Abstract
The aim of this study was to evaluate the effect of oral enrofloxacin on the epileptic status of Genetic Absence Epilepsy Rats from Strasbourg (GAERS). Five adult female GAERS rats, with implanted extradural electrodes for EEG monitoring, were declared free of clonic seizures after an 8-wk observation period. Enrofloxacin was then added to their drinking water (42.5 mg in 750 mL), and rats were observed for another 3 days. The number of spike-and-wave discharges and mean duration of a single discharge did not differ before and after treatment, but 2 of the 5 rats developed clonic seizures after treatment. Enrofloxacin should be used with caution in GAERS rats because it might induce clonic seizures.
Abbreviations: GABA, γ-aminobutyric acid; GAERS, genetic absence epilepsy in rat from Strasbourg; SWD, spike-and-wave discharge
The potential adverse effects of antibiotics on the nervous system include antibiotic-induced seizures that have not been investigated intensively. With a few exceptions, the literature includes only case reports of antibiotic-induced seizures in predisposed patients.2,5,7-10,15 Fluoroquinolones are one of the antibiotic classes most commonly associated with seizures and are thought to provoke seizures through inhibitory effects on γ-aminobutyric acid (GABA) transmission.5 Enrofloxacin is a fluoroquinolone antimicrobial drug that is rapidly bactericidal against a wide variety of clinically important bacterial organisms. Enrofloxacin is potent, well tolerated by animals, and can be administered through a variety of routes (orally by tablet and drinking water, subcutaneously, and intramuscularly).1
The GAERS (Genetic Absence Epilepsy in Rat from Strasbourg) strain is a genetic Wistar rat model of absence epilepsy that displays electroclinical, behavioral, and pharmacologic features of absence seizures and has become a ‘gold standard’ to study the mechanisms underlying absence epilepsy.6,12 The EEG of GAERS presents typical spike-and-wave discharges (SWD) of 0.3 to 1 mV in amplitude and 7 to 11 SWD per second in spike rate.6,12 Typical absence seizures originate from abnormal thalamocortical networks with cellular elements that include pyramidal cells and interneurons of different cortical layers, the thalamocortical neurons of sensory thalamic nuclei and their main inhibitory input (GABAergic neurons of the nucleus reticularis thalami).13
Consequently and for the purpose of a pilot study, we hypothesized that GAERS could be predisposed to fluoroquinolone-induced seizures and that oral administration of enrofloxacin would increase their seizure activity. During that pilot study, some rats developed clonic seizures. To our knowledge, no description of antibiotic-induced clonic seizure in GAERS has been published, the aim of the present case study was to document clonic seizures induced by oral administration of enrofloxacin in GAERS.
Case Report
Materials and Methods
The pilot study was designed as a prospective, blinded experiment. The experiments were approved by St Vincent's Hospital (Melbourne) Animal Ethics Committee and conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (2013).3
Female GAERS (Rattus norvegicus; age, 6 mo) with a clean colony pathogen status (tested for 17 different virus, 18 bacterias and fungi, and 13 ectoparasites and endoparasites) were obtained from the Royal Melbourne Hospital's Biologic Research Facilities, The University of Melbourne (Parkville, Victoria, Australia), to take part in an 8-wk experiment investigating novel techniques to deliver antiepileptic drugs. The first 5 rats randomly allocated to the control group were also used in the present pilot study.
The GAERS were housed individually in high-top rat cages (RB2, Wiretainers, Melbourne, Victoria, Australia) with recycled paper bedding (Fibrecycle, Yatala, Queensland, Australia). The temperature and humidity of the room were kept at 22 °C and 55%, respectively. The rats were maintained under a 12:12-h light:dark cycle with free access to food (Barastoc GR2 pellets, Ridley, Melbourne, Victoria, Australia) and tap water.
