell lysate or sham-infected with clarified RAW 264.7 cell lysate free of MNV. At 8 wk after MNV infection, when mice were 20 wk old, they were anesthetized with ketamine–xylazine and perfused with formalin, after which the heart was collected and stored in formalin for plaque analysis. A 2- to 3-mm portion of the descending thoracic aorta was collected and stored in RNAlater (Ambion, Austin, TX) and frozen at –80 °C. The propagation and preparation of MNV4 has been described previously.17,18
To evaluate early changes associated with atherosclerosis development, a separate group of mice was assigned to 1 of 4 treatment groups and infected as outlined earlier: (1) uninfected control mice (n = 10), (2) MNV-monoinfected mice (n = 10), (3) Cpn-monoinfected mice (n = 9), or (4) MNV–Cpn-coinfected mice (n = 9). ApoE−/− mice were euthanized by using an inhaled overdose of CO2 1 wk after MNV infection, when mice were 13 wk old. Blood was collected by cardiocentesis and heparinized (20 units heparin per 1 mL blood) for flow cytometric analysis. Peritoneal macrophages were collected by using peritoneal lavage with 10 mL of sterile PBS, and cells were resuspended in RPMI 1640 media containing 10% FBS and 1% penicillin–streptomycin and incubated in 6-well plates for 1 h at 37 °C. After incubation, adherent cells (macrophages) were lysed in RLT buffer (RNeasy kit, Qiagen, Valencia, CA) for RNA extraction. The aortic arch and a 2- to 3-mm portion of the descending thoracic aorta was dissected free from surrounding adventitial tissue and stored at –80 °C for RNA extraction.
RT-PCR.
RNA from BMDM and peritoneal macrophages was extracted by using the RNeasy kit (Qiagen) according to the manufacturer's instructions. RNA from the aortic arch was extracted by using Lysing Matrix D tubes (MP Biomedical, Santa Ana, CA) and the RNeasy Fibrous Tissue Kit (Qiagen), whereas the descending thoracic aorta was extracted by using Lysing Matrix D tubes and the RNeasy kit. RNA was converted to cDNA by using SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Real-time RT-PCR analysis was performed with Power SYBR Green Master Mix (Applied Biosystems, Warrington, UK) and a Stratagene Mx3005P analyzer (Agilent Technologies, Santa Clara, CA). The primer sequences used for various genes were previously described.9,12,14,18,22,27,29 Target gene expression in aortic arch samples was normalized to β-actin and expressed as fold change relative to a control sample (RNA from aortic tissue of an uninfected mouse) that was run on every plate as a calibrator. Target gene expression for peritoneal macrophages and BMDM was normalized to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase and presented as the fold change relative to the average value obtained from uninfected animals or uninfected cells. RT-PCR analysis for the detection of MNV4 was performed using primers as previously described18 on samples of the aortic arch and descending aorta from the mice described earlier, which were used to evaluate early changes associated with atherosclerosis development; the descending aorta samples from the mice which were used to evaluate atherosclerotic plaque development were analyzed also.
Quantification of atherosclerosis.
Quantification of plaque size in 20-wk-old ApoE−/− mice was performed as previously described by using Movat pentachrome stain and scored by a researcher blinded to treatment groups.32,34
Flow cytometric analysis of peripheral blood.
RBC in heparinized blood were lysed by using Gey solution, cell count was determined, and samples were blocked with antiCD16/CD32 antibody (BD Biosciences, San Jose, CA). Antimouse antibodies were then used to label cell surface markers including lineage (Lin; (NK1.1, CD90.2, B220, Ly6G, Ter119, and CD49b), Ly6C, CD11b, CD11c, IA/IE, and F4/80 (all obtained from BD Biosciences). Leukocytes were gated on forward-scatter A and side-scatter A, and single cells were gated on forward-scatter A and forward-scatter W parameters. Lin– cells were further gated to identify monocytes (Lin–CD11b+F4/80–class II–CD11c–). In addition, monocytes were characterized on the basis of Ly6C expression. Samples with fewer than 80 events in the monocyte gate were excluded from analysis (low-number artifact). Data were collected on a BD LSRII and analyzed by using FlowJo (Tree Star, Ashland, OR).
Statistics.