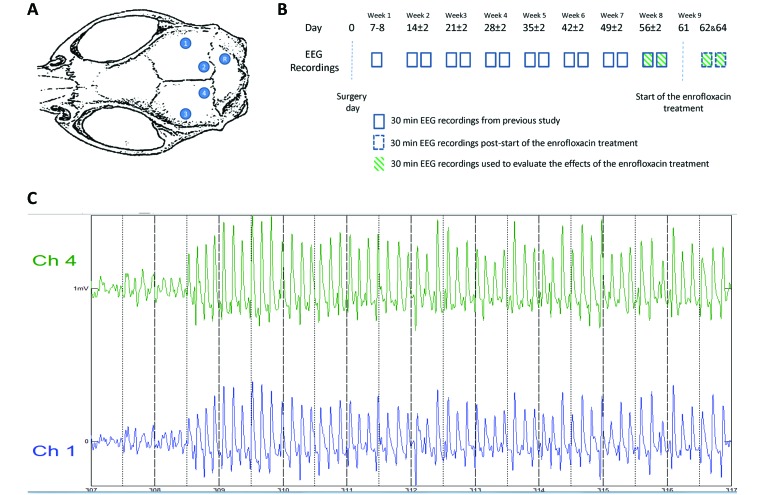
All 5 rats underwent surgery for the implantation of EEG recording electrodes (Figure 1 A). Immediately before surgery, the rats were weighed and anesthetized with an intraperitoneal injection of ketamine (75 mg/kg; ketamine hydrochloride 100 mg/mL, Ceva Animal Health, Glenorie, New South Wales, Australia) and xylazine (10 mg/kg; xylazine hydrochloride 20 mg/mL, Troy Laboratories, Glendenning, New South Wales, Australia). After the induction of anesthesia, each rat was placed in a stereotaxic apparatus (Kopf, Stoelting, Wood Dale, IL) and given isoflurane (0.5% to 1% in oxygen, 1 L/min; Isoflo, Abbott Australasia, Botany, New South Wales, Australia) by nose-cone, subcutaneous carprofen (5 mg/kg; 50 mg/mL, Parnell Australia, Alexandria, New South Wales, Australia) for pain relief, and 0.9% sodium chloride (2 mL; Baxter, Old Toongabble, New South Wales, Australia) for cardiovascular support. The EEG recording electrodes were implanted as follows: after aseptic preparation of the scalp, a single incision was made down the midline, the skull cleared of tissue, and the exposed bone dried with 3% hydrogen peroxide (Sanofi, Victoria, Queensland, Australia). Four extradural electrodes, consisting of small jewelers’ screws (diameter, 3.2 mm; Plastics One, Roanoke VA), were implanted cranial to the interaural line (2 on each side of the sagittal suture), and one screw was implanted caudal to the interaural line to the right of the sagittal suture. The electrodes were connected to an adaptor secured with dental repair material (Cold-curing acrylic, Vertex, Zeist, The Netherlands). The skin was sutured (3-0 Monosyn, B Braun, Rubi, Spain), leaving only part of the dental cement exposed, and each rat was placed on a heating pad for recovery from anesthesia. Postoperative treatment included subcutaneous buprenorphine (0.03 mg/kg BID; Temgesic, 0.324 mg/mL buprenorphine hydrochloride, Reckitt Benckiser Healthcare, Hull, United Kingdom), 0.9% sodium chloride (2 mL daily), and carprofen (5 mg/kg, daily) as needed for a maximum of 3 d.
Figure 1.
(A) Schematic diagram illustrating the positioning of the epidural recording screw electrodes (1 through 4) and reference electrode (R). (B) Experimental design. EEG recording (30-min session twice weekly) began on day 7 or 8 after surgery and continued for the following 7 wk. On day 61, the drinking water was replaced with enrofloxacin-containing water (1.7 mL of enrofloxacin 25 mg/mL in 750 mL of drinking water), and additional EEG recordings were performed on days 62 and 64. The enrofloxacin treatment was stopped on day 65. (C) The clonic seizure of rat 5 started on day 64 after 308 s of EEG recording and lasted for 40 s. The intensity of the clonic seizure was classified as stage 4 (clonic seizure in a seating position).11 The EEG recordings presented SWD that were similar in amplitude (0.3 to 0.7 mV) and frequency (average, 7 SWD per s) to that observed during absence seizure.