Data was analyzed by using Prism statistical software (GraphPad Software, La Jolla, CA). One-way ANOVA with posthoc testing (Tukey multiple comparison) was used for comparison among 4 groups, whereas a t test was used for the comparison of 2 groups. P values less than 0.05 were considered significant.
Results
Effect of MNV on gene expression of IL6, IL1β, and MCP1 in Cpn-infected ApoE−/− BMDM.
To evaluate the effects of MNV and Cpn on inflammatory cytokines and chemokines, ApoE−/− BMDM were infected in the presence of oxLDL with MNV alone, Cpn alone, or both agents combined, and gene expression related to macrophage activation and recruitment, including the genes for IL6, IL1β, and macrophage chemoattractant protein 1 (MCP1), was evaluated. Infections were performed in the presence of oxLDL to better mimic the in vivo plaque environment, which is rich in lipids. MNV infection alone resulted in a significant (P < 0.05) increase in MCP1 transcripts but not those of IL6 or IL1β as compared with their expression in uninfected BMDM. Cpn monoinfection resulted in a 170 ± 85 (mean ± SEM) fold increase (P < 0.05) in IL1β expression and a 8.0 ± 0.1 fold increase (P < 0.05) in MCP1 expression compared with that in uninfected BMDM (Figure 1). Coinfection with MNV and Cpn led to a significant (P < 0.05) increase in IL6 and MCP1 but not IL1β expression as compared with that in Cpn-monoinfected cells (Figure 1). IL6 expression increased 752 ± 51 fold and MCP1 expression increased 10.0 ± 0.3 in coinfected BMDM as compared with uninfected cells. These data suggest that MNV infection enhances IL6 and MCP1 gene expression in ApoE−/− BMDM that are already infected with Cpn.
Figure 1.
Alterations in gene expression of (A) IL6, (B) IL1β, and (C) MCP1 by MNV infection in Cpn-infected ApoE−/− BMDM. Gene expression levels in ApoE−/− bone marrow-derived macrophages infected with MNV, Cpn, or both agents (triplicate wells per group). BMDM were treated with Cpn (MOI = 5) or mock-infected for 24 h and then with MNV (MOI = 0.2) or mock-infected for another 24 h in the presence of oxLDL. At 48 h after initial Cpn infection, RNA was extracted and evaluated for gene expression by real-time RT-PCR. Expression levels are expressed as fold change compared with transcript levels in uninfected oxLDL-exposed BMDM. Data are given as mean ± SEM (error bars). One-way ANOVA with posthoc Tukey multiple-comparison testing was used to determine significant (P < 0.05) differences between groups. Value significantly (P < 0.05) different from that in uninfected BMDM (*), in MNV-monoinfected BMDM (Δ) and in Cpn-monoinfected BMDM (φ).
Effect of MNV on macrophage phenotype in Cpn-infected ApoE−/− BMDM.
To evaluate the effect of MNV and Cpn on macrophage phenotype, gene expression was measured for markers of classically activated macrophages (M1), including the genes for inducible nitric oxide synthase (iNOS), IL12β, and TNFα, as well as alternatively activated macrophages (M2), including the arginase 1 (Arg1) and IL10 genes. The M1 and M2 phenotypes, which have both been demonstrated in atherosclerotic plaques, have opposing functions in that M1 macrophages promote inflammation, whereas M2 macrophages are regulatory and help to dampen and control local inflammatory responses.10,21,24
Cpn monoinfection significantly (P < 0.05) increased IL12β gene expression by 700 ± 70 fold and TNFα expression by 10.0 ± 0.4 fold compared with that in uninfected BMDM. Cpn monoinfection did not change iNOS expression as compared with that in uninfected controls (Figure 2 A). The addition of MNV to BMDM already infected with Cpn (coinfected group) induced a significant increase (P < 0.05) in iNOS and TNFα transcripts when compared with that in BMDM infected with Cpn alone, corresponding to a 59767 ± 9610 fold increase in iNOS expression and a 13 ± 1 fold increase in TNFα expression relative to uninfected BMDM. The addition of MNV to BMDM already infected with Cpn (that is, the coinfected group) did not alter IL12β expression when compared with that associated with Cpn infection only. There were no significant changes in iNOS, IL12β, or TNFα in MNV-monoinfected BMDM as compared with uninfected controls. In addition, Cpn monoinfection elicited an M2 response, with a 11.0 ± 0.6 fold increase (P < 0.05) in Arg1 expression as well as a 2.5 ± 0.3 fold increase (P < 0.05) in IL10 expression as compared with that in uninfected BMDM (Figure 2 B). Coinfection partially dampened this M2 response, as indicated by a significant (P < 0.05) decrease in the relative expression of Arg1 as compared with that in Cpn-monoinfected BMDM. Coinfection did not alter IL10 gene expression as compared with that in Cpn-monoinfected BMDM. MNV monoinfection had no effect on M2-associated genes (Figure 2 B). These findings suggest that Cpn elicits an M1 proinflammatory macrophage phenotype that is exacerbated by concurrent MNV infection. In addition, there is evidence that, although Cpn monoinfection elicits an M2 phenotype indicated by increases in Arg1 and IL10 gene expression, coinfection may partially attenuate this regulatory M2 macrophage response through significant decreases in Arg1 gene expression.
Figure 2.
MNV exacerbates a proinflammatory macrophage phenotype in Cpn-infected ApoE−/− BMDM. Gene expression of (A) iNOS, IL12β, and TNFα (M1-associated genes) and (B) Arg1 and IL10 (M2-associated genes) in ApoE−/− bone marrow-derived macrophages (BMDM) infected with MNV, Cpn, or both agents (triplicate wells per group). BMDM were treated with Cpn (MOI = 5) or mock-infected for 24 h, and then with MNV (MOI = 0.2) or mock-infected for another 24 h in the presence of oxLDL. At 48 h after initial Cpn infection, RNA was extracted and evaluated for gene expression by real-time RT-PCR. Expression levels are expressed as fold change compared with transcript levels in uninfected oxLDL-exposed BMDM. Data are given as mean ± SEM (error bars). One-way ANOVA with posthoc Tukey multiple-comparison testing was used to determine significant (P < 0.05) differences between groups. Value significantly (P < 0.05) different from that in uninfected BMDM (*), in MNV-monoinfected BMDM (Δ), or in Cpn-monoinfected BMDM (φ).
Effect of MNV infection on Cpn-accelerated atherosclerosis in ApoE−/− mice.
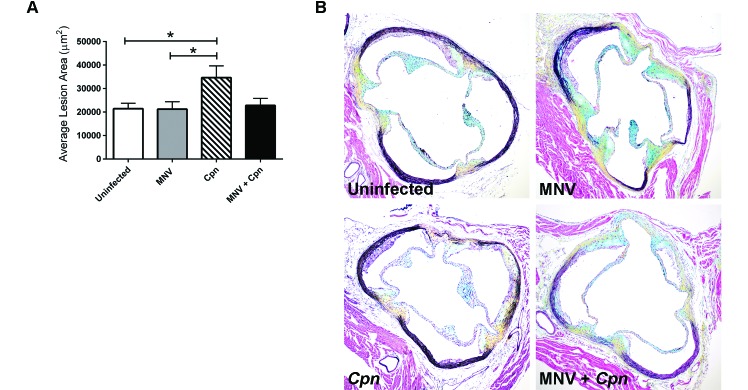
Given our in vitro findings that MNV increased MCP1 and IL6 gene expression, as well as exacerbated the proinflammatory macrophage phenotype in Cpn-infected ApoE−/− BMDM, we hypothesized that concurrent infection with MNV and Cpn would result in the exacerbation of Cpn-accelerated atherosclerosis in vivo. To test this hypothesis, ApoE−/− mice of varied infection status (no infection, MNV monoinfection, Cpn monoinfection, and coinfection) were examined at 20 wk old (12 wk after Cpn infection, 8 wk after MNV infection) for atherosclerotic lesion size in the aortic sinus. Infection with Cpn significantly (P = 0.04) increased the average aortic sinus lesion area (34,648 ± 5015 µm2) by approximately 62% when compared with that of uninfected controls (21,443 ± 2305 µm2; Figure 3). Unexpectedly, coinfected mice had smaller plaque lesions than did Cpn-monoinfected animals. Specifically, coinfected mice showed a 56% reduction in the average aortic lesion area (22,800 ± 3004 µm2) compared with that of Cpn-monoinfected animals (34,648 ± 5015 µm2), although this difference did not reach statistical significance (P = 0.057). MNV monoinfection did not increase plaque lesion size compared with that of uninfected controls.
Figure 3.