Beginning at day 7 or 8 after surgery, rats were monitored for at least 60 min (30 min for recovery from anesthesia and acclimation, 30 min for EEG recording) daily and on at least 2 d each week for the following 7 wk. At day 61, regular drinking water was replaced with water containing enrofloxacin (42.5 mg in 750 mL of drinking water; Baytril, 25 mg/mL, Bayer Australia, Pymble, New South Wales, Australia), and additional EEG recordings were obtained on days 62 and 64 (Figure 1 A). Each EEG recording session began 4 to 6 h after the start of the light cycle. Treatment with enrofloxacin stopped on day 65. The rats were weighed (sensitivity, ± 1 g; model KD-200, Tanika, Kowloon, Hong Kong) on days 61, 62, and 64.
For each monitoring session, rats were anesthetized in an induction cage by using isoflurane in oxygen for 3 min to connect the recording electrodes through shielded cables to the EEG acquisition system,4 which consisted of TDT processors (Tucker-Davis Technologies, Alachua FL) and high-impedance head stages driven by TDT's programmable software. The EEG was sampled at 3051.76 Hz, and recording began only after the rats had returned to normal behavior (moving around without ataxia, eating, cleaning itself) and presented absence seizures. During the recording sessions, the rat's behavior was observed, and the EEG was visualized by using a custom-designed MATLAB program.4 When clonic seizures occurred, their intensity was classified according to the modified Racine scale.11 At the end of a recording session, the rats were briefly anesthetized with isoflurane (4% in oxygen, 2 L/min) to be disconnected from the shielded cable.
To evaluate the effects of the enrofloxacin treatment on the GAERS SWD, the median number of SWD, duration of a single SWD, and cumulative duration of SWD during 60 min were compared between weeks 8 and 9 (Figure 1 B) by using the Wilcoxon signed ranks test (IBM SPSS Statistics, version 20, IBM Corp, Armonk, NY USA). The significance level was set at 5%. The researcher performing EEG analysis was blinded regarding treatment and used a GAERS-specific automated SWD detection algorithm.4
Results
All 5 rats were declared free of clonic seizures during the first 8 wk of EEG recordings. On day 61, the median weight of the rats was 189 g (range, 185 to 204 g). The median weight gains were 0 g (range, –1 to 2 g) and –0.2 g (range, –1 to 1 g) on days 62 and 64, respectively, when compared with day 61. Although the duration of SWD showed an increasing trend (p = 0.34), the number of SWD, duration of a single SWD, and cumulative duration of SWD during 60 min did not differ significantly (Figure 2). However, 2 rats each manifested a single episode of clonic seizures. The first observed episode occurred on day 62 (24 h after the start of treatment) and lasted 31 s, whereas the second episode occurred on day 64 (72 h after the start of treatment) and lasted 40 s. During both episodes, the affected rats had convulsions interpreted as clonic seizures while they were in a sitting position. The first signs were trembling of the whiskers, shortly followed by spasms and jerking of the neck muscles and some flexion of the legs. Both seizures were classified as intensity stage 4 seizures.
Figure 2.