MNV does not alter Cpn-induced atherosclerosis in ApoE−/− mice. ApoE−/− mice were infected with MNV, Cpn, or both agents, and their aortic sinus lesions were evaluated at 20 wk old. (A) Lesion area was determined by using computer-assisted morphometry. One-way ANOVA with post hoc Tukey multiple-comparison testing was used to determine significant (P < 0.05) differences between groups (uninfected control mice, n = 21; MNV-monoinfected mice, n = 21; Cpn-monoinfected mice, n = 18; and MNV–Cpn-coinfected mice, n = 16). (B) Representative lesions from mice of differing infection status at 20 wk old, 8 wk after MNV infection. Data are given as mean ± SEM (error bars). Movat pentachrome stain; magnification, 100×.
Effect of MNV on circulating monocyte populations in Cpn-infected ApoE−/− mice.
One proposed indirect mechanism by which pathogens alter lesion size is through an effect on circulating monocytes. Monocytes differ in their recruitment to and function within the plaque.38,40 Murine monocytes can be differentiated according to their Ly6C surface expression, and Ly6Chi monocytes are preferentially recruited to plaques, where they differentiate into proinflammatory macrophages.10,39 To determine whether MNV infection leads to alterations in the circulating monocytes of Cpn-infected mice, peripheral blood was evaluated in uninfected, MNV-monoinfected, Cpn-monoinfected, and coinfected 13-wk-old ApoE−/− mice. No differences were noted between groups in the percentage of monocytes in the peripheral blood (Figure 4 A). Furthermore, although there were no differences in the percentage of Ly6Chi monocytes in Cpn-monoinfected mice compared with uninfected controls, the percentage of Ly6Chi monocytes in mice coinfected with both MNV and Cpn (10.6% ± 3.7%) was significantly (P < 0.05) increased relative to that in uninfected mice (1.1% ± 0.3%; Figure 4 B). The percentage of Ly6Chi monocytes also differed significantly (P < 0.05) between coinfected (10.6% ± 3.7%) and MNV-monoinfected mice (2.3% ± 1.1%). These findings suggest that, although neither infection alone affects monocyte numbers, coinfection with MNV and Cpn increases the proportion of Ly6Chi monocytes.
Figure 4.
MNV alters circulating monocyte populations in Cpn-infected ApoE−/− mice. Flow cytometry was used to measure the (A) percentage of monocytes among total cells recovered from peripheral blood and (B) percentage of Ly6Chi monocytes among total monocytes from 13-wk-old ApoE−/− mice of varied infection status. Samples with fewer than 80 events in the monocyte gate were eliminated from analysis. Data are given as mean ± SEM (error bars). One-way ANOVA with posthoc Tukey multiple-comparison testing was used to determine significant (P < 0.05) differences between groups (uninfected control mice, n = 8; MNV-monoinfected mice, n = 9; Cpn-monoinfected mice, n = 6; and MNV–Cpn-coinfected mice, n = 6).
Effects of MNV on cytokine, chemokine, and adhesion molecule expression at the site of lesion development in the aorta and in peritoneal macrophages of Cpn-infected ApoE−/− mice.
To determine whether cytokines were directly altered in the local environment of plaque development in the aorta, gene expression in the aortic arch of 13-wk-old ApoE−/− mice of varied infection status (no infection, MNV monoinfection, Cpn monoinfection, and coinfection) was evaluated. In addition to looking directly at the site of lesion formation, gene expression of peritoneal macrophages from these mice was assessed because they have the potential to influence lesion development indirectly. No significant differences were detected in aortic arch MCP1, TNFα, or IL1β gene expression between any of the infection groups (Figure 5 A). Likewise, no differences in gene expression of MCP1, TNFα, or Arg1 were seen in peritoneal macrophages (Figure 5 B). Furthermore, given that we observed a robust increase in iNOS expression after in vitro coinfection of BMDM with MNV and Cpn (Figure 2 A), we evaluated iNOS expression in the aortic arch and found no differences between any groups (data not shown).
Figure 5.
MNV does not alter cytokine or chemokine gene expression in the aortic arch or peritoneal macrophages of Cpn-infected ApoE−/− mice. Gene expression of (A) MCP1, TNFα, and IL1β in the aortic arch and (B) MCP1, TNFα, and Arg1 in unelicited peritoneal macrophages from 13-wk-old ApoE−/− mice of varied infection status was measured by using real-time RT-PCR analysis. Data are given as mean ± SEM (error bars); no significant differences were detected between groups (uninfected control mice, n = 10; MNV-monoinfected mice, n = 10; Cpn-monoinfected mice, n = 9; and MNV–Cpn-coinfected mice, n = 9).