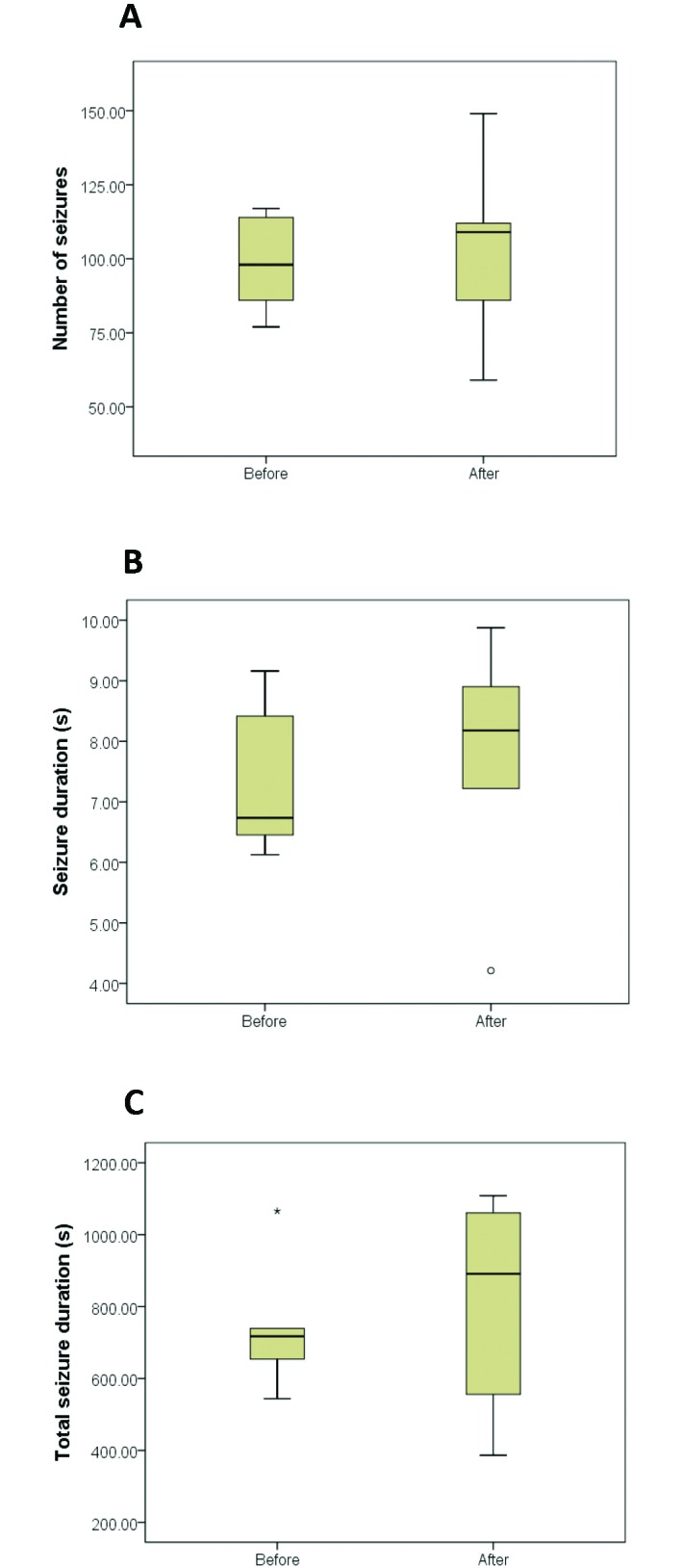
Box-and-whisker plots of the epileptic activity before enrofloxacin treatment (n = 5) and afterward (posttreatment, n = 5). (A) Number of SWD. (B) Median duration of a single SWD. (C) Total duration of SWD during 60 min of recording time each week. °, ‘yellow card zone’ outlier (that is, distance between the outlier and the interquartile range box is greater than 1.5 times the interquartile range box length); *, ‘red card zone’ outlier (distance between the outlier and the interquartile range box is greater than 3 times the interquartile range box length).
The EEG recordings obtained during the clonic seizures presented SWD of similar amplitude (0.3 to 0.7 mV) and frequency (7 SWD per s) to those observed during absence seizures (Figure 1 C).
Discussion
The GAERS strain is a genetic rat model of absence epilepsy of which all rats present recurrent generalized nonconvulsive seizures accompanied with behavioral arrest, staring, and sometimes twitching of the vibrissae.12 Although the small number of rats in the present study precluded definitive determination of the effect of enrofloxacin on SWD activity, the administration of the antibiotic coincided with the appearance of clonic seizures, which are abnormal in this model of epilepsy.12
To our knowledge, the neurologic circuits and biochemical substrates of absence seizures and clonic seizures are similar. However, why some similar patterns of abnormal brain activity result in absence compared with clonic compared with tonic–clonic seizures is unknown. The incidence of clonic seizures (n = 2) remained uncommon when compared with the number of nonconvulsive seizures recorded (n > 500) during the same recording period, but 2 of the 5 rats were affected, representing 40% of the sample population.