Adhesion molecules can contribute significantly to atherogenesis because they, in part, regulate the rate and number of monocytes and macrophages entering developing lesions.10 To this effect, we examined expression of intracellular adhesion molecule 1 in the aortic arch tissue, where MNV might exert a direct effect, as well as in peritoneal macrophages. There were no significant differences in relative expression of intracellular adhesion molecule 1 among infection groups in either the aortic arch or in peritoneal macrophages (Figure 6).
Figure 6.
MNV does not alter the expression of intracellular adhesion molecule 1 in the aortic arch or peritoneal macrophages of Cpn-infected ApoE−/− mice. Gene expression of intracellular adhesion molecule 1 in the (A) aortic arch and (B) unelicited peritoneal macrophages from 13-wk-old ApoE−/− mice was measured by using real-time RT-PCR analysis. Data are given as mean ± SEM (error bars); no significant differences were detected between groups (uninfected control mice, n = 10; MNV-infected, n = 10; Cpn-infected, n = 9; MNV–Cpn-coinfected, n = 9).
MNV is not detectable in aortic tissue of infected ApoE−/− mice.
To determine whether MNV localizes to sites of atherosclerosis in ApoE−/− mice acutely after infection and during the early lesion development process, RT-PCR for MNV was performed on the aortic arch and descending aorta tissue at 1 wk after MNV infection in 13-wk-old ApoE−/− mice. MNV was not detected in the aortic tissue of any of the mice, regardless of Cpn status. To determine whether MNV localizes to the site of lesion development during chronic stages of atherosclerosis, RT-PCR for MNV was performed on the descending aorta of 20-wk-old mice, that is, at 8 wk after MNV infection. Similarly, MNV was not detected in any mice regardless of Cpn status. To confirm that mice gavaged with MNV were infected, MNV RT-PCR was performed on pooled feces from each mouse cage. All fecal samples from cages of mice experimentally infected with MNV were positive by MNV RT-PCR at both 1 and 8 wk after infection, and feces from uninfected cages were confirmed to be MNV-negative.
Discussion
Atherosclerosis is a leading cause of morbidity in humans and involves chronic systemic vascular inflammation. The innate immune system is a major contributor to this process, and macrophages and monocytes are key components of atherosclerotic plaques.10,11,24,38 Local and systemic changes in these cells, such as those that occur in response to infection, are thought to underlie pathogen accelerated atherosclerosis. Considerable evidence in humans, as well as from animal studies, has implicated the common respiratory pathogen C. pneumoniae as a risk factor for cardiovascular disease. Hyperlipidemic animal models have been used to understand how this pathogen augments atherogenesis, and Cpn infection in ApoE−/− mice has been reported to increase the size of atherosclerotic plaque lesions.2,5,30 Our laboratory previously demonstrated that MNV, an enzootic viral infection of laboratory mice, alters atherosclerosis in both Ldlr−/− and ApoE−/− mouse models, but this effect is dependent on the timing of infection or dietary fat content.17,32,33 Given the potential of this virus to interfere with atherosclerosis, we investigated the effect of coinfection with MNV and Cpn in ApoE−/− mice and hypothesized that coinfection would lead to increased expression of inflammatory cytokines and chemokines and exacerbate atherosclerotic lesion size as compared with the results of Cpn monoinfection. We were interested in determining whether a virus and bacterium could work together to alter disease as compared with infection with either agent alone, given that previous evidence indicates that coinfection with a bacterium and virus may indeed alter disease progression in other mouse models.2,20,25
Coinfection of BMDM exposed to oxLDL, used to mimic the hyperlipidemic environment in which plaques form in vivo, resulted in significant increases in gene expression of IL6 and MCP1 and elicited an M1 proinflammatory macrophage phenotype, as evidenced by increases in iNOS and TNFα transcripts and a decrease in Arg1 gene expression. Although we did not specifically evaluate the macrophages within the vascular tissue of our infected mice, our in vitro BMDM findings are in agreement with studies demonstrating that Cpn induces M1 macrophages after pulmonary infection.19 IL6 and MCP1 are strong macrophage chemoattractants and have been correlated with increased plaque macrophage content.10,24 whereas iNOS and TNFα are potent proinflammatory factors that contribute to sustained vascular inflammation.11 Macrophage subtypes have been shown to play differing roles in plaque development.10,21,24 Classically activated macrophages (M1, characterized by iNOS, IL12β, TNFα) contribute to sustained inflammation, whereas alternatively activated macrophages (M2, characterized by Arg1 and IL10) appear to stabilize the plaque.9,12,21,24 Collectively, our in vitro findings suggest that MNV exacerbates Cpn-accelerated atherosclerosis in ApoE−/− mice through changes in either circulating or plaque-resident macrophages.
A recent publication reported that atherosclerotic lesions in ApoE−/− mice were proportional to intralesional monocyte accumulation,40 suggesting that an effect on monocytes in particular results in significant changes to plaque development. Furthermore, a subset of these cells, Ly6Chi monocytes, have been shown to both preferentially adhere to inflamed endothelium and give rise to proinflammatory macrophages.37 Here we show that, although Cpn does not affect the percentage of blood monocytes at 1 wk after infection, coinfected animals have an increase in the Ly6Chi subset at this time, suggesting that plaque lesion size would be increased in coinfected mice.
In our in vivo study, infection with Cpn significantly increased the average aortic sinus lesion area by 62% compared with that of uninfected controls, as we expected and as reported by others.2,5,30 Coinfection of ApoE−/− mice with Cpn/MNV did not significantly alter plaque lesion area as compared with infection with Cpn alone, suggesting that MNV did not alter Cpn-induced atherosclerosis. In general, our findings are in disagreement with studies in humans, which have found a positive correlation between the number of infections experienced by a patient (infectious burden) and cardiovascular disease.37,45 Furthermore, our current in vivo finding is in disagreement with our in vitro data regarding increased proinflammatory cytokines and macrophages and with our analysis of circulating Ly6Chi monocytes, which were increased after coinfection. One explanation may be that BMDM in vitro are imperfect predictors of atherosclerosis in vivo and that the disagreement between our in vitro and in vivo findings may be due to the complexity of downstream effects of the chemokines and cytokines evaluated. For example, although iNOS is typically thought of as proinflammatory, iNOS also been shown to be atheroprotective against Cpn-accelerated disease.7 Another possible explanation is that monocyte populations are responsive to many systemic factors38,39 and therefore change over time. Our finding of increased Ly6Chi monocytes at 1 wk after MNV infection may have reflected a transient effect or one perhaps subsequently altered by systemic responses.
Emerging evidence indicates that resident viruses help define immunophenotype, systemic inflammation, and even disease susceptibility.4,8 Although our in vivo findings suggest that this particular norovirus does not impact atherosclerosis in Cpn-infected ApoE−/− mice, it is intriguing to speculate that MNV may be atheroprotective, in light of the 56% reduction in plaque lesion area in coinfected mice compared with Cpn-monoinfected mice. In support of this notion, a protective effect has been reported in ApoE−/− mice coinfected with murine cytomegalovirus and Cpn: whereas Cpn led to significant increases in lesion size, concurrent infection with cytomegalovirus abrogated this effect, thus demonstrating viral protection against Cpn-accelerated atherosclerosis.2 In addition, a recent study reported that MNV conferred protection to antibiotic treated mice against pathogenic bacterial challenge with Citrobacter rodentium.20 Mice infected with MNV prior to C. rodentium challenge had reduced weight loss, diarrhea, and intestinal injury.20 More recently, MNV has been reported to play a protective role in Pseudomonas aeruginosa acute pneumonia.41 Collectively, these studies support the notion that, under certain conditions, MNV may be protective.
Taken together these data suggest that how viral infections such as MNV alter disease phenotype should be considered, given that enzootic viruses may be intercurrent variables. Although MNV did not cause changes to the atherosclerosis induced by Cpn infection in ApoE−/− mice, we show that coinfection might alter inflammatory markers in vitro and blood monocyte populations in vivo. Furthermore, in light of the high prevalence of MNV, investigators should consider the MNV infection status of mice intended for their studies.
Acknowledgments
We thank Jerry Ricks, Mark Berry, Gary Knowles, Nalini Agrawal, Amy Lee, and Tomasz Wietecha for their technical assistance. This work was possible due to support from NIH grant R21-OD011135 and the University of Washington's Nutrition Obesity Research Center P30-DK035816.