In addition, considering that alteration of the blood–brain barrier is a risk factor for antibiotic-induced seizures (by allowing for increased concentration of the antibiotic in the brain) and that certain quinolones have synergistic inhibition with NSAID at the GABA receptor, the administration of enrofloxacin perioperatively when performing craniotomies might result in a higher incidence and more severe clonic seizures than those observed in the present study.5,10
The 5 GAERS rats used in this pilot experiment were control animals in a separate study investigating novel techniques to deliver antiepileptic drugs. Because the recording period for the antiepileptic drug experiment was 8 wk, enrofloxacin couldn't be administered earlier. In addition, GAERS epileptic activity is age-dependent; therefore, the SWD activity that occurred during week 9 (after the start of enrofloxacin treatment) was compared only with week 8 (pretreatment) data.12 Specifically, the number and duration of SWD increase with age, with the maximal number of SWD at the age of 6 mo.12
One limitation of the current study is that the water consumption was not measured. However, rats were weighed daily, and all rats maintained appropriate weight during the antibiotic treatment, implying a normal hydration status.
When combined with a nonseizure-modulating dose of theophylline, ciprofloxacin and norfloxacin increased seizure activity in amygdaloid-kindled rats.16 However, when given as a sole agent in that model, enrofloxacin decreased seizure activity.16 In another experiment, ciprofloxacin (≥ 50 mg/L) showed moderate to marked epileptogenic effects when tested on slices of rat hippocampus, which have a low threshold for the development of epileptiform activity.7
The GABAA receptor plays a major role in the epileptogenic activity of fluoroquinolones, and (in vitro) the N-methyl-D-aspartate receptor is directly involved in this process as a target for the excitatory effect of fluoroquinolones.5,10,14 The substituent at the C7 position of fluoroquinolones appears to be an important predictor of GABA binding and seems to be, at least partially, responsible for the differences in seizure incidence among fluoroquinolones.10 To our knowledge, the C7 substituent of enrofloxacin has not been studied for its epileptogenic activity.
The EEG of GAERS presents typical SWD that originate from abnormal thalamocortical networks, including its GABAergic neurons.6,13 In contrast, kindling is a local process whereby an electrical stimulus typically delivered to a limbic brain structure evokes an electrographic after-discharge capable of spreading and initiating a generalized convulsive seizure.16 The kindled rat model of epilepsy doesn't involve the GABAergic neurons in the initiation of the seizure, and this might be the reason for the different effects of enrofloxacin between the GAERS and kindled rat models. In addition, amygdala-kindled events are focal as compared with the primarily generalized nature of the seizures in GAERS rats; consequently these processes have different mechanisms from the pharmacologic perspective.
Isoflurane was used briefly when connecting and disconnect the recording apparatus. In previous experiments, we found that some rats showed stress behavior (for example, vocalization) during handling for connection and disconnection; consequently we opted for brief general anesthesia or sedation to increase animal wellbeing. Rats recovered quickly from the anesthetic episodes, and it is unlikely that, after the 30-min acclimation period, isoflurane would still be present to interfere with epileptic activity.
Finally, no postmortem brain histologic analyses were performed on the rats and, although improbable, it cannot be excluded that the appearance of the clonic seizures coincided with the development of an intracranial pathology unrelated to administration of enrofloxacin. Similarly, it cannot be excluded that the isoflurane anesthesia affected the appearance of SWD; however the 30-min recovery period prior to recording likely reduced isoflurane effects to being negligible.