References
- 1.Blessing E, Campbell LA, Rosenfeld ME, Chough N, Kuo CC. 2001. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis 158:13–17. [DOI] [PubMed] [Google Scholar]
- 2.Burnett MS, Gaydos CA, Madico GE, Glad SM, Paigen B, Quinn TC, Epstein SE. 2001. Atherosclerosis in apoE knockout mice infected with multiple pathogens. J Infect Dis 183:226–231. [DOI] [PubMed] [Google Scholar]
- 3.Burnett MS, Durrani S, Stabile E, Saji M, Lee CW, Kinnaird TD, Epstein SE. 2004. Murine cytomegalovirus infection increases aortic expression of proatherosclerotic genes. Circulation 109:893–897. [DOI] [PubMed] [Google Scholar]
- 4.Cadwell K. 2014. Expanding the role of the virome—commensalism in the gut. J Virol 89:1951–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell LA, Moazed TC, Kuo CC, Grayston JT. 1998. Preclinical models for Chlamydia pneumoniae and cardiovascular disease: hypercholesterolemic mice. Clin Microbiol Infect 4:S23–S32. [PubMed] [Google Scholar]
- 6.Campbell LA, Kuo CC. 2004. Chlamydia pneumonia—an infectious risk factor for atherosclerosis? Nat Rev Microbiol 2:23–32. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro BB, Blessing E, Kuo CC, Rosenfeld ME, Puolakkainen M, Campbell LA. 2003. Nitric oxide synthase plays a role in Chlamydia pneumoniae-induced atherosclerosis. Cardiovasc Res 60:170–174. [DOI] [PubMed] [Google Scholar]
- 8.Duerkop BA, Hooper LV. 2013. Resident viruses and their interactions with the immune system. Nat Immunol 14:654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gracey E, Lin A, Akram A, Chiu B, Inman RD. 2013. Intracellular survival and persistence of Chlamydia muridarum is determined by macrophage polarization. PLoS One 8:e69421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gui T, Shimokado A, Sun Y, Akasaka T, Muragaki Y. 2012. Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery. Mediators Inflamm 2012:693083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson GK. 2005. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 12.Hayes EM, Tsaousi A, Gregoli KD, Jenkinson SR, Bond AR, Johnson JL, Bevan L, Thomas AC, Newby AC. 2014. Classical and alternative activation and metalloproteinase expression occurs in foam cell macrophages in male and female ApoE null mice in the absence of T and B lymphocytes. Front Immunol 5:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson KS. 2008. Murine norovirus, a recently discovered and highly prevalent viral agent of mice. Lab Anim (NY) 37:314–320. [DOI] [PubMed] [Google Scholar]
- 14.Henkel JS, Beers DR, Siklós L, Appel SH. 2006. The chemokine MCP1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci 31:427–437. [DOI] [PubMed] [Google Scholar]
- 15.Herrera VL, Shen L, Lopez LV, Didishvili T, Zhang YX, Ruiz-Opazo N. 2003. Chlamydia pneumoniae accelerates coronary artery disease progression in transgenic hyperlipidemia–genetic hypertension rat model. Mol Med 9:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsich E, Zhou YF, Paigen B, Johnson TM, Burnett MS, Epstein SE. 2001. Cytomegalovirus infection increases development of atherosclerosis in Apolipoprotein-E knockout mice. Atherosclerosis 156:23–28. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CC, Paik J, Brabb TL, O'Brien KD, Kim J, Sullivan BG, Hudkins KL, Seamons A, Finley JC, Meeker SM, Maggio-Price L. 2015. Murine norovirus infection variably alters atherosclerosis in apolipoprotein-E deficient mice. Comp Med.65:369–381. [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu CC, Paik J, Treuting PM, Seamons A, Meeker SM, Brabb TL, Maggio-Price L. 2014. Infection with murine norovirus 4 does not alter Helicobacter-induced inflammatory bowel disease in Il10−/− mice. Comp Med 64:256–263. [PMC free article] [PubMed] [Google Scholar]
- 19.Jupelli M, Shimada K, Chiba N, Slepenkin A, Alsabeh R, Jones HD, Peterson E, Chen S, Arditi M, Crother TR. 2013. Chlamydia pneumoniae infection in mice induces chronic lung inflammation, iBALT formation, and fibrosis. PLoS One 8:e77447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernbauer E, Ding Y, Cadwell K. 2014. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. 2010. Macrophage plasticity in experimental atherosclerosis. PLoS One 5:e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan S, Rahman HNA, Okamoto T, Matsunaga T, Fujiwara Y, Sawa T, Yoshitake J, Ono K, Ahmed KA, Rahaman MM, Oyama K, Takeya M, Ida T, Kawamura Y, Fujii S, Akaike T. 2014. Promotion of atherosclerosis by Helicobacter cinaedi infection that involves macrophage-driven proinflammatory responses. Sci Rep 4:4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo CC, Shor A, Campbell LA, Fukushi H, Patton DL, Grayston JT. 1993. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis 167:841–849. [DOI] [PubMed] [Google Scholar]
- 24.Leitinger N, Schulman IG. 2013. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol 33:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lencioni KC, Seamons A, Treuting PM, Maggio-Price L, Brabb T. 2008. Murine norovirus: an intercurrent variable in a mouse model of bacteria-induced inflammatory bowel disease. Comp Med 58:522–533. [PMC free article] [PubMed] [Google Scholar]
- 26.Lencioni KC, Drivdahl R, Seamons A, Treuting PM, Brabb T, Maggio-Price L. 2011. Lack of effect of murine norovirus infection on a mouse model of bacteria-induced colon cancer. Comp Med 61:219–226. [PMC free article] [PubMed] [Google Scholar]
- 27.Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madjid M, Awan I, Ali M, Frazier L, Casscells W. 2005. Influenza and atherosclerosis—vaccination for cardiovascular disease prevention. Expert Opin Biol Ther 5:91–96. [DOI] [PubMed] [Google Scholar]
- 29.Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM, Maggio-Price L. 2014. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res 74:4398–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo CC. 1999. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis 180:238–241. [DOI] [PubMed] [Google Scholar]
- 31.Nieto FJ. 1999. Viruses and atherosclerosis—a critical review of the epidemiologic evidence. Am Heart J 138:S453–S460. [DOI] [PubMed] [Google Scholar]
- 32.Paik J, Kwok F, Seamons A, Brabb T, Kim J, Sullivan B, Hsu C, O'Brien KD, Maggio-Price L. 2015. Effects of murine norovirus on atherosclerosis in Ldlr–/– mice depends on the timing of infection. Comp Med 65:114–122. [PMC free article] [PubMed] [Google Scholar]
- 33.Paik J, Fierce Y, Drivdahl R, Treuting PM, Seamons A, Brabb T, Maggio-Price L. 2010. Effects of murine norovirus infection on a mouse model of diet-induced obesity and insulin resistance. Comp Med 60:189–195. [PMC free article] [PubMed] [Google Scholar]
- 34.Paik J, Fierce Y, Mai PO, Phelps SR, McDonald T, Treuting P, Drivdahl R, Brabb T, LeBoeuf R, O'Brien KD, Maggio-Price L. 2011. Murine norovirus increases atherosclerotic lesion size and macrophages in Ldlr−/− mice. Comp Med 61:330–338. [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld ME, Campbell LA. 2011. Pathogens and atherosclerosis:update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost 106:858–867. [DOI] [PubMed] [Google Scholar]
- 37.Sessa R, Pietro MD, Filardo S, Turriziani O. 2014. Infectious burden and atherosclerosis: a clinical issue. World J Clin Cases 2:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swirski FK, Weissleder R, Pittet MJ. 2009. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol 29:1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. 2007. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. 2006. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci USA 103:10340–10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thepaut M, Grandjean T, Hober D, Lobert PE, Bortolotti P, Faure K, Dessein R, Kipnis E, Guery B. 2015. Protective role of murine norovirus against Pseudomonas aeruginosa acute pneumonia. Vet Res 46:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward JM, Wobus CE, Thackray LB, Erexson CR, Faucette LJ, Belliot G, Barron EL, Sosnovtsev SV, Green KY. 2006. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol Pathol 34:708–715. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization.[Internet]. 2014. Global status report on noncommunicable diseases 2014. [Cited 09 Feb 2015]. Available at: https://cspinet.org/new/pdf/who-global-status-report-on-ncds-2014__1_.pdf
- 44.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. 2000. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol 85:140–146. [DOI] [PubMed] [Google Scholar]