Within the described experimental conditions, enrofloxacin did not appear to change the duration or frequency of SWD in GAERS rats. However, this antibiotic should be administered with caution because it might induce clonic seizures in GAERS, especially during the perioperative period, when aggravating factors such as disruption of the blood–brain barrier and coadministration of NSAID may be present.
Acknowledgments
We gratefully acknowledge the sponsorship of the Victorian Government through its Science Technology and Innovation Initiative, administered by the Department of Industry, Innovation, and Regional Development, and of NHMRC (grant no. 619614). We thank the Australian Research Council (ARC) for continuing financial support (ARC grant no. DP0987344).
References
- 1.Adams HR. 2001. Veterinary pharmacology and therapeutics, 8th ed. Ames (IA): Iowa State University Press. [Google Scholar]
- 2.Arcieri GM, Becker N, Esposito B, Griffith E, Heyd A, Neumann C, O'Brien B, Schacht P. 1989. Safety of intravenous ciprofloxacin. A review. Am J Med 87 Suppl 1:S92–S97. [DOI] [PubMed] [Google Scholar]
- 3.Australian Government National Health and Medical Research Council. Australian code of practice for the care and use of animals for scientific purposes 8th ed. 2013. Available at : https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ea28_code_care_use_animals_131209.pdf.
- 4.Bauquier SH, Lai A, Jiang JL, Sui Y, Cook MJ. 2015. Evaluation of an automated spike-and-wave complex detection algorithm in the EEG from a rat model of absence epilepsy. Neurosci Bull 31:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharyya S, Darby R, Berkowitz AL. 2014. Antibiotic-induced neurotoxicity. Curr Infect Dis Rep 16:448. [DOI] [PubMed] [Google Scholar]
- 6.Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. 1998. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol 55:27–57. [DOI] [PubMed] [Google Scholar]
- 7.Grondahl TO, Langmoen IA. 1993. Epileptogenic effect of antibiotic drugs. J Neurosurg 78:938–943. [DOI] [PubMed] [Google Scholar]
- 8.Kim A, Kim JE, Paek YM, Hong KS, Cho YJ, Cho JY, Park HK, Koo HK, Song P. 2013. Cefepime-induced nonconvulsive status epilepticus (NCSE). J Epilepsy Res 3:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerner PI, Smith H, Weinstein L. 1967. Penicillin neurotoxicity. Ann N Y Acad Sci 145:310–318. [DOI] [PubMed] [Google Scholar]
- 10.Lode H. 1999. Potential interactions of the extended-spectrum fluoroquinolones with the CNS. Drug Saf 21:123–135. [DOI] [PubMed] [Google Scholar]
- 11.Luttjohann A, Fabene PF, van Luijtelaar G. 2009. A revised Racine's scale for PTZ-induced seizures in rats. Physiol Behav 98:579–586. [DOI] [PubMed] [Google Scholar]
- 12.Marescaux C, Vergnes M, Depaulis A. 1992. Genetic absence epilepsy in rats from Strasbourg—a review. J Neural Transm Suppl 35:37–69. [DOI] [PubMed] [Google Scholar]
- 13.Noebels J, Avoli M, Rogawski M, Olsen R, Delgado-Escueta A. 2012. Jasper's basic mechanisms of the epilepsies, 4th ed. New York (NY): Oxford University Press. [Google Scholar]
- 14.Schmuck G, Schurmann A, Schluter G. 1998. Determination of the excitatory potencies of fluoroquinolones in the central nervous system by an in vitro model. Antimicrob Agents Chemother 42:1831–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsubouchi K, Ikematsu Y, Hashisako M, Harada E, Miyagi H, Fujisawa N. 2014. Convulsive seizures with a therapeutic dose of isoniazid. Intern Med 53:239–242. [DOI] [PubMed] [Google Scholar]
- 16.Vancutsem PM, Schwark WS. 1992. Effects of fluoroquinolone antimicrobials alone and in conjunction with theophylline on seizures in amygdaloid-kindled rats. Mechanistic and pharmacokinetic study. Epilepsy Res 13:59–71. [DOI] [PubMed] [Google Scholar]